Abstract
Background:
Tinea pseudoimbricata, characterized by concentric scaly rings simulating Tinea imbricata is caused by dermatophytes other than Trichophyton concentricum. It is reported to occur in patients with steroid abuse and in immunocompromised individuals.
Aim:
To study the clinico-mycological profile and dermoscopic features of T. pseudoimbricata in immunocompetent patients.
Methods:
We have evaluated 14 consecutive, clinically diagnosed patients of T. pseudoimbricata with positive 10% potassium hydroxide (KOH) examination and culture, seen over a period of 6 months. Dermoscopy was performed in all patients. The demographic, clinical, and mycological features of each patient were recorded on a predesigned proforma.
Results:
There were seven male and seven female patients with a mean age of 27.6 years and a mean disease duration of 3.8 months. All patients gave a history of application of potent or super-potent topical steroid with or without oral/injectable steroid for varying duration. Culture isolates were Trichophyton mentagrophytes complex and Trichophyton rubrum in 11 and 3 patients, respectively. Dermoscopic analysis showed features of steroid abuse in majority of the patients.
Limitation:
A small sample size was the limitation of our study.
Conclusion:
T. pseudoimbricata is a special subset of Tinea incognito caused by injudicious and inappropriate use of topical steroid. The typical appearance should alert the dermatologists regarding the possible abuse of steroids. Most common species isolated on culture was T. mentagrophytes complex.
Keywords: Dermoscopy, steroid abuse, Tinea indecisiva, Tinea pseudoimbricata
Introduction
Dermatophytosis in the past few years has been increasing at a frightening rate and now accounts for approximately 50% of the outpatient attendance.[1] Patients present with extensive, recurrent, and recalcitrant disease often with atypical lesion morphology of superficial dermatophytosis. Widespread availability of over-the-counter potent and super-potent topical steroids (alone or in combination with antifungals and antibiotics) and self-medication have led to the evolution of an atypical presentation of dermatophytosis; Tinea incognito. Latter include a special lesion morphology termed as “Tinea pseudoimbricata” or “Tinea indecisiva.” T. pseudoimbricata has morphological similarity to T. imbricata which is characterized by multiple concentric rings, but is caused by dermatophytes other than Trichophyton concentricum.[2,3] It is often seen in immunocompromised individuals or in patients with a history of steroid abuse.[2] This condition has been postulated to occur as a result of local immunosuppression induced by topical steroids.[2,3] The objective of the present case series is to study the clinico-mycological, dermoscopic, and epidemiological characteristics of “now not so rare” form of this dermatophytosis.
Methods
This was a cross-sectional study conducted over a period of 6 months. Eighteen (18) patients with the clinical diagnosis of T. pseudoimbricata were examined and underwent potassium hydroxide (KOH) examination of skin scraping and culture. Exclusion criteria included patients with immune-suppression and T. concentricum isolate on mycological culture. A detailed history pertaining to the use of topical formulation (name and contents were ascertained either on seeing the tubes used or from the prescription), duration and frequency of application, oral and/or injectable drugs taken, and information about the source of prescription was noted. A thorough clinical examination and hematological investigations were carried out to look for any associated co-morbidity in all the subjects and findings were recorded on a predesigned proforma. Skin scraping from the lesions was used for direct microscopic examination in 10% KOH and for culture on the Saboraud's Dextrose Agar (SDA) with cycloheximide and chloramphenicol. Dermoscopy was done using the Universal Serial Bus (USB) dermatoscope (Dinolite AM413ZT).
Results
Fourteen of the 18 patients had both KOH and culture positivity and had been included for analysis. The mean age of the 14 patients was 27.6 ± 13.8 years (range: 5–55 years). There were seven males and seven females. The mean disease duration was 3.8 ± 1.7 months (range: 2–7 months). Most of the patients (71.4%) belonged to the upper lower and lower socioeconomic status, according to the modified Kuppuswamy's socioeconomic status scale.[4] All patients gave history of using potent and super-potent topical steroids; clobetasol proprionate (0.05%) was used by 11/14 patients (78.57%), and betamethasone diproprionate and mometasone furoate by remaining 3 patients (21.43%). Five patients (35.71%) took oral steroids, while 2 (14.28%) had a history of taking injectable steroids (injection dexamethasone and injection triamcinolone acetonide) as well. None of the patients had applied topical antifungal alone or taken oral antifungal medication. The topical steroid had been used in combination with antifungals like miconazole, clotrimazole, and terbinafine, antibiotics like ornidazole, ofloxacin in varying combinations. About 7/14 (50%) patients had done self-medication on suggestion from friends and family while steroid combination was prescribed by chemist in 4 (28.6%) and by unqualified registered medical practitioners in 3 (21.4%). None of the patients had visited a dermatologist for the treatment of their skin disease. Family history of dermatophytosis was present in five patients (35.71%) and all these patients shared towels and other clothes suggesting transmission by fomites.
All patients presented with multiple (>2) concentric erythematous rings that was associated with moderate-to-severe pruritus [Figure 1a-d]. Scaling was seen in 13 patients (92.8%). All patients except two had involvement of more than one body site. The most common clinical presentation was Tinea corporis (83.33%) followed by Tinea cruris (41.67%) and Tinea faciei (8.3%). Two patients (14.28%) had concomitant toe nail onychomycosis, while one patient had concomitant pityriasis versicolor (PV). KOH examination showed hyaline, long, branching septate hyphae in all the patients. Fungal culture on the SDA medium grew T. mentagrophytes complex in 11 patients (78.57%) and T. rubrum in 3 (21.83%) [Figure 2a and b]. The demographic, clinical, and mycological details are summarized in Table 1. Patients with T. mentagrophytes had a more widespread and multiple site infection as compared to T. rubrum. The inflammation and chronicity of the lesions were again greater in T. mentagrophytes.
Figure 1.
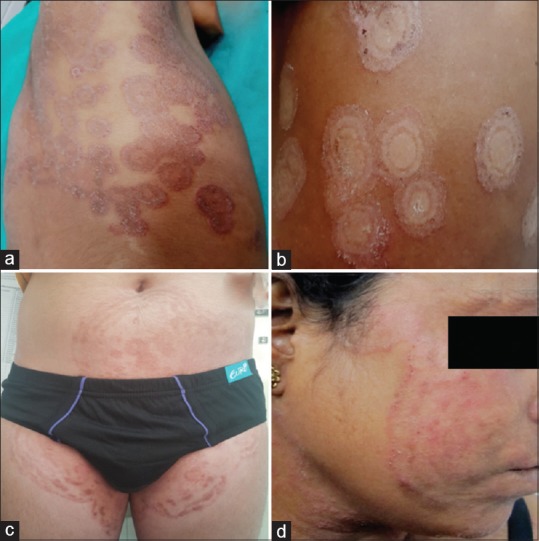
Various presentations of Tinea pseudoimbricata: (a) multiple concentric rings with scaling and pustular lesions in right axilla and chest; (b) classic “ring within ring” appearance of T. pseudoimbricata in a young girl; (c) extensive lesions on trunk and groin following topical and systemic steroid abuse; (d) T. pseudoimbricata on face after potent steroid application for over 3 months
Figure 2.
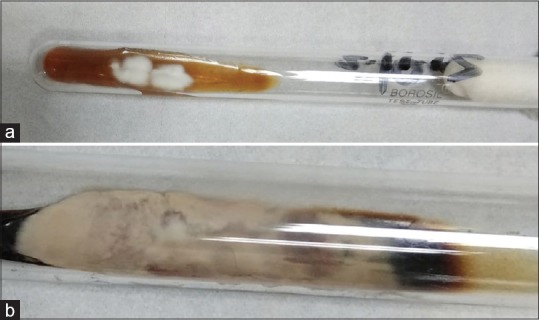
(a) White to cream colored colonies of Trichophyton mentrophytes complex; (b) downy to cottony creamy yellow to brown colored growth of T richophyton rubrum
Table 1.
Demograohic and mycological profile of the patients
| Case no. | Age (years)/sex | Disease duration (months) | Steroid abuse: topical (T), oral (O), injectable (I) | KOH examination | Fungal culture | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 55 F | 3 | T+O + I | Septate hyphae | T. mentagrophytes | T. corporis |
| 2 | 7 F | 2.5 | T | Septate hyphae | T. mentagrophytes | T. corporis |
| 3 | 5 F | 2.5 | T | Septate hyphae | T. mentagrophytes | T. corporis |
| 4 | 37 F | 5 | T+O + I | Septate hyphae | T. mentagrophytes | T. corporis |
| 5 | 27 M | 2 | T | Septate hyphae | T. mentagrophytes | T. corporis |
| 6 | 35 M | 2 | T | Septate hyphae | T. rubrum | T. corporis T. cruris, onychomycosis |
| 7 | 16 M | 3 | T | Septate hyphae | T. mentagrophytes | T. corporis |
| 8 | 40 M | 6 | T+O | Septate hyphae | T. rubrum | T. corporis T. cruris |
| 9 | 19 M | 4 | T | Septate hyphae | T. mentagrophytes | T. faciei |
| 10 | 25 F | 5 | T+O | Septate hyphae | T. mentagrophytes | T. corporis T. cruris |
| 11 | 20 F | 3 | T | Septate hyphae | T. mentagrophytes | T. corporis T. cruris |
| 12 | 42 F | 2 | T+O | Septate hyphae | T. mentagrophytes | T. cruris |
| 13 | 26 M | 7 | T | Septate hyphae | T. mentagrophytes | T. faciei |
| 14 | 32 M | 6 | T | Septate hyphae | T. rubrum | T. cruris with OM |
Dermoscopy was done for all the patients and the most recent concentric lesion was chosen to document the dermoscopic features. Dermoscopy revealed background erythema (100%); with dilated linear, tortous, and/or dotted vessels (75%); peripheral whitish scales (100%); micropustules at the borders (83.33%); tinea of vellous hairs (16.67%); reddish brown hemorrhagic spots at the periphery (66.67%) [Figure 3a-c].
Figure 3.
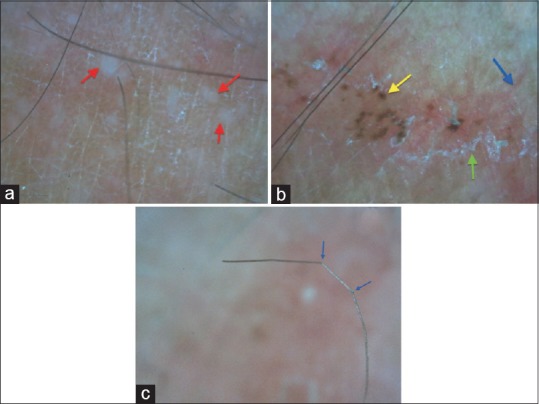
(a) Micropustules (red arrow) seen over a background of erythema and scaling; (b) dermoscopy of a ring of T. pseudoimbricata showing scaling (green arrow), linear vessel (blue arrow), and brown hemorrhagic spot (yellow arrow); (c) vellus hair in the lesion appear bent and broken due to fungal invasion of the hair shaft [Dinolite AM413ZT; 200X; Polarising]
Discussion
The term “imbricata” is derived from the Latin word imbrex, and refers to overlapping roof tiles.[5] T. imbricata is a distinct superficial mycosis caused by T. concentricum with a characteristic pattern of concentric and/or annular plaques of erythema and scales.[2,6] The disease has a restricted geographical distribution in South-East Asia, South Pacific, Central, and South America.[6] Cases clinically simulating T. imbricata but caused by species other than T. concentricum have been labeled as “T. pseudoimbricata” or “T. indecisiva.[3,5] T. pseudoimbricata has been reported to be caused by Trichophyton tonsurans, T. rubrum, T. mentagrophytes, Microsporum audouinii, and Microsporum gypseum. All our patients presented with multiple concentric or annular erythematous scaly plaques. Fungal culture in our patients revealed T. mentagrophytes to be the predominant species followed by T. rubrum. Similar observations were reported by a study from North India where T. mentagrophytes was isolated in 7 out of 9 T. pseudoimbricata patients.[7]
The exact etiopathogenesis is still not clear but such cases have been known to occur in patients with some degree of immunosuppression.[2,3,5] The clinical pattern in T. imbricata has been explained by immunologic interaction between host and pathogen. The majority of patients affected with T. imbricata are anergic to intradermal antigen of T. concentricum; the cellular immune response is impaired, with a T-lymphocyte hyporeactivity and there is a dominance of the humoral immune response.[8] Similar hypothesis has been put forward to explain the concentric rings of T. pseudoimbricata.[9,10] Fungal invasion initially activates the host immune response leading to suppression of the fungal cell growth. Topical application of potent steroid causes local immunosuppression which may diminish the host responses, specifically the cellular immune responses, against the fungus. This causes switching on of certain fungal genes and reactivation of fungal replication. Beyond a certain time and threshold of fungal replication, host responses again become active leading to inflammation. Injudicious intermittent therapy with topical steroids with or without antifungals repeats this cycle many a times leading to the appearance of concentric erythematous scaly rings.
All our patients gave a history of potent topical steroid application with/without oral or injectable steroids prior to appearance of lesions. This further substantiates the “local immunosuppression” theory. The increasing trend of self-medication and injudicious use, as seen in our study, is worsening the situation. Another alarming fact that stands out in our study is the positive family history in a significant number of patients. This fact might be responsible for the reinfection in these patients.
KOH and fungal culture have been standard and confirmatory diagnostic tools in fungal infections.[1] With the availability of dermoscopy, we further explored the diagnostic utility of a dermatosocpe in T. pseudoimbricata. Though dermoscopy has been described for dermatophytic infections,[11] we could not find any report of dermoscopy in T. pseudoimbricata. The presence of background erythema with dilated linear, tortous, and/or dotted vessels on dermoscopy may point toward the possible topical steroid abuse.[12] The polygonal arrangement of these vessels may point toward the chronicity of topical steroid abuse.[12] In addition, we suggest that the presence of micropustules at the periphery indicate disease activity while the brownish hemorrhagic spots may indicate the excoriation marks secondary to pruritus. Dermoscopy may serve as a quick and non-invasive tool for identification of cases with topical steroid abuse.
Owing to the paucity of literature on T. pseudoimbricata, there is no standard treatment protocol. According to the author's experience, systemic antifungal is mandated in such situations for a prolonged period of at least 6–8 weeks. The rationale for using longer treatment period can be derived from the fact that minimum inhibitory concentration (MIC) of azoles is higher in T. mentagrophytes than T. rubrum.[13] The prognosis is good if the patient adheres to the treatment and further steroid abuse is halted. It is of equal importance to treat the family members to decrease the chances of reinfection and to counsel regarding the separate washing of infected clothes and avoidance of sharing of towels, clothes, bed linen, and soaps.
As our study had a small sample size, the findings needs to be validated on larger sample size from different geographic area to delineate the etiological fungus of pseudoimbricata.
Conclusion
“Concentric ring” appearance in any case of dermatophytosis should alarm the possible abuse of potent steroids by the patient. The diagnosis must be confirmed by KOH and fungal culture. With the easy availability of over-the-counter high potent steroids and their inappropriate use, many more cases may surface with time. With this study, we intend to highlight the clinico-mycological features of T. pseudoimbricata; a unique subtype of T. incognito.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Panda S, Verma S. The menace of dermatophytosis in India: The evidence that we need. Indian J Dermatol Venereol Leprol. 2017;83:281–4. doi: 10.4103/ijdvl.IJDVL_224_17. [DOI] [PubMed] [Google Scholar]
- 2.Sonthalia S, Singal A, Das S. Tinea cruris and tinea corporis masquerading as tinea indecisiva: Case report and review of the literature. J Cutan Med Surg. 2014;18:1–6. [PubMed] [Google Scholar]
- 3.Batta K, Ramlogan D, Smith AG, Garrido MC, Moss C. ’Tinea indecisiva’ may mimic the concentric rings of tinea imbricata. Br J Dermatol. 2002;147:384. doi: 10.1046/j.1365-2133.2002.04767.x. [DOI] [PubMed] [Google Scholar]
- 4.Bairwa M, Rajput M, Sachdeva S. Modified Kuppuswamy's socioeconomic scale: Social researcher should include updated income criteria, 2012. Indian J Community Med. 2013;38:185–6. doi: 10.4103/0970-0218.116358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SP, Smith AG. “Tinea pseudoimbricata”: Ttinea corporis in a renaltransplant recipient mimicking the concentric rings of tinea imbricata. Clin Exp Dermatol. 2003;28:332–3. doi: 10.1046/j.1365-2230.2003.01281.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonifaz A, Vázquez-González D. tinea imbricata in the Americas. Curr Opin Infect Dis. 2011;24:106–11. doi: 10.1097/QCO.0b013e328342cbc1. [DOI] [PubMed] [Google Scholar]
- 7.Gupta V, Bhatia R, Sondhi P, Mahajan R. ’Ring-within-a-ring’ appearance: Morphological clue to topical steroid abuse in dermatophytosis. J Eur Acad Dermatol Venereol. 2017;31:e2–3. doi: 10.1111/jdv.13576. [DOI] [PubMed] [Google Scholar]
- 8.Hay RJ, Reid S, Talwat E, Macnamara K. Immune responses of patients with tineaimbricata. Br J Dermatol. 1983;108:581–6. doi: 10.1111/j.1365-2133.1983.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 9.Verma S, Hay RJ. Topical steroid-induced Tinea pseudoimbricata: A strikingform of tinea incognito. Int J Dermatol. 2015;54:e192–3. doi: 10.1111/ijd.12734. [DOI] [PubMed] [Google Scholar]
- 10.Verma SB, Zouboulis C. Indian irrational skin creams and steroid-modified dermatophytosis-an unholy nexus and alarming situation. J Eur Acad Dermatol Venereol. 2018;32:e426–7. doi: 10.1111/jdv.15025. [DOI] [PubMed] [Google Scholar]
- 11.Knöpfel N, del Pozo LJ, Escudero Mdel M, Martín-Santiago A. Dermoscopic visualization of vellus hair involvement in Tinea Corporis: A criterion for systemic antifungal therapy? Pediatr Dermatol. 2015;32:e226–7. doi: 10.1111/pde.12648. [DOI] [PubMed] [Google Scholar]
- 12.Jakhar D, Kaur I. Dermoscopy of topical steroid damaged/dependent face. Indian Dermatol Online J. 2018;9:286–7. doi: 10.4103/idoj.IDOJ_301_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatia V K, Sharma PC. Determination of minimum inhibitory concentrations of itraconazole, terbinafine and ketoconazole against dermatophyte species by broth microdilution method. Indian J Med Microbiol. 2015;33:533–7. doi: 10.4103/0255-0857.167341. [DOI] [PubMed] [Google Scholar]


