Abstract
Lumbar foraminal pathology causing entrapment of neurovascular contents and radicular symptoms are commonly associated with foraminal stenosis. Foraminal neuropathy can also be derived from inflammation of the neighboring lateral recess or extraforaminal spaces. Conservative and interventional therapies have been used for the treatment of foraminal inflammation, fibrotic adhesion, and pain. This update reviews the anatomy, pathophysiology, clinical presentation, diagnosis, and current treatment options of foraminal neuropathy.
Keywords: Constriction, Pathologic; Decompression; Electric Stimulation; Fibrosis; Foraminotomy; Ganglia, Spinal; Inflammation; Lumbosacral Region; Pain Management; Radiculopathy; Spinal Nerve Roots
INTRODUCTION
Lumbar foraminal neuropathy is a pathologic condition of neurovascular contents in the foramen causing radicular symptoms, which is associated with narrowed foramen. Foraminal stenosis is common in the elderly population [1], characterized by narrowing of the bony exit of the nerve root due to degenerative changes in the intervertebral discs, zygapophyseal joint, ligaments, and bony parts. The narrowed foramen causes irritation and compression of the entrapped nerve to develop inflammation and pain, as well as vascular congestion causing neurogenic claudication. Depending on the magnitude of neuroforaminal narrowing and the impact on the neurovascular contents, symptoms may vary from pain, tingling, and numbness to motor weakness and gait impairment.
The neural passage in the foramen can also be narrowed with fibrotic adhesion or ligament changes with or without foraminal stenosis. Inflammation in the epidural and foraminal spaces derived from chemical or mechanical irritation of the disc or zygapophyseal joint can generate fibrotic tissue that fills the foraminal space and adheres to the neurovascular contents. Subsequently, the perineural space is packed with fibrosis and granulation tissue, and the inflamed swollen nerve is entrapped in the foramen. It is unclear whether fibrotic adhesion itself causes pain while it contributes to neural irritation and compression. Literature shows that there is no direct relationship between pain and scar tissue after spinal surgeries [2]. Nonetheless, the inflamed nerve can develop pain when it is irritated and compressed by the adhered fibrotic tissue, especially during the mobility of the spine.
Foraminal neuropathy is a common source of radicular leg pain that can remain despite extensive treatment of spinal stenosis. In general, conservative and interventional pain treatments are applied, and this article is to review foraminal neuropathy and current treatment methods.
MAIN BODY
1. Anatomy
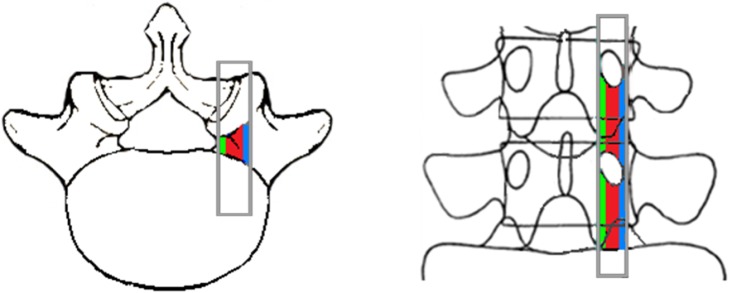
The lumbar foramen is formed by the vertebral body, pedicles, disc, superior and inferior articular processes, ligamentum flavum, and zygapophyseal joint. A foramen is an inter-pedicular osseous hole appearing with an oval or inverted teardrop shape that has three anatomical zones, the entrance ( internal), mid ( intraforaminal), and exit (extraforaminal) zones (Fig. 1).
Fig. 1.
Superoposterior view of the foramen. Internal (green), intraforaminal (red), and external (blue) zones.
A foramen is segmented into multiple subcompartments and stabilized by transforaminal ligaments, through which the nerve root, dorsal root ganglion (DRG), radicular artery and veins, and lymphatics pass. Nerve roots and the DRG exit the dural sac and course through the lateral recess to the superior and anterior region of the foramen. The 5th lumbar nerve root occupies 25%–30% of the foraminal space, while the other lumbar nerve roots occupy 7%–22% of the foramen [3].
There are two types of foraminal ligaments; radiating ligaments that connect the nerve root sleeves to the wall of the foramen and transverse processes, and transforaminal ligaments [4,5]. The ligaments in the internal zone are seen in the inferior aspect of the medial portion of the foramen, creating sub-compartments in the lower foramen where veins run through. Transforaminal ligaments in the intraforaminal zone are seen in the anterior, anterior-superior and horizontal-mid portion. The external ligaments are divided into the superior, middle, and inferior corporotransverse ligaments attaching to the transverse process (Fig. 2). The ligaments are fascial condensations with ligamentous features and are not always present at all levels or on both sides of the spine. The overall incidence of the transforaminal ligaments is approximately 47%, and the ligaments occupy as much as 30% of the foramen [6].
Fig. 2.
The lateral view of foramen with ligaments, nerve root (NR), and dorsal root ganglion (DRG).
The mid zone is a foraminal region where the nerve root and DRG pass. The lumbar DRG, lacking a protective capsule, is commonly located in the intraforaminal area. Moon et al. [7] found that at the 4th lumbar spine the DRG was 48% intraforaminal, 41% intraspinal, and 6% extraforaminal. In the 5th lumbar spine, the DRG positions were 75% intraforaminal, 10% intraspinal, and 6% extraforaminal.
2. Causes
Foraminal neuropathy is caused by foraminal stenosis, but it can also be secondary to lateral recess or extraforaminal pathology (Table 1). Foraminal stenosis is due to acquired anatomical changes, but a narrow spinal canal can also be congenital in nature. Congenital stenosis is uncommon and found in achondroplasia, and other congenital defects. Acquired stenosis is secondary to degenerative changes of the spine, such as hypertrophy of the facet joint, ligament and bone, disc disorders, and osteophyte formation [8]. Most acquired stenosis is due to gradual anatomical deterioration with the aging process and foraminal stenosis increases with after the age of 60. Risk factors include weight gain, back stress, trauma, and excessive use of alcohol and tobacco.
Table 1.
Conditions Causing Foraminal Neuropathy
| Extraforaminal etiology: foraminal stenosis |
| Congenital |
| Idiopathic |
| Achondroplasia |
| Spinal dysraphism |
| Segmentation failure |
| Osteopetrosis |
| Developmental |
| Early vertebral arch ossification |
| Shortened pedicles |
| Thoracolumbar kyphosis |
| Apical vertebral wedging |
| Morquio syndrome |
| Osseous exostosis |
| Acquired |
| Disc disorders with bulging or herniation |
| Degenerative disc disease |
| Osteoarthritis |
| Spondylolisthesis |
| Scoliosis |
| Hypertrophy of ligamentum flavum |
| Pedicular hypertrophy |
| Facet arthritis and hypertrophy |
| Facet joint cyst |
| Osteophytes |
| Compression fracture |
| Paget’s disease |
| Ankylosing spondylitis |
| Post-traumatic |
| Post-surgery |
| Neoplasm |
| Intraforaminal etiology |
| Fibrosis |
| Epidural/foraminal inflammation |
| Degenerative spinal disorders |
| Post-surgery |
| Transforaminal ligament |
| Hypertrophy |
| Calcification/ossification |
Foraminal neuropathy is also from inflammation and fibrosis of the lateral recess and extraforaminal space. Epidural inflammation from degenerative disorders of the disc, zygapophyseal joint, bone and ligament, hard scars after the surgery, or hardening of transforaminal ligaments causes foraminal neuropathy.
3. Pathogenesis
Degenerative disc changes cause compression and bulging of the discs that result in foraminal narrowing. Jenis and An [9] described foraminal stenosis characterized by degenerative disk changes that narrow disk height and permit the superior articular process (SAP) to sublux antero-superiorly. As the subluxation continues, the biomechanics of the spine is disrupted and provokes osteophytosis and ligamentum flavum hypertrophy. An abnormal weight loading pattern is generated over the functional unit of the lumbar spine at the disc, joint, and supportive tissues that increases back stress and changes the segmental mechanics progressively to result in spinal instability. Degeneration with spinal instability induces further changes with annular tears and disc distortion, facet hypertrophy, and bone spur formation [10], which further compromises the foraminal space.
Foraminal narrowing can be at different locations depending on the pathologic conditions. Incidence of foraminal stenosis and nerve root impingement increases in the lower lumbar levels due to the increased diameter of the DRG. Commonly involved nerves are the fifth lumbar nerve root (75%), followed by the fourth root (15%), the third root (5.3%), and the second root (4%) [9].
Foraminal stenosis is anatomically anteroposterior (transverse), craniocaudal (vertical), or circumferential. Anteroposterior stenosis results from the SAP and posterior vertebral body transversely, and craniocaudal stenosis is from osteophytes of the posterolateral vertebral endplate and a laterally bulging or herniated disc compressing the nerve root against the superior pedicle vertically [11]. Dynamic foraminal stenosis implies position dependent provocation of foraminal volume with intermittent lumbar extension-provoked nerve root impingement [12]. Inufusa et al. [13] reported that lumbar flexion increased the foraminal volume by 12%, whereas extension decreased by 15%. The incidence of nerve root compression on the dynamic motion was 21% in the neutral position, 15.4% in the flexion position, and 33.3% in the extended position.
Most elderly people have some degree of spinal stenosis, but only a small portion of them develops symptoms, and there is no correlation between the severity of stenosis and clinical symptoms [14]. Radicular symptoms are initiated primarily by the neural inflammation within the subcompartment of the foramen, not proportional to the degree of foraminal stenosis.
The nerve roots are fixed by fibrous attachments at the neck of the nerve root sheath as it exits the dural sac to the periosteum of the pedicle, and at the lateral aspect of the foramen to pedicles superiorly and inferiorly, where abnormal tension may impose to develop inflammation with spinal degeneration and instability [15]. The nerve roots are also predisposed to chemical and mechanical irritation due to close proximity to the disc and joint. McCarron et al. [16] observed a marked inflammatory response in nerve roots that were exposed to nucleus pulposus.
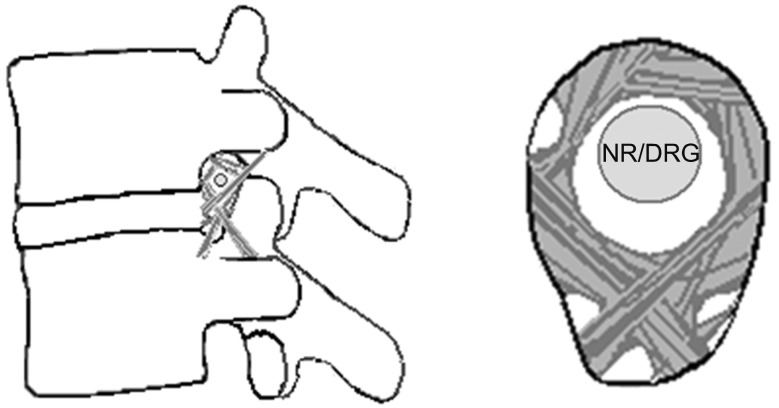
The inflammatory response was accompanied by edema, fibrin deposition, and granular tissue formation, leading to eventual fat coalescence and fibrosis. An annular tear with leakage of the nucleus pulposus into the epidural and foraminal spaces can induce significant inflammation of the nerve roots and DRG. Inflammation with fibrotic and granulation tissue in the epidural and foraminal spaces (Figs. 3, 4) inhibits the mobility of the nerve root and it is entrapped in the subcompartments of the foramen (Fig. 5).
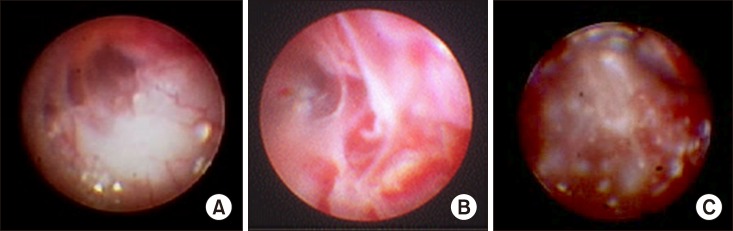
Fig. 3.
Epiduroscopic findings of inflammation. (A) Mild inflammation, (B) moderate inflammation with fibrosis, and (C) extensive inflammation with granulation and fibrosis.
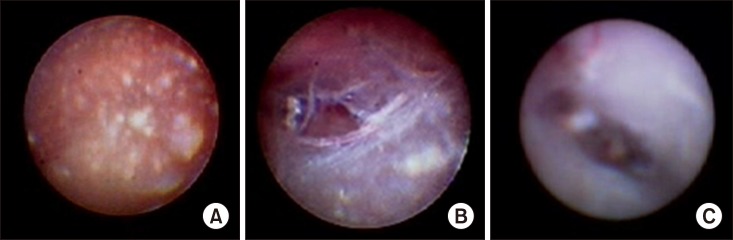
Fig. 4.
Epiduroscopic findings of inflammation fibrosis complex. (A) Epidural space is filled with gelatinous exudates and fibrosis, (B) fibrotic bands and meshes, and (C) membranous fibrosis.
Fig. 5.
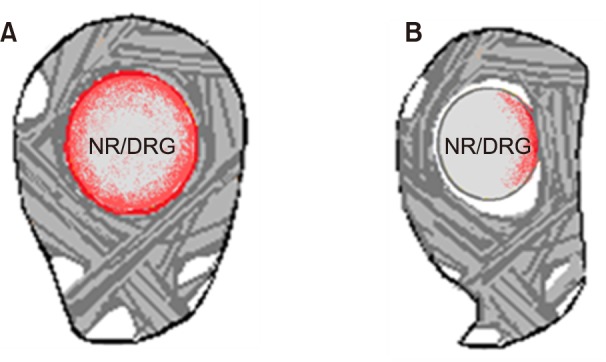
Lateral view of foraminal nerve entrapment. (A) Inflamed swollen nerve in normal foramen and (B) compressed nerve in foraminal stenosis. NR: nerve root, DRG: dorsal root ganglion.
It was postulated that transforaminal ligaments may also entrap the nerves in the foramen, but Amonoo-Kuofi et al. [5] reported that the role of transforaminal ligaments was protective of nerves and vessels. But if the nerve is inflamed with edema and fibrotic adhesion within the subcompartment surrounded by the transforaminal ligaments, the nerve can be entrapped and compressed, especially with mobility of the joints [17]. Thickened or hardened transforaminal ligaments, due to calcification or ossification, can also irritate the nerve to induce inflammation, and the nerve is entrapped.
Another potential pain mediator in the foramen is the DRG, and its active role in the generation of neuropathic pain has been documented. The DRG contains pain-mediating neuropeptides, such as substance P, and proinflammatory cytokines from glial cells, which induces neuropathic pain [18,19]. With persistent nociceptive pain signals, glial cells and Schwann cells within the DRG are activated and release a cascade of cytokines and other proinflammatory proteins that change neuronal activity. Proinflammatory cytokines sensitize and lower the threshold of glial cells to action potential firing, eventually leading to central sensitization and neuropathic pain. Prolonged afferent neural impulse through the DRG attains a sustained release of neuropeptides and inflammatory cytokines from activated glial cells, which induces spontaneous hyperactivity of the DRG and continued pain even after the inflammatory tissue reaction is resolved. The L4 and L5 DRG, located intraforaminally, are prone to stenotic compression and neuropathic pain.
Venous engorgement can trigger an inflammatory cascade, fibrosis, and increased epidural pressure that can produce neurogenic claudication and/or compression. The vascular compromise can also lead to ischemic neuritis that may contribute to developing symptoms of foraminal stenosis [20].
4. Clinical presentation
Degenerative narrowing of the lumbar foramen is a gradual process and it is unpredictable whether foraminal narrowing will or will not lead to clinical symptoms; however, it is generally understood that foraminal narrowing increases the risk to develop radicular pain and compression. Many patients can remain asymptomatic or experience only mild discomfort, but if the patient has a foraminal neuropathy with an inflamed nerve regardless of stenosis, the nerve produces pain.
Patients with foraminal neuropathy present with radicular symptoms and/or neurogenic claudication. The onset of symptoms is insidious and the symptoms progress slowly. Symptoms may be either unilateral or bilateral depending on the involvement of the foramen, including pain radiating down the leg, tingling, numbness, tightness, heaviness, muscle weakness, and spasms in the back, buttock, and leg when standing or walking.
The sensory nerve root elicits nociceptive pain that includes deep aching and throbbing with heaviness and a squeezing sensation associated with tingling and numbness. As the DRG becomes inflamed and entrapped in the foramen, the pain changes to neuropathic-type pain characterized by sharp, shooting, burning, stabbing, and lancinating sensations. The pain is frequently associated with the symptoms of central pain such as allodynia, hyperalgesia, and hyperesthesia [21], and progresses from being sensitive to becoming intolerable [22].
Generally, the pain is exacerbated when the spine is extended with decreased volume in the foramen, and relieved while the spine is flexed with increased foraminal space to reduce the pressure on the nerve. Patients may develop gait impairment with a limited range of motion and a risk of falling, and bladder and bowel disturbances. It is important to rule out any underlying vascular problems and bladder disorders.
Physical findings are usually non-specific. The straight leg raising and femoral nerve stretching tests are usually normal. A neurological examination is also normal unless the motor function is involved.
5. Diagnosis
Foraminal neuropathy presenting radicular pain and foraminal stenosis can be diagnosed with a thorough history and physical examination, not primarily based on a radiographic finding. Boden et al. [23] noted abnormal findings in 57% of asymptomatic patients sixty years or older on magnetic resonance imaging (MRI) scans. Ishimoto et al. [14] reported that 9.9% of the patients with moderate radiographic stenosis obliterating one-third to two-thirds of spinal canal showed symptoms and 17.5% of patients with severe radiographic stenosis obliterating more than two-thirds of spinal canal had symptoms.
The degree of foraminal narrowing is assessed with MRI. MRI can identify nerve compression and determine the cross-sectional area of the spinal canal. A computed tomography myelogram can be considered as an alternative if patients cannot undergo an MRI [24,25]. Standard lumbar anteroposterior and lateral X-rays are also helpful in determining the extent of spinal degeneration for disc space narrowing, facet joint hypertrophy, osteophytes, and spondylolisthesis. Dynamic flexion and extension views are taken to determine spinal instability and stenosis.
Foraminal stenosis is graded based on perineural fat obliteration or the foraminal dimension. Lee et al. [26] graded foraminal stenosis on the basis of perineural fat obliteration and nerve root morphology on sagittal MRI. Grade 1 denotes a mild degree of foraminal stenosis, showing perineural fat obliteration surrounding the nerve root in a transverse or vertical direction. Grade 2 is a moderate degree in the vertical and transverse directions. There is a narrowing of foraminal width and height, but without evidence of morphologic changes in the nerve root. Grade 3 is a severe degree, with nerve root collapse or morphologic changes.
The normal height of the foramen varies from 20 to 23 mm, and the width of the superior foramen varies from 8 to 10 mm. A height less than 15 mm and a posterior disk height of 4 mm or less are associated with significant nerve root compression and indicate foraminal stenosis [11]. Nonetheless, direct measurement of the bony canal on a radiographic image does not provide an accurate assessment of the degree of stenosis [27].
Electrodiagnostic evaluation is useful in some patients with symptoms and findings that are equivocal or in conflict with imaging results. Electromyography (EMG) is beneficial in determining peripheral versus central origin of leg pain [28].
Epiduroscopy can be applied for the diagnosis of the epidural and foraminal inflammatory status. Location, distribution, and the extent of inflammation and fibrosis are visualized directly, and the source of pain can be documented.
6. Differential diagnosis
Other causes of leg pain that mimics the symptoms of foraminal neuropathy should be differentiated. A differential diagnosis should include radiculopathy from spinal stenosis, disc disorders, or extraforaminal disorders. Neurologic disorders such as diabetic neuropathy or other types of peripheral neuropathy should also be differentiated. Neurologic disorders usually present predominant paresthesia and the pain is mild that is rather diffuse in the legs, not referred from the spinal site. EMG is useful to diagnose peripheral neuropathy.
Degenerative osteoarthritis of the hip or knee can also mimic the leg pain of foraminal neuropathy. The pain gets aggravated with the mobility of the joints and weight bearing, which is not usually associated with paresthesia. Radiological examination is required to differentiate.
Vascular claudication from peripheral vascular diseases that is common among the elderly population should also be ruled out as a potential cause of leg pain [24]. Thorough vascular examination and studies should be obtained. Patients with vascular claudication have a decreased or absent dorsalis pedis pulse and the symptoms are relieved after a short rest or standing, and sitting or bending is not required to relieve the pain. Weakness of the leg and back pain are rare with vascular disorders, and the patients experience cramping pain referred from distal side of the leg to the proximal side, while foraminal neuropathy causes a deep ache with tingling and numbness radiating from proximal side to the distal side of the leg.
7. Management
1) Conservative therapy
Conservative management is recommended with multimodal approaches for initial treatment of foraminal neuropathy, including bed rest, medications, and an exercise program. Fifty percent of patients with mild to moderate pain have pain relief with conservative treatment in less than three months [29]. Interventional therapies with nerve root or epidural steroid injections are applied for moderate to severe pain. Operative treatment is indicated for patients with severe pain and constant neurologic symptoms, and in patients where conservative treatment has failed. Patients with multiple medical comorbidities, that can be a high risk for surgery, should be treated non-operatively if possible.
Pharmacological therapy with non-steroid anti-inflammatory drugs (NSAIDs) such as ibuprofen and naproxen are commonly used as a first line of treatment to control the symptoms. These help in reducing inflammation and a mild to moderate degree of nociceptive pain, especially when it is combined with acetaminophen. Steroids may also be used for a short term and muscle relaxants can be added as an adjunct.
A non-opioid analgesic such as tramadol is helpful for the control of moderate to severe pain. Tramadol induces analgesic effects through different targets on the noradrenergic system, serotoninergic system, and opioid receptor system. Long-term use of high doses of tramadol can cause abuse, addiction, and withdrawal syndrome.
Opioid analgesics such as oxycodone, hydrocodone, and morphine are used for severe pain but are recommended only for short term pain control, due to multiple adverse effects including development of opioid induced hyperalgesia (OIH) causing increased central pain paradoxically. OIH is a state of nociceptive sensitization as a result of neuroplastic changes involving the central glutaminergic system and N-methyl-D-aspartate (NMDA) receptors [30,31]. Opioids also activate glia through Toll-like receptors-4 that induces to release neuroexcitatory proinflammatory cytokines and suppresses opioid analgesia. Opioid tolerance and addiction can be developed leading to an escalation of opioid intake [32,33]. Long term use of high doses of opioids is often associated with overdose and inadvertent death, and there is a lack of evidence supporting the efficacy of long term opioid treatment for chronic pain [34].
Neuropathic pain is treated with administration of anti-depressants and gabapentinoids. Gabapentin is widely used, but the side effects are not uncommon and the efficacy is limited. Abuse, addiction, and withdrawal syndrome relevant to long term use of gabapentin have been reported [35], and gabapentin is classified as a controlled substance in some states of the USA.
Lately, concomitant use of gabapentin with opioids has been of concern since the risk of opioid-related death is 49% greater than those taking opioids only [36].
Ketamine, an NMDA receptor antagonist, is a dissociative anesthetic that has been re-visited to use for neuropathic pain [37,38]. Ketamine reduces pain with changes of the neuroplastic condition by counteracting spinal sensitization or wind-up phenomena and has also been used for the treatment of depression. At low doses, psychotropic side effects are less apparent and well managed with benzodiazepines. Ketamine is administered by a low dose infusion but compounded oral or nasal spray preparations have also been used. Ketamine has the potential to develop tolerance and addiction as well as withdrawal syndrome that is not severe.
Lately, control of neuropathic pain exerted by glial cell activation has emerged as a potential new target, but drug therapy with microglial and astrocyte attenuators are still experimental [39].
2) Physical therapy
Physical therapy and occupational therapy are used with other modalities. A comprehensive rehabilitation program of manual therapy, stretching, and strengthening exercises for the lumbar spine and hip region has been advocated. Among many different modalities of physical therapy, classically flexion type exercises have been advocated for patients with spinal stenosis. A recent study has shown that manual physical therapy may be more effective than flexion type exercises [40].
3) Interventional therapy
(1) Steroid injection
Interventional approaches such as nerve root blocks and epidural steroid injections have been widely applied. An epidural steroid injection involves a combination of steroids and local anesthetics that are injected directly into the affected nerve root area. Although the actual mechanism of action is not fully understood, the anti-inflammatory effects of steroids with inhibition of phospholipase-2 and inhibition of neural transmission in nociceptive C-fibers have been well known [41]. Steroids are also known to stabilize cellular membranes, suppress immune responses, and enhance neuronal blood flow. A forced epidural injection of the solution also helps release fibrosis and wash out the inflammatory substances. An epidural steroid injection is beneficial when combined with other NSAIDs and a home exercise program.
Nonetheless, the efficacy of epidural steroid injections remains controversial. Systematic reviews of epidural steroid injections are confusing, as they mix different spinal disorders in the studies, such as radiculopathy, spinal stenosis, and disc herniation. Various results have been reported depending on the approaching techniques, i.e., the interlaminar, caudal, or transforaminal route, with or without fluoroscopic guidance, and the different mixtures with particulate or non-particulate steroids, local anesthetics, and normal saline [42,43].
The evidence for the effectiveness of epidural steroid injections ranges from limited to strong, but in general, the transforaminal approach under fluoroscopic guidance has shown better outcomes for spinal stenosis. Particulate and non-particulate steroids provide equal efficacy, and steroid injections show superior efficacy over the injections of local anesthetics alone [44].
The safety of epidural steroid injections has been a concern, and in April 2014, the Food and Drug Administration (FDA) of the USA issued a warning of rare, but serious adverse events from epidural steroid injections, including loss of vision, stroke, paralysis, and death. The FDA also states that the effectiveness and safety of the steroids for epidural use have not been established, and the FDA has not approved corticosteroids for such use [45]. But most of the cases with catastrophic complications were related to the transforaminal approach in the cervical spine. Careful injection techniques to avoid complications have been guided, including the use of image-guidance with a contrast medium, and non-particulate steroids for transforaminal injections [46].
Transforaminal steroid injection has been most frequently used for foraminal neuropathy, owing to the direct injection of medications into the inflamed nerve root and DRG at the foramen [47,48]. Manchikanti et al. [49] reported an excellent epidurographic filling of nerve roots and the ventral epidural space from transforaminal injections as compared to inconsistent filling from interlaminar injections. Nonetheless, a transforaminal injection may not achieve adequate epidurographic filling if there is significant perineural narrowing at the foramen with tissue swelling and fibrotic adhesion. So it is likely that the injection is applied during the early course of the inflammatory condition before the fibrosis is formed in order to obtain a better result. Cyteval et al. [50] reported the predictive factor for successful pain relief was not the cause of pain, location, and pain intensity, but the duration of symptoms before the procedure. Patients with excellent results had a mean duration of symptoms of 3.04 months versus 7.96 months in the group with poor pain relief.
(2) Percutaneous adhesiolysis
Success of steroid injections depends on the spread of the injectate into the target area of the foramen, and the injections may fail if there is not enough space for the injectate to spread through, or the spread is interrupted by fibrosis with or without foraminal stenosis.
There are two types of fibrosis sealing off the epidural and foraminal spaces; loose fibrous tissue like a spider web or mesh that is easy to release, and a hard, dense fibrotic band, strand, or membrane that is often difficult to release [51,52]. It is necessary to release fibrotic adhesion in the epidural and foraminal spaces for those who show a filling defect on epidurography and are refractory to conventional steroid injections in order to open the sealed space and reach the target area properly, as well as for decompression. So the techniques for lysis of adhesion (LOA) have been employed via the epidural or transforaminal route, and steroid injections after LOA with a catheter showed better clinical outcomes as compared to transforaminal injections only [53,54].
Percutaneous neuroplasty with LOA was introduced to release scar tissue for the pain of failed back surgery syndrome [55]. A non-steerable spring-wound Racz catheter (Tun-L-KathTM; Epimed international Inc., Dallas, TX), 0.9 mm in diameter, is inserted into the region of the filling defect on epidurography and hydrostatic pressure is applied by injecting a solution to release the post-surgery scar adhesion. As the scar is detached and the epidural space is opened up, the injectate is facilitated to spread into the target area. The catheter is inserted transforaminally as well, to reach higher levels at L4 and L5, respectively [56]. A steerable catheter (Navicath®; Myelotec Inc., Roswell, GA), 1.3 mm in diameter, was introduced later and has been used for the purpose of neuroplasty [57].
Both catheters are thin and not strong enough to penetrate or break the dense scar adhesion, and LOA is made by injecting pressure that forces open the vulnerable, split space in between the soft fibrotic tissue around the nerve. Subsequently, LOA can be made partially and the targeted nerve sealed with fibrotic adhesion is not reached down deeply, although epidurographic findings may show good visualization of the affected nerve roots. Devulder et al. [58] reported improvement of epidurographic spread was not correlated with pain relief and the pain relief was only for a limited period of 1 month using Racz’s neuroplasty.
The neuroplasty technique has been widely used and systematic reviews show that LOA is more effective than conventional epidural injections for radicular pain from spinal stenosis and disc herniation, but not more effective than caudal epidural injections in failed back surgery syndrome [59–61]. LOA also shows poor outcomes for foraminal stenosis [62].
(3) Epiduroscopy
Epiduroscopy was introduced to diagnose ongoing epidural pathology and release fibrotic adhesion [63]. Epiduroscopy shows the epidural condition, including the location, distribution, and extent of inflammation and fibrosis, and the source of pain can be confirmed. The scope is inserted caudally with a video guided catheter (Myelotec Inc.), 2.6 mm in diameter, and is advanced easily to the suspected target area. Adhesiolysis is performed thoroughly using a steerable catheter, and steroid medication is delivered to the inflamed nerve root precisely under direct visualization. The catheter is steered to the foraminal area and percutaneous foraminotomy at the subarticular and subpedicular areas can be achieved efficiently by manipulating the steering tip in a multi-directional way to release and break the durable fibrotic adhesion.
Systematic reviews show that epiduroscopic adhesiolysis is effective for failed back surgery syndrome and is useful for those who failed with multiple treatment modalities including Racz’s neuroplasty [64,65]. As the guiding catheter has a large bore, it can release dense fibrotic adhesion effectively but is difficult to advance into the foramen if it shows severe narrowing.
The use of epiduroscopy has been limited due to technical difficulty and cost containment; however, the diagnostic value of epiduroscopy has been acknowledged in that it helps physicians learn and understand the actual status of the epidural condition causing intractable back and leg pain which is refractory to conventional treatment [51].
(4) Balloon adhesiolysis
Balloon adhesiolysis using a Fogarty catheter (Edward Lifescience, Irvine, CA), 1 mm in diameter and 5 mm with the inflated balloon, was employed to release epidural adhesions [66]. Kim et al. [67] applied transforaminal balloon adhesiolysis in 62 patients with foraminal stenosis. The Fogarty catheter was placed in the medial side of lateral recess and then the control group received injection without balloon inflation. The study group received injection after balloon inflation with 0.13 mL for 5 seconds at 5 spots in the foramen. Follow-up after 12 weeks showed improvement on the visual analogue pain scale (VAS, 0–100 mm [0 = no pain and 100 = worst pain]) from 68.4 ± 13.3 (control group) and 71.7 ± 13.4 (study group) to 56.8 ± 20.8 and 41.6 ± 22.7, respectively. The Oswestry disability index (ODI) score and neurogenic claudication distance were also improved.
Transforaminal balloon adhesiolysis is a direct approach from outside of the foramen and foraminotomy is made with the insertion of the catheter and inflation of the balloon. It is difficult to engage the catheter when the foramen is narrowed with heavy adhesion, osteophytes, facet hypertrophy, or a hardened transforaminal ligament. Inflation of the balloon at the stenotic area may compromise circulation and compress the DRG to cause ischemia and neural damage.
A steerable catheter with a balloon tip (ZiNeu®; Zubenui Inc., Seoul, Korea) was introduced [68], which is inserted caudally and steered to the target foramen for balloon inflation. An epiduroscope can be inserted through the lumen of the catheter as well.
(5) Percutaneous decompressive foraminotomy
Recently, percutaneous lumbar extraforaminotomy (PLEF) was presented for foraminal adhesiolysis by detaching foraminal ligaments, particularly at the posterior and inferior quadrant of the neural foramen, using a cup-shaped curette (BS extraforaminotomy kit; BioSpine Co., Seoul, Korea), 1.6 mm in diameter. PLEF mechanically releases adhesions of the inferior transforaminal ligament, the lower part of the superior corporopedicular ligament, the mid-transforaminal ligament, and part of the anterior facet joint capsule, which compress exiting nerve roots [69]. Decompressive foraminotomy is achieved to reduce venous stasis and perineural edema, and eventually to promote the spread of injected steroid medication in the foramen.
PLEF can be applied for those with heavy adhesion and compression by abnormal ligaments at the foramen refractory to LOA. Lee et al. [70] underwent a pilot study with 20 patients that showed an overall mean pain reduction of 36.3% at 3 months and improved the ODI 20%. Manipulation of the instruments at the stenotic foramen could injure the adjacent nerves and DRG, but only minor pain was reported that was spontaneously resolved.
A percutaneous drill with a protective shield for the nerves (Claudicare®; SEAWON Meditech, Bucheon, Korea), 3.5 mm in diameter, was also introduced to release the hypertrophied capsule of the SAP and part of the thickened transforaminal ligaments [71].
(6) Pulsed radiofrequency (PRF)
PRF neurostimulation has emerged since it has been proven effective in the control of various chronic pain problems, especially for the control of neuropathic pain [72]. PRF is a pulsed mode of radiofrequency consisting of short high voltage bursts at low temperature (below 42°C). The mode of action is unclear, but references have been made to the neuromodulatory effect of the alteration of synaptic transmission of pain signals. It appears that PRF up-regulates c-fos in the DRG and modulates glial cell activation [73,74].
PRF can help patients with neuropathic pain derived from the DRG that is refractory to conservative and interventional injection therapies. PRF is applied to the nerve root or DRG with unipolar or bipolar needle electrodes either transforaminally or epidurally. Ding et al. [75] reported that transforaminal steroid injection with concomitant PRF relieved the radicular pain significantly with long-term remission.
(7) Electrical stimulation
Electrical stimulation therapy has been employed for the treatment of pain from failed back surgery syndrome. Shealy et al. [76] implanted the first spinal column stimulator (SCS) in 1967, which has shown to effectively control the pain of neuropathic origin [77,78]. Its efficacy has demonstrated successful treatment in approximately 50% of patients, but concerns with paresthesia and the development of tolerance have been raised.
Lately, burst SCS that mimics the natural neuronal firing patterns and high frequency stimulation were introduced for paresthesia-free stimulation [79]. Closed loop SCS was also introduced to mitigate the effects of positional changes and the development of tolerance [80]. A new SCS paradigm has also been developed with a 3-dimensional anatomical model of the spinal cord automatically calculating the optimal program to precisely target the selected central point of stimulation [81,82]. The use of SCS is limited due to the high cost of the stimulators.
Electrical stimulation of the DRG has also been advocated to alleviate the neuropathic pain [83,84]. In 2016 the FDA approved implantable DRG stimulators for the treatment of complex regional pain syndrome utilizing low frequency and amplitude through four implanted leads. DRG stimulation decreases hyperexcitability of the DRG and dorsal horn neurons, stabilizes microglial-releasing cytokines, and thereby decreases neuropathic pain. It also modifies the neural patterns of oscillatory and bursting activity and alters abnormal electrical activity within the DRG [85]. DRG stimulation can be used for neuropathic pain that is refractory to conservative and interventional injection therapies, but it is difficult to place the leads for post-surgery patients due to heavy scar tissue.
CONCLUSIONS
Lumbar foraminal neuropathy is a common source of radicular symptoms causing pain, weakness and paresthesia. Based on the pathologic conditions, optimal treatment can be achieved with an application of non-surgical treatment modalities. Better understanding of the foraminal status causing the neuropathy is necessary for successful management.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9:545–50. doi: 10.1016/j.spinee.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annertz M, Jönsson B, Strömqvist B, Holtås S. No relationship between epidural fibrosis and sciatica in the lumbar postdiscectomy syndrome. A study with contrast-enhanced magnetic resonance imaging in symptomatic and asymptomatic patients. Spine (Phila Pa 1976) 1995;20:449–53. doi: 10.1097/00007632-199502001-00007. [DOI] [PubMed] [Google Scholar]
- 3.Hasue M, Kikuchi S, Sakuyama Y, Ito T. Anatomic study of the interrelation between lumbosacral nerve roots and their surrounding tissues. Spine (Phila Pa 1976) 1983;8:50–8. doi: 10.1097/00007632-198301000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Q, Zhong E, Shi B, Li Y, Sun C, Ding Z. The morphology and clinical significance of the intraforaminal ligaments at the L5-S1 levels. Spine J. 2016;16:1001–6. doi: 10.1016/j.spinee.2016.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Amonoo-Kuofi HS, el-Badawi MG, Fatani JA. Ligaments associated with lumbar intervertebral foramina. 1. L1 to L4. J Anat. 1988;156:177–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan SG, Wen YL, Zhang P, Li YK. Ligament, nerve, and blood vessel anatomy of the lateral zone of the lumbar intervertebral foramina. Int Orthop. 2015;39:2135–41. doi: 10.1007/s00264-015-2831-6. [DOI] [PubMed] [Google Scholar]
- 7.Moon HS, Kim YD, Song BH, Cha YD, Song JH, Lee MH. Position of dorsal root ganglia in the lumbosacral region in patients with radiculopathy. Korean J Anesthesiol. 2010;59:398–402. doi: 10.4097/kjae.2010.59.6.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res. 1976;115:4–5. [PubMed] [Google Scholar]
- 9.Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine (Phila Pa 1976) 2000;25:389–94. doi: 10.1097/00007632-200002010-00022. [DOI] [PubMed] [Google Scholar]
- 10.Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;165:110–23. [PubMed] [Google Scholar]
- 11.Hasegawa T, An HS, Haughton VM, Nowicki BH. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J Bone Joint Surg Am. 1995;77:32–8. doi: 10.2106/00004623-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara A, An HS, Lim TH, Haughton VM. Morphologic changes in the lumbar intervertebral foramen due to flexionextension, lateral bending, and axial rotation: an in vitro anatomic and biomechanical study. Spine (Phila Pa 1976) 2001;26:876–82. doi: 10.1097/00007632-200104150-00010. [DOI] [PubMed] [Google Scholar]
- 13.Inufusa A, An HS, Lim TH, Hasegawa T, Haughton VM, Nowicki BH. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine (Phila Pa 1976) 1996;21:2412–20. doi: 10.1097/00007632-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Ishimoto Y, Yoshimura N, Muraki S, Yamada H, Nagata K, Hashizume H, et al. Associations between radiographic lumbar spinal stenosis and clinical symptoms in the general population: the Wakayama Spine Study. Osteoarthritis Cartilage. 2013;21:783–8. doi: 10.1016/j.joca.2013.02.656. [DOI] [PubMed] [Google Scholar]
- 15.de Peretti F, Micalef JP, Bourgeon A, Argenson C, Rabischong P. Biomechanics of the lumbar spinal nerve roots and the first sacral root within the intervertebral foramina. Surg Radiol Anat. 1989;11:221–5. doi: 10.1007/BF02337826. [DOI] [PubMed] [Google Scholar]
- 16.McCarron RF, Wimpee MW, Hudkins PG, Laros GS. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine (Phila Pa 1976) 1987;12:760–4. doi: 10.1097/00007632-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Qian Y, Qin A, Zheng MH. Transforaminal ligament may play a role in lumbar nerve root compression of foraminal stenosis. Med Hypotheses. 2011;77:1148–9. doi: 10.1016/j.mehy.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion - a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31–8. doi: 10.2147/JPR.S26603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein J. Report of the 1985 ISSLS Traveling Fellowship. Mechanisms of spinal pain. The dorsal root ganglion and its role as a mediator of low-back pain. Spine (Phila Pa 1976) 1986;11:999–1001. doi: 10.1097/00007632-198612000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Rydevik B, Brown MD, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine (Phila Pa 1976) 1984;9:7–15. doi: 10.1097/00007632-198401000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999;82:3347–58. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- 22.Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–95. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–8. doi: 10.2106/00004623-199072030-00013. [DOI] [PubMed] [Google Scholar]
- 24.Hilibrand AS, Rand N. Degenerative lumbar stenosis: diagnosis and management. J Am Acad Orthop Surg. 1999;7:239–49. doi: 10.5435/00124635-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Wiesel SW, Tsourmas N, Feffer HL, Citrin CM, Patronas N. A study of computer-assisted tomography. I. The incidence of positive CAT scans in an asymptomatic group of patients. Spine (Phila Pa 1976) 1984;9:549–51. doi: 10.1097/00007632-198409000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Lee JW, Yeom JS, Kim KJ, Kim HJ, Chung SK, et al. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol. 2010;194:1095–8. doi: 10.2214/AJR.09.2772. [DOI] [PubMed] [Google Scholar]
- 27.Bolender NF, Schönström NS, Spengler DM. Role of computed tomography and myelography in the diagnosis of central spinal stenosis. J Bone Joint Surg Am. 1985;67:240–6. doi: 10.2106/00004623-198567020-00009. [DOI] [PubMed] [Google Scholar]
- 28.Haig AJ, Tong HC, Yamakawa KS, Quint DJ, Hoff JT, Chiodo A, et al. The sensitivity and specificity of electrodiagnostic testing for the clinical syndrome of lumbar spinal stenosis. Spine (Phila Pa 1976) 2005;30:2667–76. doi: 10.1097/01.brs.0000188400.11490.5f. [DOI] [PubMed] [Google Scholar]
- 29.Onel D, Sari H, Dönmez C. Lumbar spinal stenosis: clinical/radiologic therapeutic evaluation in 145 patients. Conservative treatment or surgical intervention? Spine (Phila Pa 1976) 1993;18:291–8. doi: 10.1097/00007632-199302000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–96. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 31.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–87. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 32.Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–65. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kissin I. Long-term opioid treatment of chronic nonmalignant pain: unproven efficacy and neglected safety? J Pain Res. 2013;6:513–29. doi: 10.2147/JPR.S47182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonnet U, Richter EL, Isbruch K, Scherbaum N. On the addictive power of gabapentinoids: a mini-review. Psychiatr Danub. 2018;30:142–9. doi: 10.24869/psyd.2018.142. [DOI] [PubMed] [Google Scholar]
- 36.Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14:e1002396. doi: 10.1371/journal.pmed.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77:357–67. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sigtermans MJ, van Hilten JJ, Bauer MC, Arbous MS, Marinus J, Sarton EY, et al. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304–11. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Jo D, Chapman CR, Light AR. Glial mechanisms of neuropathic pain and emerging interventions. Korean J Pain. 2009;22:1–15. doi: 10.3344/kjp.2009.22.1.1. [DOI] [Google Scholar]
- 40.Whitman JM, Flynn TW, Childs JD, Wainner RS, Gill HE, Ryder MG, et al. A comparison between two physical therapy treatment programs for patients with lumbar spinal stenosis: a randomized clinical trial. Spine (Phila Pa 1976) 2006;31:2541–9. doi: 10.1097/01.brs.0000241136.98159.8c. [DOI] [PubMed] [Google Scholar]
- 41.Johansson A, Hao J, Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990;34:335–8. doi: 10.1111/j.1399-6576.1990.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 42.Abdi S, Datta S, Trescot AM, Schultz DM, Adlaka R, Atluri SL, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician. 2007;10:185–212. [PubMed] [Google Scholar]
- 43.Kim D, Brown J. Efficacy and safety of lumbar epidural dexamethasone versus methylprednisolone in the treatment of lumbar radiculopathy: a comparison of soluble versus particulate steroids. Clin J Pain. 2011;27:518–22. doi: 10.1097/AJP.0b013e31820c53e0. [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, Kim DH, Kim DH, Shin KH, Park SJ, Lee GJ, et al. Comparison of clinical efficacy of epidural injection with or without steroid in lumbosacral disc herniation: a systematic review and meta-analysis. Pain Physician. 2018;21:449–68. [PubMed] [Google Scholar]
- 45.U.S. Food and Drug Administration. FDA drug safety communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain [Internet] Silver Spring: U.S. FDA; 2014. [cited 2019 May 3]. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-label-changes-warn-rare-seriousneurologic-problems-after. [Google Scholar]
- 46.Rathmell JP, Benzon HT, Dreyfuss P, Huntoon M, Wallace M, Baker R, et al. Safeguards to prevent neurologic complications after epidural steroid injections: consensus opinions from a multidisciplinary working group and national organizations. Anesthesiology. 2015;122:974–84. doi: 10.1097/ALN.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 47.Cooper G, Lutz GE, Boachie-Adjei O, Lin J. Effectiveness of transforaminal epidural steroid injections in patients with degenerative lumbar scoliotic stenosis and radiculopathy. Pain Physician. 2004;7:311–7. [PubMed] [Google Scholar]
- 48.Rahatli FK, Harman A, Boyvat F, Zararsiz G. Comparison of transforaminal and interlaminar epidural steroid injection in managing lumbar radiculopathy. Biomed Res. 2017;28:2204–8. [Google Scholar]
- 49.Manchikanti L, Cash KA, Pampati V, Damron KS, McManus CD. Evaluation of lumbar transforaminal epidural injections with needle placement and contrast flow patterns: a prospective, descriptive report. Pain Physician. 2004;7:217–23. [PubMed] [Google Scholar]
- 50.Cyteval C, Fescquet N, Thomas E, Decoux E, Blotman F, Taourel P. Predictive factors of efficacy of periradicular corticosteroid injections for lumbar radiculopathy. AJNR Am J Neuroradiol. 2006;27:978–82. [PMC free article] [PubMed] [Google Scholar]
- 51.Choi YK. Spinal epiduroscopy as an educational tool. Korean J Pain. 2018;31:132–4. doi: 10.3344/kjp.2018.31.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosscher HA, Heavner JE. Incidence and severity of epidural fibrosis after back surgery: an endoscopic study. Pain Pract. 2010;10:18–24. doi: 10.1111/j.1533-2500.2009.00311.x. [DOI] [PubMed] [Google Scholar]
- 53.Park Y, Lee WY, Ahn JK, Nam HS, Lee KH. Percutaneous adhesiolysis versus transforaminal epidural steroid injection for the treatment of chronic radicular pain caused by lumbar foraminal spinal stenosis: a retrospective comparative study. Ann Rehabil Med. 2015;39:941–9. doi: 10.5535/arm.2015.39.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park CH, Lee SH. Effectiveness of percutaneous transforaminal adhesiolysis in patients with lumbar neuroforaminal spinal stenosis. Pain Physician. 2013;16:E37–43. [PubMed] [Google Scholar]
- 55.Racz GB, Holubec JT. Lysis of adhesions in the epidural space. In: Racz GB, editor. Techniques of neurolysis. Boston: Springer; 1989. pp. 57–72. [DOI] [Google Scholar]
- 56.Anderson SR, Racz GB, Heavner J. Evolution of epidural lysis of adhesions. Pain Physician. 2000;3:262–70. [PubMed] [Google Scholar]
- 57.Lee JH, Lee SH. Clinical effectiveness of percutaneous adhesiolysis using Navicath for the management of chronic pain due to lumbosacral disc herniation. Pain Physician. 2012;15:213–21. [PubMed] [Google Scholar]
- 58.Devulder J, Bogaert L, Castille F, Moerman A, Rolly G. Relevance of epidurography and epidural adhesiolysis in chronic failed back surgery patients. Clin J Pain. 1995;11:147–50. doi: 10.1097/00002508-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Helm Ii S, Benyamin RM, Chopra P, Deer TR, Justiz R. Percutaneous adhesiolysis in the management of chronic low back pain in post lumbar surgery syndrome and spinal stenosis: a systematic review. Pain Physician. 2012;15:E435–62. [PubMed] [Google Scholar]
- 60.Gerdesmeyer L, Wagenpfeil S, Birkenmaier C, Veihelmann A, Hauschild M, Wagner K, et al. Percutaneous epidural lysis of adhesions in chronic lumbar radicular pain: a randomized, double-blind, placebo-controlled trial. Pain Physician. 2013;16:185–96. [PubMed] [Google Scholar]
- 61.Jamison DE, Hsu E, Cohen SP. Epidural adhesiolysis: an evidence-based review. J Neurosurg Sci. 2014;58:65–76. [PubMed] [Google Scholar]
- 62.Lee JH, Lee SH. Clinical effectiveness of percutaneous adhesiolysis and predictive factors of treatment efficacy in patients with lumbosacral spinal stenosis. Pain Med. 2013;14:1497–504. doi: 10.1111/pme.12180. [DOI] [PubMed] [Google Scholar]
- 63.Saberski LR, Kitahata LM. Direct visualization of the lumbosacral epidural space through the sacral hiatus. Anesth Analg. 1995;80:839–40. doi: 10.1097/00000539-199504000-00035. [DOI] [PubMed] [Google Scholar]
- 64.Hayek SM, Helm S, Benyamin RM, Singh V, Bryce DA, Smith HS. Effectiveness of spinal endoscopic adhesiolysis in post lumbar surgery syndrome: a systematic review. Pain Physician. 2009;12:419–35. [PubMed] [Google Scholar]
- 65.Takeshima N, Miyakawa H, Okuda K, Hattori S, Hagiwara S, Takatani J, et al. Evaluation of the therapeutic results of epiduroscopic adhesiolysis for failed back surgery syndrome. Br J Anaesth. 2009;102:400–7. doi: 10.1093/bja/aen383. [DOI] [PubMed] [Google Scholar]
- 66.Raffaeli W, Righetti D, Andruccioli J, Sarti D. Epiduroscopy and radiofrequency technique: the Raffaeli-Righetti technique. Pain Clin. 2007;19:185–91. doi: 10.1179/016911107X376927. [DOI] [Google Scholar]
- 67.Kim SH, Choi WJ, Suh JH, Jeon SR, Hwang CJ, Koh WU, et al. Effects of transforaminal balloon treatment in patients with lumbar foraminal stenosis: a randomized, controlled, double-blind trial. Pain Physician. 2013;16:213–24. [PubMed] [Google Scholar]
- 68.Choi SS, Joo EY, Hwang BS, Lee JH, Lee G, Suh JH, et al. A novel balloon-inflatable catheter for percutaneous epidural adhesiolysis and decompression. Korean J Pain. 2014;27:178–85. doi: 10.3344/kjp.2014.27.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Transfeldt EE, Robertson D, Bradford DS. Ligaments of the lumbosacral spine and their role in possible extraforaminal spinal nerve entrapment and tethering. J Spinal Disord. 1993;6:507–12. doi: 10.1097/00002517-199306060-00006. [DOI] [PubMed] [Google Scholar]
- 70.Lee SC, Kim WJ, Lee CS, Moon JY. Effectiveness of percutaneous lumbar extraforaminotomy in patients with lumbar foraminal spinal stenosis: a prospective, singlearmed, observational pilot study. Pain Med. 2017;18:1975–86. doi: 10.1093/pm/pnw355. [DOI] [PubMed] [Google Scholar]
- 71.Yoo Y, Moon JY, Yoon S, Kwon SM, Sim SE. Clinical outcome of percutaneous lumbar foraminoplasty using a safety-improved device in patients with lumbar foraminal spinal stenosis. Medicine (Baltimore) 2019;98:e15169. doi: 10.1097/MD.0000000000015169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sluijter ME, Cosman ER, Rittman WB, III, van Kleef M. The effects of pulsed radiofrequency fields applied to the dorsal root ganglion: a preliminary report. Pain Clin. 1998;11:109–17. [Google Scholar]
- 73.Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir (Wien) 2011;153:763–71. doi: 10.1007/s00701-010-0881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Higuchi Y, Nashold BS, Jr, Sluijter M, Cosman E, Pearlstein RD. Exposure of the dorsal root ganglion in rats to pulsed radiofrequency currents activates dorsal horn lamina I and II neurons. Neurosurgery. 2002;50:850–5. doi: 10.1097/00006123-200204000-00030. [DOI] [PubMed] [Google Scholar]
- 75.Ding Y, Li H, Zhu Y, Yao P, Zhao G. Transforaminal epidural steroid injection combined with pulsed radio frequency on spinal nerve root for the treatment of lumbar disc herniation. J Pain Res. 2018;11:1531–9. doi: 10.2147/JPR.S174318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489–91. doi: 10.1213/00000539-196707000-00025. [DOI] [PubMed] [Google Scholar]
- 77.Song JJ, Popescu A, Bell RL. Present and potential use of spinal cord stimulation to control chronic pain. Pain Physician. 2014;17:235–46. [PubMed] [Google Scholar]
- 78.Kamihara M, Nakano S, Fukunaga T, Ikeda K, Tsunetoh T, Tanada D, et al. Spinal cord stimulation for treatment of leg pain associated with lumbar spinal stenosis. Neuromodulation. 2014;17:340–4. doi: 10.1111/ner.12092. [DOI] [PubMed] [Google Scholar]
- 79.Arle JE, Mei L, Carlson KW, Shils JL. High-frequency stimulation of dorsal column axons: potential underlying mechanism of paresthesia-free neuropathic pain relief. Neuromodulation. 2016;19:385–97. doi: 10.1111/ner.12436. [DOI] [PubMed] [Google Scholar]
- 80.Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics. 2014;11:553–63. doi: 10.1007/s13311-014-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veizi E, Hayek SM, North J, Brent Chafin T, Yearwood TL, Raso L, et al. Spinal Cord Stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS-LUMINA study. Pain Med. 2017;18:1534–48. doi: 10.1093/pm/pnw286. [DOI] [PubMed] [Google Scholar]
- 82.Remacle T, Gilis N, Mauviel S, Remacle JM. Treating low back pain in failed back surgery patients with multicolumn-lead spinal cord stimulation. J Vis Exp. 2018;(136):56804. doi: 10.3791/56804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation. 2013;16:67–71. doi: 10.1111/ner.12013. [DOI] [PubMed] [Google Scholar]
- 84.Liem L, Russo M, Huygen FJ, Van Buyten JP, Smet I, Verrills P, et al. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation. 2015;18:41–8. 48–9. doi: 10.1111/ner.12228. [DOI] [PubMed] [Google Scholar]
- 85.Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. 2015;18:24–32. doi: 10.1111/ner.12247. [DOI] [PubMed] [Google Scholar]