Abstract
Purpose:
Mutations in the RAS/RAF/MEK/ERK signaling pathway are commonly found in biliary tract cancer (BTC). Binimetinib, a selective inhibitor of MEK1/2, has single-agent activity. Preclinical data support binimetinib combination with chemotherapy, when given in an interrupted dosing schedule.
Patients and Methods:
A phase I/II trial evaluated bini-metinib in combination with gemcitabine and cisplatin in patients with untreated advanced BTC. The primary endpoints were to determine the MTD (phase I), and PFS 6 and RR (phase II). Tumor tissue for targeted gene sequencing and blood samples for peripheral blood pERK expression were evaluated. Patients received oral binimetinib twice daily with gemcitabine and cisplatin on day 8 and 15 of a 21-day cycle. Binimetinib was held for 2 days prior to and on day of each chemotherapy treatment.
Results:
Twelve patients enrolled in the phase I showed the MTD of binimetinib at 45 mg orally twice daily with gemcitabine 800 and cisplatin 20 mg/m2. Twenty-nine patients were treated in the phase II. Six patients treated at MTD in phase I were evaluable as part of phase II. PFS 6 months was 54% and RR was 36%. Median overall survival was 13.3 months (95% CI, 9.8–16.5). MSK-IMPACT 410-gene panel showed aberrations in the RAS–RAF–MEK–ERK pathway and mutations in PIK3CA, AKT2, PIK3CG, BRAF, and MAP3K1 in responding patients.
Conclusions:
Binimetinib with gemcitabine and cisplatin did not show an improvement in PFS 6 and RR. Molecular profiling may help select patients who may benefit from this triplet therapy, which is not planned at this time.
Introduction
Biliary tract cancer (BTC) includes intrahepatic, extrahepatic cholangiocarcinoma, and gallbladder cancer; the majority of patients present with advanced disease and survive less than one year from diagnosis. The standard-of-care treatment for patients with advanced disease was established by the phase III ABC-02 study, which randomized 410 patients with locally advanced or metastatic cholangiocarcinoma, gallbladder cancer, or ampullary cancer to receive either gemcitabine and cisplatin or gemcitabine alone (1). The median overall survival (OS) was 11.7 months among the cisplatin–gemcitabine group and 8.1 months among the gemcitabine group (P < 0.001), without substantial increase in toxicity with the combination regimen. Activation of the MAP kinase signaling cascade is commonly observed in BTCs and occurs by multiple mechanisms including ERBB2 over-expression, KRAS, BRAF, and NRAS mutations (2). Two prior studies have assessed therapeutic targeting of the MEK pathway in patients with biliary tract cancer. A phase II trial evaluating the MEK inhibitor selumetinib in a mixed population of pretreated and treatment-naïve patients with biliary tract cancer reported an objective response rate (RR) of 12%, and a median progression-free survival of 3.7 months (3). A phase I study of binimetinib included 30 patients with advanced biliary tract cancer and reported an objective RR of 10% (4). In vivo studies performed on human tumor explants have shown that binimetinib activity is potentiated by gemcitabine and cisplatin, when given in an interrupted dosing schedule, providing a rationale for the combination evaluated in this phase I/II trial.
The primary objective of the phase I part of the study was to evaluate the safety and the recommended phase II dose (RP2D) of binimetinib in combination with gemcitabine and cisplatin. The dose-limiting toxicities (DLT) were defined as listed in Table 1. The primary endpoints of the phase II study were to determine progression-free survival (PFS) 6 and RR once the RP2D of binimetinib in combination with gemcitabine and cisplatin was established. Patients treated at the MTD during the phase I were included in the analysis for the primary and secondary endpoints of phase II.
Table 1.
DLT criteria
DLT Criteria:
|
Patients and Methods
This was a single arm, single institution nonrandomized, open-label, phase I/II study.
Patients
Patients (age ≥ 18 years), ECOG 0–1 with metastatic BTC, and measurable disease per RECIST (version 1.1) were enrolled (5). No prior systemic therapy for metastatic BTC was permitted. Patients who received prior chemotherapy in the adjuvant setting were eligible if more than 6 months had passed since completion of the adjuvant treatment. Patients were required to have adequate bone marrow (hemoglobin ≥ 8 g/dL, absolute neutrophil count ≥ 1,500/mcL, platelets ≥ 100,000/mcL), renal (serumcreatinine < 1.6 mg/dL and/or measured creatinine clearance from 24-hour urine collection of ≥ 60 mL/minute), and hepatic functions (total bilirubin ≤ 2 mg/dL, ALT/AST ≤ 5 × ULN). Patients with biliary obstruction could join if bilirubin was corrected to required limit after adequate biliary drainage. Patients with symptomatic uncontrolled brain metastases or gastrointestinal malabsorption were excluded.
In view of the binimetinib ophthalmologic serous retinopathy toxicity (6), patients with known evidence of central serous retinopathy (CSR), retinal vein occlusion (RVO) or ophthalmopathy at baseline that would be considered a risk factor for CSR or RVO, were excluded.
Patients provided written informed consent. The study was registered with ClinicalTrials.gov NCT01828034. The study was conducted in accordance with International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki and was approved by the Institutional Review Board (IRB protocol 13–004).
Treatment
In the phase I component of the study, a classic 3 + 3 cohort dose escalation scheme was used to identify the MTD of binimetinib when administered with gemcitabine and cisplatin. Three dose levels were planned and evaluated. Patients were treated with oral binimetinib 30 mg twice daily (cohort 1) or 45 mg twice daily (cohorts 2 and 3) in combination with gemcitabine 800 mg/m2 (cohorts 1 and 2) or 1,000 mg/m2 (cohort 3) and cisplatin 20 mg/m2 both given weekly for 2 out of 3 weeks. Binimetinib was self-administered orally twice daily continuously. Gemcitabine and cisplatin were administered intravenously on day 8 and 15 of a 21-day cycle. Patients were instructed to hold binimetinib 2 days prior to gemcitabine and cisplatin administration and resume one day after. Imaging studies were obtained every 3 cycles (9 weeks) for patients on both the phase I and II study portions. Adverse event monitoring was completed at each visit. If treatment was held for toxicity, radiographic imaging and cardiac assessments continued per original treatment schedule (i.e., every 9 weeks ± 1 week from initiation of study treatment).
Safety evaluations
In each dose level, subjects were enrolled in cohorts of 3 to 6 patients. Subjects were monitored for DLT during the first cycle (21 days) and escalation could not occur until all subjects in the cohort had received at least 21 days of treatment. A DLT was defined as any of the events listed in Table 1 occurring during the first cycle attributed as possibly, probably or definitely related to the study treatment.
Safety and tolerability were assessed by adverse events (AE) and changes in laboratory parameters according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4; ref. 7). The number of events and number of subjects with any AEs were displayed by preferred term for each treatment group. Within each preferred term, subjects were counted only once if they had more than one event reported during the treatment period, and events were summarized using descriptive statistics. The percentage of subjects who experienced toxicity at each dose level was calculated.
Targeted gene sequencing
The secondary objectives of the phase I and II studies were to perform next-generation sequencing (NGS) on tissue samples to look for genotypic differences between tumors and correlate findings with response and resistance to therapy and anatomic/histologic classification. Previously collected samples (e.g., archival tissue from prior resection or biopsy) were used in all cases. A pathologist reviewed all tumor samples and macrodissection was performed as needed to enrich for tumor content. Tumors were profiled for somatic genomic alterations using MSK-IMPACT, an in-house, targeted NGS assay that analyzes all exons and selected introns of 410 cancer-associated genes (8, 9). Matched germline DNA from prospectively collected blood samples was analyzed in all patients. Although paired germline sequencing was employed for somatic mutation calling, we did not analyze samples for pathogenic germline mutations in this study.
Flow cytometric analysis of phosphorylated ERK
Peripheral blood mononuclear cells (PBMC) from serial blood samples, collected at baseline, cycle 1 day 3 (on binimetinib), cycle 1 day 8 (off binimetinib), and cycle 2 day 3 (on binimetinib) were analyzed for their ability to signal via the T-cell receptor (TCR) by phosphoflow measurement of pERK expression on stimulated CD4 and CD8 T cells. Study patients PBMC samples were thawed, counted, and allowed to rest overnight in a humidified tissue culture incubator at 37°C, 5% CO2 in RPMI culture media containing 30% autologous plasma to maintain presence of any circulating in vivo administered binimetinib. On the following day, cells were washed and resuspended in fresh 30% autologous plasma media and counted. Subsequently, cells were evaluated for their ability to induce pERK after brief T-cell stimulation with either anti-CD3 (OKT3, eBioscience) or 100 ng/mL phorbol-12-myristate 13-acetate (PMA, EMD Millipore) at 37°C. After stimulation for varying lengths of time (0–30 minutes), 4% paraformaldehyde (Alfa Aesar) was added to the cells and they were incubated for 10 minutes at 37°C to terminate the reaction and fix cells. Next, cells were permeabilized by addition of ice cold 95% methanol and incubation at 20°C for 30 minutes. Fixed and permeabilized cells were washed three times with PBS (Mediatech) containing 2% FBS (Gemini Bio-Products) and then stained with rabbit anti-pERK1/2 (D13.14.4E, Cell Signaling Technology) at room temperature for 30 minutes. After another wash, cells were stained overnight at 4°C with anti–CD3-Pacific Blue (UCHT1, BD Biosciences), anti–CD4-FITC (SK3, BD Biosciences), anti–CD8-PE (SK1, BD Biosciences), and donkey anti-rabbit IgG-APC (Jackson ImmunoResearch). Cells were washed three times the following day with PBS/2% FBS and acquired on a BD Fortessa flow cytometer, where they were evaluated for CD4 and CD8 T-cell expression of pERK. Flow cytometric analyses were performed using FlowJo software (FlowJo, LLC).
Statistical analysis
The primary objective of the dose escalation phase I portion of the study was to determine the safety and tolerability of binimetinib in combination with gemcitabine and cisplatin for advanced BTC and to determine the MTD and RP2D of binimetinib in combination with gemcitabine and cisplatin. Six patients treated at the MTD on the phase I study were eligible for inclusion in analysis of the primary and secondary endpoints of the phase II study. In the phase II study, an exact binomial single stage design was used to discriminate between true PFS 6 of 59% versus 82%, and between true response rates of 50% versus 26%. Of 35 patients to be enrolled to the phase II part, if at least 26 patients were observed to remain free of progression and survive for at least 6 months, or at least 14 responses were observed over one year, this agent would be considered worthy of further testing in biliary tract cancers. This design yields 91% power to detect a true PFS 6 rate of at least 82%, and/or a true RR of at least 50%. It yields a 0.05 probability of a positive PFS result if the true PFS 6 rate is no more than 59%, and/or a 0.05 probability of a positive RR result if the true RR is no more than 26%. Therefore, considering that PFS and RR are independent, the overall type 1 error is 0.10. Patients who dropped out of the study before 6 months due to toxicity or other reason without documented disease progression were counted as events for the primary endpoints.
PFS was calculated from study entry to documented disease progression or death from any cause, whichever occurred first. Patients who progressed at the 26-week planned scan, regardless of the actual calendar date of that scan, were declared as have progressed at 6 months. OS was calculated from study entry to death. A patient lost to follow-up was censored at last follow up. PFS and OS were estimated using the Kaplan–Meier methodology and compared between disease types (gallbladder vs. cholangio-carcinoma) using log-rank test. Fisher exact test was used to assess associations between categorical variables.
For the exploratory correlative study endpoints, molecular sequencing and determination of pERK expression on PBMCs was summarized descriptively by time point.
Results
Forty-five patients were enrolled to the study between September 2013 and September 2015. Among the 45 patients, 4 were deemed nonevaluable due to withdrawal of consent and not being treated. Demographics are summarized in Table 2. The study database cutoff for data analysis was June 2017. All patients had completed study treatment at that time.
Table 2.
Patient demographics
| Characteristic | Phase I (n =12) |
Phase II (n = 35)a |
All (n = 41) |
|---|---|---|---|
| Age | |||
| Median (SD) | 69 (9.7) | 66 (9.7) | 66 (9.6) |
| Range | (47–76) | (45–83) | (45–83) |
| Gender, n (%) | |||
| Male | 5 (41) | 19 (86) | 21 |
| Female | 7 (58) | 16 80) | 20 |
| Karnofsky status, n (%) | |||
| 80 | 6 (21) | 22 (79) | 27 |
| 90 | 1 (10) | 9 (90) | 10 |
| 100 | 0 (0) | 4 (100) | 4 |
| Disease, n (%) | |||
| Gallbladder ca | 1 (8) | 11 (82) | 12 |
| IHC | 5 (20) | 20 (80) | 24 |
| EHC | 1 (20) | 4 (80) | 5 |
Twenty-nine patients were enrolled into phase II, but 6 patients from phase I continued into phase II for a total of 35.
Dose escalation phase
Patients were treated with oral binimetinib 30 mg twice daily (cohort 1) or 45 mg twice daily (cohorts 2 and 3) in combination with gemcitabine 800 mg/m2 (cohorts 1 and 2) or 1,000 mg/m2 (cohort 3) and cisplatin 20 mg/m2 both given weekly for 2 out of 3 weeks. Three patients were sequentially enrolled to each of cohorts 1 and 2; no DLTs were observed. Three patients were enrolled to cohort 3; 2 patients had a DLT of grade 4 neutropenia. Thus, an additional 3 patients were enrolled to cohort 2; no further DLTs were observed at this dose level. The MTD and RP2D of binimetinib in combination with gemcitabine 800 mg/m2 and cisplatin 20 mg/m2 was 45 mg orally twice daily, with binimetinib held 2 days prior to and resumed one day following chemotherapy. These 6 patients were eligible for assessment of the primary and secondary endpoints of the phase II study.
Phase II study
Twenty-nine patients along with the 6 patients from phase I described above, for a total of 35 patients, were enrolled and treated with oral binimetinib 45 mg twice daily in combination with gemcitabine 800 and cisplatin 20 mg/m2, with binimetinib held 2 days prior to and resumed one day following chemotherapy. In the event of toxicity, dose reductions and interruptions were permitted per protocol.
Toxicity
Hematologic toxicities mainly due to the gemcitabine and/or cisplatin, and liver dysfunction possibly related to the combination were the most common reported adverse events. Skin toxicity due to binimetinib was also commonly noted (Table 3). Ninety percent of patients experienced grade 3 to 4 AEs. The most frequent grade 3 to 4 toxicities included leukopenia and anemia, which occurred at rates higher than expected with chemotherapy alone (1). Although hyponatremia has been reported from the use of cisplatin, this plus the hyperlipasemia may have been contributed by the combination (Table 4).
Table 3.
Most frequent AEs, regardless of relationship to any drugs
| Most frequent events (occurring in ≥ 10% patients) | Cohort 1(%) n =12 |
Phase II (%) n = 35a |
All n = 41) |
|---|---|---|---|
| Hypoalbuminemia | 12 (100) | 32 (91) | 38 (93) |
| Anemia | 12 (100) | 32 (91) | 38 (93) |
| Alkaline phosphatase increased | 12 (100) | 31 (89) | 37 (88) |
| Thrombocytopenia | 10 (83) | 30 (86) | 36 (88) |
| Aspartate aminotransferase increased | 7 (57) | 28 (80) | 31 (76) |
| Rash acneiform | 8 (67) | 19 (54) | 27 (66) |
Table 4.
Most frequent grade 3–4 adverse events
| Most frequent grade 3–4 events (occurring in ≥ 10% patients) | Phase I (%) n =12 | Phase II (%) n = 35 | Total (%) N = 41 |
|---|---|---|---|
| Neutropenia | 5(42) | 17 (49) | 22 (54) |
| Leukopenia | 4 (33) | 16 (46) | 20 (49) |
| Anemia | 3 (25) | 14 (40) | 17 (41) |
| Elevated lipase | 4 (33) | 11 (31) | 15 (37) |
| Hyperbilirubinemia | 1 (8) | 7 (20) | 8 (20) |
| Hyponatremia | 0 (0) | 6 (17) | 6 (15) |
Response
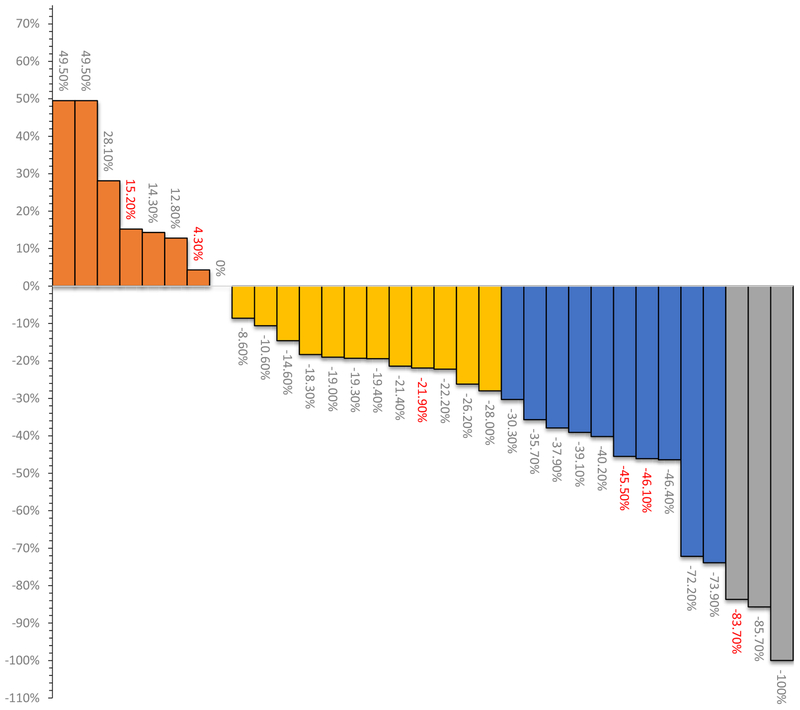
Of the 35 patients evaluable for the primary endpoints of the phase II study, the overall response rate was 36% [12 complete response (CR)/partial response (PR) among 35 patients; 95% confidence interval (CI), 20.4%–52.7%]. Therefore, this study did not meet the prespecified primary endpoint of at least 14 responses (40%) observed over one year. The best radiographic responses were as follows: 3 had CR (8.6%), 9 PR, 14 had stable disease (SD; 28.6%), and 7 (20%) had progressive disease (PD; Fig. 1). Two patients were not evaluable for radiologic response due to clinical progression or withdrawal prior to first scan and were counted as progression events. Radiographic responses by disease type were gallbladder 45%, and cholangio-carcinoma 32% CR/PR, respectively. Two of the 3 CRs were considered as such, as RECIST 1.1 implies CR in case of disappearance of all target lesions, while target pathologic lymph nodes could have a reduction in short axis to <10 mm.
Figure 1.
Waterfall plots indicating best percentage change from baseline in overall response according to RECIST 1.1 (n = 35). Two patients were not evaluable for response due to clinical progression and withdrawal before the first scan. Green bars represent a complete response (CR), blue bars represent partial response (PR), purple bars represent stable disease (SD), and red bars represent progression of disease (PD). Indicated in red are patients who were permitted to move to single-agent binimetinib following at least one cycle of the triplet regimen.
PFS and OS
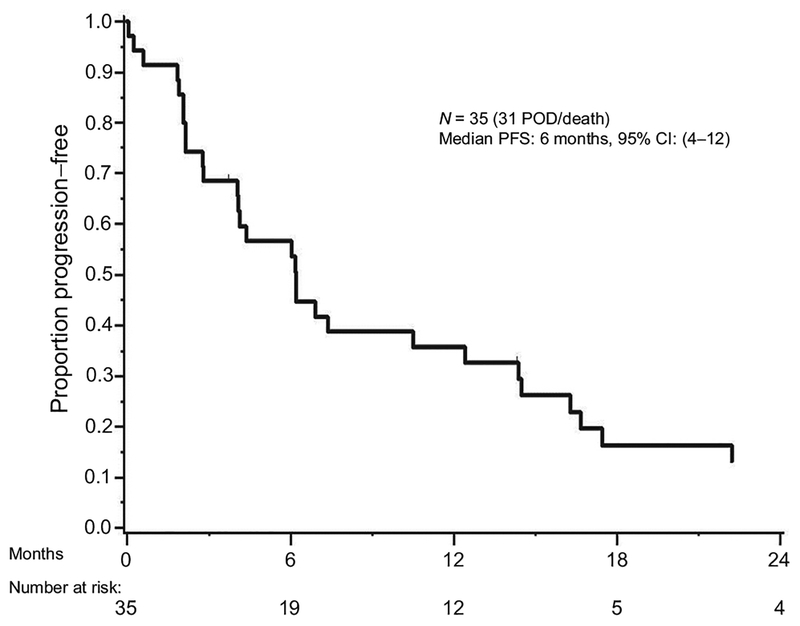
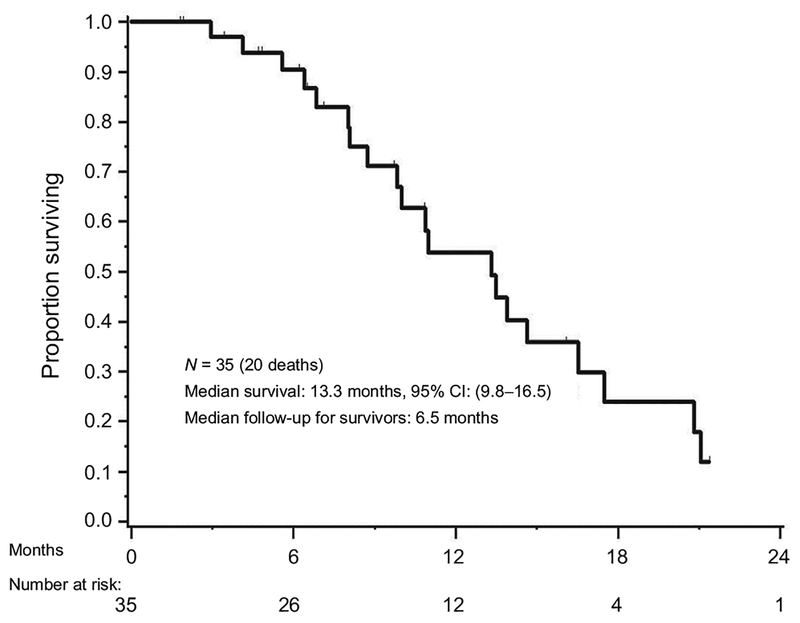
With the median PFS from treatment of 6 (95% CI, 4–12) months, we observed total of 31 with disease progression or death (Fig. 2). Nineteen of 35 patients (54%) were progression free at 6 months. Therefore, this study did not meet the prespecified primary endpoint where at least 26 patients (74%) were required to remain free of progression for at least 6 months. PFS did not differ significantly by disease type (P = 0.416); PFS at 6 months was 63.6% (95% CI, 40.7–99.5] for gallbladder cancer and 53.5% (95% CI, 36.7–78.1) for cholangiocarcinoma, respectively. Twelve patients and 4 patients were alive and progression free at 12 months, and 24 months from treatment, respectively. Median OS from start of study treatment was 13.3 months (95% CI, 9.8–16.5; Fig. 3).
Figure 2.
Overall PFS of patients treated with binimetinib in combination with gemcitabine/cisplatin chemotherapy. Median PFS is 6 months.
Figure 3.
Kaplan–Meier curve for median OS in patients treated with binimetinib in combination with gemcitabine/cisplatin chemotherapy. Median OS is 13.3 months.
Targeted gene sequencing
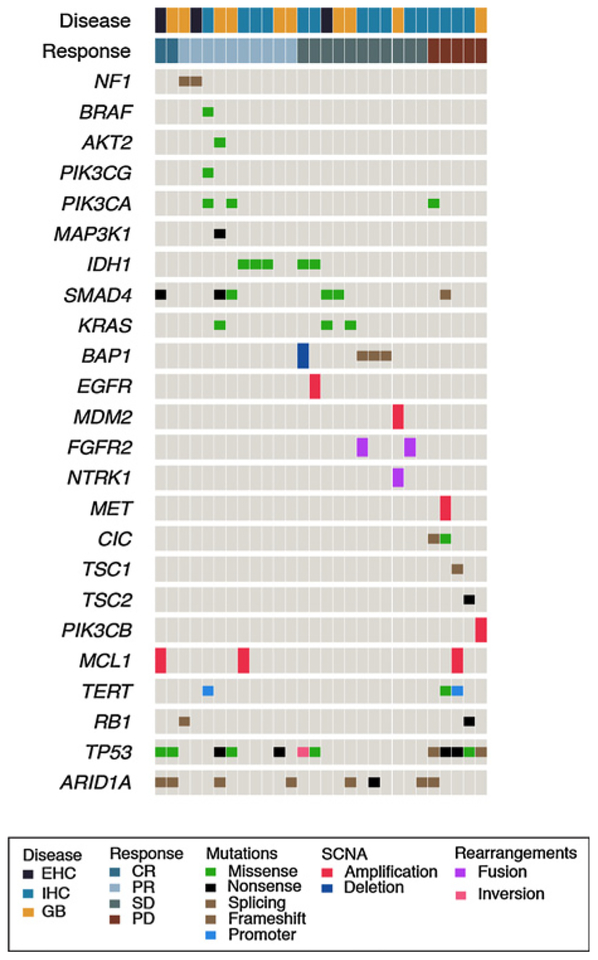
Archival tumors samples for targeted NGS using the MSK-IMPACT platform were available from 28 patients treated on the phase I/II study (Fig. 4). An additional 2 patients who did not have archival tissue available but achieved a CR to study therapy had a tumor biopsy performed at time of disease progression for NGS to evaluate for potential markers of acquired resistance to binimetinib. Among patients with CR or PR, alterations in the MAPK pathway were seen in 4 of 12 (33.3%) patients (KRAS L19F, NF1 del, NF1 splice, BRAF G111S), whereas 2 of 16 (12.5%) patients with SD or PD had KRAS Q61H mutations. Alterations in the PI3K pathway were seen in 4 of 12 patients (33.3%) who responded and 2 of 16 patients (12.5%) who progressed.
Figure 4.
IMPACT oncoprint. Note that in both CR patients, tumor samples for sequencing were obtained at time of disease progression and no pretreatment archival tissue was available.
Flow cytometric analysis of phosphorylated ERK
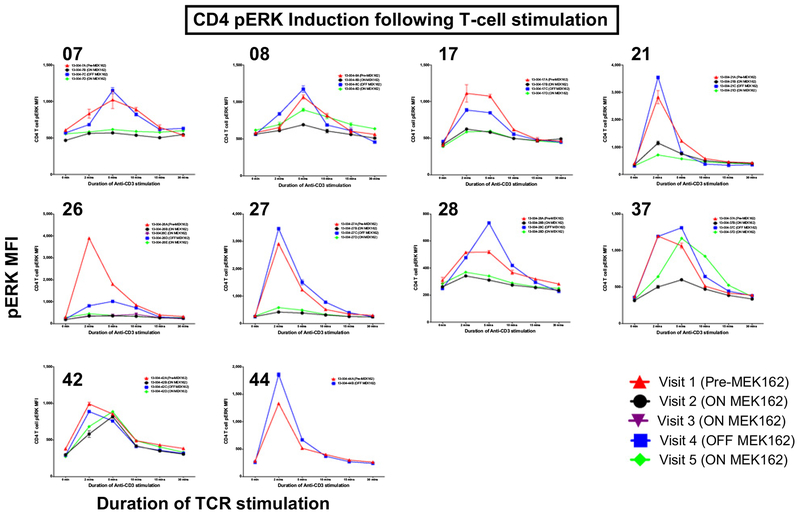
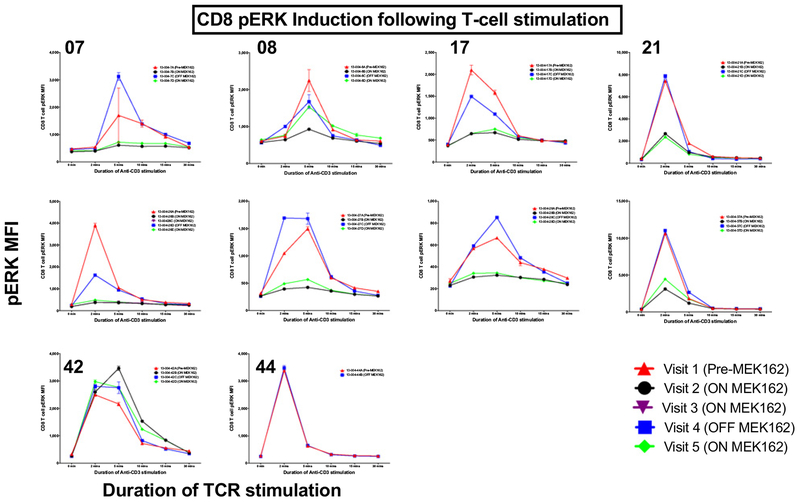
Thirty-nine samples from 10 patients (5 with PD as best response, 5 with PR as best response) underwent kinetic analysis of pERK induction after TCR stimulation (Fig. 5). For 8 of 10 (80%) patient samples, decreased phosphorylation of ERK after TCR stimulation was observed on C1D3 of binimetinib treatment for both CD4+ and CD8+ T cells; this was followed shortly by recovery of pERK levels on C1D8 (when patients were off binimetinib 48 hours) and then further inhibition of pERK signaling on C2D3 of binimetinib treatment in the majority of patients. In 1 of 10 patient, who progressed on treatment, no change in pERK was observed. One additional patient who progressed on treatment was off binimetinib at the 2 time points evaluated, and therefore, no change in pERK induction was expected or observed. It was observed that the patient with little change in pERK induction following binimetinib administration exhibited disease progression. However, there were also samples from patients who progressed that showed decreased pERK on treatment.
Figure 5.
Phospho flow combined results: Summary kinetics graphs of CD4 (top) and CD8 (bottom) phospho-ERK expression (mean fluorescence intensity values of pERK staining) at 0, 2, 5, 10, 15, and 30 minutes after anti-CD3 (OKT3) stimulation of patient PBMC samples collected at the visits indicated (red triangle = visit 1; black circle = visit 2; purple upside down triangle = visit 3; blue square = visit 4; green diamond = visit 5). Numbers on the top left corner of each graph refer to the studied patient sequential number on study.
Discussion
In this phase I/II study, we identified the MTD of binimetinib in combination with gemcitabine 800 mg/m2 and cisplatin 20 mg/m2 as 45 mg orally twice daily. Per prespecified primary endpoints of the study, if at least 26 of 35 patients enrolled to the phase II study remained free of progression for at least 6 months, or at least 14 responses were observed over one year, the combination would be considered worthy of further testing in biliary tract cancers. This design yielded 91% power to detect a true PFS 6 rate of at least 82%, and/or a true RR of at least 50%. Although significant responses to therapy were observed in a minority of patients, the phase II study did not meet either of its prespecified primary endpoints and further development of this combination is not planned or anticipated at this time.
We observed on-target effect of decreased phosphorylation of ERK in patients while receiving binimetinib treatment and recovery of pERK levels following discontinuation of MEK inhibition for 48 hours. These observations however remain descriptive.
Comprehensive molecular profiling of archival tissue from 28 patients treated on study identified somatic alterations in the MAP kinase pathway at baseline in a subgroup of responders to the combination therapy, including alterations in KRAS, BRAF, and NF1, a finding that requires prospective evaluation.
The activity of gemcitabine and cisplatin in patients with advanced biliary tract cancer was confirmed in the ABC-02 trial(1), a randomized phase III trial comparing the combination with gemcitabine alone. In this large study, gemcitabine 1,000 mg/m2 and cisplatin 25 mg/m2 were given weekly for 2 weeks followed by a week of rest for a total of 8 cycles over 6 months. Compared with gemcitabine, combination therapy significantly improved OS (11.7 vs. 8.1 months, P < 0.001), PFS (8.0 vs. 5.0 months, P < 0.001), and disease control (81.4% vs. 71.8%, P = 0.049). Differential activity was not seen among the various primary tumor sites along the biliary tree (1). In this study, we gave a lower dose of gemcitabine and cisplatin than in the ABC-02 (1) study, and continued chemotherapy beyond 8 cycles in patients without toxicity necessitating discontinuation. We noted increased hematologic toxicity with the triplet combination than was noted in the ABC-02 study. The rate of neutropenia, leukopenia, and anemia, respectively, were 54%, 49%, and 41% in this study, as compared with rates of 25%, 15.7%, and 7.6% in the gemcitabine-cisplatin arm of the ABC-02 study (1). This may indicate increased hematologic toxicity with the addition of binimetinib as was noted in the phase I dose escalation portion of the study. Alternatively, this may reflect the cumulative hematologic toxicity of chemotherapy as treatment on our study was continued in the absence of significant toxicity or progression rather than discontinued after 8 cycles as per the ABC-02 study. Although it is possible that lack of benefit with the triplet regimen may have been due to decreased dose intensity of the known active chemotherapy agents, we consider it more likely that the addition of MEK inhibitor simply did not augment the activity of chemotherapy in the majority of patients on the study. Supporting this hypothesis, the response rates, progression and overall survival reported here compare favorably with the ABC-02 data to indicate that no detrimental effect of the lower dose of gemcitabine and cisplatin was observed.
The relatively deep and occasionally durable responses to therapy observed in this study may reflect efficacy of the cytotoxic regimen alone; however, two prior studies have reported radio-graphic responses to single-agent MEK inhibition in patients with previously treated advanced biliary cancer (3, 4). This indicates that a subset of patients with biliary tract cancer are inherently sensitive to MEK inhibition, and the durability of response may indicate the combination therapy with addition of chemotherapy delays time to resistance and enhances sensitivity. Our approach for treating all patients may prove in retrospect to be wrong, and prior selection of patients may be required.
Because we initiated the study of the combination of gemcitabine, cisplatin and binimetinib, a phase I study evaluated the same combination of gemcitabine and cisplatin plus selumetinib (10) at the standard doses of ABC-02 (1), with no dose interruptions. They identified selumetinib 75 mg twice daily as the recommended phase II dose in combination, with acceptable toxicity. They noted a radiographic response rate of 20% among 8 patients evaluable for response. It is unclear whether there will be a follow-up to that study, additional molecular correlative studies are underway.
To summarize, binimetinib can be safely combined with gemcitabine and cisplatin in advanced BTC. However, the efficacy signals observed were modest and not superior to gemcitabine and cisplatin alone, and further development of this combination is not planned or anticipated at this time.
Translational Relevance.
The commonly noted activating mutations in the RAS/RAF/MEK/ERK signaling pathway in biliary tract cancers make them a putative target for therapy. Preclinical data support antitumor synergy for binimetinib (MEK162) when combined with chemotherapy. Previous work reported in CCR has demonstrated that the combination of a MEK inhibitor plus gemcitabine is highly schedule-dependent, and is predicted to be more effective in the clinic using sequential rather than simultaneous dosing protocols. On the basis of these arguments, the combination of binimetinib and gemcitabine plus cisplatin, the latter a standard-of-care regimen for biliary tract cancer, was studied in a similar schedule-dependent fashion, yet did not show any improvement in 6-month progression-free survival (PFS 6) and response rate (RR). Targeted gene sequencing showed that alterations in the PI3K pathway were seen in one third of responders and a limited number of nonresponders. Flow cytometric analysis of phosphorylated ERK in peripheral blood mononuclear cells showed on-target effect of decreased phosphorylation of ERK in patients while receiving binimetinib treatment, and recovery of pERK levels following discontinuation of MEK inhibition for 48 hours was noted. This, however, failed to show or suggest that binimetinib target engagement informs clinical prognosis.
Acknowledgments
This work was supported by Novartis and Array Biopharma.
Disclosure of Potential Conflicts of Interest
M.A. Lowery is a consultant/advisory board member for Agios Pharmaceuticals, Celgene, EMD Serono, and Roche. J.J. Harding is a consultant/advisory board member for Bristol-Myers Squibb, CytomX, Eisai, and Eli Lilly, and reports receiving commercial research grants from Bristol-Myers Squibb. M. Berger is a consultant/advisory board member for Roche and reports receiving commercial research support from Illumina. K.H. Yu is a consultant/advisory board member for Halozyme and Ipsen, reports receiving commercial research support from Halozyme, and was an expert witness for West-Ward Pharmaceuticals in Novartis Pharmaceuticals Corporation et al v. West-Ward Pharmaceuticals International Limited (District Court of Delaware, Civil Action No. 15-cv-474-RGA). A. Cercek is a consultant/advisory board member for Bayer, and reports receiving commercial research support from AbbVie, Amgen, and Seattle Genetics. E.M. O’Reilly and G.K. Abou-Alfa are consultants/advisory board members for 3DMedcare, Agios, Alignmed, Amgen, Antengene, Aptus, Aslan, Astellas, AstraZeneca, Bayer, Beigene, Bioline, BMS, Boston Scientifc, Bridgebio, Carsgen, Celgene, Casi, Cipla, CytomX, Daiichi, Debio, Delcath, Eisai, Exelixis, Genoscience, Gilead, Halozyme, Hengrui, Incyte, Inovio, Ipsen, Jazz, Jansen, Kyowa Kirin, LAM, Lilly, Loxo, Merck, Mina, NewLink Genetics, Novella, Onxeo, PCI Biotech, Pfizer, Pharmacyte, Pharmacyclics, Pieris, QED, Redhill, Sanofi, Servier, Silenseed, Sillajen, Sobi, Targovax, Tekmira, Twoxar, Vicus, Yakult, and Yiviva. No potential conflicts of interest were disclosed by the other authors.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–81. [DOI] [PubMed] [Google Scholar]
- 2.Tannapfel A, Sommerer F, Benicke M, Katalinic A, Uhlmann D, Witzigmann H, et al. Mutations of the BRAF gene in cholangio-carcinoma but not in hepatocellular carcinoma. Gut 2003;52:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekaii-Saab T, Phelps MA, Li X, Saji M, Goff L, Kauh JS, et al. Multi-Institutional Phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 2011;29:2357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendell JC, Javle M, Bekaii-Saab TS, Finn RS, Wainberg ZA, Laheru DA, et al. A phase 1 dose-escalation and expansion study of bini-metinib (MEK162), a potent and selective oral MEK1/2 inhibitor. Br J Cancer 2017;116:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RECIST Working Group. RECIST 1.1. Available from: http://recist.eortc.org/recist-1-1-2/.
- 6.van Dijk EHC, van Herpen CML, Marinkovic M, Haanen JB, Amundson D, Luyten GP, et al. Serous retinopathy associated with mitogen-activated protein kinase kinase inhibition (Binimetinib) for metastatic cutaneous and uveal melanoma. Ophthalmology 2015;122:1907–16. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. CTCAE Version 4. Available from: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf. [Google Scholar]
- 8.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagnost 2015;17:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res 2018;24:4154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridgewater J, Lopes A, Beare S, Duggan M, Lee D, Ricamara M, et al. A phase 1b study of selumetinib in combination with cisplatin and gemcitabine in advanced or metastatic biliary tract cancer: the ABC-04 study. BMC Cancer 2016;16:153. [DOI] [PMC free article] [PubMed] [Google Scholar]