Abstract
Background
Hidradenitis suppurativa (HS) is a chronic and painful skin disease. In addition, HS lesions may be associated with pus and odour, potentially leading to significant stigma and, consequently, greatly affected quality of life (QOL). QOL is a multidimensional construct, which can be measured in various ways. However, generic or dermatologic QOL measures may not capture changes in QOL particularly affected in HS. Accordingly, patients and experts included in the HIdradenitis SuppuraTiva cORe outcomes set International Collaboration (HISTORIC) agreed that future clinical HS trials should measure HS-specific QOL.
Objectives
To develop an HS-specific QOL instrument (HiSQOL, Hidradenitis Suppurativa Quality of life).
Method
The initial phases of the questionnaire development, described in this study, included item generation by patient interviews, development of a pilot questionnaire, questionnaire refinement, and pilot testing.
Results
For item generation, 21 patients were interviewed individually or in focus groups. Analysis of the interviews identified 105 candidate items and, next, a pilot questionnaire was developed. Finally, item reduction and two rounds of pilot testing resulted in a 23-item questionnaire representing physical, psychological, and social QOL dimensions.
Conclusions
We have comprehensively explored on HS's possible effect on the QOL of the affected individuals and identified a 23-item HS-specific QOL questionnaire. The questionnaire proved to be feasible, acceptable, and comprehensible in the second round of pilot testing. With HiSQOL, researchers can measure HS-specific QOL in future clinical trials, potentially enabling them to discover more effective treatment options. It is envisaged, that after thorough validation in a trial setting, a streamlined version of HISQOL may also become available for clinical use in daily practice.
Key Words: Hidradenitis suppurativa, Quality of life, Questionnaire development, Patient-reported outcome measures
Introduction
Quality of life (QOL) is a multidimensional construct, which can be measured in various ways. Hidradenitis suppurativa (HS) is a chronic, painful, and debilitating skin disease with an estimated prevalence of 0.1–4% worldwide [1, 2]. The primary lesions are inflammatory nodules that may develop into abscesses and sinus tracts with subsequent scarring, affecting flexural sites such as the axillae and groins [1, 3].
A central characteristic of HS is pain. Pain during the disease course is experienced by 97% of patients with HS [4], and frequently, patients report that pain limits their physical functioning and activities of daily living, such as doing household, exercising, or moving around [5]. HS lesions may, furthermore, be associated with a malodorous discharge. Most patients are well aware of this fact and find their symptoms embarrassing and repulsive and often fear people's reaction. In addition, patients may feel embarrassed about visible active disease or scars, and HS may, consequently, lead to significant stigma [5, 6]. Furthermore, patients with HS report of emotional reactions such as sadness, anger, irritation, and loss of control, and all in all, HS may greatly affect physical, psychological, and social QOL dimensions [5, 6, 7, 8, 9, 10, 11]. Notably, HS patients are at a greater risk of completed suicide than the background population [12], and although no causality has been established, this fact does emphasise the potentially profound consequences of HS.
Interventions for HS should thus aim to improve QOL, and clinical trials should measure QOL as a matter of routine. However, QOL can be measured with different types of outcome measurement instruments. Dermatologic-specific QOL instruments, such as Dermatology Life Quality Index (DLQI) [13] and Skindex [14], were used to measure QOL in several HS trials [15]. These instruments, however, were developed with the broader dermatologic patient in mind, and the instruments may not capture changes in QOL particularly affected in HS. Accordingly, patients and experts included in the HIdradenitis SuppuraTiva cORe outcomes set International Collaboration (HISTORIC) agreed that future clinical HS trials should measure HS-specific QOL [16].
No validated HS-specific QOL instrument exists to date, and the purpose of the present study is thus to launch the development of such an instrument (HiSQOL, Hidradenitis Suppurativa Quality of Life). With HiSQOL, researchers can measure HS-specific QOL in future clinical trials, potentially enabling them to discover more effective treatment options.
Materials and Methods
The study is reported in accordance with the EQUATOR network recommendations, using the Standards for Reporting Qualitative Research (SRQR) statement [17].
All interviews and pilot tests were conducted by an experienced interviewer (MA in psychology and education [S.E.]). Data analysis was performed by S.E. using interpretative phenomenological analysis, where the researcher's own conceptions and interpretative activity are seen as playing an active role in the dynamic process of interpreting and analysing.
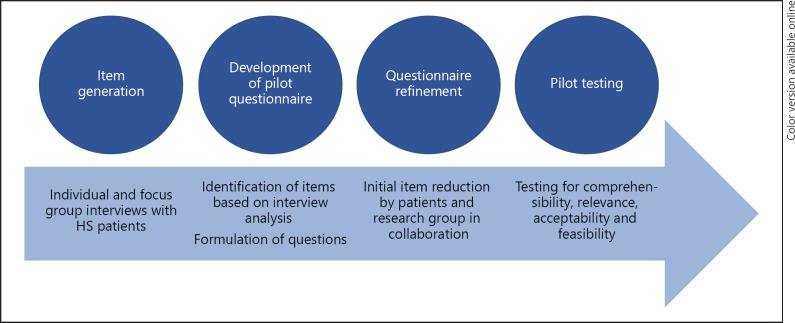
The initial phases of the questionnaire development, described in this study, included item generation, development of a pilot questionnaire, questionnaire refinement (initial item reduction), and pilot testing (Fig. 1). All patients were identified from our outpatient clinic, and all patients with a physician-confirmed diagnosis of HS and older than 18 were eligible. A representative diversity of age, sex, disease severities, and treatment types were invited.
Fig. 1.
Study overview. Flowchart of the methods used. See the Methods section for details.
Item Generation
Both individual and focus group interviews were conducted. All interviews were initiated with questions about HS location and severity, pain due to HS, disease duration, and completion of the DLQI questionnaire [13]. The individual interviews were initially open-ended and included questions such as “How does HS affect your daily life?,” “Do any activities trouble you or do you even avoid certain activities?” (2008–2010). Later, the interviews were more centred on QOL with questions such as “What is QOL?,” “What is QOL to you?,” “Does HS impact your QOL?,” “What is needed to change your QOL?,” “What is most important to change?” (2014–2016).
The focus group interviews were semi-structured based on the running analysis of the individual interviews. A mixture of former and new participants facilitated the generation of new items. All interviews were tape recorded and transcribed verbatim. Interviews continued until the point of saturation, i.e. when no further items were identified.
In addition to the interviews, a review of the literature was done to check whether previous studies had identified any items that were not identified in the interviews. A systematic search of studies published before January 19, 2015, was performed in collaboration with an information specialist using the databases PubMed, Embase, Cinahl, and Web of Science. The search strategy was constructed to include HS and QOL and terms related to QOL (see online suppl. material; for all online suppl. material, see www. karger.com/doi/10.1159/000496234). All studies on HS and topics related to QOL were included and screened for relevance by three authors (S.E., L.T., I.M.).
Development of the Pilot Questionnaire
The pilot questionnaire was developed based on the interview analysis. The qualitative method used was an iterative process of moving back and forth between interviews, transcription, analysis, and new interviews to expand the interviewer's knowledge on the impact of HS. This qualitative method provided a depth of insight into the complexity of meaning and reasoning in relation to HS. The analysis aimed at identifying central topics of general relevance to HS patients. The interviews were initially examined for units of meaning, coded as items and grouped into categories whenever a topic was identified as having HS-specific importance. To enhance trustworthiness, some of the interviews (n = 5) were analysed by two researchers (S.E. and I.M.). Based on the analysis, a list of items covering a whole range of perspectives on HS and QOL was developed.
In the preparation of the questionnaire, each item was used in series of questions initiated with “Over the last week, ….” in one or more varying versions. The 1-week timeframe was chosen given the structure of surveys that measure changes over time. Each question was evaluated on a Likert scale of “Not at all, A little, A lot, Very much, or Do not know/Not relevant.”
Questionnaire Refinement (Initial Item Reduction)
First, the questions were presented to groups of patients, doctors, nurses, and the interviewer in three rounds. All participants were asked to select the questions that they found most relevant to pose in a questionnaire. Rephrasing was possible. It was underlined that questions should be case and time sensitive. After each round, non-chosen questions were removed, and reformulated questions were added. Following round three, questions that were judged relevant by 4 out of 8 were kept; the remaining questions were removed.
Secondly, the questionnaire was tested among participants of an HS information meeting. Preliminary “work domains” were created, and responses were statistically analysed for correlations, redundancy, and missing answers. This information was used in further discussions, but no decisions were made based on this information alone, since the number of responses was limited.
Thirdly, the questionnaire was tested for relevance in a smaller sample of patients in a fourth round. The patients were asked to divide the questions into categories of “very important, less important, should be reformulated, or should be removed.”
Finally, the questionnaire was presented at a focus group meeting with patients, doctors, and the interviewer. Here, each question was assessed in the light of “uniqueness, responsiveness, relevance, diversity, and importance.” Based on this discussion, the questionnaire was reduced.
Pilot Testing
The reduced questionnaire was pilot tested using “the Three Step Test Interview” [18]. The three-step test interview combines the “think aloud” method and the “probing method” and has been described as a very powerful tool with which to establish whether the patients understand the questions, whether they do so in a consistent way, and in the way the researcher intended [19].
Here, the patients responded to the questions aloud in front of the interviewer and were asked to clarify any unclear responses and comment on each question. Finally, the interviewer asked the patients to detail their response to each question. The interviewer took notes from the tape-recorded interviews and made an overview of the comments for evaluation.
Following evaluation in the project group, some questions were reformulated and others were left out. A new version of the questionnaire was then pilot tested again using the same method.
Results
Item Generation
Twenty-one patients (range 19–63 years) were interviewed individually or in focus groups. Demographic and patient characteristics data are found in Table 1. Patients reported experiences with a variety of treatments including topical treatments, antibiotic treatments, biologic treatment, and surgery.
Table 1.
Patient characteristics
| Variables | Item generation (n = 21) | Questionnaire refinement (n = 9) | Pilot testing 1 (n= 20) | Pilot testing 2 (n= 22) |
|---|---|---|---|---|
| Age, years | 37.9+10.8 | 43.8 | 39.2 | 37.2 |
| Female | 13 (62) | 6 (67) | 18 (90) | 15 (68) |
| Hurley stage | 2.1+0.6 | |||
| 1 | 3 (14) | n.a. | n.a. | n.a. |
| 2 | 12 (57) | n.a. | n.a. | n.a. |
| 3 | 6 (29) | n.a. | n.a. | n.a. |
| Disease durationa, years | 19.8+10.0 | |||
| 0–3 years | 1 (5) | − | 2 (10) | 4 |
| 4–10 years | 3 (14) | − | 1 (5) | 2 |
| 11–19 years | 9 (43) | 2 (22) | 3 (15) | 5 |
| 20 years or more | 8 (38) | 7 (78) | 14 (70) | 11 |
| HS location | ||||
| Axillae | 17 (81) | 8 (89) | 12 (60) | 18 (82) |
| Groin, genital area | 17(81) | 8(89) | 14(70) | 16(73) |
| Other locations | 9 (43) | 4 (44) | 7 (35) | 8 (36) |
| DLQI scorea (0–30) | 10.0+8.2 | n.a. | n.a. | n.a. |
| 0–1 | 3 (17) | n.a. | n.a. | n.a. |
| 2–5 | 3 (17) | n.a. | n.a. | n.a. |
| 6–10 | 3 (17) | n.a. | n.a. | n.a. |
| 11–20 | 7 (39) | n.a. | n.a. | n.a. |
| 21–30 | 2 (11) | n.a. | n.a. | n.a. |
| Treatment | ||||
| Local | 21 | n.a. | n.a. | n.a. |
| Systemic | 6 | n.a. | n.a. | n.a. |
| Biologic | 5 | n.a. | n.a. | n.a. |
| Surgery | 12 | n.a. | n.a. | n.a. |
Data are presented as mean ± standard deviation or n (%). DLQI, Dermatology Life Quality Index; n.a., not assessed.
Missing values for 3 patients.
Fifteen individual interviews and five focus group interviews were performed. Some patients participated only in individual interviews or focus groups, while some participated in both. The interviews were conducted from 2008 to 2016. Duration of the individual interviews was 1–1½ h, a few 2 h; the focus group interviews lasted 2– 3 h. Saturation was reached before the last focus group interview.
A total of 415 studies were identified in the literature review. No items that were not identified in the interviews were found.
The Pilot Questionnaire
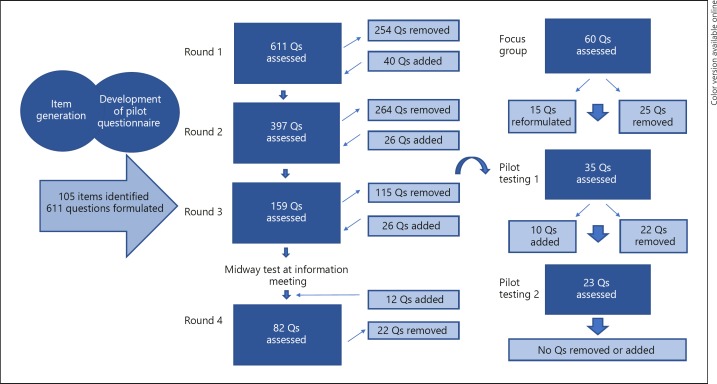
Analysis of the interviews identified 105 candidate items for the questionnaire (Table 2). Questions were formulated covering each of the items. In the primary round, the 105 items were represented with 1–18 different versions/formulations of questions resulting in a total of 611 questions.
Table 2.
Items generated
|
Psychological Emotional reactions because of HS Powerlessness due of HS Irritation because of HS Embarrassed because of HS Anger because of HS Sorrowful because of HS Depression because of HS Fear because of HS |
Desperate because of HS Suicidal thoughts because of HS Inconsolable because of HS Apathy because of HS Own fear of contagiousness Stressed because of HS HS impact on self-esteem HS impact on identity HS impact on mood |
Hopelessness because of HS HS impact on quality of life Feelings of lack of control because of HS Coping with HS Uncontrollable Unpredictable Acceptance of life with HS Will power in spite of HS Will to live despite HS Effort to do self-support because of HS |
|
Psychosocial Self-stigmatization because of HS Embarrassed because of HS Avoidance of nakedness in front of others because of HS Avoidance of common baths because of taboo linked to HS |
Hides the disease because of taboo linked to HS Feel disgusting because of HS Feel different from others because of HS People stare because of scars from HS |
Other people's acceptance in spite of HS Expectance of stigmatization because of smell from HS Expectance of prejudices because of boils and scars from HS Other people's fear of contagiousness |
|
Social Explanation of the disease to other people White lies about visible scars and boils Support from meeting other people with HS Support from surroundings to live with HS Helplessness because of HS Dependent on others because of HS Cancellations of appointments because of HS |
Isolation because of HS Nervous to go out to meet other people because of HS Avoidance of social contact because of HS Unconfident among other people because of HS flares HS impact on holiday planning |
HS impact on sexuality HS impact on relationship to partner Support from partner concerning HS Neglect of family because of HS Understanding from family concerning HS Genetics – considerations about having children or not because of HS |
|
Social – job – economy HS impact on job situation in general HS impact on getting hired for a job Impact of HS on relationships to colleagues Impact of HS on choice of job Dismissal because of HS |
Early retirement because of HS Unemployment because of HS Sick leave – absence because of HS HS impact on career Lack of social support |
Chances of rehabilitation because of HS Ability to study with HS flares Monetary cost of disease |
|
Physical Presence of ruptured boils Localization Severity Pain because of HS Number of flares |
Disturbed sleep because of HS Presence of blood Presence of secretions Presence of itching Appearance of scars |
Self-incision of boils to obtain pain relief Fatigued because of HS Appearance of lumpy skin Tired because of HS Presence of smell – odor |
|
Treatment Need for acute treatment The doctor's knowledge of HS Influence/participatory on own treatment Need for surgery / laser |
Self-treatment Effect of treatment Side effects of treatment Time spent on treatment |
Need to bandage Difficulties in bandaging |
|
Daily life with HS Secretions – spots – leaves reminiscences Need to use perfume because of HS HS impact on transportation possibilities |
Restricted ability for exercising Pain at toileting because of HS Frequent visits to bathroom to wash |
Prognosis – expected evolution Need to change habits HS impact on clothing (to avoid flare ups and to hide the disease) |
Questionnaire Refinement (Initial Item Reduction)
A total of 9 patients (Table 1), 4 doctors, 1 nurse, and the interviewer participated in the first four rounds of the initial item reduction. Following each round, some questions were removed, e.g. because they were very similar to other questions, and some questions were excluded or reformulated (Fig. 2).
Fig. 2.
Overview of questionnaire refinement (initial item reduction) and pilot testing results.
Seventy questions were tested among 55 participating patients at the HS information meeting. Analysis of the responses showed that 38 patients used the possibility to answer “not relevant” to one or more questions, indicating the necessity to pay special attention to questions concerning civil and employment status as the “not relevant” answers were often related to these topics.
The final focus group meeting included 4 patients, 2 doctors, and the interviewer; 60 questions were presented. Following discussions, the group agreed to remove 25 questions and then reformulate 15 questions resulting in 35 remaining questions.
Pilot Testing
In total, 42 patients participated in pilot testing the questionnaire (Table 1). Following evaluation of the first round of pilot testing, 22 questions were removed due to acceptability or feasibility considerations or because they were not interpreted in a consistent way. Ten questions were reformulated resulting in 23 remaining questions. The new version with 23 questions were then pilot tested in a second round. Evaluation of the second round of pilot testing did not result in reformulation or removal of any questions.
Discussion
Our study aimed to launch the development of a HS-specific QOL instrument through item generation, pilot questionnaire generation, questionnaire refinement, and pilot testing. In line with other previous studies [5, 7, 8, 9, 10, 11], our results show that HS truly can affect both physical, psychological, and social QOL dimensions profoundly. Furthermore, we have comprehensively explored on HS's possible effect on, e.g., stigmatisation, self-esteem, identity, physical functioning, and social relationships and have identified a 23-item questionnaire.
During the item reduction process, we focused on keeping the most important categories active. Items were always removed in collaboration with patients with HS. Initially, several questions were removed simply because other formulations covering the same subjects were preferred. Later, questions were removed, e.g. because they were judged as unlikely to be sensitive to change. Questions about identity, own and other people's fear of contagiousness, and satisfaction with medical personnel, fell in the category. We also excluded several questions which were very specific, such as questions about self-help, childcare, planning for children, or fear of getting fired, as they were unlikely relevant for the majority of patients with HS. Finally, following the pilot testing, some questions were removed or reformulated because they were not consistently interpreted. As an example, one question asked about three different symptoms, and this confused the respondents. In the second round of pilot testing, the 23-item questionnaire proved to be feasible, acceptable and comprehensible, and the questions were interpreted in a consistent way.
Until now, clinical studies in HS have measured QOL with either dermatologic-specific or generic questionnaires [11, 15]. Generic instruments are designed to cover a wide range of dimensions and to be applicable in a wide range of conditions. Accordingly, they can be of particular use when you wish to compare across groups of diseases. Nonetheless, our results show that HS affects the QOL of the individuals on many different levels and also in ways that are not represented in generic questionnaires, such as EQ-5D [20]. Dermatologic-specific questionnaires, such as DLQI [13], do include more of these dimensions, but still, many of the items identified in this study are not represented. As a result, it is possible that an HS patient can experience a meaningful improvement in his or her perceived QOL following a given intervention, without this improvement being reflected in, e.g., an improved DLQI score [21]. A HS-specific instrument, in contrast, has the potential of being more sensitive to change. As a consequence, an HS-specific instrument may enable future researchers to discover treatment options that improve patients' QOL more effectively.
Parallel to this study, similar initiatives have been taken in North America [22]. This study does differ slightly from ours in the sense that no focus groups, only individual interviews, were performed. In addition, the qualitative method used was somewhat different, where Sisic et al.[22] used a more quantitative approach in their interview analysis. Nevertheless, our two processes identified very comparable items (Table 3). The independent identification of the themes volunteered in two populations adds significant strength to the validity of the results, and opens the possibility for the development of an international instrument. The creation of such an instrument would have obvious benefits not only in trials and scientific studies, but most likely also in daily practice by providing an international benchmark Patient-Reported Outcome Measure (PROM). Therefore, a close collaboration has been established within the framework of the HISTORIC collaboration to develop and validate an international version of the HISQOL, and the next step in the development process will be field testing. Here, the aim is further item reduction, examination of the dimensionality, and deciding on the definitive selection of items per dimension. In addition, the PROM will have to undergo further psychometric testing for assessing reliability, validity, responsiveness, and interpretation.
Table 3.
Summary of major themes generated from patient interviews in the US and Denmark
| Concept | Examples US [22] | Examples Denmark (present study) |
|---|---|---|
| Daily activities | Walking, sitting | Restricted ability for exercising |
| Symptoms due to HS | Fatigue, pain | Tired because of HS, fatigued because of HS, pain because of HS |
| Emotional consequences | Depression, anger | Depression because of HS, fear because of HS, suicidal thoughts because of HS |
| Psychosocial consequences | Isolation from others | Isolation because of HS, nervous to go out to meet other people because of HS, avoidance of social contact because of HS |
| Restricted clothing choices | Clothing choices to avoid flare-ups | HS impact on clothing (to avoid flare-ups and to hide the disease) |
| Coping | Social coping | Coping with HS |
| Sexual functioning | Embarrassment, pain during sex | HS impact on sexuality, embarrassed because of HS |
| Work/economic consequences | Financial burden of HS management and treatment | HS impact on job situation in general, HS impact on getting hired for a job, monetary cost of disease |
| Interactions with medical personnel | Inaccessibility to medical professionals | The doctor's knowledge of HS, influence/participatory on own treatment |
| Symptoms due to treatment | Treatment side effects | Side effects of treatment |
| Concentration issues | Trouble concentrating at work | Ability to study with HS flares |
Limitations to the present study include possible cultural bias related to the monocultural development of the questionnaire. However, this is a potential limitation found in most questionnaire development processes. Despite the HS sample population being hospital-based, thus a potential selection bias, we reduced potential selection and cultural bias by recruiting our sample to represent a general HS population with regard to sex, age, disease severity, and treatment experiences.
Strengths of our study include the broad inclusion of HS patients with a physician-verified diagnosis in all phases of the questionnaire development. The involvement of patients in the reduction of the initial exhaustive questionnaire was crucial, since the patients could act as experts of what was essential and what reasonably could be left out.
In conclusion, we have comprehensively explored on HS's possible effect on the QOL of the affected individuals and identified a 23-item questionnaire called HiSQOL, Hidradenitis Suppurativa Quality of Life. The questionnaire proved to be feasible, acceptable, and comprehensible in the second round of pilot testing. With HiSQOL, researchers can measure HS-specific QOL in future clinical trials, potentially enabling them to discover more effective treatment options.
Statement of Ethics
The study was approved by The Danish Data Protection Agency. No involvement of the Medical Research Ethics committee is required for qualitative interviews and questionnaire studies in Denmark. All participants were informed about their rights and signed a consent statement.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work is supported by the Region Zealand Research Foundation.
Author Contributions
Esmann had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Esmann, Miller, Vinding, Thorlacius, Jemec. Acquisition, analysis, and interpretation of data: Esmann, Thorlacius, Miller, Bang, Vinding, Jemec. Drafting of the manuscript: Thorlacius. Critical revision of the manuscript for important intellectual content: Esmann, Miller, Bang, Vinding, Jemec. Statistical analysis: Bang. Obtained funding: Jemec. Study supervision: Jemec.
Acknowledgement
We thank all the included patients for their willingness to participate, time, and patience. We also thank Penille Pless, information specialist, and Karl Bang, biostatistician, for their assistance.
References
- 1.Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012 Jan;366((2)):158–64. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 2.Garg A, Lavian J, Lin G, Strunk A, Alloo A. Incidence of hidradenitis suppurativa in the United States: A sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017 Jul;77((1)):118–22. doi: 10.1016/j.jaad.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology. 2015;231((2)):184–90. doi: 10.1159/000431175. [DOI] [PubMed] [Google Scholar]
- 4.Matusiak Ł, Szczęch J, Kaaz K, Lelonek E, Szepietowski JC. Clinical Characteristics of Pruritus and Pain in Patients with Hidradenitis Suppurativa. Acta Derm Venereol. 2018 Feb;98((2)):191–4. doi: 10.2340/00015555-2815. [DOI] [PubMed] [Google Scholar]
- 5.Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011 May;91((3)):328–32. doi: 10.2340/00015555-1082. [DOI] [PubMed] [Google Scholar]
- 6.Matusiak Ł. Profound consequences of hidradenitis suppurativa: a review. Br J Dermatol. 2018 May; doi: 10.1111/bjd.16603. [DOI] [PubMed] [Google Scholar]
- 7.Kouris A, Platsidaki E, Christodoulou C, Efstathiou V, Dessinioti C, Tzanetakou V, et al. Quality of Life and Psychosocial Implications in Patients with Hidradenitis Suppurativa. Dermatology. 2016;232((6)):687–91. doi: 10.1159/000453355. [DOI] [PubMed] [Google Scholar]
- 8.Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010 May;90((3)):264–8. doi: 10.2340/00015555-0866. [DOI] [PubMed] [Google Scholar]
- 9.Deckers IE, Kimball AB. The Handicap of Hidradenitis Suppurativa. Dermatol Clin. 2016 Jan;34((1)):17–22. doi: 10.1016/j.det.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Tugnoli S, Bettoli V, Agnoli C, Caracciolo S. [Emotions and bodily experience in Hidradenitis Suppurative-Acne Inversa] Clin Ter. 2016 May-Jun;167((3)):e55–62. doi: 10.7417/CT.2016.1934. [DOI] [PubMed] [Google Scholar]
- 11.Riis PT, Vinding GR, Ring HC, Jemec GB. Disutility in Patients with Hidradenitis Suppurativa: A Cross-sectional Study Using EuroQoL-5D. Acta Derm Venereol. 2016 Feb;96((2)):222–6. doi: 10.2340/00015555-2129. [DOI] [PubMed] [Google Scholar]
- 12.Thorlacius L, Cohen AD, Gislason GH, Jemec GB, Egeberg A. Increased Suicide Risk in Patients with Hidradenitis Suppurativa. J Invest Dermatol. 2018 Jan;138((1)):52–7. doi: 10.1016/j.jid.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994 May;19((3)):210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 14.Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997 Nov;133((11)):1433–40. [PubMed] [Google Scholar]
- 15.Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol. 2016 Aug;175((2)):263–72. doi: 10.1111/bjd.14475. [DOI] [PubMed] [Google Scholar]
- 16.Thorlacius L, Ingram JR, Villumsen B, Esmann S, Kirby JS, Gottlieb AB, et al. HIdradenitis SuppuraTiva cORe outcomes set International Collaboration (HISTORIC) A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018 Sep;179((3)):642–50. doi: 10.1111/bjd.16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014 Sep;89((9)):1245–51. doi: 10.1097/ACM.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 18.Van der Veer K, Ommundsen R, Hak T. Larsen, K. S.: meaning shift of items in different language versions. A cross-national validation study of the illegal aliens scale. Qual Quant. 2003;37((2)):193–206. [Google Scholar]
- 19.de Vet HC, Terwee CB, Mokkink LB, Knol DL. Measurement in Medicine. Cambridge University Press; 2011. [Google Scholar]
- 20.EuroQol–a new facility for the measurement of health-related quality of life Health policy (Amsterdam, Netherlands) 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 21.Thorlacius L, Theut Riis P, Jemec GB. Severe hidradenitis suppurativa responding to treatment with secukinumab: a case report. Br J Dermatol. 2018 Jul;179((1)):182–5. doi: 10.1111/bjd.15769. [DOI] [PubMed] [Google Scholar]
- 22.Sisic M, Kirby JS, Boyal S, Plant L, McLellan C, Tan J. Development of a Quality-of-Life Measure for Hidradenitis Suppurativa. J Cutan Med Surg. 2017 Mar-Apr;21((2)):152–5. doi: 10.1177/1203475416677721. [DOI] [PMC free article] [PubMed] [Google Scholar]