ABSTRACT
The aim of this study was to investigate the effect of protocatechuic acid (PCA) on the growth performance, meat quality, and intestinal health of Chinese yellow-feathered broilers. Growing broilers were fed the basal diet or diets supplemented with 300 or 600 mg/kg PCA, or 200 mg/kg enramycin for 52 D. We found that addition of 300 mg/kg PCA significantly increased body weight, live weight, and carcass weight and decreased the feed to gain ratio of broilers; PCA improved meat quality through reducing shear force, and increasing a* (relative redness) and decreasing b* (relative yellowness) at 24 h after slaughter. The activities of alkaline phosphatase and diamine oxidase in plasma were significantly decreased by administration of 300 mg/kg PCA; PCA also significantly increased total antioxidant capability and decreased malondialdehyde content and activity of xanthine oxidase in liver. Meanwhile, it enhanced activities of total superoxide dismutase, glutathione s-transferase, and glutathione peroxidase in the jejunal mucosa. Interleukin-10 and transforming growth factor-β were significantly increased in jejunal mucosa and plasma of 300 mg/kg PCA diet group, whereas interluekin-2 and interferon-γ dropped dramatically. Moreover, relative expression of apoptosis-related genes decreased in liver, whereas that of intestinal barrier-related and immunity-related genes increased in jejunum. Furthermore, 300 mg/kg PCA treatment significantly changed α-diversity and structure of the cecal microflora in broilers, with increasing relative abundance of Firmicutes and Actinobacteria while reducing Bacteroidetes and Proteobacteria. These results indicated that PCA improved the feed efficiency, growth performance, meat quality of broilers, and antioxidant capacity. It also enhanced intestinal immune function and improved the structure of intestinal flora to favor improved intestinal health in Chinese yellow-feathered broilers.
Keywords: protocatechuic acid, broiler, intestinal health, antibiotic status, meat quality
INTRODUCTION
China is the world's second largest producer of chicken meat, half of which is from Chinese yellow-feathered broilers (Gou et al., 2016). At present, intensive poultry production confronts many stressors such as high stocking density, heat stress, inter alia, which frequently result in inflammation, oxidative damage, increased nutrient consumption, and decreased feed efficiency (Ju et al., 2015). Intestinal health is increasingly recognized as important for maximizing the health, welfare, and performance of poultry. Intestinal sickness or injury has devastating financial impact for producers, and food safety concerns for consumers (Roberts et al., 2015). There is, therefore, a growing need for appropriate supplementation of broilers to promote intestinal health and further reduce losses caused by stress.
Recently, functional feeds have been used to enhance intestinal health and to obtain safe, reliable, and high-quality animal products without any in-feed medication or antibiotics (Jayaraman et al., 2013). As an example of a widely distributed plant component, protocatechuic acid (PCA, 3,4-dihydroxybenzoic acid) is used as an active component of many Chinese herbal medicines because of the benefits from its dietary ingestion and a variety of pharmacological properties including antibacterial, anti-inflammatory, and antioxidant activities (Kakkar et al., 2014). For example, PCA content in freeze-dried roselle calyx aqueous extracts inhibits the growth of Salmonella Typhimurium DT104, Escherichia coli O157: H7, Staphylococcus aureus, Listeria monocytogenes, and Bacillus cereus (Chao and Yin, 2009). Studies showed that PCA had significant protective effect against intestinal barrier dysfunction induced by bile duct ligation in mice (Ning et al., 2013). PCA protected rats against oxidative damage induced by tert-butylhydroperoxide (t-BHP); pretreatment with PCA significantly lowered serum levels of the hepatic enzyme markers lactate dehydrogenase (LDH), alanine aminotransferase (ALT), and aspartate aminotransferase (AST), as well as reducing oxidative stress of the liver (Liu et al., 2002; Kakkar et al., 2014).
Recent work in this laboratory has also shown that PCA improved intestinal barrier and immune function, regulated the immune system, and improved the makeup of intestinal flora in mice (unpublished). There has been no related research on the administration of PCA to broilers. The goal of this study was to assess the effects of dietary supplementation of Chinese yellow-feathered broilers with PCA on their growth performance, meat quality, and indicators of antioxidant status, immune function, and composition of the intestinal microflora.
MATERIALS AND METHODS
Chicken Husbandry
The experimental protocol was approved by the Animal Care Committee of the Institute of Animal Science, Guangdong Academy of Agriculture Science, Guangzhou, P. R. China. Male hatching (Lingnan, an improved meat-type breed, obtained from Guangdong Academy of Agriculture Science, Guangzhou, China) was used. A total of 720 broilers with initial body weight (BW) 41.27 g were randomly assigned to 4 dietary treatments, each with 6 replicate pens of 30 birds (stocking density 0.38 m2/bird). Water and mashed diets were given ad libitum to broilers during the 2 phases (1 to 21 and 22 to 50 D). Daylight was eliminated and replaced with 18-h lighting from incandescent bulbs. The temperature of the room was maintained at 32 to 34°C for the first 3 D and then reduced by 2 to 3°C per week to a final temperature of 26°C.
Experimental Diets
The basal corn-soybean meal mashed diets were based on the recommendations of Chinese Feeding Standard of Chicken (NY/T815–2004) for the 2 growth phases. Birds in treatment 1 (control) were fed the basal diet and those in treatments 2 and 3 (P300, P600) were fed the basal diet supplemented with 300 or 600 mg/kg PCA, and treatment 4 (ER) was the basal diet supplemented with 200 mg/kg enramycin (Sigma, St Louis, MO). High purity (> 97%) PCA was purchased from Aladdin Biochemical Technology (Shanghai, China). The composition and nutrient levels of the basal diets, the premix of vitamin additives and trace-element additives are shown in Tables 1–3.
Table 1.
Composition and nutrient levels of the basal diets.
| Phases | 1 to 21 D | 22 to 52 D |
|---|---|---|
| Ingredients, % | ||
| Corn | 60.50 | 64.10 |
| Soybean meal | 28.50 | 23.00 |
| Soybean oil | 1.70 | 3.65 |
| Feather meal | 3.00 | 3.50 |
| Limestone | 1.22 | 1.17 |
| Dicalcium phosphate | 1.93 | 1.65 |
| Methionine | 0.17 | 0.09 |
| Lysine | 0.16 | 0.15 |
| Sodium chloride | 0.30 | 0.30 |
| Zeolite powder | 1.52 | 1.39 |
| Vitamin-mineral premix | 1.00 | 1.00 |
| Diet nutrition level, % | ||
| ME (Mcal/kg) | 2.90 | 3.05 |
| Lysine | 1.13 | 0.98 |
| Methionine | 0.45 | 0.36 |
| Methionine + cysteine | 0.87 | 0.73 |
| Threonine | 0.83 | 0.75 |
| Tryptophan | 0.25 | 0.21 |
| Isoleucine | 0.86 | 0.77 |
| CP | 21.00 | 18.95 |
| Calcium | 1.00 | 0.90 |
| Nonphytate phosphorus | 0.45 | 0.40 |
Table 3.
The Premix of trace-element additives.
| Phases | 1 to 21 D | 22 to 52 D |
|---|---|---|
| Ingredients, % | ||
| ZnSO4.H2O | 212.52 | 180.93 |
| CuSO4.H2O | 33.18 | 33.18 |
| FeSO4.H2O | 247.75 | 247.75 |
| MnSO4.H2O | 263.98 | 248.45 |
| Ca(IO3)2 | 85.80 | 85.80 |
| Na2SeO3 | 37.60 | 37.60 |
| Zeolite powder | 119.17 | 166.29 |
| Total | 1,000.00 | 1,000.00 |
Table 2.
The Premix of vitamin additives.
| Phases | 1 to 21 D | 22 to 52 D |
|---|---|---|
| Ingredients, % | ||
| Vitamin A | 120.00 | 90.00 |
| Vitamin D3 | 30.00 | 25.00 |
| Vitamin E | 40.00 | 80.00 |
| Vitamin K | 0.98 | 2.94 |
| Vitamin B1 | 2.50 | 2.50 |
| Vitamin B2 | 10.00 | 6.25 |
| pantothenic acid | 30.30 | 30.30 |
| Nicotinic acid | 50.51 | 30.30 |
| pyridoxine | 9.09 | 9.09 |
| biotin | 22.50 | 22.50 |
| folic acid | 1.67 | 1.67 |
| Vitamin B12 | 3.00 | 3.00 |
| Corncob powder | 679.45 | 697.45 |
| Total | 1,000.00 | 1,000.00 |
Measurement of Growth Performance
Feed intake was recorded daily on a per replicate basis. Birds were weighed at the beginning (day 1) and end (day 22 and 52) of each phase. Mortality was checked daily, and dead birds were recorded and weighed to adjust estimates of gain, intake, and feed conversion ratio, as appropriate. The average daily gain (ADG), average daily feed intake (ADFI), and feed to gain ratio (F:G) were calculated. At 52 D of age, birds were deprived of feed overnight and weighed immediately prior to slaughter.
Analysis of Carcass Characteristics
The birds were electrically stunned and exsanguinated, and then processed by standard method to obtain carcass trait data. Carcass weight (CW) and live weight (LW) were recorded. Dressing percentage (D%) = 100% × carcass weight (with only the blood and feathers removed)/LW. Immune organ indices = 100% × the immune organ weight/LW.
Determination of Meat Quality
Carcasses were dissected, and indices of meat quality including color, shear force, drip loss, and pH were determined on breast muscle, with the methods described previously by Jiang et al. (2011)
pH. The pH was measured at 45 min and 24 h postmortem in the right pectoralis major muscle with a portable pH meter equipped with an insertion glass electrode.
Water-holding Capacity
The water-holding capacity of breast muscle was estimated at 1 h postmortem by determining drip loss using the method described by Rasmussen and Andersson (1996) as follows. Broiler musculus pectoralis major was taken from the carcass and samples were cut using a 25-mm cork borer at a right angle to the muscle fiber direction. Samples were placed in a plastic bag filled with air and fastened to avoid evaporation, left at 4 to 6°C for 24 h, and drip loss was determined by weighing. Muscle fiber direction of the samples was horizontal to gravity, not vertical, as described in the original method (Rasmussen et al., 1996).
Color
Meat color was measured at 45 min and 24 h postmortem using a chroma meter to measure CIE LAB values (where L* measures relative lightness, a* relative redness, and b* relative yellowness). A reading was made from the surface of the sample, representing the whole surface of the muscle. A white tile (L* 92.30, a* 0.32, and b* 0.33) was used as standard.
Shear Force
The breast muscles were refrigerated overnight at 4°C and then brought to room temperature and finally cooked to an internal temperature of 70°C on a digital thermostat water bath. Endpoint internal temperature was monitored with a thermometer. After cooling to room temperature, segments 1 cm2 were cut perpendicular to the fiber orientation of the muscle then 10 sections about 3 cm thick were cut parallel to the fiber orientation through the thickest portion of the cooked muscle. Warner-Bratzler shear force was determined using an Instron Universal Mechanical Machine (Instron model 4411, Instron corp., Canton, MA). A Warner-Bratzler apparatus was attached to a 50-kg load cell, and tests were performed at a cross head speed of 127 mm/min; signals were processed with the Instron Series 9th software package.
Blood and Tissue Sampling
Subsamples of 2 birds per pen (total of 72) were weighed individually. The birds were bled from the brachial vein into evacuated EDTA-K2 tubes before slaughter by a trained team. Blood samples were held on ice for < 1 h then centrifuged at 860 g for 15 min at 4°C, and were collected, snap-frozen in liquid nitrogen, and stored at −70°C until analysis. The middle segment of the jejunum was cut for about 10 cm. Then, the jejunum was opened length-wide on an ice-cooled surface and the mucosa was gently scraped off and collected. Liver and cecal contents were collected, and snap-frozen as well. In addition, pieces of 1 mm were cut from the jejunal segments using a sharp blade and quickly fixed in 6% solution of phosphate-buffered glutaraldehyde pH 7.4 for 6 h at 4°C (McDowell and Trump, 1976) by transmission electron microscopy (TEM).
Biochemical Determinations
Frozen tissue (0.2 g) in 2 mL homogenization buffer (0.9% normal saline) was homogenized on ice with an Ultra-Turrax homogenizer for 5 s at 12,000 rpm. The homogenate was centrifuged at 3,000 g for 10 min at 4°C, and the supernatant was stored at –70°C until analysis.
The activities of aspartate aminotransferase (AST/GOT), alanine aminotransferase (ALT/GPT), alkaline phosphatase (AKP), diamine oxidase (DAO), and the content of uric acid (UA) in plasma, total superoxide dismutase (T-SOD), total antioxidant capability (T-AOC), activities of glutathione peroxidase (GSH-Px), catalase (CAT), glutathione S-transferase (GSH-ST), Na+-K+-ATPase and the content of malondialdehyde (MDA) in homogenates of intestinal mucosa, as well as T-SOD, MDA, activities of GSH-Px, CAT, and xanthine oxidase (XOD) in liver homogenates were assayed using colorimetric kits (Nanjing Jiancheng Insititute of Bioengineering, Nanjing, China) and a spectrophotometer.
Enzyme Linked ImmunoSorbent Assay
Forty milligrams of jejunum in 4 mL of homogenization buffer (0.9% normal saline) was homogenized on ice for 5 s at 12,000 rpm. The homogenate was centrifuged at 3,000 g for 10 min at 4°C. Levels of interleukin (IL)-2, IL-12, interferon (IFN)-γ, transforming growth factor (TGF)-β, and secretory immunoglobulin A (sIgA) in plasma and homogenates of intestinal mucosa were quantified using sandwich ELISA kits (Bio-function Technology Co., Ltd, Beijing, China) as per the manufacturer's instructions. Briefly, 10 μL of samples, including a standard containing protein of interest, control specimens, and unknowns, were pipetted into wells of microplates coated with the capture antibody, specific for a protein of interest. In this step, the protein antigen bounded to the capture antibody. After washing 5 times with washing solution, 50 μL of detection antibody was added to the wells, and this antibody bounded to the immobilized protein captured during the first incubation. After removal of excess detection antibody, 50 μL of HRP conjugate (secondary antibody or streptavidin) was added and bounded to the detection antibody. After the third incubation and washing to remove the excess HRP conjugate, 50 μL of substrate solution was added and converted by the enzyme to a detectable form (color signal). The intensity of this colored product was directly proportional to the concentration of antigen present in the original specimen.
RT-PCR and Quantitative PCR
Total RNA was extracted from powdered frozen liver and intestinal mucosa (RNAiso plus, TAKARA, Tokyo, Japan) and reverse-transcribed using PrimeScript II 1st Strand cDNA Synthesis Kit (TAKARA).
Real-time PCR was performed using SYBR PremixExTaq II (TAKARA) and the ABI 7500 real-time PCR system (Applied Biosystems, Carlsbad, CA) (Satoh et al., 2010). The primers used are shown in Table 4. Results were normalized to the abundance of β-actin transcripts and relative quantification was calculated using the 2−ΔΔCT method.
Table 4.
Primers for real-time PCR.
| Gene | GenBank | Primers (5′-3′) | Annealing temperature (°C) |
|---|---|---|---|
| MUC-2 | NM_0,013,18434.1 | F: GCCTGCCCAGGAAATCAAG | 56 |
| R: CGACAAGTTTGCTGGCACAT | |||
| p53 | NM_205264.1 | F: CCCATCCTCACCATCCTTACA | 58 |
| R: CTTCAGCATCTCATAGCGGC | |||
| CLDN | NM_001013611.2 | F: AGGGCTGTAGCGGCGGAAAG | 56 |
| R: GAGGATGACCAGGTGAAGAAG | |||
| Nrf2 | NM_205117.1 | F: ATCACCTCTTCTGCACCGAA | 60 |
| R: GCTTTCTCCCGCTCTTTCTG | |||
| TLR-4 | NM_001030693 | F: AGTCTGAAATTGCTGAGCTCAAAT | 57 |
| R: GCGACGTTAAGCCATGGAAG | |||
| Caspase-3 | NM_204725.1 | F: ACTCTGGAAATTCTGCCTGATGACA | 56 |
| R: CATCTGCATCCGTGCCTGA | |||
| BAX | XM_015274882.1 | F: GTGATGGCATGGGACATAGCTC | 58 |
| R: TGGCGTAGACCTTGCGGATAA | |||
| HO-1 | NM_205345.1 | F: CTCAAGGGCATTCATTCG | 56 |
| R: ACCCTGTCTATGCTCCTGTT | |||
| CD3 | NM_205512.1 | F: ATGTTGTCGCCACTGTCTTG | 58 |
| R: GGCTATCAGGTTCTGCCTGT | |||
| COX2 | NC_001323.1 | F: TGCAACGATATGGCTGAGAG | 57 |
| R: CTGCGATTCGGTTCTGGTAT | |||
| β-Gal9 | NM_001001611.2 | F: ACCACGCAGTTAAGAGCCCT | 60 |
| R: CACCAGCAAATGTCAGCAATG | |||
| OCLN | NM_205128.1 | F: TCATCCTGCTCTGCCTCATCT | 56 |
| R: CATCCGCCACGTTCTTCAC | |||
| β -actin | NM 205518 | F: GAGAAATTGTGCGTGACATCA | 60 |
| R: CCTGAACCTCTCATTGCCA |
BAX, Bcl-2 associated X protein; CD3, cluster of differentiation 3; CLDN, occudin; OCLN, claudin; COX-2, cyclo-oxygenase 2; HO-1, heme oxygenase1; Nrf2, nuclear factor 2; p53, tumor protein 53; TLR-4, Toll-like receptor 4; β-Gal9, β-Galectin-9.
Transmission Electron Microscopy
After initial fixation, the tissues were washed several times in cold (4°C) 0.1 M phosphate buffer every 15 min for 2 h. The tissues were post-fixed in a 1% solution of cold osmium tetroxide (OsO4) in 0.1 M buffer pH 7.2 for 2 h. After rapidly dehydrating through an ascending series of ethanol, blocks were transferred to a 1:1 mixture of propylene oxide and epoxy araldite. After embedding, semithin sections (1 μm) were initially cut, stained with toludine blue, and viewed with light microscopy to select suitable areas for ultrastructural examination. Ultrathin sections (60 to 100 nm) were cut with a glass knife and stained with uranyl acetate followed by lead citrate (Hayat, 1986; Okle et al., 2016). These sections were examined with the transmission electron microscope (JEOL 100cx, Akishima, Tokyo, Japan) operating at 80 kV (Okle, Derbalah and Euony, 2016).
DNA Extraction, V4-V5 16S rRNA Gene Amplification and Microbiota Community Analysis
Genomic DNA was extracted from cecal contents of 6 broilers in each treatment using TIANamp Stool DNA Kits from Tiangen Biotech (Beijing, China). PCR amplification and sequencing were performed by the G-BIO Inc. (Hangzhou, China). The V3 and V4 regions were amplified using forward primers containing the sequence 5′-CCTACGGGNGGCWGCAG-3′ and reverse primers containing the sequence 5′-GACTACHVGGGTATCTAATCC-3′.
The processed pair-end reads were assembled using PandaSeq v2.8 with default parameters. Chimeras were identified and removed using USEARCH 6.1 within QIIME. The QIIME script “add_qiime_labels.py” was used to combine the non-chimeric sequences from each sample into 1 file. OTU picking and taxonomic assignments was performed using the open-reference OTU picking workflow in Qiime with the Greengenes reference database August 2013 release (Lee et al., 2012). OTUs with abundance below 0.005% of the total number of sequences were discarded (Bokulich et al., 2013). Alpha diversity measurements, including Shannon (Chao and Shen, 2003), Chao1 (Chao, 1984), observed species, and Good's coverage (Good, 1953), were calculated using the alpha_rarefaction.py script in QIIME. Weighted and unweighted unifrac distances (Lozupone and Knight, 2005) were calculated from the rarefied OTU table using the beta_diversity_through_plots.py script in QIIME.3.2.5.1.
Statistical Analysis
Effects of treatment were examined by 1-way analysis of variance (ANOVA) in SPSS 20.0 for Windows; all percentage data were arcsine transformed before analysis. When treatment effects were significant (P < 0.05), Tukey's multiple range tests were used to compare the individual means. Tabulated results are shown as means with SEMs derived from the ANOVA error mean square.
RESULTS AND DISCUSSION
Growth Performance of Broilers
The effects of supplemental PCA or ER on growth performance of Chinese yellow-feathered broilers are presented in Table 5. During the starter phase from 1 to 21 D, there was no significant effect on ADG, ADFI, and F:G due to treatment with PCA or ER. In contrast, from 22 to 52 D, treatment with 300 mg/kg PCA (P300) increased BW of broilers (P < 0.05) and decreased F:G (P < 0.01) compared to the control group, just as ER did. Moreover, considering the whole growth period, F:G of broilers in treatments P300 and P600 decreased (P < 0.05), whereas BW of broilers was increased (P < 0.05) only by 300 mg/kg PCA compared to the control group. These results indicated that supplementation with 300 mg/kg PCA or 200 mg/kg ER improved growth performance of the broilers.
Table 5.
Effect of PCA on growth performance of 1- to 52-day-old Chinese Yellow broilers.
| Variable | CK | ER | P300 | P600 | SEM | P-value |
|---|---|---|---|---|---|---|
| 1 to 21 D | ||||||
| BW at day 21 (g) | 536.52 | 536.63 | 541.07 | 521.99 | 3.386 | 0.21 |
| ADFI (g/d) | 39.80 | 39.31 | 38.85 | 38.20 | 0.392 | 0.51 |
| ADG (g/d) | 25.90 | 25.77 | 25.99 | 25.04 | 0.167 | 0.18 |
| F:G (g:g) | 1.60 | 1.63 | 1.59 | 1.62 | 0.007 | 0.24 |
| Mortality (%) | 2.22 | 1.11 | 1.11 | 1.67 | 0.491 | 0.85 |
| 22 to 52 D | ||||||
| BW at day 52 (g) | 2,312.06a | 2,413.61b | 2,421.49b | 2,301.73a,b | 23.252 | 0.04 |
| ADFI (g/d) | 159.18a | 132.69b | 143.14a,b | 152.00a,b | 4.085 | 0.04 |
| ADG (g/d) | 59.16 | 62.02 | 62.18 | 59.43 | 0.672 | 0.23 |
| F:G (g:g) | 2.69a | 2.16b | 2.16b | 2.64a | 0.075 | <0.01 |
| Mortality (%) | 10.00 | 11.67 | 11.67 | 9.44 | 1.220 | 0.90 |
| 1 to 52 D | ||||||
| ADFI (g/d) | 109.60 | 97.95 | 105.60 | 108.75 | 1.978 | 0.01 |
| ADG (g/d) | 45.46 | 46.42 | 46.57 | 44.36 | 0.426 | 0.10 |
| F:G (g:g) | 2.455a | 2.132b | 2.245b | 2.462a | 0.046 | 0.01 |
| Mortality (%) | 12.22 | 12.78 | 12.78 | 11.11 | 1.310 | 0.97 |
a,bMeans with common letters within a main effect do not differ significantly (P < 0.05).
Each value is the mean of 6 replicates.
SEM = standard error of the mean; BW = body weight; ADFI = average daily feed intake; ADG = average daily gain; F: G = feed: gain ratio.
Both LW and CW of 52-day-old broilers (Table 6) were increased in treatments P300 (P < 0.01) and ER (P < 0.05) compared to the control group, whereas the treatments had no effect on dressing percentage (D%). Supplementation with 300 mg/kg PCA increased (P < 0.05) the spleen index of broilers but, unexpectedly, 600 mg/kg PCA sharply increased (P < 0.001) the bursa of Fabricius index compared with the controls.
Table 6.
Effects of PCA on slaughter performance of 52-day-old Chinese Yellow broilers.
| Variable | CK | ER | P300 | P600 | SEM | P-value |
|---|---|---|---|---|---|---|
| Slaughter performance | ||||||
| LW (kg) | 2.32a | 2.43b | 2.43b | 2.33a,b | 0.015 | <0.01 |
| CW (kg) | 2.13a | 2.23b | 2.23b | 2.14a,b | 0.014 | 0.04 |
| D% | 92.25 | 91.85 | 90.22 | 92.06 | 0.274 | 0.02 |
| Immune organs indices | ||||||
| Spleen | 0.12a | 0.16a,b | 0.17b | 0.13a,b | 0.001 | 0.03 |
| Thymus | 0.33 | 0.34 | 0.34 | 0.30 | 0.003 | 0.87 |
| Bursa of Fabricius | 0.09a | 0.10a | 0.09a | 0.14b | 0.001 | <0.001 |
a,bMeans with common letters within a main effect do not differ significantly (P < 0.05).
Each value is the mean, n = 12.
SEM = standard error of the mean; LW = live weight; CW = carcass weight; D% = dressing percentage (D%), 100 × CW/LW.
China is the world's second largest producer of chicken meat, and almost half is from Chinese yellow-feathered broilers, which satisfy consumer preferences regarding flavor in China and some other countries in South-East Asia (Gou et al., 2016). The major cost in poultry feeding enterprises is that of feed, which is becoming a significant issue because the prices of feed ingredients continue to rise (Gou et al., 2016). Any reduction in the F:G effectively saves costs. In the present research, supplementing the diet with 300 mg/kg PCA significantly increased BW of broilers (approximately 5%) and decreased F:G from 22 to 52 D (approximately 80% that of controls), indicating that treatment with PCA improved the growth performance and efficiency of broilers.
Meat Quality
The data in Table 7 clearly show that shear force of breast muscle, harvested at day 52, was significantly decreased in broilers supplemented with 300 mg/kg PCA compared to the control group. Furthermore, 300 mg/kg PCA increased meat a* value and b* value at 24 h postmortem, compared with the controls; there were no differences in pH or drip loss due to treatment. The results indicated that treatment with 300 mg/kg PCA improved indices related to meat quality of breast muscles. Considering the effect on both growth performance and meat quality, the P300 treatment was chosen to perform subsequent analyses.
Table 7.
Effects of PCA on indices related to meat quality of 52-day-old Chinese Yellow broilers.
| Variable | CK | ER | P300 | P600 | SEM | P-value |
|---|---|---|---|---|---|---|
| Shear force (N) | 23.57a | 20.67a,b | 17.37b | 25.24a,b | 0.990 | 0.04 |
| 24-h drip loss (%) | 1.90 | 2.02 | 2.12 | 2.06 | 0.052 | 0.26 |
| pH-45 min | 6.23 | 6.22 | 6.29 | 6.28 | 0.036 | 0.23 |
| a* value-45 min | 55.90 | 55.97 | 55.52 | 56.09 | 0.201 | 0.10 |
| b* value-45 min | 15.14 | 15.79 | 15.31 | 15.56 | 0.118 | 0.23 |
| L* value-45 min | 12.96a,c | 15.26b,c | 12.30a | 15.05b | 0.301 | <0.01 |
| pH-24 h | 5.68 | 5.67 | 5.71 | 5.63 | 0.010 | 0.09 |
| a* value-24 h | 58.54a | 60.13a,b | 60.58b | 61.71b | 0.319 | <0.01 |
| b* value-24 h | 13.58a | 13.29a,b | 12.69b | 12.91a,b | 0.130 | 0.04 |
| L* value-24 h | 15.89 | 15.94 | 13.62 | 15.43 | 0.278 | 0.08 |
a,bMeans with common letters within a main effect do not differ significantly (P < 0.05).
Each value is the mean, n = 12.
SEM = standard error of the mean; a* = redness; b* = yellowness; L* = lightness.
In the last decade, the need in European Union countries for improving the quality of the raw meat has been emphasized (Maiorano et al., 2012). Meat quality control procedures were carried out in order to reduce economic losses and also to supply high-quality products for the consumers (Ghazali and Rahim, 2016). Most highlighted value of meat production mainly depends on the appearance, juiciness, flavor, nutritional value, wholesomeness, and texture of meat (Rahima et al., 2013). Breast meat from broilers supplemented with PCA had a lower average shear force than that from the controls. Dietary PCA also increased the a* value and decreased the b* value of breast meat at 24 h after slaughter. Shear force of breast muscle, an important objective index indicative of meat tenderness, is one of the main sensorial attributes that determine global acceptability (Chen et al., 2007). Meat color is an important food quality attribute associated with acceptability at purchase and a higher a* value of meat is always favored by consumers. Reduction of L* or b* values of meat indicates less pale meat (Jiang et al., 2014). The results obtained here indicated that supplementation with PCA increased these indices related to meat quality of broiler breast muscles.
Antioxidant Status
As shown in Table 8, the activity of AKP and DAO in plasma was significantly decreased by PCA, indicating that broilers in the P300 treatment had less indication of inflammation than did the controls. Also, there were no significant effects of PCA on plasma content of UA nor activities of ALT/GPT or AST/GOT compared to the control group.
Table 8.
Effect of PCA on biochemical indices in plasma of 52-day-old Chinese Yellow broilers.
| Variable | CK | PCA | ER | SEM | P-value |
|---|---|---|---|---|---|
| UA (mmol/mL) | 178.15 | 166.22 | 152.83 | 4.524 | 0.08 |
| AKP (U/mL) | 55.33a | 33.51b | 59.78a | 4.891 | 0.04 |
| DAO (U/mL) | 8.18a | 5.80b | 7.32a,b | 0.617 | 0.05 |
| ALT/GPT (U/mL) | 9.05 | 7.97 | 8.68 | 0.393 | 0.54 |
| AST/GOT (U/mL) | 147.58 | 146.53 | 152.60 | 1.910 | 0.40 |
a,bMeans with common letters within a main effect do not differ significantly (P < 0.05).
Each value is the mean, n = 12.
SEM = standard error of the mean; UA = Uric acid; AKP = alkaline phosphatase; DAO = diamine oxidase; ALT/GPT = alanine aminotransferase; AST/GOT = aspartate aminotransferase.
Table 9 shows that, compared with the controls, supplementation with PCA and ER both decreased hepatic MDA. Also, PCA increased hepatic T-AOC (P < 0.01) and decreased (P < 0.05) XOD activity, and ER increased the activity of GSH-Px in liver.
Table 9.
Effects of PCA on biochemical indices in liver of 52-day-old Chinese Yellow broilers.
| Variable | CK | PCA | ER | SEM | p-value |
|---|---|---|---|---|---|
| T-AOC (U/mg prot) | 0.37a | 0.55b | 0.42a | 0.024 | <0.01 |
| GSH-Px (U/mg prot) | 15.06a | 15.68a | 19.23b | 0.946 | 0.04 |
| MDA (nmol/mg prot) | 1.45a | 1.02b | 1.16b | 0.103 | 0.08 |
| XOD (U/mg prot) | 10.68a | 9.45b | 9.52b | 0.228 | 0.04 |
a,bMeans with common letters within a main effect do not differ significantly (P < 0.05).
Each value is the mean, n = 12. SEM = standard error of the mean; T-AOC = Total antioxidant capability; GSH-Px = Glutathione Peroxidase; MDA = Malondialdehyde; XOD = Xanthine Oxidase.
Table 10.
Effect of PCA on biochemical indices in jejunal mucosa of 52-day-old Chinese Yellow broilers.
| Variable | CK | PCA | ER | SEM | P-value |
|---|---|---|---|---|---|
| T-SOD (U/mg prot) | 31.63a | 37.64b | 36.16b | 1.101 | 0.04 |
| T-AOC (U/mg prot) | 1.35 | 1.27 | 1.24 | 0.039 | 0.50 |
| GST-ST (U/mg prot) | 13.60a | 18.59b | 21.61b | 1.167 | 0.01 |
| GSH-Px (U/mg prot) | 173.05a | 225.65b | 259.13b | 12.655 | 0.01 |
| CAT (U/mg prot) | 89.01 | 98.30 | 99.99 | 2.246 | 0.14 |
| MDA (nmol/mg prot) | 2.44 | 2.75 | 2.29 | 0.162 | 0.52 |
| Na+-K+-ATP (U/mg prot) | 10.68 | 11.71 | 11.04 | 0.495 | 0.70 |
a,bMeans with common letters within a main effect do not differ significantly (P < 0.05).
Each value is the mean, n = 12.
SEM = standard error of the mean; T-AOC = total antioxidant capability; T-SOD = total superoxide capability; GSH-Px = glutathione Peroxidase; GSH-ST = glutathione S-transferase; MDA = malondialdehyde; CAT = catalase; Na+K+-ATP = Na+K+–ATPase.
As shown in table 10, treatments with PCA or ER increased jejunal mucosal activities of T-SOD (P < 0.05), GST-ST (P < 0.01), and GSH-Px (P < 0.01), compared to the controls but there were no differences in Na+-K+-ATP activity, T-AOC, or MDA content. These results together indicated that treatment with PCA induced a partial antioxidative effect and compromised the broiler's oxidant status.
The antioxidant status is an important factor affecting host health, and the antioxidant system is mainly composed of antioxidase and chain reaction inhibitor. These 2 cooperatively maintain balance between free radical generation and scavenging. Antioxidant capacity, reflected in T-AOC, results from interaction with various oxidative and antioxidant substances. The important antioxidant enzymes include GSH-Px, SOD, and CAT. Specifically, GSH-Px removes peroxide and hydroxyl radicals from cellular respiration, thereby reducing peroxidation of membrane polyunsaturated fatty acids; SOD scavenges superoxide anion free radicals and protects structure and function of cell membranes; CAT catalyzes the decomposition of hydrogen peroxide, reducing the formation of harmful hydroxyl radicals. Collectively, elevated activity of GSH-Px, SOD, T-AOC, and CAT reflects increased antioxidant capacity (Ju et al., 2015; wang et al., 2016a). Dietary treatment with PCA enhanced the activities in jejunal mucosa of T-SOD, GST-ST, and GSH-Px compared to those in controls. In addition, the relative expression of apoptosis-related genes BAX, HO-1, and p53 decreased in PCA-treated broilers compared with controls, indicating that PCA suppressed hepatic apoptosis, which is consistent with reduced oxidative stress and the improved antioxidant status.
Immune System and Intestinal Barrier Function
Cytokines and Immunoglobulin
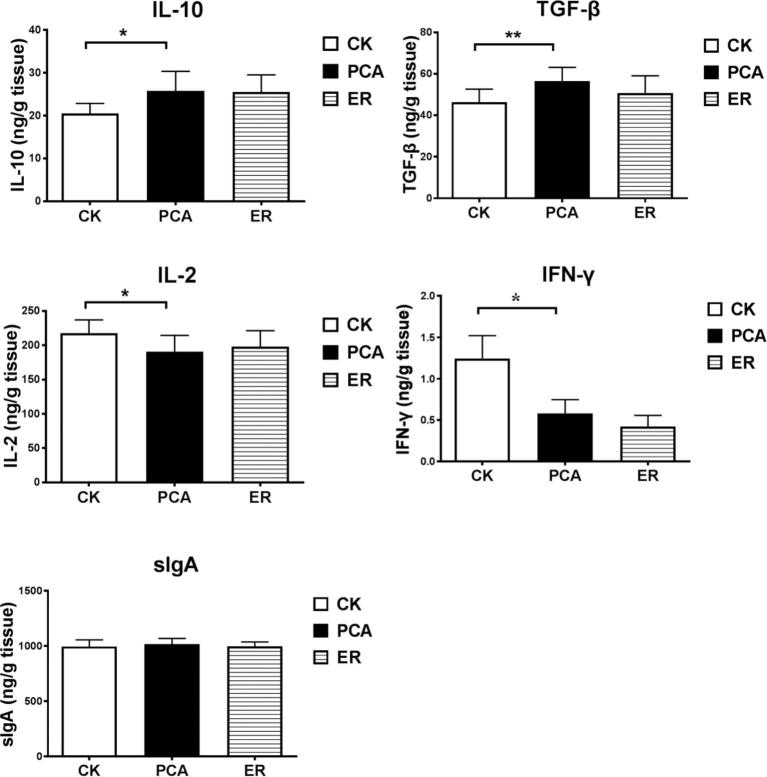
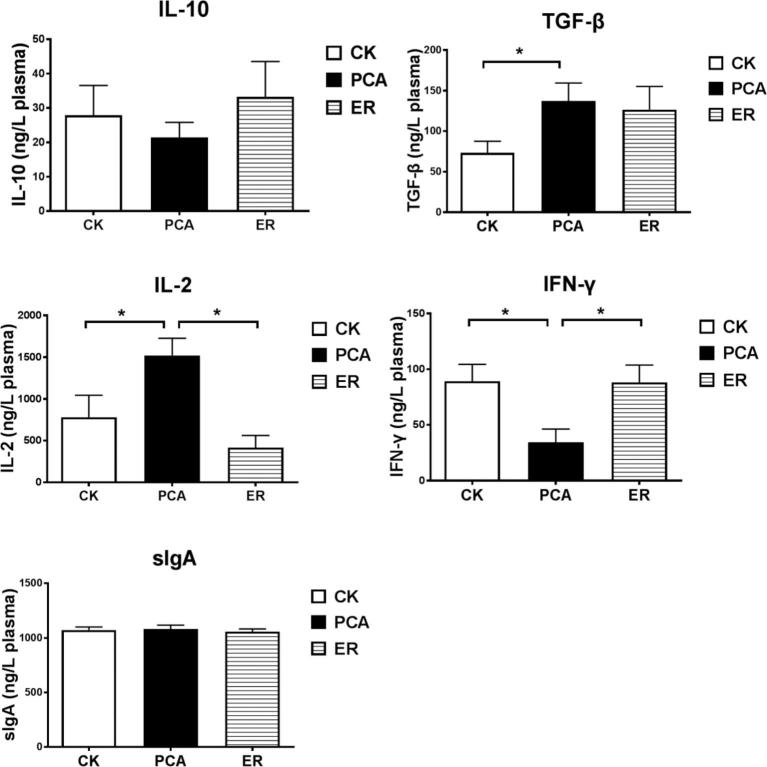
As shown in Figure 1, supplementation with PCA significantly increased anti-inflammatory cytokines IL-10 and TGF-β, and reduced the proinflammatory cytokine IL-2 in jejunal mucosa, and also reduced IFN-γ content; it had no effect on jejunal sIgA. Comparable changes occurred in plasma, as shown in Figure 2, where treatment with PCA increased TGF-β and reduced IFN-γ, compared with those in broilers in the control or ER group. As important cytokines, IL-10, TGF-β, IFN-γ, and IL-2 play essential roles in immunity in broilers, and these results together demonstrate that treatment with PCA caused changes that were consistent with improving intestinal immune function, inflammation, and relief from inflammation at the whole body level.
Figure 1.
Effect of PCA on cytokines and sIgA in jejunum. Contents of IL-2, IL-10, IFN-γ, TGF-β, and sIgA in mucosal supernatants were quantified using sandwich ELISA kits. Data are means ± SEM. IL-2, interleukin-2; IL-10, interleukin-10; IFN-γ, interferon (IFN)-γ; TGF-β, transforming growth factor-β; sIgA, secretory immunoglobulin A. *P < 0.05, **P < 0.01 (ANOVA).
Figure 2.
Plasma concentrations of cytokines and sIgA. Contents of IL-2, IL-10, IFN-γ, TGF-β, and sIgA in plasma were quantified using sandwich ELISA kits. Data are means ± SEM. *P < 0.05, **P < 0.01 (ANOVA).
Transmission Electron Microscopy
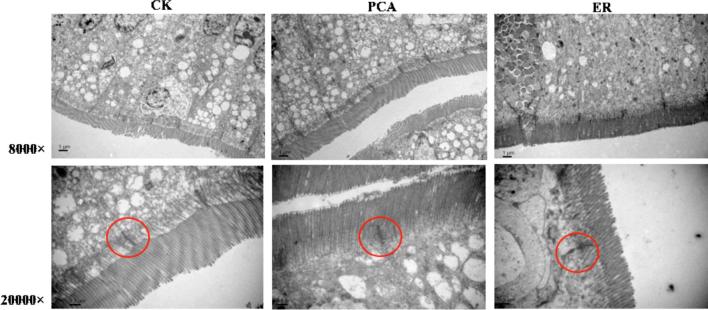
Images of TEM of the jejunum are presented in Figure 3, showing that broilers treated with dietary PCA or ER showed normal arrangement of microvilli, compared with that in the controls. These together suggested that treatment with PCA was not observed to affect the normal morphology of the jejunal mucosa, indicating that PCA did not show negative effects on intercellular connectivity, evident at the level of tight junctions, adhesion belts, and desmosomes.
Figure 3.
Transmission electron micrographs of jejunal brush border in broilers. Magnification: 8000 ×, 20,000 ×. Red circle, tight junction and adhesion belt.
Gene Expression
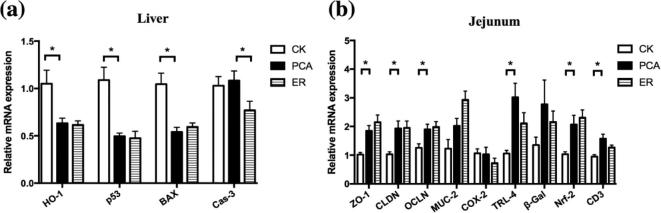
The effect of PCA on hepatic gene expression was examined (Figure 4a), and relative expression of apoptosis-related genes, such as BAX, HO-1, and p53, significantly decreased in PCA-supplemented broilers compared with the controls, whereas there were no difference compared with ER group, indicating that PCA and ER resulted in suppression of apoptosis in the liver. Jejunal mucosal gene expression (Figure 4b) of ZO-1, CLDN, and OCLN, encoding proteins related to the intestinal barrier, was significantly increased by treatment with PCA compared with those in controls. In addition, dietary PCA also increased mucosal expression of TLR-4, Nrf-2, and CD3.
Figure 4.
Effect of dietary PCA on relative gene expression in liver (a) and jejunal mucosa (b) of 52-day-old Chinese yellow broilers. Data are means ± SEM for n = 12. Differences by ANOVA are indicated by * (P < 0.05). BAX, Bcl-2 associated X protein; CD3, cluster of differentiation 3; CLDN, occudin; OCLN, claudin; COX-2, cyclo-oxygenase 2; HO-1, heme oxygenase1; Nrf2, nuclear factor 2; p53, tumor protein 53; TLR-4, toll-like receptor 4; β-Gal9, β-Galectin-9.
The immune system is the most effective defense against the invasion of pathogens. The thymus is the central organ of cellular immunity, whereas both thymus and spleen produce a large number of lymphocytes, contributing to cellular and humoral immunity (Wang et al., 2016). The bursa of Fabricius is the specific central immune organ of birds, producing B lymphocytes and antibody (Ju et al., 2015; Wang et al., 2016a). The BW-normalized indices of these 3 organs are used to evaluate the immune state of broilers (Rivas and Fabricant, 1988); larger organ indices indicate stronger cellular and humoral immune capacity. In the present study, supplementation with PCA increased indices of spleen and bursa of Fabricius compared to those in controls, indicating enhanced immune capacity.
The cytokine IL-2 has key functions in the immune system, affecting tolerance and immunity, primarily via its direct effects on T cells (Malek and Castro, 2010; Liao et al., 2011). As a proinflammatory cytokine, the reduction in IL-2 indicated less inflammation in PCA-treated broilers, which is consistent with the increased levels of the anti-inflammatory cytokines IL-10 and TGF-β. In addition, PCA decreased INF-γ, excess of which is associated with auto-inflammation. Dietary PCA also increased the expression of some immunity-related genes in jejunal mucosa, such as TLR-4, Nrf-2, and CD3. These genes either transduce the activation signals produced by T-cell receptor recognition antigens or regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, so as to play a fundamental role in pathogen recognition and activation of innate immunity.
The intestinal barrier consists of an intrinsic layer, including epithelial cells with tight junctions, an extrinsic layer of resident bacteria, and a coating of mucus containing high concentrations of sIgA (Walker and Sanderson, 1992). Dietary supplementation of broilers with PCA here did not affect the content of sIgA in jejunal mucosa. The ultrastructural images suggested that treatment with PCA did not affect the normal morphology of jejunum, but enhanced intercellular connectivity, including tight junctions, adhesion belts, and desmosomes. Tight junctions between mucosal cells are vital for the intestinal mucosal barrier resisting invasion by viruses and bacteria (Anderson and Van Itallie, 1995; Ulluwishewa et al., 2011; Lin et al., 2016) Claudins, the main proteins of the tight junction complex, contribute to their integrity; any impairment increases mucosal barrier defects (Kinugasa et al., 2000; Kucharzik et al., 2001; Berkes et al., 2003). Gene expression of proteins related to the intestinal barrier, such as ZO-1, CLDN, and OCLN, was increased by dietary PCA, which is consistent with PCA improving barrier function in addition to favoring local immune function.
Structure of Intestinal Flora
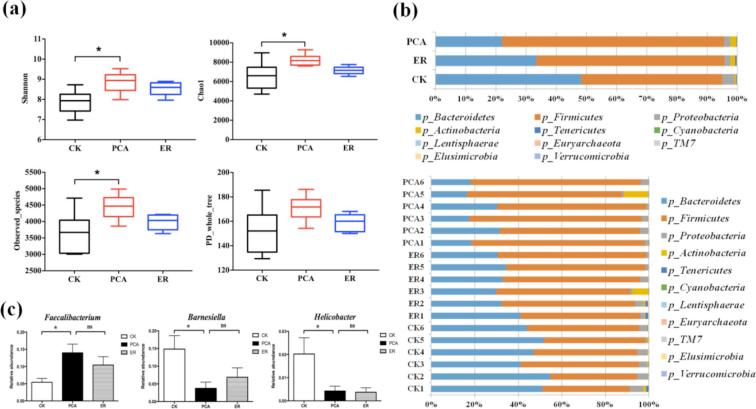
As shown in Figure 5a, α-diversity (Chao1, Shannon, and Observed_species) in cecal contents of birds supplemented with PCA differed from the controls (CK), whereas there was no significant difference in the PD_whole_tree, indicating that PCA treatment changed diversity of the microflora.
Figure 5.
Microflora analysis of 52-day-old Chinese yellow broilers. (a) α diversity analysis for samples from the 3 treatments (n = 6). (b) Phylum distributions of gut microbiota (n = 6). (c) Genus distributions of Barnesiella, Helicobacter, and Faecalibacterium.*P < 0.05 (ANOVA).
Phylum distributions of gut microbiome for the different treatments are shown in Figure 5b. Compared with the controls, relative abundance of Firmicutes, and Actinobacteria in broilers supplemented with PCA and ER increased, whereas that of Bacteroidetes and Proteobacteria decreased (P < 0.01). Furthermore, the relative abundance of Firmicutes (P < 0.01), Actinobacteria (P < 0.05) increased in birds supplemented with PCA and that of Bacteroidetes (P < 0.01) decreased compared with birds fed ER. Concerning genus distributions (Figure 4c), treatment with PCA markedly reduced the relative abundance of Barnesiella (P < 0.05) and Helicobacter (P < 0.05), while increasing the relative abundance of Faecalibacterium (P < 0.05) compared with that in the controls.
The complex gut microbial flora (microbiota) harbored by individuals has long been proposed to contribute to intestinal health and disease (Mai and Draganov, 2009). The present study showed that dietary PCA changed microflora diversity. Compared with controls, there were more Firmicutes and Actinobacteria and fewer Bacteroidetes and Proteobacteria in broilers treated with PCA. Firmicutes are predominant bacteria in the intestinal tract and capable of oxidizing sugars by lactic acid fermentation (Hold et al., 2002; Vaughan et al., 2002; Eckburg et al., 2005). A relative increase in Firmicutes could benefit gut health (Wang et al., 2016). Proteobacteria, Gram-negative bacteria, include many enteric pathogens, such as E. coli and Salmonella (Bergey et al., 1984; Madigan and Martinko, 2005). At the level of genus distributions, dietary PCA markedly reduced relative abundance of Barnesiella and Helicobacter, while increasing that of Faecalibacterium. Faecalibacterium are among the most abundant and important commensal bacteria and mainly produce butyrate and other short-chain fatty acids by fermentation of dietary fiber (Lopez-Siles et al., 2012); with other actions, they boost the immune system (Miquel et al., 2013). Strains of Helicobacter are pathogenic to humans or animals, as they are strongly associated with peptic ulcers, chronic gastritis, duodenitis, and stomach cancer (Adeniyi et al., 2012). The present results together suggested that dietary PCA improved the population makeup of intestinal flora in favor of improved intestinal health.
Even though there was no study on the administration of PCA to animals to focus on its growth promoting or meat quality improving effect, there were some familiar investigation on broilers with administration with plant extracts. Administration with extract from willow leaves improved production performance and slaughter performance of broilers (Wang et al., 2018). Adding plant extracts from thyme and rosemary to diet effectively regulated microflora and improve intestinal tract quality (Wen et al., 2017). The consistent results of these researches and ours indicated that plant extracts had great potentialities as new feed additives for broilers.
In conclusion, dietary supplementation with PCA increased efficiency and growth performance of Chinese yellow-feathered broilers and caused changes consistent with improved meat quality. PCA also improved antioxidant capacity and reduced oxidative damage in broilers. Finally, PCA enhanced indices of intestinal immune function and improved the structure of intestinal flora, favoring intestinal health.
ACKNOWLEDGMENTS
W. Bruce Currie (Emeritus Professor, Cornell University) made suggestions on presentation. This work was financially supported by National Key R&D Project (2018YFD0500600), Research System (CARS-41) from the Ministry of Agriculture, “Twelve-Five” National Science and Technology Support Program (2014BAD13B02), President's Fund of Guangdong Academy of Agricultural Sciences (201908), National Natural Science Foundation of China (31802104), and Science and Technology Project of Guangdong Province (2017B020202003), P. R. China. The authors thank all workers of the study for their participation.
Author's contributions
Yibing Wang, Shouqun Jiang and Weifen Li designed the research; Yibing Wang and Yuanyuan Wang conducted the research; Yibing Wang and Baikui Wang analysed the data and had primary responsibility for the final content; Yibing Wang and Xiaoqiang Mei wrote the paper. All authors read and approved the final manuscript.
Conflict of interest: None of the authors has any conflicts of interest to declare.
REFERENCES
- Adeniyi B. A., Lawal T. O., Otegbayo J. A., Oluwasola O. A., Odaibo G. N., Ola S. O., Okolo C. A., Akere A., Kehinde A. O.. 2012. Cultural characteristics and antibiotic susceptibility pattern of Helicobacter pylori isolated from dyspepsia patients. Gastroenterol. Insights 4:21. [Google Scholar]
- Anderson J. M., Van Itallie C. M.. 1995. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 269:G467–G475. [DOI] [PubMed] [Google Scholar]
- Bergey D. H., Holt J. G., Krieg N. R.. 1984. Bergey's Manual of Systematic Bacteriology. The Williams and Wilkins Co., Baltimore: 38:443–491. [Google Scholar]
- Berkes J., Viswanathan V. K., Savkovic S. D., Hecht G.. 2003. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52:439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich N. A., Subramanian S., Faith J. J., Gevers D., Gordon J. I., Knight R., Mills D. A., Caporaso J. G.. 2013. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10:57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A. 1984. Nonparametric-estimation of the number of classes in a population. Scand. J. Stat. 11:265–270. [Google Scholar]
- Chao A., Shen T. J.. 2003. Nonparametric estimation of Shannon's index of diversity when there are unseen species in sample. Environ. Ecol. Stat. 10:429–443. [Google Scholar]
- Chao C. Y., Yin M. C.. 2009. Antibacterial effects of roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog. Dis. 6:201–206. [DOI] [PubMed] [Google Scholar]
- Chen K. L., Chen T. T., Lin K. J., Chiou P. W. S.. 2007. The effects of caponization age on muscle characteristics in male chicken. Asian Australas. J. Anim. Sci. 20:1684–1688. [Google Scholar]
- Eckburg P. B., Bik E. M., Bernstein C. N., Purdom E., Dethlefsen L., Sargent M., Gill S. R., Nelson K. E., Relman D. A.. 2005. Diversity of the human intestinal microbial flora. Science 308:1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazali R., Rahim H. A.. 2016. First derivative prediction of raw broiler shear force using visible short wave near infrared spectroscopy. J. Teknol. 78:1–6. [Google Scholar]
- Good I. J. 1953. The population frequencies of species and the estimation of population parameters. Biometrika 40:237–264. [Google Scholar]
- Gou Z. Y., Jiang S. Q., Jiang Z. Y., Zheng C. T., Li L., Ruan D., Chen F., Lin X. J.. 2016. Effects of high peanut meal with different crude protein level supplemented with amino acids on performance, carcass traits and nitrogen retention of Chinese Yellow broilers. J. Anim. Physiol. Anim. Nutr. 100:657–664. [DOI] [PubMed] [Google Scholar]
- Hayat M. A. 1986. Basic Techniques for Transmission Electron Microscopy. BioScience. [Google Scholar]
- Hold G. L., Pryde S. E., Russell V. J., Furrie E., Flint H. J.. 2002. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis. FEMS Microbiol. Ecol. 39:33–39. [DOI] [PubMed] [Google Scholar]
- Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H.. 2013. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 92:370–374. [DOI] [PubMed] [Google Scholar]
- Jiang S., Jiang Z., Lin Y., Zhou G., Chen F., Zheng C.. 2011. Effects of different rearing and feeding methods on meat quality and antioxidative properties in Chinese Yellow male broilers. Br. Poult. Sci. 52:352–358. [DOI] [PubMed] [Google Scholar]
- Jiang S. Q., Jiang Z. Y., Zhou G. L., Lin Y. C., Zheng C. T.. 2014. Effects of dietary isoflavone supplementation on meat quality and oxidative stability during storage in lingnan yellow broilers. J. Integr. Agr. 13:387–393. [Google Scholar]
- Ju T., Guo T. X. Y, Sui J. J., Xiao X., Zhan X.. 2015. Effects of different dosage forms of sodium butyrate on serum biochemical indexes, antioxidant and anti-inflammatory functions in broilers under lipopolysaccharide stress. Chin. J. Anim. Nutr. 12:3146–3154. [Google Scholar]
- Kakkar S., Bais S.. 2014. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinugasa T., Sakaguchi T., Gu X., Reinecker H. C.. 2000. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 118:1001–1011. [DOI] [PubMed] [Google Scholar]
- Kucharzik T., Walsh S. V., Chen J., Parkos C. A., Nusrat A.. 2001. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 159:2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Herbold C. W., Polson S. W., Wommack K. E., Williamson S. J., McDonald I. R., Cary S. C.. 2012. Groundtruthing next-gen sequencing for microbial ecology-biases and errors in community structure estimates from PCR amplicon pyrosequencing. PLoS One 7:e44224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Lin J. X., Leonard W. J.. 2011. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 23:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Jiang S., Jiang Z., Zheng C., Gou Z.. 2016. Effects of equol on H2O2-induced oxidative stress in primary chicken intestinal epithelial cells. Poult. Sci. 95:1380–1386. [DOI] [PubMed] [Google Scholar]
- Liu C. L., Wang J. M., Chu C. Y., Cheng M. T., Tseng T. H.. 2002. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem. Toxicol. 40:635–641. [DOI] [PubMed] [Google Scholar]
- Lopez-Siles M., Khan T. M., Duncan S. H., Harmsen H. J., Garcia-Gil L. J., Flint H. J.. 2012. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl. Environ. Microbiol. 78:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., Knight R.. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M., Martinko J.. 2005. Brock Biology of Microorganisms. 11th edn. SciELO Espana.
- Mai V., Draganov P. V.. 2009. Recent advances and remaining gaps in our knowledge of associations between gut microbiota and human health. World J. Gastroenterol. 15:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorano G., Sobolewska A., Cianciullo D., Walasik K., Elminowska-Wenda G., Slawinska A., Tavaniello S., Zylinska J., Bardowski J., Bednarczyk M.. 2012. Influence of in ovo prebiotic and synbiotic administration on meat quality of broiler chickens. Poult. Sci. 91:2963–2969. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Castro I.. 2010. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F.. 1976. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch. Pathol. Lab. Med. 100:405–414. [PubMed] [Google Scholar]
- Miquel S., Martin R., Rossi O., Bermudez-Humaran L. G., Chatel J. M., Sokol H., Thomas M., Wells J. M., Langella P.. 2013. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 16:255–261. [DOI] [PubMed] [Google Scholar]
- Ning Q., Peng D., Gao Z., University L. M.. 2013. Protective effects and mechanisms of protocatechuic acid on common bile duct obstruction induced intestinal barrier dysfunction in mice. Pharmacol. Clin. Chin. Mater. Med. 29:27–30. [Google Scholar]
- Okle O. S. E., Derbalah A., Euony O. E.. 2016. Hepatic damage associated with fatal zinc phosphide poisoning in broiler chicks. Int. J. Vet. Sci. Med. 4:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahima H. A., Ghazalia R., Sahlana S., Maidinb M. S.. 2013. Prediction of texture of raw poultry meat by visible and near–infrared reflectance spectroscopy. j. teknol. 64:5. [Google Scholar]
- Rasmussen A. J., Andersson M.. 1996. New method for determination of drip loss in pork muscles. Proceedings of the 42nd International Congress of Meat Science and Technology, Lillehammer, Norway, Elsevier Applied Science; 42:286–287. [Google Scholar]
- Rivas A. L., Fabricant J.. 1988. Indications of immunodepression in chickens infected with various strains of Marek's Disease virus. Avian Dis. 32:1–8. [PubMed] [Google Scholar]
- Roberts T., Wilson J., Guthrie A., Cookson K., Vancraeynest D., Schaeffer J., Moody R., Clark S.. 2015. New issues and science in broiler chicken intestinal health: intestinal microbial composition, shifts, and impacts. Worlds Poult. Sci. J. 71:259–270. [Google Scholar]
- Satoh T., Takeuchi O., Vandenbon A., Yasuda K., Tanaka Y., Kumagai Y., Miyake T., Matsushita K., Okazaki T., Saitoh T., Honma K., Matsuyama T., Yui K., Tsujimura T., Standley D. M., Nakanishi K., Nakai K., Akira S.. 2010. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 11:936–944. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R. C., McNabb W. C., Moughan P. J., Wells J. M., Roy N. C.. 2011. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 141:769–776. [DOI] [PubMed] [Google Scholar]
- Vaughan E. E., de Vries M. C., Zoetendal E. G., Ben-Amor K., Akkermans A. D., de Vos W. M.. 2002. The intestinal LABs. Antonie Van Leeuwenhoek 82:341–352. [PubMed] [Google Scholar]
- Walker W. A., Sanderson I. R.. 1992. Epithelial barrier function to antigens an overview. Ann. NY Acad. Sci. 664:10–17. [DOI] [PubMed] [Google Scholar]
- Wang Y. B., Du W., Fu A. K., Zhang X. P., Huang Y., Lee K. H., Yu K., Li W. F., Li Y. L.. 2016. Intestinal microbiota and oral administration of Enterococcus faecium associated with the growth performance of new-born piglets. Beneficial Microbes 7:529–538. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang X. M. D, Su J. H.. 2018. Effects of extracts from willow leaves on the performance of AA∼+ broiler chickens. Heilongjiang Animal Husbandry and Veterinary Medicine, 2.
- Wen A., Hu Y. H, Bai X., Wang J. X. 2017. Effects of Plant Extracts on Early Intestinal Development and Microbial Community in Broilers. Journal of Anhui Science and Technology University, 6.