Abstract
Summary:
Objective:
Few bone mineral density (BMD) data are available in men with anorexia nervosa (AN), and none in those with atypical AN (ATYP) (AN psychological symptoms without low weight) or avoidant/restrictive food intake disorder (ARFID) (restrictive eating without AN psychological symptoms). We investigated the prevalence and determinants of low BMD and estimated hip strength in men with these disorders.
Design:
Cross-sectional: two centres.
Patients:
A total of 103 men, 18–63 years: AN (n = 26), ARFID (n = 11), ATYP (n = 18), healthy controls (HC) (n = 48).
Measurements:
Body composition, BMD and estimated hip strength (section modulus and buckling ratio) by DXA (Hologic). Serum 25OH vitamin D was quantified, as was daily calcium intake in a subset of subjects.
Results:
Mean BMI was lowest in AN and ARFID, higher in ATYP and highest in HC (AN 14.7 ± 1.8, ARFID 15.3 ± 1.5, ATYP 20.6 ± 2.0, HC 23.7 ± 3.3 kg/m2) (P < 0.0005). Mean BMD Z-scores at spine and hip were lower in AN and ARFID, but not ATYP, than HC (postero-anterior (PA) spine AN −2.05 ± 1.58, ARFID −1.33 ± 1.21, ATYP −0.59 ± 1.77, HC −0.12 ± 1.17) (P < 0.05). 65% AN, 18% ARFID, 33% ATYP and 6% HC had BMD Z-scores <−2 at ≥1 site (AN and ATYP vs HC, P < 0.01). Mean section modulus Z-scores were lower in AN than HC (P < 0.01). Lower BMI, muscle mass and vitamin D levels (R = 0.33–0.64), as well as longer disease duration (R = −0.51 to −0.58), were associated with lower BMD (P < 0.05).
Conclusions:
Men with AN, ARFID and ATYP are at risk for low BMD. Men with these eating disorders who are low weight, or who have low muscle mass, long illness duration and/or vitamin D deficiency, may be at particularly high risk.
Keywords: anorexia nervosa, bone density, feeding and eating disorders, vitamin D deficiency
1 |. INTRODUCTION
Low bone mineral density (BMD) and increased fracture risk are well-known complications of anorexia nervosa (AN) in females,1–4 but little is known about BMD and bone strength in men with AN or other eating disorders. In 2013, psychiatric diagnostic criteria were revised, including adding atypical AN, diagnosed in individuals who meet psychological criteria of AN, but are not low weight despite significant weight loss, and avoidant/restrictive food intake disorder (ARFID), diagnosed in individuals who demonstrate a pattern of avoidant or restrictive eating in the absence of psychological symptoms of AN.5 Whether low BMD and estimated hip strength, measured by hip structural analysis (HSA) and associated with incident fractures independent of BMD,4 are significant concerns in men with DSM-5 AN, atypical AN or ARFID is unknown.
Prior studies have reported an elevated fracture risk in men with AN,2 and a high prevalence of spine BMD Z-scores <−1 in adolescent boys6 and young men7 with AN diagnosed using DSM-IV criteria. No study has investigated the prevalence of low BMD at multiple sites, or estimated hip strength, in men across a wide age range with AN. Nor has BMD or estimated hip strength in men with atypical AN or ARFID been reported. Moreover, few studies have reported determinants of low BMD or bone strength in men with AN,7,8 and no analysis of the determinants of bone mass in men with atypical AN or ARFID has been published. Understanding risk factors for low BMD is requisite to identify those at highest fracture risk, as well as potential therapeutic targets to reduce risk.
We hypothesized the following: (a) men with DSM-5 AN, ARFID and atypical AN will have lower mean spine and hip BMD Z-scores, and more impaired estimated hip strength by HSA, than controls, with increasing severity from atypical AN to ARFID to AN, and (b) lower BMI, muscle mass and vitamin D levels will be associated with lower BMD Z-scores and estimated hip strength.
2 |. MATERIALS AND METHODS
This study was approved by the Partners Healthcare Institutional Review Board (IRB), the Sponsored Programs and Research Office at Denver Health and the Colorado Multiple IRB. We studied 103 men, aged 18–63 years. Thirty-four ambulatory men with AN, ARFID or atypical AN were consecutively recruited between 2001 and 2008 for a study at Massachusetts General Hospital (Boston, MA); all subjects provided written informed consent before study participation. Data from the MGH site were prospectively collected, and blood samples frozen for future analysis. Twenty-one men were consecutively admitted between 2009 and 2017 to an inpatient medical stabilization programme at ACUTE Center for Eating Disorders (Denver Health Medical Center, Denver, CO). Data from subjects at ACUTE were collected retrospectively. For the entire cohort, exclusion criteria included any medical condition (other than an eating disorder) or medication known to affect bone metabolism, or no dual-energy x-ray absorptiometry (DXA) data. Eight subjects from ACUTE did not have a current DXA scan because they had had one outside the system within the past 2 years. Data from either site have not been reported previously. Forty-eight healthy race- and age-matched (±2 years) men were blindly matched from a previously published randomized controlled trial of 400 men at MGH9,10 to be included as healthy controls in this study. These men met rigorous exclusion criteria, including no current diagnoses or medications known to affect bone metabolism, abnormal serum calcium or parathyroid hormone (PTH) levels, and serum testosterone level <270 ng/dL. DSM-5 eating disorder diagnoses were determined by a psychologist on unstructured clinical interview:
Anorexia nervosa (AN; n = 26): Low weight (BMI < 18.5 kg/m2) and psychological symptoms characteristic of AN (fat phobia and body image disturbance).
Atypical AN (ATYP; n = 18): Psychological symptom characteristic of AN and significant weight loss, but not currently low weight (BMI ≥ 18.5 kg/m2).
Avoidant/restrictive food intake disorder (ARFID; n = 11): Restricted eating but without psychological symptoms characteristic of AN; no weight criterion is included in the diagnostic criteria.
All subjects underwent a history and physical exam. Percent ideal body weight was calculated.11 Highest and lowest weight, duration of illness, current tobacco and alcohol use, hours of physical activity a week and a history of fracture were obtained by self-report in eating disorder subjects. Face, skull, finger, toe and heel fractures were excluded.12 Total daily calcium intake (dietary + supplemental) was assessed (n = 19; 5 AN and 14 atypical AN) via a calcium questionnaire.
2.1 |. Dual-energy x-ray absorptiometry and hip structural analysis
BMD Z-scores at the postero-anterior (PA) spine, total hip and femoral neck (FN), and body composition, were assessed by Hologic QDR 4500 densitometer at MGH [coefficient of variation (CV) for BMD, fat mass and lean mass were 1.1%, 2.1% and 1.0%, respectively] and Hologic Discovery QDR densitometer at ACUTE (CV for BMD 1.5%; Hologic, Waltham, MA). Scans were reanalysed using Hologic QDR Horizon, Software Verizon APEX 5.6.0.4. Sex- and race-specific BMD Z-scores were calculated from 2012 National Health and Nutrition Examination Survey (NHANES) reference data. Proximal femur DXA scans were analysed using HSA software.13 Section modulus (cm3) (Z, indicator of strength of bone to resist bending and torsion) and buckling ratio (BR, index of susceptibility to cortical buckling under compressive loads) were reported at the narrow neck (NN), intertrochanteric (IT) and femoral shaft (FS) regions.14 Both section modulus and buckling ratio are associated with incident hip fracture in men,15 and women independent of BMD.4 Sex- and age-specific NN and FS section modulus Z-scores were calculated from NHANES III reference data,16 which included only non-Hispanic white men (3 subjects were excluded [2 Hispanic,1 Asian] for this analysis).
2.2 |. Biochemical analysis
ACUTE and MGH serum samples were evaluated at the Denver Health Core Laboratory. Serum samples from ACUTE were run in real time. MGH samples were stored at −80°C, and later run in a single batch using the same assays. Healthy controls did not have serum samples available. Serum 25OH vitamin D was measured by CLIA (ADVIA Centaur, intra-assay CV ≤7%, sensitivity 3.2 ng/mL) traceable to LC/MS/MS. Vitamin D deficiency was defined as 25OH vitamin D <20 ng/mL.
2.3 |. Data Analysis
We conducted analyses using JMP Statistical Discovery Software, v12 Professional (SAS Institute, Cary, NC). Variables were assessed for normality using the Shapiro-Wilk test, and log-transformed if non-normal. For Table 1, we compared continuous variables across the four groups using ANOVA and Tukey-Kramer to adjust for multiple comparisons. For all other analyses, we compared continuous variables between each eating disorder group and controls using Dunnett’s test to adjust for multiple comparisons. We compared the frequency of categorical variables across groups (Table 1) or between each eating disorder group and controls (all other analyses) using Fisher’s exact test and Holm-Bonferroni to adjust for multiple comparisons. Linear regression analyses were used to investigate associations between bone variables with clinical, biochemical or body composition variables; Pearson’s correlation coefficients are reported. Multivariate least-square analyses were performed to further investigate determinants of bone variables. Significance was defined as a two-tailed P ≤ 0.05. Data are reported as mean ± SEM or n(%), unless otherwise noted.
TABLE 1.
Clinical characteristics of men with anorexia nervosa, avoidant/restrictive food intake disorder (ARFID) and atypical anorexia nervosa compared to healthy controls (mean ± SD)
| Anorexia nervosa (n = 26) |
ARFID (n = 11) | Atypical anorexia nervosa (n = 18) |
Healthy controls (n = 48) |
|
|---|---|---|---|---|
| Age (y) | 30.7 ± 14.2 | 27.5 ± 12.4 | 26.2 ± 8.3 | 28.9 ± 10.9 |
| Race/ethnicity, Caucasian | 94% | 100% | 100% | 96% |
| Current smoker | 16% | 18% | 17% | n/a |
| Alcohol use >1 drink/d | 4% | 0% | 6% | n/a |
| Physical activity (hrs/wk) | 6.2 ± 11.5 | 2.9 ± 4.9 | 18.8 ± 19.2 | n/a |
| Body mass index (BMI) (kg/m2) | 14.7 ± 1.8a | 15.3 ± 1.5a | 20.6 ± 2.0b | 23.7 ± 3.3c |
| Percent ideal body weight (%) | 62.0 ± 7.8a | 63.9 ± 6.9a | 86.6 ± 7.9b | 99.2 ± 13.8c |
| Total fat mass (%) | 13.0 ± 2.5a | 14.8 ± 2.4ab | 16.3 ± 4.6b | 22.7 ± 5.3c |
| Appendicular lean mass (kg) | 17.1 ± 2.8a | 18.2 ± 2.4a | 24.4 ± 4.2b | 25.0 ± 3.0b |
| Duration of illness (patient report, y) | 9.8 ± 10.7 | 3.4 ± 3.1 | 5.3 ± 5.5 | n/a |
| Highest past BMI (kg/m2) | 24.3 ± 7.2 | 22.6 ± 3.4 | 26.8 ± 6.3 | n/a |
| History of BMI ≥25 kg/m2 | 30% | 30% | 50% | n/a |
| Lowest past BMI (kg/m2) | 14.3 ± 1.6a | 14.7 ± 2.0a | 18.3 ± 2.5b | n/a |
| History of BMI <18.5 kg/m2 | 100%a | 89%ab | 50%b | n/a |
| 25OH vitamin D (ng/mL) | 28.3 ± 16.2 | 33.0 ± 14.0 | 25.6 ± 8.5 | n/a |
| 25OH vitamin D <20 ng/mL | 24% | 0% | 21% | n/a |
| Daily calcium intake <1000 mg* | 40% | n/a | 36% | n/a |
| Vitamin supplementation use | 16% | 18% | 47% | n/a |
| History of fracture | 17% | 9% | 17% | n/a |
Groups with different superscripts letters differ significantly at P ≤ 0.05.
Data available for a subset of subjects.
3 |. RESULTS
3.1 |. Clinical characteristics
Clinical characteristics are shown in Table 1. Mean age, race, tobacco and alcohol use, physical activity, illness duration, highest past BMI, 25OH vitamin D levels and vitamin supplementation use were similar across groups. Differences in BMI, body composition and lowest past BMI are shown in Table 1. In the subset of subjects with available data (n = 19), the percentage of subjects with daily calcium intake <1000 mg was similar between AN and ATYP (Table 1). The prevalence of prior fracture(s), all traumatic, was similar across groups.
3.2 |. BMD and estimated hip strength
Mean PA spine and total hip BMD Z-scores were lower in AN and ARFID, and FN BMD Z-scores were lower in AN, than controls (P < 0.05, Figure 1A). ATYP had similar mean BMD Z-scores compared to controls at all sites (Figure 1A).
FIGURE 1.
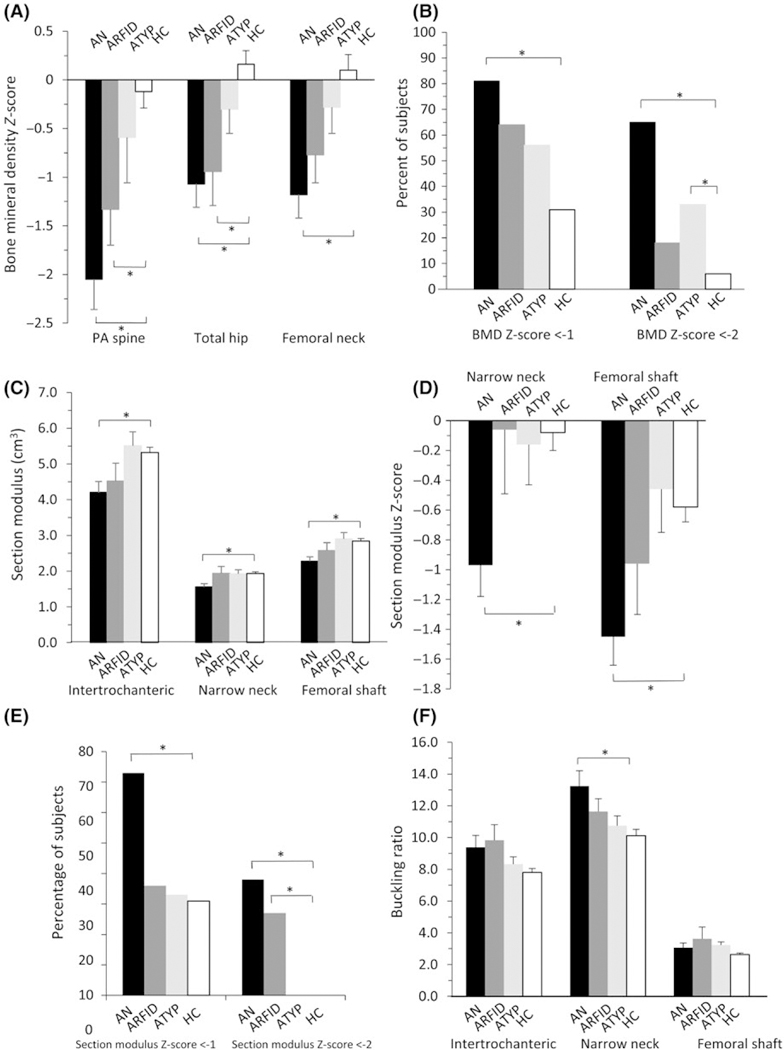
(A) Mean bone mineral density (BMD) Z-scores at the postero-anterior (PA) spine and total hip were lower in men with anorexia nervosa (AN) and avoidant/restrictive food intake disorder (ARFID) compared to healthy controls (HC). (B) The prevalence of any BMD Z-score <−1 was higher in AN than HC, while the prevalence of any BMD Z-score <−2 was higher in AN and ATYP than HC. (C) Estimated hip strength as assessed by mean section modulus was lower in men with AN compared to HC. (D) Mean section modulus Z-scores at the narrow neck and femoral shaft were lower in AN than HC. (E) The prevalence of any section modulus Z-score <−1 was higher in AN, while the prevalence of any section modulus Z-score <−2 was higher in AN and ARFID, than HC. (F) Mean buckling ratio, with a higher buckling ratio denoting more impaired estimated hip strength, was higher in AN than HC at the narrow neck. Mean ± SEM. *P ≤ 0.05
The prevalence of any BMD Z-score <−1 was higher in AN than controls (81% vs 31%, P < 0.0001), but similar in ARFID (64%) and ATYP (56%) compared to controls (Figure 1B). The prevalence of any BMD Z-score <−2 was higher in AN (65%) and ATYP (33%) than controls (6%) (P < 0.01), but similar in ARFID (18%) compared to controls (Figure 1B).
The prevalence of BMD Z-scores <−1 and <−2 per skeletal site is shown in Table 2. In AN, the prevalence of BMD Z-score <−1 and <−2 was higher at all sites than controls. In ARFID, the prevalence of BMD Z-score <−1 was higher at the hip and FN than controls. In ATYP, the prevalence of BMD Z-score <−1, and in ARFID and ATYP, the prevalence of BMD Z-score <−2, at any site was not statistically different than controls.
TABLE 2.
Prevalence of bone mineral density (BMD) Z-score <−1 and <−2 in men with eating disorders and healthy controls
| Anorexia nervosa (n = 26) |
Avoidant/restrictive food intake disorder (n = 11) |
Atypical anorexia nervosa (n = 18) |
Healthy controls (n = 48) |
|
|---|---|---|---|---|
| PA spine BMD Z-score | ||||
| <−1 | 77%a | 64% | 43% | 23% |
| <−2 | 62%a | 18% | 29% | 6% |
| Total hip BMD Z-score | ||||
| <−1 | 50%a | 64%a | 33% | 8% |
| <−2 | 15% | 9% | 0% | 2% |
| Femoral neck BMD Z-score | ||||
| <−1 | 58%a | 45%a | 33% | 10% |
| <−2 | 23%a | 0% | 0% | 2% |
Differed significantly from healthy controls at P ≤ 0.05
Mean NN and FS section modulus (Figure 1C) and section modulus Z-scores (Figure 1D) were lower, and mean NN buckling ratio (Figure 1F) was higher, in AN compared to controls (P < 0.01), but ARFID and ATYP had similar mean section modulus (Figure 1C), section modulus Z-scores (Figure 1D) and buckling ratio (Figure 1F) compared to controls. The prevalence of any section modulus Z-score <−1 was higher in AN, and any section modulus Z-score <−2 was higher in AN and ARFID, than controls (Figure 1E). In men with eating disorders, there was no difference in mean BMD Z-scores, section modulus or buckling ratio between men with and without a history of fracture.
3.3 |. Determinants of BMD and estimated hip strength
Within the entire group, there were linear univariate associations between current BMI and BMD Z-scores at all sites (Figures 2A–C), section modulus at all sites (R = 0.44–0.51) and buckling ratio at IT and FS (R = −0.37 to −0.49) (P ≤ 0.0001). There were linear univariate associations between appendicular lean mass (ALM) and BMD Z-scores at all sites (Figures 2D–F), section modulus at all sites (R = 0.63–0.70) and buckling ratio at IT and NN (R = −0.36 to −0.51) (P ≤ 0.0002). The relationships between ALM and BMD Z-scores or section modulus remained significant after controlling for BMI (P < 0.05).
FIGURE 2.
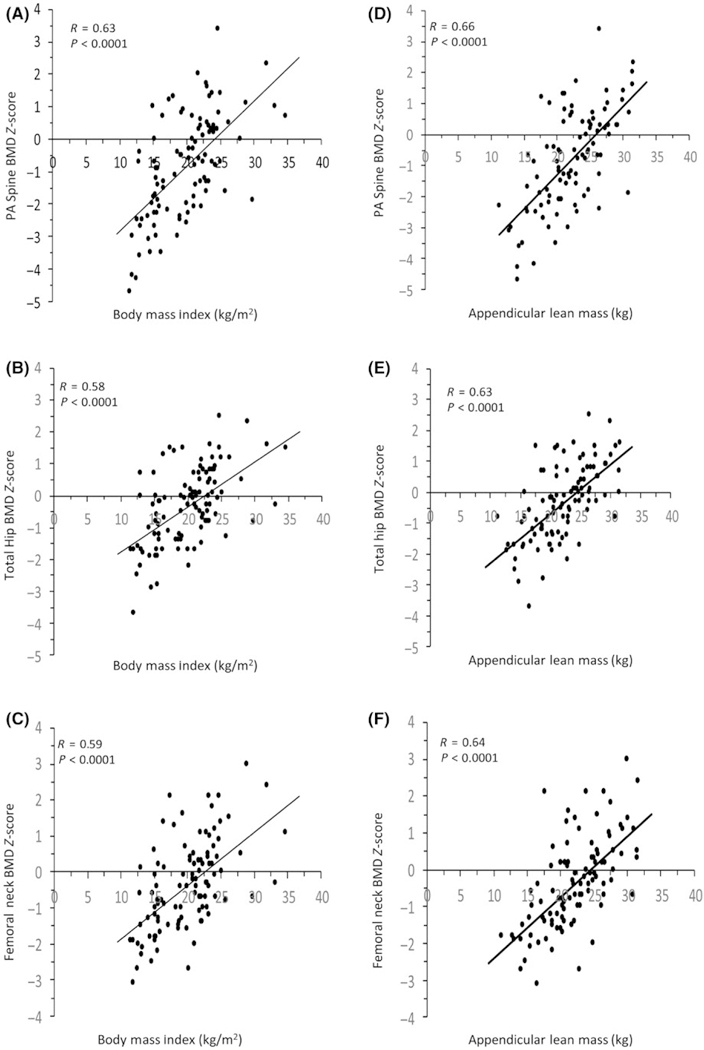
Positive linear relationship between BMI and (A) postero-anterior (PA) spine, (B) total hip and (C) femoral neck bone mineral density (BMD) Z-scores, as well as appendicular lean mass and (D) PA spine, (E) total hip and (F) femoral neck BMD Z-scores, in men with anorexia nervosa, avoidant/ restrictive food intake disorder, atypical anorexia nervosa and healthy controls
In men with eating disorders, illness duration was negatively associated with BMD Z-scores and section modulus and positively associated with buckling ratio, at all sites, which remained significant after controlling for BMI (Table 3). Highest past BMI was positively associated with BMD Z-scores and section modulus at all sites, which remained significant after controlling for current BMI (Table 3). In addition, the lower the BMI in the past, the lower the current BMD Z-score or section modulus (Table 3). Highest past BMI was also negatively associated with buckling ratio at ≥1 site (Table 3). Men with a history of low weight (BMI < 18.5 kg/m2, n = 35) had lower mean BMD Z-scores and section modulus at most sites than men who were never low weight (n = 6, P < 0.05). Men with a history of overweight (BMI ≥25 kg/m2, n = 18) had higher mean BMD Z-scores and section modulus at all sites, and lower IT and NN buckling ratio, than men who were never overweight (n = 29, P < 0.001). A higher number of hours of physical activity a week trended towards being associated with higher total hip (R = 0.32, P = 0.08) and femoral neck (R = 0.31, P = 0.09) BMD Z-scores, but not with estimated hip strength.
TABLE 3.
Associations between disease history and bone mineral density (BMD) or estimated hip strength in men with eating disorders
| PA spine BMD Z-score |
Total hip BMD Z-score |
Femoral neck BMD Z-score |
Intertrochanteric section modulus (cm3) |
Narrow neck section modulus (cm3) |
Femoral shaft section modulus (cm3) |
Intertrochanteric buckling ratio |
Narrow neck buckling ratio |
Femoral shaft buckling ratio |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | P | R | P | R | P | R | P | R | P | R | P | R | P | R | P | R | P | |
| Duration of illness (patient report, y) | −0.53 | 0.0002a | −0.58 | <0.0001a | −0.51 | 0.0003a | −0.47 | 0.0009a | −0.54 | 0.0001a | −0.37 | 0.01a | 0.59 | <0.0001a | 0.55 | <0.0001a | 0.57 | 0.001a |
| Highest past BMI (kg/m2) |
0.60 | <0.0001a | 0.55 | <0.0001a | 0.54 | <0.0001a | 0.70 | <0.0001a | 0.60 | <0.0001a | 0.75 | <0.0001a | −0.32 | 0.02 | −0.38 | 0.007 | −0.25 | 0.08a |
| Lowest past BMI (kg/m2) |
0.57 | <0.0001 | 0.44 | 0.002 | 0.43 | 0.003 | 0.53 | 0.0002 | 0.46 | 0.001 | 0.59 | <0.0001a | −0.22 | 0.15 | −0.23 | 0.12 | 0.08 | 0.6 |
Remained significant after controlling for BMI at P ≤ 0.05.
3.4 |. Associations of calcium intake and vitamin D levels with BMD and estimated hip strength
In men with eating disorders, 25OH vitamin D levels were significantly associated with BMD Z-scores (R = 0.33–0.35), section modulus (R = 0.31–0.37) and buckling ratio (R = −0.29 to −0.36) at all sites (P ≤ 0.05), which remained significant after controlling for BMI (P < 0.05).
Men with 25OH vitamin D deficiency had lower mean BMD Z-scores and section modulus at all sites, and higher mean NN buckling ratio, than men with 25OH vitamin D sufficiency (P < 0.05, Figure 3). Men with daily calcium intake <1000 mg trended towards having a lower mean total hip BMD Z-score (−0.80 ± 0.34 vs 0.03 ± 0.32, P = 0.09) and had significantly higher mean IT (2.19 ± 0.07 vs 1.96 ± 0.06) and FS (1.36 ± 0.18 vs 1.03 ± 0.06) buckling ratio (P < 0.05). Men with and without vitamin D deficiency, or low daily calcium intake, had similar clinical characteristics.
FIGURE 3.
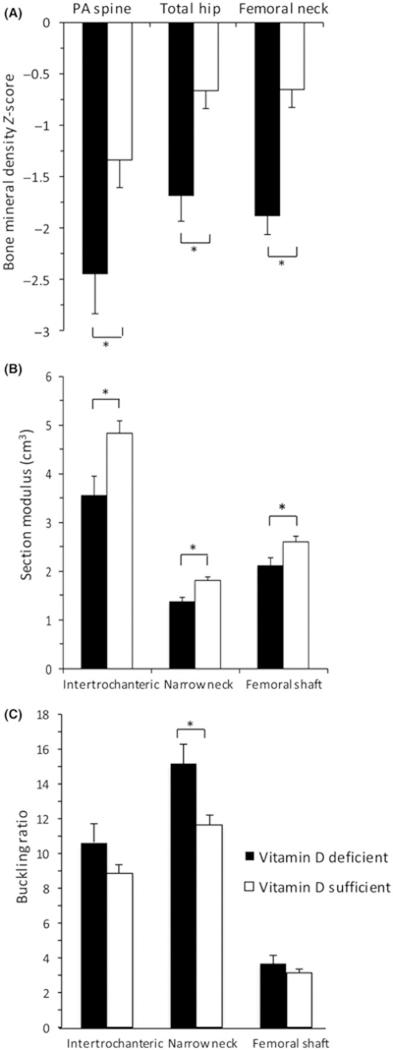
Men with eating disorders and vitamin D deficiency (25OH vitamin D level <20 ng/mL) had lower mean (A) bone mineral density (BMD) Z-scores and (B) section modulus at all sites, as well as (C) higher narrow neck buckling ratio compared to those with 25OH vitamin D sufficiency. Mean ± SEM. *P ≤ 0.05
4 |. DISCUSSION
Our data suggest that men with AN are at high risk for low BMD and estimated hip strength. Men with ARFID (despite comparably low weight) and atypical AN (who are higher weight) are at lower, but significant, risk of low BMD, but have preserved estimated hip strength. Men with a long disease duration or who have low weight, muscle mass and/or vitamin D levels may be at particularly high risk for impaired BMD and hip strength. We found that a history of overweight or no history of low weight may be relatively protective or a marker of less severe undernutrition. This is the first report of the prevalence and determinants of low BMD and estimated hip strength in men across a wide age range with DSM-5 eating disorders AN, ARFID and atypical AN.
Women with DSM-5 AN are at risk for low BMD, especially at the spine.1,17 We demonstrated that men with DSM-5 AN are also at high risk, with prevalent BMD Z-scores <−1 and <−2 similar to women.1 We reported that the spine is more commonly and severely affected than the hip in men, similar to women. Our results are consistent with spine BMD results from a prior study in young men with DSM-IV AN,7 although hip BMD was not reported in that study. Our data suggest that men with DSM-5 AN have low hip BMD and estimated hip strength compared to controls, which had not been published. This is clinically relevant because impaired estimated hip strength is associated with incident hip fracture.4,15
This is the first report of BMD and estimated hip strength in men with atypical AN and ARFID. We demonstrated that both groups are at risk for low BMD, although not as high a risk as AN. Similar to men with AN, we reported that the spine is more severely affected than the hip in atypical AN and ARFID. Unlike in men with AN, estimated hip strength is generally not impaired. It is possible that the non-significantly shorter disease duration in men with ARFID, and the higher weight in men with atypical AN, could explain this.
Similar to what is reported in women,1,18–20 low current BMI, low muscle mass and long disease duration were associated with low BMD and estimated hip strength in men in our study. Food restriction and weight loss have the unintended consequence of muscle loss, which is particularly detrimental given muscle’s anabolic effect on bone. Long disease duration may be especially deleterious to bone in young adults, given it is a time of peak bone mass accrual. A high percentage of men were overweight in the past, much higher than in a study of women with AN and atypical AN.1 Our data suggest that men with a history of overweight may be relatively protected from low BMD and impaired hip strength. It is also possible that a history of overweight is simply a marker of less severe undernutrition, thus resulting in less impaired BMD and bone strength. This may be true for men with no history of low weight as well. Our finding that higher physical activity trended towards being associated with higher hip BMD was surprising, given that excessive exercise during active disease has been associated with lower BMD.21 This association may have been confounded by the relatively higher physical activity level in the atypical AN group compared to the AN group, as the atypical AN group also had relatively higher BMD than the AN group. Future studies could include more detailed exercise and activity questionnaires.
We report a high prevalence of vitamin D deficiency and low daily calcium intake in men with AN and atypical AN. In females with AN, the prevalence of vitamin D deficiency largely depends on the use of supplements, as 36% of females with AN, not on vitamin D supplementation, were reported to have vitamin D deficiency in one study,22 compared with 2% of females with AN who were encouraged to take vitamin D supplementation in another study.23 In our study, vitamin D deficiency and inadequate daily calcium intake were associated with low BMD and/or estimated hip strength, which had previously been reported in females with AN,22 but not men with eating disorders. Whether other differences in dietary micro- or macronutrient content may contribute to differences in bone mass in men with eating disorders warrants further study.
Limitations of the study design include its cross-sectional nature, such that causality cannot be determined. We were not able to control for variability between densitometers at the two sites, although we did analyse densitometer data using the same software. Although the AN and atypical AN groups were a combination of ambulatory and inpatient subjects, the ARFID group were inpatients, suggestive of more severe psychiatric disease. Future studies investigating BMD in men with ARFID should include ambulatory subjects. Some data were self-reported, such as physical activity and disease duration, although the latter may be more accurate than the formal diagnosis date as delays in diagnosis are common. The healthy controls in this study had a higher than expected prevalence of PA spine BMD Z-score <−1 despite being rigorously evaluated for inclusion. This may be because the serum testosterone cut-off level for inclusion was relatively low. Despite this, we were able to detect significant differences in mean PA spine BMD Z-scores between eating disorder groups and controls; these differences would have been even larger had fewer of the controls had BMD Z-scores <−1. We did not have complete data on endocrine hormone variables, such as testosterone,24,25 estradiol,10 insulin-like growth factor 126 and cortisol, 27,28 that are known to be associated with BMD in other male and female populations. Future longitudinal studies that include BMD, endocrine hormone variables and fracture data across the natural history of disease, as well as fracture data, could inform recommendations for BMD screening in men with eating disorders.
In conclusion, impairments in BMD and estimated hip strength are prevalent and severe in men with AN, while men with ARFID (despite similarly low weight) and atypical AN (who are normal weight) are at a lower, but still significant, risk of low BMD. The prevalence of low BMD in men with AN and atypical AN is commensurate to women with these diseases. Men with low BMI, low muscle mass, long duration of illness and vitamin D deficiency are at particular risk, whereas a history of overweight or no history of low weight may be relatively protective or a marker of less severe undernutrition. Whether weight recovery, increasing muscle mass and/or treatment of vitamin D deficiency improve BMD and bone strength warrants further study.
Acknowledgments
Funding information
National Institutes of Health, Grant/Award Number: K23 DK115903, K24 DK02759, K24 HL092902, MO1 RR01066, R01 AG030545 and RR-1066; AbbVie
Footnotes
CONFLICT OF INTEREST
KTE reports future royalties for Cognitive Behavioral Therapy for Avoidant/Restrictive Food Intake Disorder: Children, Adolescents and Adults published by Cambridge University Press. Margherita Mascolo reports equity in Alsana: An Eating Recovery Community. KKM reports that she is the recipient of an investigator-initiated grant from Amgen. MS, AD, MSR, EM, GTL, AW, TMH, KS, EWY, Madhusmita Misra, AK and PM report no conflict of interests.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Schorr M, Thomas JJ, Eddy KT, et al. Bone density, body composition, and psychopathology of anorexia nervosa spectrum disorders in DSM-IV vs DSM-5. Int J Eat Disord. 2017;50(4):343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagata JM, Golden NH, Leonard MB, Copelovitch L, Denburg MR. Assessment of sex differences in fracture risk among patients with anorexia nervosa: a population-based cohort study using the health improvement network. J Bone Miner Res. 2017;32(5):1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melton LJ, Beck TJ, Amin S, et al. Contributions of bone density and structure to fracture risk assessment in men and women. Osteoporos Int. 2005;16(5):460–467. [DOI] [PubMed] [Google Scholar]
- 4.Kaptoge S, Beck TJ, Reeve J, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23(12):1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association Publishing. Diagnostic and statistical manual of mental disorders (5th ed). In: Feeding and Eating Disorders. Washington, DC: American Psychiatric Publishing;2013:329–354 [Google Scholar]
- 6.Nagata JM, Golden NH, Peebles R, et al. Assessment of sex differences in bone deficits among adolescents with anorexia nervosa. The International journal of eating disorders. 2017;50(4):352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehler PS, Sabel AL, Watson T, Andersen AE. High risk of osteoporosis in male patients with eating disorders. Int J Eat Disord. 2008;41(7):666–672. [DOI] [PubMed] [Google Scholar]
- 8.Misra M, Katzman DK, Clarke H, et al. Hip structural analysis in adolescent boys with anorexia nervosa and controls. J Clin Endocrinol Metab. 2013;98(7):2952–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein JS, Lee H, Burnett-Bowie S-A, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkelstein JS, Lee H, Leder BZ, et al. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Investig. 2016;126(3):1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamwi G Therapy: changing dietary concepts. New York: American Diabetes Association; 1964. [Google Scholar]
- 12.Mackey DC, Lui LY, Cawthon PM, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298(20):2381–2388. [DOI] [PubMed] [Google Scholar]
- 13.Beck TJ, Ruff CB, Warden KE, Scott WW, Rao GU. Predicting femoral neck strength from bone mineral data. Invest Radiol. 1990;25(1):6–18. [DOI] [PubMed] [Google Scholar]
- 14.Beck TJ, Broy SB. Measurement of Hip Geometry-Technical Background. J Clin Densitom. 2015;18(3):331–337. [DOI] [PubMed] [Google Scholar]
- 15.Rivadeneira F, Zillikens MC, De Laet CE, et al. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam study. J Bone Miner Res. 2007;22(11):1781–1790. [DOI] [PubMed] [Google Scholar]
- 16.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW. Structural trends in the aging femoral neck and proximal shaft: analysis of the third national health and nutrition examination survey dual-energy x-ray absorptiometry data. J Bone Miner Res. 2000;15(12):2297–2304. [DOI] [PubMed] [Google Scholar]
- 17.Mehler PS, Blalock DV, Walden K, et al. Medical findings in 1,026 consecutive adult inpatient-residential eating disordered patients. Int J Eat Disord. 2018;51(4):305–313. [DOI] [PubMed] [Google Scholar]
- 18.Solmi M, Veronese N, Correll Cu, et al. Bone mineral density, osteoporosis, and fractures among people with eating disorders: a systematic review and meta-analysis. Acta Psychiatr Scand. 2016;133(5):341–351. [DOI] [PubMed] [Google Scholar]
- 19.Bachmann KN, Fazeli PK, Lawson EA, et al. Comparison of hip geometry, strength, and estimated fracture risk in women with anorexia nervosa and overweight/obese women. J Clin Endocrinol Metab. 2014;99(12):4664–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachmann KN, Schorr M, Bruno AG, et al. Vertebral volumetric bone density and strength are impaired in women with low-weight and atypical anorexia nervosa. J Clin Endocrinol Metab. 2017;102(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waugh EJ, Woodside DB, Beaton DE, Cote P, Hawker GA. Effects of exercise on bone mass in young women with anorexia nervosa. Med Sci Sports Exerc. 2011;43(5):755–763. [DOI] [PubMed] [Google Scholar]
- 22.Gatti D, El Ghoch M, Viapiana O, et al. Strong relationship between vitamin D status and bone mineral density in anorexia nervosa. Bone. 2015;78:212–215. [DOI] [PubMed] [Google Scholar]
- 23.Haagensen AL, Feldman HA, Ringelheim J, Gordon CM. Low prevalence of vitamin D deficiency among adolescents with anorexia nervosa. Osteoporos Int. 2008;19(3):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81(12):4358–4365. [DOI] [PubMed] [Google Scholar]
- 25.Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82(8):2386–2390. [DOI] [PubMed] [Google Scholar]
- 26.Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87(9):4177–4185. [DOI] [PubMed] [Google Scholar]
- 27.Lawson EA, Donoho D, Miller KK, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94(12):4710–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schorr M, Lawson EA, Dichtel LE, Klibanski A, Miller KK. Cortisol Measures Across the Weight Spectrum. J Clin Endocrinol Metab. 2015;100(9):3313–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]


