Abstract
Background/Objective:
To determine the impact of vascular burden on rates of decline in episodic memory and executive function. We hypothesize that greater vascular burden will have an additive negative impact on cognition after accounting for baseline cognitive impairment, positron emission tomography (PET) amyloid burden, and magnetic resonance imaging (MRI) measures.
Methods:
Individuals were followed an average of 5 years with serial cognitive assessments. Predictor variables include vascular burden score (VBS), quantitative brain MRI assessment, and amyloid imaging. Subjects consisted of 65 individuals, 53% of whom were male, aged 73.2 ± 7.2 years on average with an average of 15.5 ± 3.3 years of educational achievement.
Results:
Baseline cognitive impairment was significantly associated poorer episodic memory (p < 0.0001), smaller hippocampal volume (p < 0.0001), smaller brain volume (p = 0.0026), and greater global Pittsburg Imaging Compound B (PiB) index (p = 0.0008). Greater amyloid burden was associated with greater decline in episodic memory over time (β = −0.20 ± 0.07, p < 0.005). VBS was significantly associated with the level of executive function performance (β = −0.14 ± 0.05, p < 0.005) and there was a significant negative interaction between VBS, cognitive impairment, and PiB index (β = −0.065 ± 0.03, p = 0.03).
Conclusions:
Our results find a significant influence of VBS independent of standard MRI measures and cerebral amyloid burden on executive function. In addition, VBS reduced the amount of cerebral amyloid burden needed to result in cognitive impairment. We conclude that the systemic effects of vascular disease as reflected by the VBS independently influence cognitive ability.
Keywords: Alzheimer’s disease, cerebrovascular disorders, cognition, neuroimaging
INTRODUCTION
Advancing age is associated with cognitive decline and dementia. While this is most commonly due to Alzheimer’s disease (AD) pathology [1], most individuals with dementia have mixed pathologies, particularly the mixture of cerebrovascular and AD [2, 3].
Advances in neuroimaging methods now allow for the comprehensive assessment of both cerebral amyloid burden [4] and cerebrovascular brain injury. Current data suggest that cerebral amyloid burden accumulates rapidly with age [5], is associated with poorer memory performance [6], and places individuals at increased risk for incident cognitive impairment [7] and dementia [8]. Asymptomatic cerebrovascular disease is similarly common [9] and also associated with cognitive decline [10], incident mild cognitive impairment (MCI), and dementia [11].
Recent studies have examined the relationship between magnetic resonance imaging (MRI) measures of vascular brain injury (white matter hyperintensities (WMH) and infarcts), cerebral amyloid burden, and cognition [12, 13]. These studies suggest that MRI measures of vascular brain injury have an early and independent effect on cognition, even among those who have associated cerebral amyloid burden. Further studies find that vascular risk factors (VRFs) can accelerate age-related brain atrophy [14] even in cortical areas affected by AD pathology [15]. Finally, VRFs or clinically manifest vascular disease (VDx) negatively affect cognition independent of WMH and cerebral infarctions [16].
These findings support the notion that VRFs and VDx are common and may have considerable impact on cognitive function among older individuals [17], particularly when the extent of AD is relatively modest [18]. To date, however, we are not aware of any studies that have comprehensively examined the individual and combined impact of vascular burden scores (VBS = VRFs + VDx), MRI measures of WMH (a recognized measure of small vessel vascular disease [19]), infarcts, regional atrophy, and cerebral amyloid burden on the trajectory of cognitive performance in multiple cognitive domains. Given current literature, we hypothesized that VBS would have an additional impact on trajectories of cognitive performance. To test this hypothesis, we studied participants of the UC Davis Alzheimer’s Disease Center Diversity Cohort who had a spectrum of cerebral amyloid, VBS, and had repeated cognitive assessment for approximately 5 years on average.
MATERIALS AND METHODS
Subjects
The sample consisted of 65 participants evaluated at the UC Davis Alzheimer’s Disease Center (ADC) [20], who had amyloid imaging utilizing Pittsburg Imaging Compound B (PiB). All participants were evaluated approximately annually a median number of 5 times with a maximum of 11 evaluations, performed over a median of 4.87 years. All participants were recruited according to previously described methods [20]. Subjects included individuals who were classified as cognitively normal, MCI, or dementia based on detailed medical history, neurological examination, and neuropsychological testing using the Uniform Data Set battery [21, 22]. Because of the limited number of demented individuals with PiB imaging, subjects were dichotomized as impaired (MCI or Dementia) versus unimpaired (cognitively normal).
Inclusion and exclusion criteria have been previously described [20]. This study was approved by the institutional review board at UC Davis and all study participants provided written informed consent.
Vascular burden score
The presence and number of VRFs (e.g., hypertension, diabetes, or hyperlipidemia) for each individual was based on thorough review of the subject’s medical history, medical records, and medications brought into the clinic at the time of initial evaluation. VDx defined as a history of coronary artery disease (angina, coronary stent, coronary artery bypass graph, myocardial infarction, atrial fibrillation, pace maker, congestive heart failure) or cerebrovascular disease (transient ischemic attack, stroke, carotid endarterectomy, or carotid stent) also was assessed. VBS for each individual was the sum of VRFs and VDx and could vary from 0 to 5 (e.g., the sum of hypertension, diabetes, hyperlipidemia, coronary artery, and cerebrovascular disease).
Neuropsychological measures
Outcome measures for this study consisted of the Spanish and English Neuropsychological Assessment Scale (SENAS) [23, 24] subtests of episodic memory and executive function. These measures do not have appreciable floor or ceiling effects for participants in this sample and have linear measurement properties across a broad ability range [23, 24].
MRI
Image acquisition
Brain imaging was obtained at the University of California at Davis MRI research center on a 1.5T GE Signa Horizon LX Echospeed system or the Veterans Administration at Martinez on a 1.5 T Marconi system using compatible imaging parameters described fully in the Supplementary Material. Image quantification, was performed by raters who were blind to age, gender, race, educational achievement, ethnicity, and diagnostic status.
MRI analysis
For this study, we quantified MR images obtained at, or near to, initial evaluation. The mean time lag from initial assessment to MRI was 0.23 ± 0.93 years.
MRI tissue classification
MRI baseline measurements were made as part of our in-house processing pipeline described previously [25]. Briefly, structural MRI images were processed to remove the skull using an atlas-based method [26]. Gray, white, and cerebrospinal fluid tissues segmentation was performed using an algorithm designed to enhance accuracy at likely tissue boundaries [27].
Further segmentation of WMHs utilized a Bayesian approach where the likelihood of WMH was estimated from FLAIR signal characteristics the prior probability of WMH occurrence was calculated from previous supervised segmentations of independent FLAIR images and additional posterior probability constraints were applied at each image voxel.
MRI hippocampal segmentation
Hippocampal volume was computed by a multiatlas hippocampal segmentation algorithm [26].
MRI infarction detection
The presence of MRI infarction was determined from the size, location, and imaging characteristics of the lesion [9]. An image viewing system allowed for superimposition of the 3DT1, FLAIR, and T2 weighted image at three times magnified view to assist in interpretation of lesion characteristics. Signal void, best seen on the T2 weighted image was interpreted to indicate a vessel. Only lesions 3mm or larger qualified for consideration as cerebral infarcts.
PET
Image acquisition
All PiB-PET images were acquired at Lawrence Berkeley National Laboratory (LBNL) on a Siemens ECAT EXACT HR PET scanner in 3D acquisition mode. PiB radiotracer was synthesized at this facility using a standard protocol [28] where 10 to 15 mCi of [11C] PiB was injected into an antecubital vein. Dynamic acquisition frames (34 to 35 frames total) were obtained over 90 min. Distribution volume ratio (DVR) images were created with PiB frames corresponding to 35–90 min post-injection and a gray matter cerebellar mask as reference region [29, 30].
PET analysis
For each subject, a DVR PiB index was derived from the native-space image [15] after registration to MRI by averaging the weighted mean value from FreeSurfer-derived ROIs in frontal (cortical regions anterior to the precentral gyrus), temporal (middle and superior temporal regions), parietal (supramarginal gyrus, inferior/superior parietal lobules and precuneus), and posterior cingulate cortex using the Desikan-Killiany atlas [31].
Timing of PET to cognitive assessments
PET imaging was acquired through ancillary research funding after the longitudinal study of cognition in a diverse sample of older adults had commenced. At the time of this analysis, most subjects had PET imaging near to their final observation (0.77±1.2 years prior to final cognitive testing; median difference of 6 months).
Statistical analyses
The aim of this study was to evaluate effects of VBSon cognitive trajectories independent of cerebral amyloid burden, brain atrophy, WMH, and infarcts measured by MRI. We used a series of mixed-effects regression models with person specific slopes and intercepts as random effects to evaluate how baseline cognitive performance and change over time were influenced by VBS, cerebral amyloid, brain volumes, WMH, and infarcts. We chose an analytical approach similar to that described by Zheng et al. [16] using serial regression analyses in a sequence consistent with a published biological markers model [32], utilizing PiB imaging as the measure of amyloid burden. Consequently, the initial model assessed fixed effects of baseline demographic variables as predictors of level of and change in cognitive performance with the added fixed effect of global PiB burden (PiB index). Given that the timing of PiB imaging varied in relationship to cognitive testing, we also included a PET imaging time-lag variable computed as the difference in years between the date of PiB imaging and the date of the first neuropsychological testing. The second model evaluated the added fixed effects of baseline MRI variables of brain, WMH and hippocampal volumes as well as the number of infarcts identified on MRI. The third model included the added fixed effect of baseline level of cognitive impairment (dichotomized as impaired versus unimpaired). The final model included the additional fixed effect of baseline VBS. Age, education, and gender were included in each model. Only variables that were significantly associated with cognitive level or change were carried forward into subsequent models. In all models, the fixed effects on longitudinal change were modeled by an interaction term with time since initial assessment. To assist in interpretation of the results, age was centered at 70 years, and educational achievement at 12 years. Cognitive impairment was coded to reflect differences from cognitively normal. Amyloid burden and MRI measures were centered on the mean of cognitively normal individuals.
RESULTS
Subject demographics as well as mean VBS, cognition, MRI, and PiB measures according to baseline clinical diagnosis are summarized in (Table 1).
Table 1.
Subject demographics and baseline vascular burden, cognition, MRI, and amyloid measures. Continuous measures are summarized as mean±standard deviation. Mean cognitive performance are baseline measures. All quantitative MRI variables are adjusted for differences in head size × 100 (%TCV). WMH is log transformed to normalize distribution
| Normal (N = 37) | MCI (N = 25) | Demented (N = 3) | |
|---|---|---|---|
| Years of Observation | 5.7±3.0 | 3.0±2.7 | 4.4±2.8 |
| Age | 72±7 | 75±7 | 80±5 |
| Gender (%F) | 51% | 44% | 33% |
| Ethnicity (% White non-Hispanic) | 51% | 80% | 100% |
| Education (years) | 15±4 | 16±2 | 17±4 |
| Vascular Burden (number) | 1.7±1.2 | 1.7±1.2 | 1.3±1.2 |
| Episodic Memory (SD units) | 0.30±0.81 | −0.96±0.54 | −1.24±0.24 |
| Executive Function (SD units) | 0.13±0.57 | −0.07±0.43 | −0.51±0.45 |
| Brain Volume (%TCV) | 77.9±2.4 | 75.7±2.5 | 75.8±0.6 |
| Hippocampus (% TCV) | 0.58±0.06 | 0.47±0.06 | 0.42±0.06 |
| WMH (log %TCV) | −5.3±1.4 | −5.2±1.26 | −5.6±1.25 |
| MRI Infarcts (%) | 39% | 20% | 0% |
| Global PiB Index | 1.19±0.22 | 1.41±0.36 | 1.75±0.17 |
MCI, mild cognitive impairment; TCV, total cranial volume; WMH, white matter hyperintensities; PiB, Pittsburg Imaging Compound B.
At baseline, subjects were 73.2±7.2 years of age on average, 53% male, and had an average of 15.5±3.3 years of educational achievement. The cohort had a median VBS of 2. The prevalence of the components that made up the VBS are described in the Supplementary Material. The prevalence of MRI detected infarcts did not differ significantly by degree of baseline cognitive impairment with a trend for more prevalent infarcts in the cognitively normal group.
As expected, there were significant group differences in baseline episodic memory (p < 0.0001), hippocampal volume (p < 0.0001), brain volume (p = 0.0026), and global PiB index (p = 0.0008) based on degree of baseline cognitive impairment. Using the previously reported PiB cutoff of 1.08 [12], we also found that 48% of all subjects were considered to have a high amyloid burden with a prevalence of 72% among those identified as cognitively impaired at baseline. There was no significant association between WMH burden (p = 0.46) or MRI Infarcts (p = 0.86) and PiB index adjusting for age and gender.
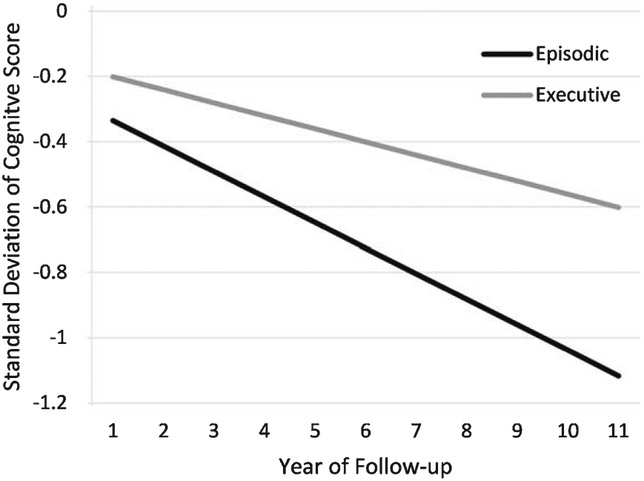
Subjects were followed 4.9±3.0 years on average, although the cognitively normal individuals in the cohort were followed significantly longer (6.3 years) than cognitively impaired individuals (3.1 years), p < 0.0001. Over the course of observation, there was modest, but significant group average decline in both episodic memory (−0.08 sd/year; p < 0.0001) and executive function (−0.04 sd/year; p < 0.01, Fig. 1).
Fig. 1.
Graphic depiction of cognitive trajectories based on mixed effects models adjusting for age, gender and education. Episodic memory performance declined 0.08 sd per year (p < 0.001) whereas executive function performance declined 0.04 sd per year (p < 0.01).
Separate fixed effects models that examined the impact of demographics, amyloid status, significant MRI measures, degree of cognitive impairment, and VBS (including separate effects of VRS and VDx) on longitudinal cognitive performance are summarized in Supplementary Table 1, but briefly reviewed here. Impaired individuals performed worse on both cognitive tests at baseline and rate of executive function decline over time. Global PiB index was also significantly associated with extent of baseline memory performance and rate of change in both memory and executive function. Baseline hippocampal volume was significantly associated with baseline memory performance and change in executive function. Baseline WMH volume was significantly associated with change in executive function. Baseline brain volume was significantly associated with baseline memory performance and change in executive function performance. There was no relationship between the presence of infarction on MRI with baseline or change in cognition (data not shown). VBS was significantly associated with baseline executive function. The findings were similar when VRF or VDx scores were tested separately.
Sequential modeling is summarized in (Table 2). The results essentially recapitulated the previous independent analyses with the exception that WMH and brain volume did not significantly associate with cognitive performance when combined with amyloid and hippocampal measures. For memory performance, amyloid burden remained a significant effect on rate of change across all models. As expected, hippocampal volume was significantly associated with baseline memory performance initially, but the impact was attenuated when cognitive ability was added at a subsequent model. VBS were not associated with memory performance, either individually or in the full model. Predictors of executive performance (Table 3), however, were slightly different. For example, education had a substantial and sustained effect on baseline level of executive function performance across all models, whereas baseline hippocampal volume and degree of cognitive impairment contributed no significant explanatory variance to the trajectories of executive function performance in the final model. This difference could reflect the overall mild degree of cognitive impairment in this community based group. VBS, however, was significantly associated with the level of executive function performance. Moreover, level of performance and the strength of this effect (as measured by the beta coefficient) was essentially unchanged in comparison with the model where this variable was entered individually (see Supplementary Table 1).
Table 2.
Estimates of memory trajectories. All models include demographic variables (see text for details)
| Variable¶ | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Time | −0.078±0.016** | −0.076±0.015*** | −0.069±0.022** | −0.054±0.030 |
| Gender (M) | −0.19±0.10 | −0.11±0.10 | −0.14±0.09 | −0.11±0.09 |
| Age | −0.02±0.02 | −0.02±0.01 | −0.02±0.01 | −0.02±0.01 |
| Education | 0.06±0.03 | 0.07±0.03* | 0.08±0.03** | 0.07±0.03* |
| PET Image Time Lag | 0.14±0.04** | 0.09±0.04* | 0.06±0.04 | 0.05±0.04 |
| PiB Index | −0.88±0.37* | −0.69±0.35 | −0.51±0.32 | −0.63±0.33 |
| PiB Index * Time | −0.21±0.07** | −0.19±0.07** | −0.20±0.07** | −0.20±0.07** |
| Hippocampus | 411±143** | 124±149 | 153±149 | |
| Hippocampus * Time | 13.1±21 | 19.4±26 | 20.2±26 | |
| Impairment | −0.46±0.12*** | −0.45±0.11*** | ||
| Impairment * Time | 0.011±0.02 | 0.013±0.02 | ||
| Vascular Burden | −0.10±0.07 | |||
| Vascular Burden * Time | −0.008±0.01 |
Age centered at 70 years, male gender and education centered at 12 years was included in all models. Hippocampus and brain volume expressed as %TCV centered to non-impaired mean volume, WMH volume expressed as log WMH %TCV centered to non-impaired mean volume. PiB Index centered to non-impaired mean DVR. p values: <0.0005***, <0.005**, <0.05*. TCV, total cranial volume; WMH, white matter hyperintensities; PiB, Pittsburg Imaging Compound B.
Table 3.
Estimates of executive function trajectories
| Variable¶ | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Time | −0.036±0.013** | −0.030±0.011* | −0.046±0.016** | −0.07±0.02* |
| Gender (M) | −0.10±0.06 | −0.07±0.07 | −0.08±0.06 | −0.05±0.06 |
| Age | −0.02±0.01* | −0.02±0.01* | −0.02±0.01* | −0.02±0.01 |
| Education | 0.10±0.02*** | 0.10±0.02*** | 0.10±0.02*** | 0.10±0.02*** |
| PET Imaging Time Lag | 0.07±0.03** | 0.05±0.03 | 0.05±0.03 | 0.03±0.03 |
| PiB Index | 0.21±0.23 | 0.25±0.24 | 0.29±0.23 | 0.13±0.23 |
| PiB Index * Time | −0.20±0.05*** | −0.16±0.05** | −0.14±0.05** | −0.14±0.05* |
| Hippocampus | 105±95 | 26.2±108 | 65±104 | |
| Hippocampus * Time | 46±15.6* | 31.1±17.7 | 28.8±19.1 | |
| Impairment | −0.12±0.09 | −0.10±0.08 | ||
| Impairment * Time | −0.03±0.02 | −0.03±0.02 | ||
| Vascular Burden | −0.14±0.05** | |||
| Vascular Burden * Time | 0.011±0.01 |
Age centered at 70 years, male gender and education centered at 12 years was included in all models. Hippocampus and brain volume expressed as %TCV centered to non-impaired mean volume, WMH volume expressed as log WMH %TCV centered to non-impaired mean volume. PiB Index centered to non-impaired mean DVR. p values: <0.0005***, <0.005**, <0.05*. TCV, total cranial volume; WMH, white matter hyperintensities; PiB, Pittsburg Imaging Compound B.
Post-hoc analyses that included individual components of the VBS (e.g., VRFs alone and VDx alone), found a significant negative effect of extent of baseline vascular disease on level of executive function (β = −1.71, p = 0.0006). There was no significant negative association between level of executive function and number of baseline VRFs. VDx, therefore, appears to have a more substantial impact on cognition in this cohort.
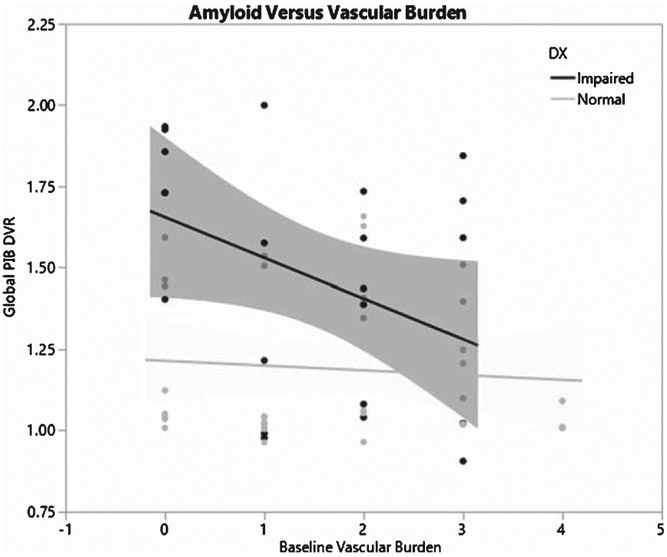
Finally, given recent evidence suggesting a relationship between VRFs and amyloid status [33-35], we performed additional post-hoc analysis to investigate this phenomenon. There was a weak negative relationship between VBS and PiB index (β = −0.05±0.03, p = 0.07) when adjusting for age and gender. Further analysis, however, found that this effect differed by baseline level of cognitive impairment. We found a significant interaction between baseline degree of cognitive impairment (impaired versus unimpaired) and VBS on amyloid level (β = −0.065±0.03, p = 0.03) indicating that, among impaired individuals, VBS was negatively associated with amyloid level, whereas for unimpaired individuals there was no relationship between VBS and amyloid levels (Fig. 2). Sub-analysis of VBS found that the extent of baseline VDx drove this interaction (β = −0.70 × 0.27, p = 0.02).
Fig. 2.
Graphic depiction of the relationship between vascular burden and cerebral amyloid burden for cognitively impaired versus cognitively normal individuals. For cognitively normal individuals, there was not significant relationship. For cognitively impaired individuals, however, there was a significant inverse relationship, signifying that less amyloid was required to achieve a state of impairment amongst individuals with higher levels of vascular burden (i.e., an additive effect of vascular disease and cerebral amyloid burden).
DISCUSSION
Results of this longitudinal study confirm prior cross-sectional studies [12, 13] suggesting that WMH have an effect independent of cerebral amyloid burden on cognition, specifically executive function. Our results, however, also extend these studies by showing that VBS has an effect on the level of executive function performance even after accounting for all other measured factors (Table 3). Moreover, analysis of the relationship between VBS and cerebral amyloid burden showed that increasing levels of VBS (particularly due to VDx) result in similar degrees of cognitive impairment at lower levels of cerebral amyloid burden (Fig. 2) confirming the additive negative effect of cerebrovascular disease on cognition [18, 36], particularly when individuals are mildly impaired [12, 13]. Our results also confirm recent published evidence that shows that coronary artery disease negatively affects cognition independent of WMH and cerebral infarction [16]. In summary, our results find that VBS influences baseline executive function and that this effect is present, and not at all diminished, after accounting for the effects of a comprehensive set of measures.
The observed adverse effect of VBS was significant only for baseline executive function and not baseline episodic memory. We also found no significant associations between VBS and change in either cognitive domain studied. There is a substantial body of literature showing that executive function is especially sensitive to effects of VRF, VDx, WMH, and infarcts [17]. Finding an effect of vascular burden on baseline executive function but not change in executive function most likely reflects that vascular risk factors likely promote vascular disease over a timespan of decades starting in middle adulthood [37, 38] and that the proximate effects of VRFs may be diminished amongst older individuals [39]. Follow-up over 5 years, therefore, may not be sufficient to detect effects of a slow but insidious disease process. Some support for this hypothesis can be derived from our analysis of the relationship between PiB index and VBS for the impaired individuals in our study as summarized in Fig. 2. Individuals with high VBS (significantly associated with worse executive function at baseline) required less amyloid burden to develop cognitive impairment at baseline evaluation. Given that the VBS is the sum of both vascular risk factors and clinically evident vascular disease, this measure likely represents the results of lifetime vascular health. The relatively mild degree of cognitive impairment in this group, however, could also have influenced these results. That is, VBS may only have an independent impact on executive function for those in whom AD pathology is relatively mild (i.e., non-demented) [12]. Further work that includes a larger group of demented individuals is necessary to test this hypothesis.
There are at least two reasons for the apparent discrepancy between MRI evidence of vascular brain injury (i.e., WMH and infarcts) and the influence of VBS on cognition. First are the limitations to our measures of vascular brain injury. Our analysis focused on the commonly used MRI measures of WMH and MR infarction [40]. Conventional MRI does not detect microinfarcts [41]. Cortical microinfarcts are recognized as important contributors to dementia risk, particularly among those with relatively low AD pathological burden [42]. Microinfarcts also are significantly associated with underlying athero- and arteriosclerosis, which are both likely to be present in this study [43]. Microinfarcts, therefore, may be an additional pathology not identified by the MRI technology used in this study.
In addition, significant reductions in cerebral blood flow (CBF) surrounding areas of WMH [44] have been identified, suggesting that CBF also may be a more sensitive measure of vascular brain injury than WMH. Individuals with vascular cognitive impairment have lower CBF measures in cortical and subcortical gray matter without differences in gray matter density [45]. Regional CBF measures also correlated with cognitive performance. Alterations in regional CBF, therefore, may have cognitive consequences in the absence of structural brain injury measures. Coincident Alzheimer’s disease, however, may also influence CBF measures thereby limiting the conclusions that can be rendered from this work. Future studies that combine cerebral blood flow and amyloid measures may clarify this issue.
The systemic effects of vascular disease, however, is a second consideration that could explain cognitive effects of VBS in the absence of visible brain injury. It is now recognized that atherosclerosis leads to increased systemic inflammation [46]. Whether this is a consequence of VRFs on endothelial function or vice versa, there is substantial evidence the microvascular dysfunction occurs in association with subcortical ischemic vascular disease that may consequently influence cognition [47]. A variety of systemic inflammatory factors are associated with brain injury [48] and cognitive impairment [49]. Further studies that include measures of inflammation in addition to those done here may further advance our understanding of this phenomenon.
Our study has a number of additional limitations beyond measurement of vascular brain injury. The sample size of this study was relatively small and did not ascertain all causes of cardiovascular disease such as elevated serum homocysteine or sleep disordered breathing. As such, it may not fully exemplify the general community. Secondly, amyloid PET was obtained later in the course of follow-up, thereby reducing the ability of PET to estimate level of cognitive performance at baseline. We believe, however, that both of these limitations are mitigated by the comprehensive baseline clinical and MRI evaluation as well as yearly longitudinal cognitive assessments over approximately 5 years of observation, during which time amyloid accumulation is likely to be relatively modest given the generally mild degree of cognitive impairment among the cohort [50].
Conclusion
Our results extend previous findings confirming the independent influence of VBS on cognition¾specifically executive function. These independent effects additionally reduce the amount of cerebral amyloid burden needed to result in clinically relevant cognitive impairment (Fig. 2). Limitations to our study include the lack of highly sophisticated measures of subtle vascular brain injury that may or may not explain further variance in cognitive ability. Alternatively, our study may point to the possibility that the systemic effects of vascular disease independently influence cognitive ability [47] emphasizing possible alternative pathways for treatment. The addition of amyloid imaging was a necessary requirement to draw such conclusions and will likely serve as an important covariate for further studies of VRF, VDx, and cognitive impairment.
Supplementary Material
ACKNOWLEDGMENTS
We are extremely grateful to the individuals who participated in this study and staff who support our research.
This work was supported by: P30 AG10129, R01 AG010220, R01 AG031563, and R01 AG021028.
This article was prepared while Dr. Reed was employed at U.C. Davis. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0965r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-180965.
REFERENCES
- [1].Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA (2006) Memory complaints are related to Alzheimer disease pathology in older persons. Neurology 67, 1581–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204. [DOI] [PubMed] [Google Scholar]
- [3].Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA (2007) Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol 62, 59–66. [DOI] [PubMed] [Google Scholar]
- [4].Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55, 306–319. [DOI] [PubMed] [Google Scholar]
- [5].Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA (2010) APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wirth M, Oh H, Mormino EC, Markley C, Landau SM, Jagust WJ (2013) The effect of amyloid beta on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimers Dement 9, 687–698e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA (2009) Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol 66, 1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, Aizenstein HJ, Cohen AD, Weissfeld LA, Mathis CA, Klunk WE, De-Kosky ST (2009) Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol 65, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA (2005) Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiol Aging 26, 491–510. [DOI] [PubMed] [Google Scholar]
- [10].Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C (2011) Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S (2010) Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The Framingham Offspring Study. Stroke 41, 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marchant NL, Reed BR, DeCarli CS, Madison CM, Weiner MW, Chui HC, Jagust WJ (2012) Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol Aging 33, 1006e1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Marchant NL, Reed BR, Sanossian N, Madison CM, Kriger S, Dhada R, Mack WJ, Decarli C, Weiner MW, Mungas DM, Chui HC, Jagust WJ (2013) The aging brain and cognition: Contribution of vascular injury and Abeta to mild cognitive dysfunction. JAMA Neurol 70, 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, D’Agostino RB, Decarli C (2004) Stroke risk profile, brain volume, and cognitive function: The Framingham Offspring Study. Neurology 63, 1591–1599. [DOI] [PubMed] [Google Scholar]
- [15].Villeneuve S, Reed BR, Madison CM, Wirth M, Marchant NL, Kriger S, Mack WJ, Sanossian N, DeCarli C, Chui HC, Weiner MW, Jagust WJ (2014) Vascular risk and Abeta interact to reduce cortical thickness in AD vulnerable brain regions. Neurology 83, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zheng L, Mack WJ, Chui HC, Heflin L, Mungas D, Reed B, DeCarli C, Weiner MW, Kramer JH (2012) Coronary artery disease is associated with cognitive decline independent of changes on magnetic resonance imaging in cognitively normal elderly adults. J Am Geriatr Soc 60, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].DeCarli C (2013) Clinically asymptomatic vascular brain injury: A potent cause of cognitive impairment among older individuals. J Alzheimers Dis 33(Suppl 1), S417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA (2004) Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 62, 1148–1155. [DOI] [PubMed] [Google Scholar]
- [19].Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C (2004) Stroke risk profile predicts white matter hyperintensity volume: The Framingham Study. Stroke 35, 1857–1861. [DOI] [PubMed] [Google Scholar]
- [20].Hinton L, Carter K, Reed BR, Beckett L, Lara E, DeCarli C, Mungas D (2010) Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Dis Assoc Disord 24, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA (2006) The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 20, 210–216. [DOI] [PubMed] [Google Scholar]
- [22].Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC (2009) The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord 23, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Crane PK, Narasimhalu K, Gibbons LE, Pedraza O, Mehta KM, Tang Y, Manly JJ, Reed BR, Mungas DM (2008) Composite scores for executive function items: Demographic heterogeneity and relationships with quantitative magnetic resonance imaging. J Int Neuropsychol Soc 14, 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H (2004) Spanish and English Neuropsychological Assessment Scales (SENAS): Further development and psychometric characteristics. Psychol Assess 16, 347–359. [DOI] [PubMed] [Google Scholar]
- [25].Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C (2010) Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer disease. Stroke 41, 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Aljabar P, Heckemann RA, Hammers A, Hajnal JV, Rueckert D (2009) Multi-atlas based segmentation of brain images: Atlas selection and its effect on accuracy. Neuroimage 46, 726–738. [DOI] [PubMed] [Google Scholar]
- [27].Fletcher E, Singh B, Harvey D, Carmichael O, DeCarli C (2012) Adaptive image segmentation for robust measurement of longitudinal brain tissue change. Conf Proc IEEE Eng Med Biol Soc 2012, 5319–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE (2003) Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem 46, 2740–2754. [DOI] [PubMed] [Google Scholar]
- [29].Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16, 834–840. [DOI] [PubMed] [Google Scholar]
- [30].Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA (2005) Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 25, 1528–1547. [DOI] [PubMed] [Google Scholar]
- [31].Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- [32].Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ (2013) Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 12, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Reed BR, Marchant NL, Jagust WJ, DeCarli CC, Mack W, Chui HC (2012) Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging 33, 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reed B, Villeneuve S, Mack W, DeCarli C, Chui HC, Jagust W (2014) Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol 71, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yassine HN, Feng Q, Azizkhanian I, Rawat V, Castor K, Fonteh AN, Harrington MG, Zheng L, DeCarli C, Jagust WJ, Chui HC (2016) Association of serum docosa-hexaenoic acid with cerebral amyloidosis. JAMA Neurol 73, 1208–1216. [DOI] [PubMed] [Google Scholar]
- [36].Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA (2003) Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology 60, 1082–1088. [DOI] [PubMed] [Google Scholar]
- [37].Weinstein G, Maillard P, Himali JJ, Beiser AS, Au R, Wolf PA, Seshadri S, DeCarli C (2015) Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology 84, 2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S (2016) Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: The Framingham Third Generation Cohort Study. Hypertension 67, 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pase MP, Davis-Plourde K, Himali JJ, Satizabal CL, Aparicio H, Seshadri S, Beiser AS, DeCarli C (2018) Vascular risk at younger ages most strongly associates with current and future brain volume. Neurology 91, e1479–e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12, 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Smith EE, Schneider JA, Wardlaw JM, Greenberg SM (2012) Cerebral microinfarcts: The invisible lesions. Lancet Neurol 11, 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, Giannakopoulos P (2007) Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology 68, 927–931. [DOI] [PubMed] [Google Scholar]
- [43].Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA (2017) The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol 27, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Promjunyakul NO, Lahna DL, Kaye JA, Dodge HH, Erten-Lyons D, Rooney WD, Silbert LC (2016) Comparison of cerebral blood flow and structural penumbras in relation to white matter hyperintensities: A multi-modal magnetic resonance imaging study. J Cereb Blood Flow Metab 36, 1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sun Y, Cao W, Ding W, Wang Y, Han X, Zhou Y, Xu Q, Zhang Y, Xu J (2016) Cerebral blood flow alterations as assessed by 3D ASL in cognitive impairment in patients with subcortical vascular cognitive impairment: A marker for disease severity. Front Aging Neurosci 8, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352, 1685–1695. [DOI] [PubMed] [Google Scholar]
- [47].Thompson CS, Hakim AM (2009) Living beyond our physiological means: Small vessel disease of the brain is an expression of a systemic failure in arteriolar function: A unifying hypothesis. Stroke 40, e322–330. [DOI] [PubMed] [Google Scholar]
- [48].Jefferson AL, Massaro JM, Wolf PA, Seshadri S, Au R, Vasan RS, Larson MG, Meigs JB, Keaney JF Jr, Lipinska I, Kathiresan S, Benjamin EJ, DeCarli C (2007) Inflammatory biomarkers are associated with total brain volume: The Framingham Heart Study. Neurology 68, 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Andersson C, Preis SR, Beiser A, DeCarli C, Wollert KC, Wang TJ, Januzzi JL Jr, Vasan RS, Seshadri S (2015) Associations of circulating growth differentiation factor-15 and ST2 concentrations with subclinical vascular brain injury and incident stroke. Stroke 46, 2568–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O’Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC (2011) Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol 69, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.