Abstract
Controlling morphologies of nanomaterials such as their shapes and surface features has been a major endeavor in the field of nanoscale science and engineering, because the morphology is a major determining factor for functional properties of nanomaterials. Compared with conventional capping ligands based on organic molecules or polymers, the programmability of biomolecules makes them attractive alternatives for morphology-controlled nanomaterials synthesis. Towards the goal of predictable control of the synthesis, many studies have been performed on using different sequences of biomolecules to generate specific nanomaterial morphology. In this review, we summarize recent studies in the past few years on using DNA and peptide sequences to control inorganic nanomaterial morphologies, focusing on both case studies and mechanistic investigations. The functional properties resulting from such a sequence-specific control are also discussed, along with strengths and limitations of different approaches to achieving the goal.
Keywords: Shape-control, Sequence specificity, biomimetic synthesis, bio-inorganic interface
Graphic abstract
1. Introduction
Inorganic nanomaterials have found wide applications in catalysis, electronics, sensing, imaging and medicine [1–4]. The performance of inorganic nanomaterials in these applications depends on the precise control of their properties, which are derived from their physiochemical parameters. Among all the parameters, morphology, such as shapes and surface features, has always been a major determining factor. Therefore, much effort has been devoted to developing synthetic methods to achieve various nanostructure morphologies. Despite progress made, it remains a significant challenge to achieve programmable synthesis towards a desired nanomaterial morphology.
To meet the challenge, biomolecules, such as DNA [5, 6] and peptides [7–9], have been used for the synthesis of diverse inorganic nanomaterials to generate desired nanomaterial morphologies, to mimic the precision and intricacy of nature’s control of biomaterials. A major advantage of these biomolecules is that they are programmable through variation of their sequence, resulting a myriad of ligands that are tunable in length, charges, functional groups and conformations to enhance the synthetic tool box.
In this review, we will summarize the recent efforts in discovering biomolecules with different sequences to control inorganic nanomaterial morphologies. Our discussion will mainly focus on using DNA molecules and peptides as the capping agents for inorganic nanomaterial synthesis, as the most recent developments have occurred in these areas.
2. Sequence Specific Control of Inorganic Nanomaterial morphologies by Biomolecules
2.1. Role of biomolecules in morphology-controlled synthesis of inorganic nanomaterials
In the synthesis of inorganic nanomaterials, capping agents play an important role in determining the size, shape and growth kinetics of the nanostructures. Biomolecules are a special class of capping agents that can bind to the surface of the nanomaterials, lower their surface energy, and thus affect the nanomaterials growth at different stages, both kinetically and thermodynamically, resulting in different morphologies. For example, in the synthesis of metal nanoparticles, metal ions are firstly reduced by either an exogenous reductant or endogenous reducing functional groups on biomolecules themselves; Biomolecules may also interact with the precursors by forming a complex, which can be the direct template for the following nucleation and growth events [10]. During the metal particle formation, biomolecules may interact with the forming nanocrystals based on their specific molecular recognition and hence produce specific-faceted nanostructures.
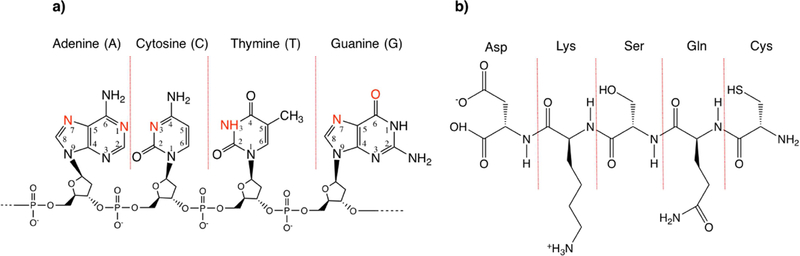
Nature has demonstrated it is possible to control the synthesis of biomaterials such as bones and sea shells, at the molecular level, using biomolecules with specific sequences that form different structures [11]. Therefore, mimicking the natural process to achieve sequence-specific control of nanomaterials morphology has been a major endeavor in the field of nanomaterial synthesis. Morphology-controlled synthesis is known to be related to facet-specific interactions of capping ligands with inorganic materials [12]. The interactions are mainly driven by soft epitaxial matching, which involves the coordination of polarizable functional groups in the ligands (e.g., O, N, C) with hollow sites on different facets of nanomaterials [13, 14]. Biomolecules have diverse functional groups that contain these polarizable atoms, such as the phosphodiester backbone and the nitrogen or oxygen atoms in nucleobases, including N7 and N1 of adenine, N7 and O6 of guanine, and N3 of cytosine and thymine [15] (Fig. 1a) of DNA, as well as -NH2, -CO2H, -OH, -SH in the side chains of peptides [16] (Fig. 1b). These functional groups in different sequences of biomolecules allow different coordination modes onto the materials’ surfaces, directing the nanocrystals to grow into different morphologies.
Figure 1.
Structures of a) DNA and b) peptide with representative sequences. The potential coordination sites in DNA bases are highlighted in red.
Apart from the functional groups, other properties such as length and conformation, including secondary and tertiary structures, can also affect the interaction between biomolecules and nanomaterials [17]. For example, Hamad-Schifferli and coworkers [18] showed that the location of the DNA bases with high or low affinity towards gold nanoparticles determines whether they have “laid down” or exhibited random coil conformation. Peptides also exhibited sequence-specific conformational change on inorganic materials. For example, Ruan et al. [19] explored the effect of compositional and conformational changes of a platinum (111) facet-specific peptide on its facet-binding properties and identified phenylalanine as the key to facet selectivity. In addition, the importance of conformation of CTPR3 protein that binds to gold ion in determining the initial nucleation step of gold nanoparticle formation has also been established [10]. All these studies have clearly demonstrated the importance of sequence-specific biomolecules in controlling the morphologies of inorganic materials.
2.2. Sequence-specific control of inorganic nanomaterial morphologies by DNA
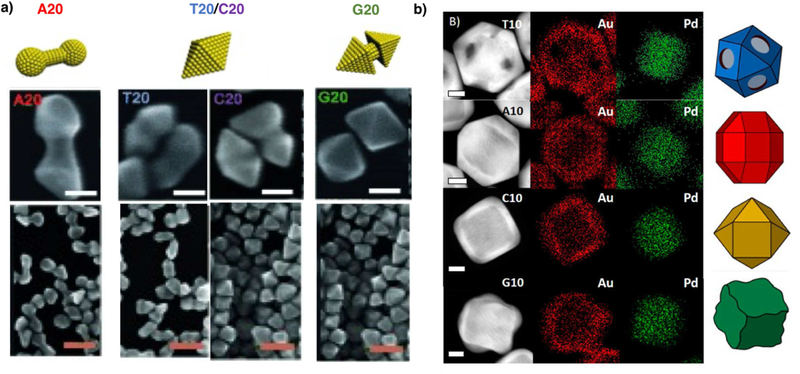
DNA sequences have been screened for synthesis of various metal nanoparticles, including Au [20–23**], Ag [24] and Pd@Au [25**] core-shell nanoparticles. In the case of Au nanoparticles, Song et al. [26**] screened homo-oligo nucleotides as well as sequences with different base combinations for controlling the morphology of Au nanorod overgrowth. The structures synthesized in the presence of homo-oligo nucleotides with 20 adenine bases (A20) exhibited dumbbell-shaped nanostructures, whereas G20 (G = guanine) yielded octahedron-shaped nanostructures. Meanwhile, the particles synthesized in the presence of T20 (T = thymine) or C20 (C = cytosine) grew into cracked octahedron with smooth facets (Fig. 2a). When the sequences with two base combinations were applied in the synthesis, A and G bases exhibited dominating effects over C and T in determining the final morphology. Whereas if A and G bases are combined, the final structure could be precisely tuned from dumbbell shape to octahedron shape, depending on the ratio of A and G in the sequence applied. The authors have also conducted a detailed mechanistic study, and proposed that the differences in nanoparticle morphologies resulted from the different binding affinity of DNA bases to the gold surface. Adenine has the highest binding affinity; Therefore, it could stabilize the high-energy side facets ({110} and {100} facets) during the nanoparticle growth, which resulted in longitudinal growth from the end of gold nanorod into a dumbbell shape. On the other hand, G has a low affinity, which cannot stabilize side facets, resulting in transversal growth into the octahedral shape. C and T have intermediate binding affinity, between A and G, hence can form the transitional cracked octahedron morphology. Finally, the authors also investigated the effect of phosphorothioate modified A20 sequence on the final morphology of the particles. The results showed that by varying the number of phosphorothioate modifications on the strand, the intra-particle gap between the two spheres in the A20 dumbbell could be controlled. As a result of these morphological differences, the localized surface plasmon resonance (LSPR) peaks can be tuned from visible to second near-infrared (NIR-II) region.
Figure 2.
Sequence-specific control of inorganic nanomaterial morphologies by DNA. a) SEM images of gold nanoparticle morphologies produced with A20, T20, C20, and G20 at different magnifications and corresponding models. Adapted with permission from reference [26**]. Copyright 2015, John Wiley and Sons. Sacle bars = 30 nm for the top row. Scale bars = 100nm for the bottom row. b) STEM and elemental mapping images of the Pd−Au nanoparticles synthesized in the presence of T10, A10, C10, and G10 and models of corresponding particles. Adapted with permission from reference [25**]. Copyright 2016 American Chemical Society. Scale bars = 25 nm.
To extend the above studies of morphological controls from monometallic to bimetallic nanoparticles systems, Satyavolu et al. [25**] screened four homo-oligo nucleotide sequences, A10, G10, C10, T10 for Pd-Au core-shell structural synthesis using Pd cubic seed. As a result, A10 produced rhombicuboctahedron shape, and C10 produced cuboctahedron shape, while G10 and T10 promoted an undulated structure and a core-frame structure, respectively (Fig. 2b). The authors proposed that the final morphologies of the nanoparticles depend on the interaction of DNA molecules with both the seed surface and the precursor, which determines the deposition and diffusion rate of atoms on the surface. In the case of T10, because of its low binding affinity to both the precursor and the surface in comparison with the other sequences, the deposition rate of atoms was much faster than the diffusion rate of atoms. Therefore, the metal atoms would preferentially grow on the high-energy site (edge and vertices of cubic seed), which resulted in {100} facet exposed core-frame structure. On the other hand, C10 has a stronger binding affinity than T10, hence C10 could slow down the gold atom deposition rate by its surface passivation effect, as well as the interaction with precursor. In the presence of C10, the growth of nanoparticles was also initiated from the edge and vertices. However, because of the low deposition rate, the nanoparticles would eventually reach a thermodynamically more stable cuboctahedral morphology through atom diffusion. The growth in the presence of G10 and A10 adopted mechanisms that were more unique to the DNA sequences. As supported by circular dichroism, G10 could form G-quadruplex, which could passivate the surface to a larger extend. The formation of secondary structures led to island growth of the shell which eventually evolved into undulated structure. The growth in the presence of A10 adopted a sigmoidal growth curve as showed by the UV-Vis kinetic study. In addition, transmission electron microscopy (TEM) demonstrated that A10 would promote the self-nucleation of gold precursors to form smaller nanocrystals at the initial stage. Therefore, the authors proposed that the growth was achieved by the aggregation and Ostwald ripening of the smaller nanoparticles onto seed crystals. More recently, the same DNA sequences were utilized in a follow up study where a high-indexed Pd concave nanocube was used as the seed [27]. The role of the seed in a DNA-mediated synthesis was elucidated in this study.
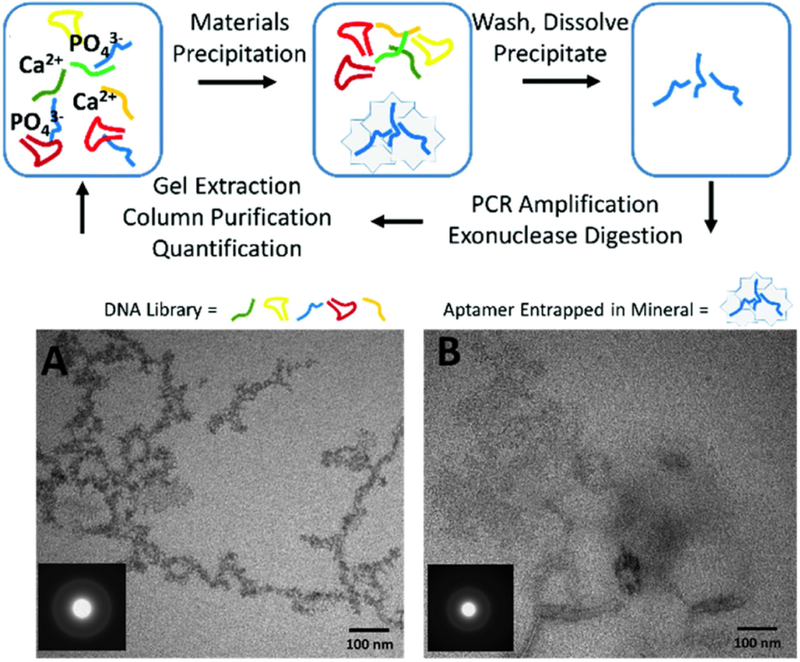
In addition to screening the selected sequences for inorganic nanomaterial synthesis, DNA sequences can also be selected toward specific inorganic material surface using in vitro selection. In vitro selection is a combinatorial method conventionally used for selecting biomolecules that bind to specific molecular targets, where a random DNA library of 1014 to 1015 sequences are mixed with the target molecules, and DNA sequences that are bound to the targets would then be isolated and amplified by polymerase chain reaction (PCR). The selection cycle would be repeated under increasingly stringent conditions until the sequence with the highest binding affinity is identified [28, 29]. DNA sequence selection toward inorganic nanomaterials could be achieved by replacing the molecular target with target nanomaterials. For example, Morse and coworkers [30*] performed a single round of DNA sequence selection on polycrystalline ZnO nanoparticles, and used sequencing to analyze the results. They showed that T30 has the strongest binding affinity for ZnO nanoparticles, and it could assist the synthesis of ZnO nanoparticles with an amorphous-crystalline core-shell structure. Furthermore, based on the binding affinity of DNA towards inorganic material surface, selection has also been performed based on in situ synthesis assay. Baillargeon et al. [31**] used systematic evolution of ligands by exponential enrichment (SELEX) combined with precipitation assay to identify the DNA sequences that are specific for calcium phosphate. In this case, DNA directly participated in the nanomaterial formation process in selection and was incorporated into the final calcium phosphate structures, which were analyzed by sequencing (Fig. 3). The selected sequence was used for the synthesis of calcium phosphate nanoparticles. The resulting structure from selected DNA sequence is a loose network of weakly associated amorphous nanoparticles, whereas the structures synthesized without DNA sequence did not form the network (Fig. 3). The idea of using the ability to template nanomaterial synthesis as the criteria for selection is valuable in providing better predictability of the performance of selected sequence in the actual nanomaterial synthesis. There have been very limited reports about selection on metal nanomaterials. This could potentially be attributed to the strong non-specific interaction of metal surface and DNA sequences, which would generate high background noise and false positive signals [29]. Moreover, even in the reported case, the relative binding affinity of different DNA sequences towards inorganic materials surface is debatable. For example, oligo-C DNA has later been demonstrated to have stronger binding affinity towards ZnO than oligo-T DNA [32],[33]. No facet specific DNA selection has been reported.
Figure 3.
A generalized protocol of precipitation SELEX and TEM images of CaCO3 formed in the presence of (A) 50 nM DNA sequence from the selection and (B) no DNA. Inset shows selected-area electron diffraction. Adapted from reference [31**] with permission from The Royal Society of Chemistry.
2.3. Sequence-specific control of inorganic nanomaterial morphologies by peptides
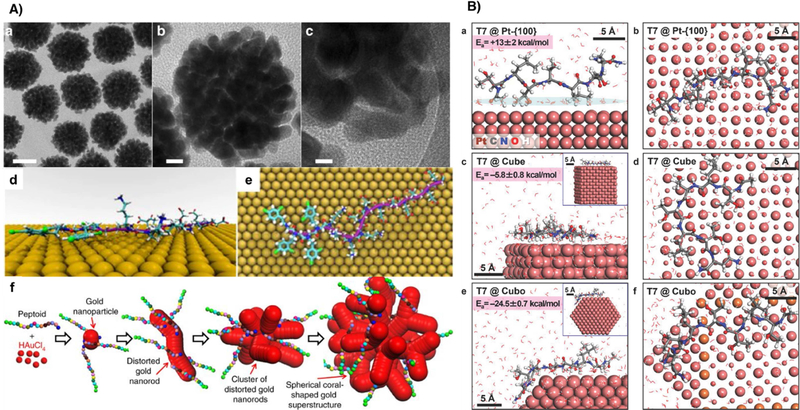
In comparison to DNA molecules, peptides contain more diverse functional groups and therefore have also been employed for various inorganic nanomaterial synthesis. For example, Tang and coworkers [34] used peptide R5 (SSKKSGSYSGSKGSKRRIL) for the synthesis of Au and Pt nanoparticles. As a result, when the synthesis was performed under lower metal-to-peptide ratios, both Au and Pt nanoparticles grew into spherical shapes, whereas higher metal-to-peptide ratios yielded nanoribbons or networked nanochains. In addition to peptides, peptoids, which are peptidomimetics whose side chains are appended to the nitrogen atom of the peptide backbone, rather than to the α-carbons, have also been studied for controlling the morphology of inorganic nanomaterials. Because peptoids do not contain backbone hydrogen bonding interactions found in peptides, using peptoids for controlling nanomaterials syntheses would allow the focus on the effect from the side chains instead of peptide bonds, potentially making the system less complicated and more predictable. Yan et al. [35**] screened ten peptoids with different sequences containing different side chains for gold nanoparticle synthesis. The resulting morphologies varied from spherical to coral-shape, depending on the sequences used (Fig. 4A). By systematically varying the hydrophobicity, number of carboxylate and amino groups, and the side-chain positions of the peptoids, the authors found that side chains containing amino group, high hydrophobicity, as well as the specific arrangement of the hydrophobic groups were all critical elements in enhancing the binding affinity of peptoids towards gold nanoparticle surfaces.
Figure 4.
Sequence-specific control of inorganic nanomaterials morphologies by peptides. A) a-c TEM image of Pep-1-induced formation of spherical coral-shaped gold nanoparticles. d, e Representative structures of Pep-1 adsorbed at the aqueous Au (111) interface predicted from REST-MD simulations. f Mechanism of peptide induced formation of spherical coral-shaped gold nanoparticles. Adapted with permission from reference [35**]. Copyright 2018 Nature Publishing Group. B) Differences in average binding conformation of a single peptide T7 on extended Pt {100} surfaces, cubes, and cuboctahedra according to molecular dynamics simulation. Adapted with permission from reference [41*]. Copyright 2015, John Wiley and Sons.
A mechanistic study on the nanoparticle growth was conducted with in situ TEM, molecular simulations and time-of-flight secondary ion mass spectrometry. As a result, the peptoid with higher binding affinity would induce the growth of distorted gold nanorods, which aggregated into spherical coral-shaped particles. In contrast, the peptoids with lower binding affinity could not stabilize the high surface energy of the aggregated cluster of distorted nanorods. Therefore, the particles would relax to spheres. Furthermore, the authors also demonstrated the same principles can be applied to the controlled synthesis of coral-shaped Pd- and Pt- nanoparticles.
Instead of screening different peptide sequences for morphological controls, specific peptide sequence could also be selected for inorganic nanomaterial synthesis by phage display. In the phage display method, randomized DNA sequences were inserted into the phage virus genome, which would create the peptide library by expressing the corresponding peptides on the coat protein of the phage. Then a target surface would be brought in contact to the phage. The unbound or weakly bound phage would be washed away whereas the tightly bound phage would be retained, amplified and sequenced to find the corresponding sequences that code for the peptides [36]. This method allows for the simultaneous testing of up to 1011 different peptides [37]. This technique has been used to identify peptide sequences that can specifically bind to metals, oxides and semiconductors [38, 39]. Furthermore, an improvement of phage display method has enabled facet-specific peptide selection toward certain materials. For example, Chiu et al. [40**] selected peptides that bind either Pt-{100} or Pt-{111} by using Pt cubes and Pt octahedrons as targets in phage display selection. The selected peptides could produce Pt cubes and Pt tetrahedrons correspondingly. A follow up study on the recognition mechanism of the peptides on different Pt nanoparticle facets was performed using molecular dynamics simulation (MD) [41*]. The simulated results showed excellent matching between peptide T7 and the epitaxial sites on the edge of {100} facet (Fig. 4B). In addition, the concentration of the T7 peptide used during nanoparticle synthesis also affected the relative coverage of peptide sequence on different facets. The experimental results demonstrated that only intermediate concentration of T7 can lead to high yields of Pt nanocubes, which agreed with the simulated results. In a special case, as an extension of phage display method, Mao and coworkers [42] selected a peptide sequence that specifically binds to tetragonal barium titanate (BaTiO3) using phage display and then expressed the peptide sequence on the phage. The peptide was then used as the direct template for in situ synthesis of BaTiO3 polycrystalline nanowires.
3. Mechanism for sequence-specific control of inorganic nanomaterials morphologies
3.1. Sequence-specific interaction with nanomaterials surface
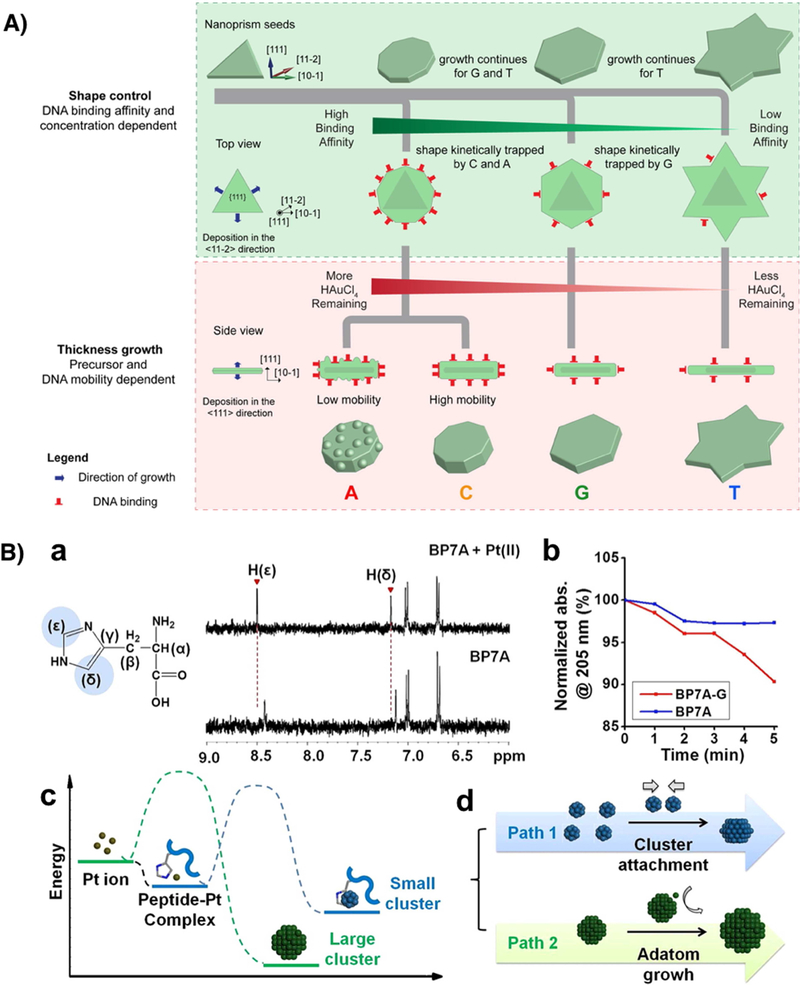
It is generally believed that the interaction of biomolecules with inorganic nanomaterials shares some common features with that of conventional capping agents such as small organic molecules. Both could modify the surface energy and surface structure of inorganic nanomaterials during the growth process. However, the modification by biomolecules is sequence dependent. For example, DNA could interact with gold nanoparticles through ring nitrogen, exocyclic amino and keto groups of the nucleobases. The exocyclic amino group and the ring nitrogen in adenine enables exceptionally high adsorption affinity towards gold surface even in the range of covalent attachment [43]. On the other hand, the delocalization of the lone pair electrons of the imine group in cytosine and guanine contributed to their non-covalent attachment onto the gold nanoparticles. Finally, thymine is thought to have the lowest affinity towards gold surface among all nucleobases, because it does not have imine group [44]. In addition to the aforementioned interactions, DNA could also interact with the gold surface through electrostatic and hydrophobic forces, which depend highly on the protonation state of the nucleobases and conformation of DNA molecules with different sequences [43]. As a result, DNA molecules with different sequences have different binding affinity as well as different mobility on the surface of gold nanoparticles, which affect the growth process of nanomaterials. For instance, Tan et al. [23**] investigated DNA-mediated morphological evolution of gold nanoprism seeds into nonagon with rough surface, nonagon with smooth surface, hexagon, and six-pointed stars in the presence of A30, C30, G20 and T30, respectively (Fig. 5A). After characterizing the growth process of nanoparticles using various analytical methods including UV-vis, SEM, TEM, zeta potential, fluorescence, and cyclic voltammetry, the authors observed that the growth always started with the expansion of diameter of the prism seed, followed by the increase of thickness. The authors proposed that, in the first stage, the binding affinity and concentration of DNA molecules with different sequences determined the desorption rate as well as the density of the DNA onto the seed. T30 has the lowest binding affinity for the gold surface, and therefore the incoming precursor could freely deposit on the seed surface and let the shape to fully develop into six-pointed star with the largest diameter amongst all four shapes. While A30, C30 and G20 have higher binding affinity than T30, those DNA molecules could stabilize the high-energy side facets (reentrant groves in the stacking defaults), thus kinetically trapped the intermediate morphologies during the shape evolution of AuNPs. And the stronger the affinity, the earlier the growth would be arrested. In the second stage, the growth in thickness depends on both the remaining precursor concentration and DNA mobility. The thickness of the nanoparticles in the second stage has an inverse trend with the diameter development in the first stage. The nanoparticles that grew larger in diameter in the first stage would consume more gold precursor than the nanoparticles that have smaller diameter. As a result, there would be less remaining gold precursor for thickness increase in the second stage for large nanoparticles. On the other hand, the roughness of the gold nanoparticles depends on the mobility of DNA sequences on gold surface. For example, because A30 has lower mobility than C30 on gold surface, A30 resulted in roughened nonagon, while C30 resulted in smooth nonagon.
Figure 5.
Mechanism for sequence-specific control of inorganic nanomaterials morphologies by DNA and peptides. A) Proposed mechanism of growth of Au nanoprisms controlled by DNA. Adapted with permission from reference [23**], copyright 2015 American Chemical Society. B) Interaction of BP7A peptide with Pt precursor and cluster. a) NMR spectra of peptide-Pt(II) complex; b) UV vis spectra of K2PtCl4 reduction in the presence of BP7A and BP7A-G peptides; c) Scheme showing the complex formation between histidine-containing peptides and Pt ions and following Pt cluster formation. d) Scheme showing two possible NC growth pathways: cluster attachment and adatom deposition. Adapted with permission from reference [47**], copyright 2014 American Chemical Society.
3.2. Sequence-specific interaction with precursor
In addition to sequence-specific interactions with inorganic nanomaterial surface, biomolecules could also interact with the precursor during the synthesis. Previous studies have demonstrated that certain biomolecule sequences could form complexes with metal ions or stabilize small clusters under various conditions [6, 45, 46]. Those complexes and clusters could also be important reaction intermediates in biomolecule-controlled synthesis of inorganic nanomaterials. For example, Ruan et al. [47**] reported that the histidine residue in BP7A peptide could form complexes with Pt ions, which reduced the reduction rate of the Pt precursors and modified the growth pathway of Pt nanocrystals towards cluster attachment. The strong interaction between histidine and Pt ions and the preferential stabilization of {111} facets by BP7A peptide contributed to the formation of twinned seed in Pt nanocrystals.
The exact mechanism for nucleation and growth of inorganic nanomaterials is still debatable. In classical nucleation theory, nucleation and growth are mediated by monomer-by-monomer addition. However, in recent years, more and more studies have indicated an alternative nucleation pathway through cluster or particle addition [48]. Direct experimental evidence of the cluster intermediates captured during crystal formation is still rather limited. With the well-controlled reaction kinetics and stabilization of clusters, biomolecule-controlled inorganic material nucleation and growth are very likely to adopt a non-classical nucleation pathway, and hence could be a model system to elucidate the unknown nucleation and growth mechanism. A close multidisciplinary collaboration involving chemistry, biology and physics is required for fully understand the mechanism for biomolecule sequence specific control of inorganic nanomaterial synthesis.
4. Properties resulting from the sequence-specific control
A major motivation behind exploring new synthetic routes to control morphologies of inorganic nanoparticles is the resultant physiochemical properties, which can be exploited in many applications including, but are not limited to, catalysis, optical spectroscopy and biological applications [49]. Since the synthetic condition using biomolecules are normally more benign or “green”, the study is a step towards the production of more sustainable materials that can potentially replace existing materials that are otherwise synthesized under harsher conditions.
4.1. Optical properties including surface enhanced Raman spectroscopy (SERS)
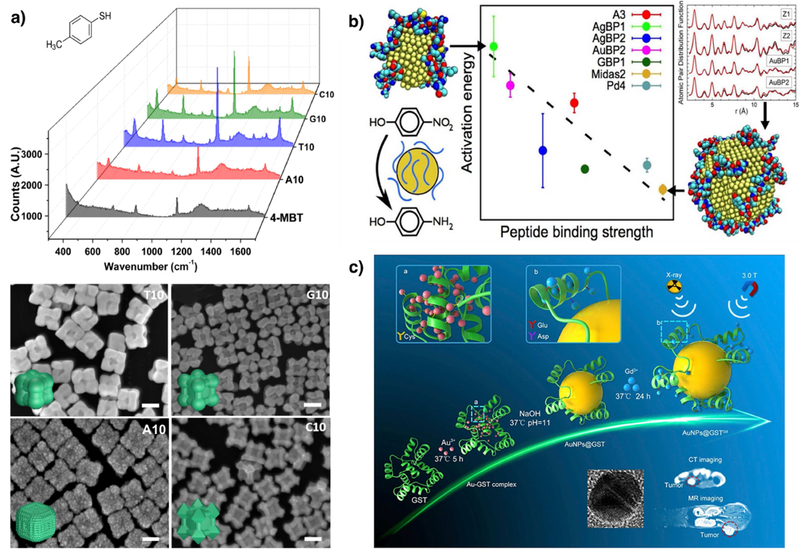
The morphology of plasmonic metal nanoparticles such as Au and Ag strongly determines the optical absorption cross-section of the nanoparticles. The Lu lab has reported the synthesis of DNA-sequence dependent morphology control of both Au [50] and Pd@Au [27] nanoparticles, which display sequence-dependent plasmonic particles. The Pd@Au particles showed different enhancement factors as SERS probes for 4-mercaptobenzenethiol [27] (Fig. 6a). Using a slightly different synthetic approach, Nam and coworkers [51] were able to manipulate “nano-gaps” by anchoring thiolated DNA with difference sequences onto the Au seed to control the shell growth. They reported the relationships between the width of the interior plasmonic nanogap and surface-enhanced Raman scattering efficiencies. On the other hand, Pu et al. [52] reported a protein-mediated synthesis of Ag nanoparticles that exhibited specific localized surface plasmon resonance (LSPR) for each protein used. This method involved multiple proteins that produced multiple readouts of LSPR signals of AgNPs to further construct sensor arrays for pattern recognition of proteins.
Figure 6.
Examples of sequence-specific properties of nanomaterials with different morphologies. a) SERS spectra of 4-MBT functionalized on the DNA-mediated nanoparticles and Raman spectra of a concentrated solution of 4-MBT. The corresponding SEM images of the DNA mediated Pd@Au core shell nanoparticles. Scale bar = 100 nm. Adapted with permission from reference [27], copyright 2018 Tsinghua University Press and Springer-Verlag GmbH Germany, part of Springer Nature. b) Peptide sequence dependent structure-function relationship in peptide mediated AuNP synthesis. The Au NPs exhibit varying degrees of catalytic activity for 4-nitrophenol reduction. The pair distribution function (PDF) analysis revealed sequence-dependent differences in structural order at the NP surface. Adapted with permission from reference [55], copyright 2016 American Chemical Society c) Schematic illustration of GST-mediated AuNPs that anchor Gd ions and biomedical imaging functions of material as a MR/CT dual-modal imaging contrast agent. Adapted with permission from reference [58*], copyright 2018 Elsevier Ltd.
4.2. Catalytic properties
The synthesis of nanomaterials with different morphologies through sequence-specific control can give rise to the ability to tune catalytic efficiencies of these materials. For example, Bedford et al. [53**] have demonstrated that peptides with different sequences play an important role in determining the catalytic efficiencies of Pd nanocatalysts for the Stille coupling reaction and olefin hydrogenation. These Pd nanoparticles were synthesized in the presence of combinatorially derived peptides that influence the surface disorder of the nanoparticles. Atomic pair distribution function (PDF) analysis of high-energy X-ray diffraction (HE-XRD) patterns and X-ray absorption fine-structure spectroscopy (XAFS) were used to probe atomic-scale structure details of peptide-derived Pd nanocatalysts, backed by MD simulation. The overall analysis shows clear atomic-scale structural differences associated with changes in the peptide sequences. In agreement to this result, sequence-specific peptides have been used for metal composition control in Pd-Au bimetallic nanoparticles to achieve ultimate tunability of catalytic efficiency in electrocatalytic methanol oxidation reactions [54]. Similar effects were observed in the case of 4-nitrophenol reduction by Au nanoparticles synthesized in the presence of different peptide sequences [55]. (Fig. 6b).
4.3. Biomedical imaging and nanomedicine
Given that the nanoparticle surfaces are functionalized with biomolecules such as DNA, peptide or proteins, the biocompatibility of the particles increases compared to nanoparticles synthesized with other ligands. The DNA-mediated Ag nanoparticles have been utilized for antimicrobial activity in the past. On the other hand, peptide-coated nanomaterials have been used as implant materials, for regenerative medicine, and for medical imaging [56]. Wang et al. [57] reported BSA protein mediated synthesis of Au core/shell nanoparticles loaded with drugs, which were further used for simultaneous CT imaging and drug delivery. Similarly, Zhang and coworkers [58*] reported glutathione S-transferase (GST) protein mediated in-situ synthesis of Au NPs. The protein also had the functional groups to anchor Gd ions and this set-up enabled them to perform in vivo MR and CT imaging (Fig. 6c). Peptide nanofibers have also been used for the synthesis of Ag nanoparticles, which were utilized for antimicrobial applications [59].
5. Summary and outlook
As discussed in the sections above, impressive progress has been made in the past few years toward sequence-specific controlled morphologies of inorganic nanomaterials. The programmability and unique chemical and structural characteristics of biomolecules allow screening of sequences to direct the growth of nanostructures into specific morphology, which in some cases cannot be achieved by conventional methods using inorganic anions or organic molecules. To complement the rational design method, combinatorial molecular selection or evolution method has also been developed to discover specific sequences towards different inorganic material surfaces as well as specific nanostructure morphology. Each of the two methods has its own strengths and limitations.
Screening of biomolecules for nanomaterials synthesis is the most straight forward method. It starts with a relatively small library (usually tens to hundreds of sequences). Each sequence is assayed separately, where in situ nanomaterial synthesis is performed in each assay. The sequences that produce desired nanostructures will be retained and the material characterized further. Screening is performed exactly in the synthesis condition. Therefore, it has better predictability for the actual performance of biomolecules in nanomaterial synthesis. However, the throughput of screening is relatively low (several to hundreds of sequences). Each sequence need to be assayed separately, and each synthesis may take some time to finish, which makes this method time consuming and labor intensive. Moreover, because of the relatively small library, the best sequence could be left out considering the numerous combinations of biomolecule building blocks.
Selection, on the other hand, enables examination of a much larger library (1011 to 1015 sequences) where all sequences are mixed together and assayed simultaneously. For most studies so far, selection is essentially a binding assay, where the sequence with the strongest binding affinity will be selected. However, capping agent assisted nanocrystal growth process is more than a simple binding event, it involves a dynamic adsorption and desorption process of molecules on the surface. Any shapes and sizes of the nanocrystals used as target in the selection is eventually a snapshot of a growing crystal and therefore the affinity for those targets does not guarantee the final yield of the same crystal. For example, a molecule with too strong an affinity might quench the crystal growth at a much earlier time point, which can result in crystals that never evolve into the desired shape. Moreover, the affinity-based selection has the interference from non-sequence specific binding to nanomaterial surface, such as electrostatic interaction, and this issue is especially serious with inorganic materials [29]. If the background noise is too high and the difference between different sequences is too little, it is highly possible to generate a false positive result. Additionally, most of the reported cases are on metal nanomaterial synthesis. The generality of these methods need to be demonstrated on other nanomaterials. New methods that have combine the benefits of both better predictability in screening and high throughput in selection should be developed to accelerate discovery of new biomolecules with sequences that can control novel morphologies.
The successful cases of using rationally designed biomolecules to control synthesis toward certain nanostructure morphology is still relatively rare. Most of the studies are based on trial and error. Mechanistic insight is essential in truly achieving sequence specific controlled synthesis of inorganic nanomaterials. However, challenges remain in multiple perspectives in understanding biomolecule-inorganic nanomaterial interaction in the synthesis. For biomolecules, different sequences generate more than one conformation or three-dimensional structures, and they often dynamically interchange between each other in aqueous solution. For inorganic material surfaces, varies facets as well as lattice defects, together with the limited choice of atomic level characterization technique made their structures difficult to characterize and simulate. Therefore, these factors make understanding biomolecule-inorganic interface difficult. Currently, direct experimental tool to resolve the configuration and packing of biomolecules on inorganic nanomaterial surfaces is quite rare [12]. We have to largely rely on computational tool to understand and predict the behavior of biomolecules on inorganic surfaces [56]. Moreover, in the actual nanomaterial synthesis, the surface of the growing crystal is always changing, and the interaction of biomolecules with precursors and other biomolecules should also be considered. Both experimental and computational methods need to be developed in order to provide greater insight of biomolecule controlled inorganic nanomaterial synthesis, which would eventually help us to obtain programmable nanomaterial morphologies using different biomolecule sequences.
Highlights.
Recent progress in sequence-specific control of inorganic nanomaterial morphologies by DNA and peptides are reviewed.
Mechanisms for the sequence-specific control of inorganic nanomaterial morphologies are discussed.
Properties resulting from the sequence-specific controlled inorganic nanomaterial morphologies are presented.
Strengths and limitations of the methods to discover biomolecular controlled inorganic material morphologies are summarized.
Acknowledgements:
We wish to thank all Lu group members who have made significant contributions to work described in this review. N. S. R. S. would like to thank the Beckman Graduate Fellowship for financial support. US National Institute of Health (GM124316 and MH110975) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- [1].Ryabchuk P, Agostini G, Pohl M-M, Lund H, Agapova A, Junge H, et al. Intermetallic nickel silicide nanocatalyst—A non-noble metal–based general hydrogenation catalyst. Science Advances 2018;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xia Y, Gilroy Kyle D, Peng HC, Xia X. Seed-Mediated Growth of Colloidal Metal Nanocrystals. Angew Chem Int Ed 2016;56:60–95. [DOI] [PubMed] [Google Scholar]
- [3].Chen Z, Liu C, Cao F, Ren J, Qu X. DNA metallization: principles, methods, structures, and applications. Chem Soc Rev 2018. [DOI] [PubMed]
- [4].Pu F, Ren J, Qu X. Nucleobases, nucleosides, and nucleotides: versatile biomolecules for generating functional nanomaterials. Chem Soc Rev 2018;47:1285–306. [DOI] [PubMed] [Google Scholar]
- [5].Coffer JL, Bigham SR, Pinizzotto RF, Yang H. Characterization of quantum-confined CdS nanocrystallites stabilized by deoxyribonucleic acid (DNA). Nanotechnology 1992;3:69. [Google Scholar]
- [6].Petty JT, Zheng J, Hud NV, Dickson RM. DNA-Templated Ag Nanocluster Formation. JACS 2004;126:5207–12. [DOI] [PubMed] [Google Scholar]
- [7].Whaley SR, English DS, Hu EL, Barbara PF, Belcher AM. Selection of peptides with semiconductor binding specificity for directed nanocrystal assembly. Nature 2000;405:665. [DOI] [PubMed] [Google Scholar]
- [8].Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO. Biomimetic synthesis and patterning of silver nanoparticles. Nature Materials 2002;1:169. [DOI] [PubMed] [Google Scholar]
- [9].Reiss BD, Mao C, Solis DJ, Ryan KS, Thomson T, Belcher AM. Biological Routes to Metal Alloy Ferromagnetic Nanostructures. Nano Lett 2004;4:1127–32. [Google Scholar]
- [10].Roth KL, Geng X, Grove TZ. Bioinorganic Interface: Mechanistic Studies of Protein-Directed Nanomaterial Synthesis. The Journal of Physical Chemistry C 2016;120:10951–60. [Google Scholar]
- [11].Zan G, Wu Q. Biomimetic and Bioinspired Synthesis of Nanomaterials/Nanostructures. Adv Mater 2016;28:2099–147. [DOI] [PubMed] [Google Scholar]
- [12].Xia Y, Xia X, Peng H-C. Shape-Controlled Synthesis of Colloidal Metal Nanocrystals: Thermodynamic versus Kinetic Products. JACS 2015;137:7947–66. [DOI] [PubMed] [Google Scholar]
- [13].Ramezani-Dakhel H, Ruan L, Huang Y, Heinz H. Molecular Mechanism of Specific Recognition of Cubic Pt Nanocrystals by Peptides and of the Concentration-Dependent Formation from Seed Crystals. Adv Funct Mater 2015;25:1374–84. [Google Scholar]
- [14].Heinz H, Ramezani-Dakhel H. Simulations of inorganic-bioorganic interfaces to discover new materials: insights, comparisons to experiment, challenges, and opportunities. Chem Soc Rev 2016;45:412–48. [DOI] [PubMed] [Google Scholar]
- [15].Carnerero Jose M, Jimenez-Ruiz A, Castillo Paula M, Prado-Gotor R. Covalent and Non-Covalent DNA–Gold-Nanoparticle Interactions: New Avenues of Research. ChemPhysChem 2016;18:17–33. [DOI] [PubMed] [Google Scholar]
- [16].Zheng XT, Xu HV, Tan YN. Bioinspired Design and Engineering of Functional Nanostructured Materials for Biomedical Applications. Advances in Bioinspired and Biomedical Materials Volume 2: American Chemical Society; 2017. p. 123–52. [Google Scholar]
- [17].Li Y, Tang Z, Prasad PN, Knecht MR, Swihart MT. Peptide-mediated synthesis of gold nanoparticles: effects of peptide sequence and nature of binding on physicochemical properties. Nanoscale 2014;6:3165–72. [DOI] [PubMed] [Google Scholar]
- [18].Brown KA, Park S, Hamad-Schifferli K. Nucleotide−Surface Interactions in DNA-Modified Au−Nanoparticle Conjugates : Sequence Effects on Reactivity and Hybridization. The Journal of Physical Chemistry C 2008;112:7517–21. [Google Scholar]
- [19].Ruan L, Ramezani-Dakhel H, Chiu C-Y, Zhu E, Li Y, Heinz H, et al. Tailoring Molecular Specificity Toward a Crystal Facet: a Lesson From Biorecognition Toward Pt{111}. Nano Lett 2013;13:840–6. [DOI] [PubMed] [Google Scholar]
- [20].Wang Z, Zhang J, Ekman JM, Kenis PJA, Lu Y. DNA-Mediated Control of Metal Nanoparticle Shape: One-Pot Synthesis and Cellular Uptake of Highly Stable and Functional Gold Nanoflowers. Nano Lett 2010;10:1886–91. [DOI] [PubMed] [Google Scholar]
- [21].Wang Z, Tang L, Tan Li H, Li J, Lu Y. Discovery of the DNA “Genetic Code” for Abiological Gold Nanoparticle Morphologies. Angew Chem Int Ed 2012;51:9078–82. [DOI] [PubMed] [Google Scholar]
- [22].Wang Z, Bharathi MS, Hariharaputran R, Xing H, Tang L, Li J, et al. pH-Dependent Evolution of Five-Star Gold Nanostructures: An Experimental and Computational Study. ACS Nano 2013;7:2258–65. [DOI] [PubMed] [Google Scholar]
- [23**].Tan LH, Yue Y, Satyavolu NSR, Ali AS, Wang Z, Wu Y, et al. Mechanistic Insight into DNA-Guided Control of Nanoparticle Morphologies. JACS 2015;137:14456–64.Highlight: This paper discussed the interaction of DNA with the surface of Au NPs and their role in control the growth of Au NPs.
- [24].Wu J, Tan LH, Hwang K, Xing H, Wu P, Li W, et al. DNA Sequence-Dependent Morphological Evolution of Silver Nanoparticles and Their Optical and Hybridization Properties. JACS 2014;136:15195–202. [DOI] [PubMed] [Google Scholar]
- [25**].Satyavolu NSR, Tan LH, Lu Y. DNA-Mediated Morphological Control of Pd–Au Bimetallic Nanoparticles. JACS 2016;138:16542–8.Highlight: This paper is the first DNA mediated synthesis of bimetallic nanoparticles system
- [26**].Song T, Tang L, Tan Li H, Wang X, Satyavolu Nitya Sai R, Xing H, et al. DNA-Encoded Tuning of Geometric and Plasmonic Properties of Nanoparticles Growing from Gold Nanorod Seeds. Angew Chem Int Ed 2015;54:8114–8Highlight: This paper explored the effect of modified DNA sequences with phosphorothioate on the morphology of Au nanoparticles.
- [27].Satyavolu NSR, Pishevaresfahani N, Tan LH, Lu Y. DNA-encoded morphological evolution of bimetallic Pd@Au core-shell nanoparticles from a high-indexed core. Nano Research 2018. [DOI] [PMC free article] [PubMed]
- [28].McGhee CE, Loh KY, Lu Y. DNAzyme sensors for detection of metal ions in the environment and imaging them in living cells. Curr Opin Biotechnol 2017;45:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou Y, Huang Z, Yang R, Liu J. Selection and Screening of DNA Aptamers for Inorganic Nanomaterials. Chemistry – A European Journal 2017;24:2525–32. [DOI] [PubMed] [Google Scholar]
- [30*].Bawazer LA, Newman AM, Gu Q, Ibish A, Arcila M, Cooper JB, et al. Efficient Selection of Biomineralizing DNA Aptamers Using Deep Sequencing and Population Clustering. ACS Nano 2014;8:387–95.Highlight: This paper used deep sequencing to assisted the selection towards ZnO nanoparticles to make the selection more efficient.
- [31**].Baillargeon KR, Meserve K, Faulkner S, Watson S, Butts H, Deighan P, et al. Precipitation SELEX: identification of DNA aptamers for calcium phosphate materials synthesis. Chem Commun 2017;53:1092–5.Highlight: This paper selected calcium phosphate binding DNA sequences in actual nanoparticle synthesis condition, which provided better predictability in the performance of the selected sequence in actual nanomaterial synthesis.
- [32].Lu C, Huang Z, Liu B, Liu Y, Ying Y, Liu J. Poly-cytosine DNA as a High-Affinity Ligand for Inorganic Nanomaterials. Angew Chem Int Ed 2017;56:6208–12. [DOI] [PubMed] [Google Scholar]
- [33].Ma L, Liu B, Huang P-JJ, Zhang X, Liu J. DNA Adsorption by ZnO Nanoparticles near Its Solubility Limit: Implications for DNA Fluorescence Quenching and DNAzyme Activity Assays. Langmuir 2016;32:5672–80. [DOI] [PubMed] [Google Scholar]
- [34].Wang Q, Tang Z, Wang L, Yang H, Yan W, Chen S. Morphology Control and Electro catalytic Activity towards Oxygen Reduction of Peptide-Templated Metal Nanomaterials: A Comparison between Au and Pt. ChemistrySelect 2016;1:6044–52. [Google Scholar]
- [35**].Yan F, Liu L, Walsh TR, Gong Y, El-Khoury PZ, Zhang Y, et al. Controlled synthesis of highly-branched plasmonic gold nanoparticles through peptoid engineering. Nature Communications 2018;9:2327.Highlight: This paper used peptoids in the morphology control of gold nanoparticles. Peptoids are ideal biomolecule platform for studying nanoparticle growth because the lack of backbone hydrogen bonding. The effect from the side chains could be isolated and make the system more predictable.
- [36].Chiu C-Y, Ruan L, Huang Y. Biomolecular specificity controlled nanomaterial synthesis. Chem Soc Rev 2013;42:2512–27. [DOI] [PubMed] [Google Scholar]
- [37].Corra S, Shoshan MS, Wennemers H. Peptide mediated formation of noble metal nanoparticles—controlling size and spatial arrangement. Curr Opin Chem Biol 2017;40:138–44. [DOI] [PubMed] [Google Scholar]
- [38].Rawlings AE, Bramble JP, Tang AAS, Somner LA, Monnington AE, Cooke DJ, et al. Phage display selected magnetite interacting Adhirons for shape controlled nanoparticle synthesis. Chemical Science 2015;6:5586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].You F, Yin G, Pu X, Li Y, Hu Y, Huang Z, et al. Biopanning and characterization of peptides with Fe3O4 nanoparticles-binding capability via phage display random peptide library technique. Colloids and Surfaces B: Biointerfaces 2016;141:537–45. [DOI] [PubMed] [Google Scholar]
- [40**].Chiu C-Y, Li Y, Ruan L, Ye X, Murray CB, Huang Y. Platinum nanocrystals selectively shaped using facet-specific peptide sequences. Nature Chemistry 2011;3:393.Highlight: This paper is one of the first example of using phage display to for facet specific peptide selection, the resulting peptide sequence has good specificity in controlling the synthesis of Pt nanocrystal.
- [41*].Ramezani-Dakhel H, Ruan L, Huang Y, Heinz H. Molecular Mechanism of Specific Recognition of Cubic Pt Nanocrystals by Peptides and of the Concentration-Dependent Formation from Seed Crystals. Adv Funct Mater 2015;25:1374–84.Highlight: This paper provided mechanistic insight for the molecular recognition of peptide toward certain facet of Pt nanoparticles.
- [42].Li Y, Cao B, Yang M, Zhu Y, Suh J, Mao C. Identification of Novel Short BaTiO3-Binding/Nucleating Peptides for Phage-Templated in Situ Synthesis of BaTiO3 Polycrystalline Nanowires at Room Temperature. ACS Applied Materials & Interfaces 2016;8:30714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Koo KM, Sina AAI, Carrascosa LG, Shiddiky MJA, Trau M. DNA–bare gold affinity interactions: mechanism and applications in biosensing. Analytical Methods 2015;7:7042–54. [Google Scholar]
- [44].Carnerero JM, Jimenez-Ruiz A, Castillo PM, Prado-Gotor R. Covalent and Non-Covalent DNA-Gold-Nanoparticle Interactions: New Avenues of Research. Chemphyschem 2017;18:17–33. [DOI] [PubMed] [Google Scholar]
- [45].Kondo J, Tada Y, Dairaku T, Hattori Y, Saneyoshi H, Ono A, et al. A metallo-DNA nanowire with uninterrupted one-dimensional silver array. Nature Chemistry 2017;9:956. [DOI] [PubMed] [Google Scholar]
- [46].Weng B, Lu K-Q, Tang Z, Chen HM, Xu Y-J. Stabilizing ultrasmall Au clusters for enhanced photoredox catalysis. Nature Communications 2018;9:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47**].Ruan L, Ramezani-Dakhel H, Lee C, Li Y, Duan X, Heinz H, et al. A Rational Biomimetic Approach to Structure Defect Generation in Colloidal Nanocrystals. ACS Nano 2014;8:6934–44.Highlight: This paper discussed the interaction of peptide with Pt precursor and the formation of Pt clusters in peptide controlled Pt crystal nucleaiton
- [48].De Yoreo JJ, Gilbert PU, Sommerdijk NA, Penn RL, Whitelam S, Joester D, et al. CRYSTAL GROWTH. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015;349:aaa6760. [DOI] [PubMed] [Google Scholar]
- [49].Walsh TR. Pathways to Structure–Property Relationships of Peptide–Materials Interfaces: Challenges in Predicting Molecular Structures. Acc Chem Res 2017;50:1617–24. [DOI] [PubMed] [Google Scholar]
- [50].Song T, Tang L, Tan Li H, Wang X, Satyavolu Nitya Sai R, Xing H, et al. DNA-Encoded Tuning of Geometric and Plasmonic Properties of Nanoparticles Growing from Gold Nanorod Seeds. Angew Chem Int Ed 2015;54:8114–8. [DOI] [PubMed] [Google Scholar]
- [51].Oh J-W, Lim D-K, Kim G-H, Suh YD, Nam J-M. Thiolated DNA-Based Chemistry and Control in the Structure and Optical Properties of Plasmonic Nanoparticles with Ultrasmall Interior Nanogap. JACS 2014;136:14052–9. [DOI] [PubMed] [Google Scholar]
- [52].Pu F, Ran X, Guan M, Huang Y, Ren J, Qu X. Biomolecule-templated photochemical synthesis of silver nanoparticles: Multiple readouts of localized surface plasmon resonance for pattern recognition. Nano Research 2017. [Google Scholar]
- [53**].Bedford NM, Ramezani-Dakhel H, Slocik JM, Briggs BD, Ren Y, Frenkel AI, et al. Elucidation of Peptide-Directed Palladium Surface Structure for Biologically Tunable Nanocatalysts. ACS Nano 2015;9:5082–92.Highlight: An important study that adopts a hybrid experimental and computational approach to examine sequence dependent structure/function relationships at the atomic level by using extensive structural characterization methods.
- [54].Bedford NM, Showalter AR, Woehl TJ, Hughes ZE, Lee S, Reinhart B, et al. Peptide-Directed PdAu Nanoscale Surface Segregation: Toward Controlled Bimetallic Architecture for Catalytic Materials. ACS Nano 2016;10:8645–59. [DOI] [PubMed] [Google Scholar]
- [55].Bedford NM, Hughes ZE, Tang Z, Li Y, Briggs BD, Ren Y, et al. Sequence-Dependent Structure/Function Relationships of Catalytic Peptide-Enabled Gold Nanoparticles Generated under Ambient Synthetic Conditions. JACS 2016;138:540–8. [DOI] [PubMed] [Google Scholar]
- [56].Walsh TR, Knecht MR. Biointerface Structural Effects on the Properties and Applications of Bioinspired Peptide-Based Nanomaterials. Chem Rev 2017;117:12641–704. [DOI] [PubMed] [Google Scholar]
- [57].Huang H, Yang D-P, Liu M, Wang X, Zhang Z, Zhou G, et al. pH-sensitive Au–BSA– DOX–FA nanocomposites for combined CT imaging and targeted drug delivery. International Journal of Nanomedicine 2017;12:2829–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58*].Yang W, Wu X, Dou Y, Chang J, Xiang C, Yu J, et al. A human endogenous protein exerts multi-role biomimetic chemistry in synthesis of paramagnetic gold nanostructures for tumor bimodal imaging. Biomaterials 2018;161:256–69.Highlight: A report that uses a Human endogenous protein for the synthesis of Au NP and anchoring Gd ions for bimodal imaging. Highlights the advantages of using naturally biomolecules in NP synthesis.
- [59].Pazos E, Sleep E, Rubert Pérez CM, Lee SS, Tantakitti F, Stupp SI. Nucleation and Growth of Ordered Arrays of Silver Nanoparticles on Peptide Nanofibers: Hybrid Nanostructures with Antimicrobial Properties. JACS 2016;138:5507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]