Abstract
Purpose of Review
In this review, we discuss the recent advancements in liver bioengineering and cell therapy and future advancements to improve the field towards clinical applications.
Recent Findings
3D printing, hydrogel-based tissue fabrication, and the use of native decellularized liver extracellular matrix as a scaffold are used to develop whole or partial liver substitutes. The current focus is on developing a functional liver graft through achieving a non-leaky endothelium and a fully constructed bile duct. Use of cell therapy as a treatment is less invasive and less costly compared to transplantation, however, lack of readily available cell sources with low or no immunogenicity and contradicting outcomes of clinical trials are yet to be overcome.
Summary
Liver bioengineering is advancing rapidly through the development of in vitro and in vivo tissue and organ models. Although there are major challenges to overcome, through optimization of the current methods and successful integration of induced pluripotent stem cells, the development of readily available, patient-specific liver substitutes can be achieved.
Keywords: Liver, Tissue engineering, Decellularization, Cell therapy, Induced pluripotent stem cells
Introduction
Liver diseases are recognized as the second leading cause of mortality among all digestive diseases and eighth among all leading causes of death in the USA [1]. It is reported that more than one million deaths were caused by cirrhosis in 2010 [2]. Current clinical remedies just minimally improve the patient’s survival and the only treatment available for patients with end-stage liver failure is orthotopic liver transplantation (OLT). Although this approach yields high survival rates, the number of acceptable donor livers falls short of the worldwide need. The primary reason behind this scarcity is that the majority of donor livers are considered not eligible for transplantation due to conditions such as high-fat content and ischemic damage after cardiac death. To improve long-term patient survival, alternative strategies are proposed through liver bioengineering, which aims to restore the essential hepatic functions. The main applications of liver tissue engineering are development of in vitro platforms to conduct preclinical drug toxicity testing and disease modeling [3–6], development of partial grafts, induction of liver regeneration through cell therapies, and development of artificial or natural whole organ substitutes to replace the damaged tissue/organ [7, 8] (Fig. 1). In this review, we will focus on the recent advancements in the development of partial or whole liver grafts and cell therapies addressing their potential and the improvements necessary for their future clinical use.
Fig. 1.
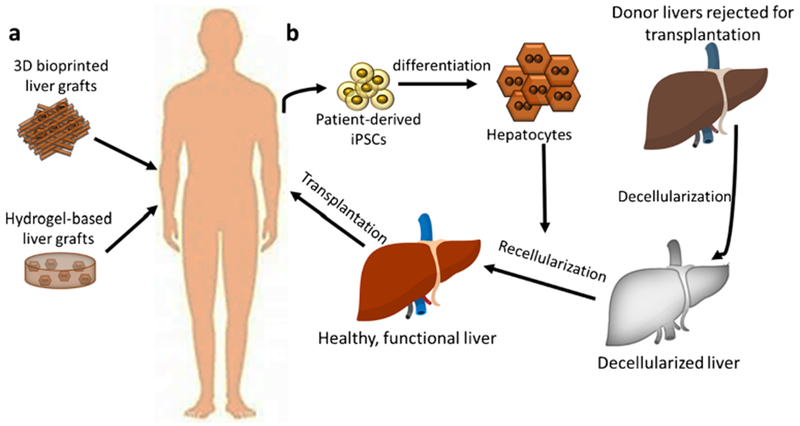
Current applications of liver bioengineering include fabricating partial liver grafts through 3D bioprinting and using hydrogel-based tissues for repairing the lost liver function (a). Recently, the focus has been shifted towards optimizing the decellularization of donor livers rejected for transplantation and their recellularization with iPSC-derived hepatocytes to render them eligible for transplantation (b).
Liver Engineering for Implantable Grafts
The field of tissue and organ engineering has shown promising progress as evidenced with clinical applications of the engineered trachea and bladder [9, 10]. However, the same success has not been reached for complex organs such as the heart and liver as it is challenging to mimic the structural and functional complexity of these organs in vitro [11]. The liver is one of the most complex organs owing to its highly complex and interwoven vascular, lymph, and biliary network. Thus, both the cellular and biochemical composition, as well as the structural organization of the liver is crucial for the development of fully functional liver substitutes [8].
One approach towards developing a partial liver graft is the fabrication of cell-laden constructs in vitro using molding or 3D printing. Successful culture of hepatocytes and hepatocyte-like cells has been shown in tissue models comprised of synthetic materials [12], as well as natural materials [13–15]. A recent study [16] showed the fabrication of a seed liver tissue which expanded upon ectopic implantation to immunodeficient mice with liver injury, benefiting from the regenerative cues secreted in response to injury. The constructs were fabricated in a spatially controlled manner by seeding stripes of hepatic aggregates, which were comprised of human hepatocytes and human fibroblasts, and endothelial cells in a fibrin hydrogel. It was reported that the human liver graft had grown, and human endothelial cells formed functional blood vessels. It was discussed, however, that the engraftmentofthis model would be limited in patients with severe conditions such as cirrhosis, due to fibrosis which would interfere with the ECM remodeling potential for seed liver expansion. In another study [14], a 3D printed in vitro liver model was developed using alginate hydrogel and hepatocyte-like cells that were directly converted from mouse fibroblasts using retroviral transduction. When transplanted in vivo, higher rates of proliferation, albumin secretion, and integration with host liver were reported. However, the safety of the retroviral manipulation as well as more detailed liver function and integration studies need to be performed for translation of this tissue model to the clinic.
Although materials such as fibrin and alginate support the successful culture of hepatocytes, the effect of liver-specific microenvironmental cues is overlooked in such models. As an alternative material, the study by Lee et al. investigated the use of porcine liver decellularized ECM bioink for bioprinting liver tissues [15]. The ECM bioink was prepared by decellularizing and solubilizing porcine liver slices. When HepG2 cells were printed with the ECM bioink, they showed improved liver-specific functionality as evidenced by higher albumin and urea secretion in vitro, compared to collagen bioink. In addition, better differentiation of human bone marrow-derived mesenchymal stem cells to hepatocytes was achieved with ECM bioink. However, durable 3D construct fabrication required incorporating polycaprolactone, showing the limitations of 3D bioprinting towards mimicking both the biochemical composition and complex 3D organization of liver. Decellularized porcine liver ECM was also used as hydrogels for immortalized murine small cholangiocyte culture [17]. Duct formation and branching were observed when the cells were encapsulated in decellularized ECM matrix while this formation was not observed in collagen type I and Matrigel controls, suggesting that the liver microenvironment plays a crucial role in bile duct formation, branching, and function.
An alternative approach to provide organ-specific micro- and macroenvironmental cues can be achieved through whole-organ decellularization. This method allows for washing off of all the cellular components while preserving the original ECM composition and organizational structure, including the vascular network. The concept of whole-organ decellularization was introduced by Ott and colleagues using mice hearts [18], which was later adapted for liver engineering for the first time by our group in rats [19]. This was then applied to larger animals such as pigs [20, 21], sheep [22], and human [23]. The recent focus has been the recellularization of the whole or partial livers to yield a fully functional tissue/organ substitute. Robertson et al. [24] showed recellularization of decellularized rat liver caudate lobes through portal vein with rat or human liver cells. The recellularized livers were cultured in a bioreactor for 28 days and showed liver-specific functions such as albumin and urea production and drug metabolism. In another study, Ogiso and colleagues have compared the perfusion seeding of hepatocytes through portal vein versus bile duct to mice whole decellularized livers [25]. They have observed that when the cells were introduced through portal vein the cell clusters clogged the vasculature leading to a non-homogenous parenchymal coverage following 60 h of perfusion. When bile duct was used for cell seeding, cells reached parenchyma of the liver more successfully within 33 h of perfusion. They have also shown that fetal hepatocytes showed higher proliferation and higher albumin production in decellularized livers, compared to adult hepatocytes. Although not shown in this study, the seeding method was discussed to allow for seeding of non-parenchymal cells through the portal vein, such as endothelial cells, enabling a secondary route for vascularization [25]. The combined seeding of hepatocytes and endothelial cells was shown by Kojima et al. [26]. They used decellularized rat livers which were recellularized with primary hepatocytes through bile duct perfusion and subsequently with liver sinusoidal endothelial cells through the portal vein perfusion. When integrated for extracorporeal perfusion for 3 h, the endothelialized liver grafts showed no severe blood clotting. Researchers also reported that the recellularized livers were durable for 8-h-long extracorporeal perfusion.
To achieve clinical acceptance, however, long-term blood perfusion without thrombus formation is crucial. A recent attempt was reported by our group [27•] aiming to decrease thrombus formation through immobilization of elastin-like peptide (ELP) conjugated with a fibronectin-derived peptide, REDV, (ELP-REDV) to the decellularized liver vasculature. The immobilization of ELP-REDV was designed to enhance the endothelial cell attachment and proliferation through REDV peptide while maintaining the native-like mechanical properties of the ECM and facilitating elastin expression through ELP without blocking cell interactions with the native liver ECM. Human endothelial cells showed higher attachment and proliferation on 100-μm-thick decellularized liver slices when conjugated with ELP-REDV. The conjugation of this peptide was applied to whole rat livers and an improved cell attachment was observed compared to untreated livers, and endothelial mono-layer formation was achieved throughout the liver vasculature. ELP-REDV conjugation was also shown to facilitate higher expression of endothelial markers such as endothelial nitric oxide synthase, vascular endothelial growth factor and vascular endothelial cadherin in whole livers. Finally, ELP-REDV conjugated livers showed a significantly lower platelet activation upon perfusion with platelet-rich plasma compared to untreated livers [27•]. The longest perfusion of decellularized livers was reported by Mao et al. [28••]. They showed endothelialization of decellularized porcine whole livers that can be perfused with blood without substantial clotting for 3 days. They performed perfusion decellularization to juvenile porcine livers and perfused them with porcine umbilical cord vein endothelial cells (PUVECs) first through a hepatic vein for 24–48 h followed by seeding through the portal vein. The endothelialized livers were then implanted to recipient pigs as auxiliary grafts and connected to host circulation. Both before and after the 72 h of in vivo perfusion, the endothelialized grafts showed vascular patency and significantly lower thrombogenesis compared to non-endothelialized controls [28••]. A different approach to improve the vascular potency as well as the overall regenerative capacity of decellularized livers was proposed by Yang and colleagues [29]. They utilized the regenerative capacity of the body to develop decellularized liver matrices enhanced with regenerative bioactive cues to induce vascularization and achieve improved cell survival and function. The liver matrices were collected from mice that underwent hepatectomy and seeded with primary mouse hepatocytes and liver-derived progenitor cells. The regenerative matrices showed improved hepatocyte survival and proliferation, as well as enhanced differentiation of progenitor cells to endothelial cells when compared to non-regenerative liver matrices. The regenerative matrix was also shown to induce blood vessel formation in pigs [29].
Despite the successful examples of hepatocyte and non-parenchymal cell culture in animal decellularized livers, the physiological species-to-species differences, and potential immunological problems with xenogeneic grafts still pose a concern [30]. To address this issue, Wang et al. investigated the effect of cross-linking the decellularized porcine livers on the immunogenic response of the host [31]. They used glutaraldehyde and genipin as cross-linking agents and showed that upon implantation to the abdominal wall of rats, the genipin cross-linked porcine livers evoked a significantly less immune response. Although it was reported that the hepatocytes were viable and functional on cross-linked decellularized livers, the change in structural characteristics such as tissue stiffness and, importantly, the immune response in the human body remains to be investigated.
As an ultimate solution to overcome cross-species differences, recent studies focus on optimizing human liver decellularization and recellularization. Whole human liver decellularization was first shown by Mazza et al. [23]. They performed perfusion decellularization of whole human livers and determined that the 3D architecture and essential structural proteins were preserved. They showed successful recellularization of 5 × 5 × 5 mm cubic sections of the decellularized human liver with human hepatic stellate, endothelial, and epithelial cell lines. The first machine perfusion decellularization of whole human livers was reported by Verstegen and colleagues [32] for establishing a platform that provides easier adaptation to clinical settings. Using dual perfusion through the portal vein and hepatic artery, they showed the preservation of biliary and vascular structure and biochemical composition of the livers. Sections of decellularized liver were shown to support HUVEC attachment and culture; however, seeding of whole livers or liver lobes was not performed.
Pitfalls and Hurdles to Overcome in Liver Bioengineering
The ideal engineered human liver substitute should be comprised of functional human hepatocytes, have leak-proof vasculature, and a functional biliary network, and should not evoke an immune response. Until now, the attempts suggest that the decellularized livers hold great potential for successful seeding and culture of hepatocytes. However, populating the bile duct with functional cells and achieving an intact vascular network remains to be perfected. Studies so far report at most 3-days-long blood perfusion after endothelialization, which was not thrombus free. This shows that the main challenge to reach the clinical stage is not being able to develop liver substitutes that can withstand long-term blood perfusion without clotting. Through optimization of endothelial cell seeding and maintaining the intact endothelium during perfusion, engineered livers that can be sustained in vivo can be achieved.
Another important concern before engineered liver substitutes can be translated to the clinic is the lack of a readily available and renewable cell source. Successful recellularization of livers, even at small animal scale, requires hundreds of millions of cells. The limited availability and inability to expand primary hepatocytes has led the field to search for a new cell source. Induced pluripotent stem cells have proved as a potential candidate as their successful differentiation to hepatocyte-like cells has already been documented [33–35]. Importantly, studies have shown that the presence of native porcine [36] and human [37] liver ECM promotes better hepatic functionality in iPSC-derived cells in 2D. Park etal. [36] also showed the perfusion seeding of porcine iPSC-Heps to decellularized whole rat livers through the portal vein and subsequently were cultured in a closed perfusion culture system for 5 days. The recellularized livers were implanted to rats and recellularized livers were connected to host circulation through the renal artery; however, coagulation occurred even though heparin was introduced to recipient animals [36]. To reach the clinic, optimization of iPSC differentiation to mature and functional hepatocytes along with endothelial cells and cholangiocytes is necessary [38]. In addition, batch-batch consistency and potential teratoma formation are still concerns regarding the use of iPSCs in long-term clinical studies. Although iPSC-based, fully functional organs need significant progress and safety confirmation, such organ substitutes will pave the way for personalized organ construction which will minimize the risks for rejection and necessity for immunosuppressive drugs.
Another potential hurdle in using the discarded livers efficiently after decellularization is the sample to sample variation due to the unique condition of each donor. A recent study [39] attempting to develop a generic matrix for liver tissue engineering concluded that the biochemical composition of the livers from different donors varied significantly. Thus, recellularization efficiency might vary with the donor liver’s condition. More research is needed on different age groups and disease states of donor livers to determine their suitability for decellularization/recellularization process. In addition, further optimization of the decellularization process would improve the quality of liver substitutes through improving the preservation of bioactive factors [30].
Cell Therapy for Liver Regeneration
Cell therapy can be an alternative to whole-organ transplantation. Introduction of healthy cells into the body to replace the functions of the failing organ has numerous potential advantages over OLT. This procedure is less invasive, less technically challenging and expensive, can be repeated over time, and does not contraindicate or preclude OLT [40]. The earliest attempts made in this field involved the allogeneic hepatocyte transplantation (Fig. 2a) [41, 42]. To date, hepatocyte transplantation has been documented in many patients worldwide, with indications including metabolic liver disease, chronic liver failure, and acute liver failure [41, 43, 44]. Although the safety of clinical hepatocyte transplantation has been very well established, the therapeutic benefit has been modest and only temporary so far. Its application remains restricted in monogenic diseases where primary hepatic expression of a single gene is missing without major parenchymal damage. Its application in metabolic diseases has been far less successful where only partial correction of metabolic disorders has been achieved. In acute liver failure, hepatocyte transplantation has not been able to eliminate the need for OLT.
Fig. 2.
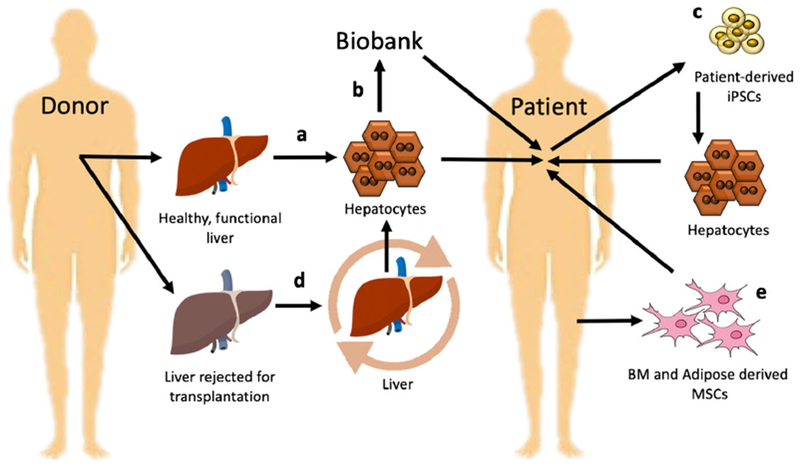
Transplantation of human hepatic cells (a). Establishment of banked cryopreserved hepatocytes for long-term storage would be of great benefit, allowing on-demand usage for emergency cases (b). iPSCs can be considered as a promising solution since they have advantages such as unlimited self-renewal potential (c). To increase the efficiency of hepatocytes isolation from discarded livers, machine perfusion methods were developed (d). Mesenchymal stem cells (MSC) seem to be a valuable substitute for human primary hepatocytes (e)
Pitfalls and Hurdles to Overcome in Liver Cell Therapies
There are several factors affecting the success of cell transplantation for liver regeneration. One of these factors is the availability of a high-quality and functional cell source. Currently, the primary sources for hepatocyte transplantation remain livers that have been rejected for OLT, surplus tissue resulting from reduced graft transplants and tissue resulting from benign tumor resection procedures [45] To increase the pool of available human hepatocytes, Uygun et al. developed a new method based on machine perfusion to utilize discarded livers as a hepatocyte cell source [46••]. It was shown that marginal donor livers damaged due to warm ischemia could be treated to yield 39 million viable hepatocytes per gram of liver similar to fresh livers. These hepatocytes had characteristic epithelial morphology but exhibited slightly lower protein synthesis functions, which was attributed to residual endoplasmic reticulum stress (Fig. 2d).
Cryopreservation of hepatocytes is a key to cell therapy for emergency transplantation in patients. Adult liver cells obtained from a single donor can be cryopreserved and used in the future for multiple patients. Establishment of banked cryopreserved hepatocytes for long-term storage would be of great benefit, allowing on-demand usage for emergency cases (Fig. 2b). However, it was shown that even cryopreservation has a considerable effect on the viability and metabolic function of these cells due to cells being subjected to damaging conditions during both freezing and thawing steps [47–50]. To increase cryopreserved cell viability, Jitraruch et al. developed an optimized protocol for cryopreservation of hepatocyte microbeads using modified freezing solutions [47].
The limited availability and inability of in vitro expansion of primary hepatocytes have led the field to search for new cell sources. Mesenchymal stem cells (MSC) seem to be a valuable substitute of human primary hepatocytes because MSCs are widely available, can be easily cultured in vitro, and are less immunogenic compared to hepatocytes [51]. These cells can be harvested from bone marrow, cord blood, adipose tissue, and amniotic fluid [40]. MSC treatment had a positive effect on rats and pigs with acute liver failure after direct or systemic transplantation as few as 5 million cells [52, 53••, 54–58]. In addition, there is evidence that MSCs can also decrease fat accumulation in the liver, preventing pathological steatotic overload after the injury and decrease the frequency of apoptotic events (Fig. 2e) [59, 60].
However, the therapeutic effect of MSCs can be still unpredictable. Gilsanz et al. reported no signs of hepatic regeneration specifically around the site of direct intraparenchymal MSCs injection [61]. Moreover, it was shown that MSC infusion can even accelerate the progress of liver fibrosis via the conversion of MSCs into fibrous scar-produced myofibroblast [62]. This phenomenon was also seen in a model of fibrosis induced by bile duct ligation, engrafted MSCs assumed a myofibroblast-like phenotype, aiding the establishment of ductal fibrosis [63]. To understand the process of fibrosis, the mechanism of MSC therapeutic action on liver failure was investigated [54]. Two possible mechanisms of MSC action were suggested. Huang et al. suggested that direct interplay between MSCs and various immune cells may be the main mechanism by which MSC infusion prevented the death of mice in the first 2 days [60]. MSCs are known to secrete the hepatocyte growth factor (HGF), which decreases stellate cell proliferation and collagen production. Neutralization of HGF partially blocked the inhibitory effect of adult-derived human liver stem cells on the proliferation and secretion profile of hepatic stellate cells [52]. Bone marrow MSCs in the direct co-culture system significantly decreased the viability of activated hepatic stellate cells [64–67]. MSC-conditioned medium inhibited hepatic stellate cell activation and delayed the development of liver fibrosis in animal models [52, 64, 68, 69].
To avoid fibrogenic MSCs features, Huang et al. studied the effect of MSC-conditioned media treatment in a model of fulminant hepatic failure. It was shown that conditioned media reduces activated hepatic stellate cell-derived myofibroblasts, which are considered to be an important contributing factor to liver fibrosis [52]. Liang et al. developed nanoparticles containing MSCs conditioned media coated with red blood cell membrane vesicles. Application of this therapy reduced the levels of proinflammatory cytokines suggesting an anti-inflammatory role of this therapy [70].
Unpredictable therapeutic effect of MSCs and their fibrogenic features has led the field to search for new cell sources such as induced pluripotent stem cells (Fig. 2c). iPSCs can be considered as a promising solution since they have advantages such as unlimited self-renewal potential [71, 72]. Cell reprogramming for the production of autologous hepatocytes potentially allows these therapies to bypass the scarcity of human donor livers, as well as avoid allogeneic rejection [73]. Moreover, their successful differentiation to hepatocyte-like cells has already been documented [33–35]. For example, Nagamoto et al. showed that iPSC-derived hepatocytes significantly increased albumin production and ameliorated lethal acute liver injury induced by the infusion of carbon tetrachloride [68]. However, as we mentioned before, there are still concerns regarding the safety and immunogenicity of iPSCs. Zhao et al. showed that iPSCs can have immunogenic features and the immunogenicity of therapeutically valuable cells derived from patient-specific iPSCs should be evaluated before any clinical application of these autologous cells into the patients [53••].
Another hurdle to overcome in cell therapy for liver regeneration is the low cell engraftment and survival post-transplantation [74]. Babaei et al. showed, for example, that even direct injection of bone marrow-derived aggregates into acute damaged liver did not show long-lasting incorporation of the bone marrow cells [75]. Moreover, allogenic hepatocyte transplantation should be still followed by an immunosuppressive therapy used for OLT [44, 74, 76]. To overcome these limitations, cells can be transplanted within a biodegradable scaffold, such as microcapsules and microbeads, to provide a microenvironment suitable for cell attachment and also protect cells from host’s immune system [47, 77]. The fact that hepatocytes are typically harvested from livers not suitable for transplantation makes quantity and quality of cells obtained highly variable [78]. Machaidze et al. tried to overcome these challenges using microencapsulated swine hepatocytes transplanted intraperitoneally in a baboon model of the fulminant liver. However, the results were not statistically significant; authors propose microencapsulated swine hepatocytes as a cell therapy product for temporary liver function support in patients with ALF because of the essentially unlimited donor liver supply. Jitraruch et al. utilized encapsulation of hepatocytes in alginate microbeads to protect the cells from host’s immune system, but also to support cell function in vivo aiming the problem of low rates of in vivo survival of transplanted hepatocytes [47, 79]. Similarly, Song et al. demonstrated successful intraperitoneal engraftment of iPSC-derived hepatocyte-like cells encapsulation in hydrogel capsules [80].
Conclusion
While cell-based bioengineered therapies for liver regeneration hold great promise to provide alternatives to orthotopic liver transplantation, cell-only therapies suffer several limitations. The ideal cell source to provide high-quality and functional cells and the route of delivery for optimal engraftment and long-term survival are the major factors to be determined for successful clinical application. The recent advances in bioengineering approaches including cell preservation, microencapsulation, liver tissue fabrication all merge to yield innovative solutions as liver replacement therapies.
Footnotes
This article is part of the Topical Collection on Cellular Transplants
Conflict of Interest Basak Uygun reports grants from National Institutes of Health (R01DK084053) and has a financial interest in Organ Solutions, LLC (reviewed and arranged by MGH and Partners HealthCare in accordance with their conflict of interest policies).
Aylin Acun and Ruben Oganesyan declare no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12(145). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ware BR, Khetani SR. Engineered liver platforms for different phases of drug development. Trends Biotechnol. 2017;35:172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Y, et al. Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip. Lab Chip. 2017;17:782–94. [DOI] [PubMed] [Google Scholar]
- 5.Mi S, Yi X, Du Z, Xu Y, Sun W. Construction of a liver sinusoid based on the laminar flow on chip and self-assembly of endothelial cells. Biofabrication. 2018;10:025010. [DOI] [PubMed] [Google Scholar]
- 6.Wu Q, et al. Establishment of an ex vivo model of nonalcoholic fatty liver disease using a tissue-engineered liver. ACS Biomater SciEng. 2018;4:3016–26. [DOI] [PubMed] [Google Scholar]
- 7.Griffith LG, Wells A, Stolz DB. Engineering Liver. Hepatol Baltim Md. 2014;60:1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazza G, Al-Akkad W, Rombouts K, Pinzani M. Liver tissue engineering: from implantable tissue to whole organ engineering. Hepatol Commun. 2017;2:131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–6. [DOI] [PubMed] [Google Scholar]
- 10.Gonfiotti A, Jaus MO, Barale D, Baiguera S, Comin C, Lavorini F, et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet Lond Engl. 2014;383:238–44. [DOI] [PubMed] [Google Scholar]
- 11.Shafiee A, Atala A. Tissue engineering: toward a new era of medicine. Annu Rev Med. 2017;68:29–40. [DOI] [PubMed] [Google Scholar]
- 12.Mohanty S, Sanger K, Heiskanen A, Trifol J, Szabo P, Dufva M, et al. Fabrication of scalable tissue engineering scaffolds with dualpore microarchitecture by combining 3D printing and particle leaching. Mater Sci Eng C. 2016;61:180–9. [DOI] [PubMed] [Google Scholar]
- 13.Lee JW, Choi YJ, Yong WJ, Pati F, Shim JH, Kang KS, et al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication. 2016;8:015007. [DOI] [PubMed] [Google Scholar]
- 14.Kang K, Kim Y, Jeon H, Lee SB, Kim JS, Park SA, et al. Three-dimensional bioprinting of hepatic structures with directly converted hepatocyte-like cells. Tissue Eng Part A. 2018;24:576–83. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Han W, Kim H, Ha DH, Jang J, Kim BS, et al. Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules. 2017;18:1229–37. [DOI] [PubMed] [Google Scholar]
- 16.Stevens KR, Scull MA, Ramanan V, Fortin CL, Chaturvedi RR, Knouse KA, et al. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci Transl Med. 2017;9:eaah5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis PL, Su J, Yan M, Meng F, Glaser SS, Alpini GD, et al. Complex bile duct network formation within liver decellularized extracellular matrix hydrogels. Sci Rep. 2018;8:12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. [DOI] [PubMed] [Google Scholar]
- 19.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, et al. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 2012;173:e11–25. [DOI] [PubMed] [Google Scholar]
- 21.Lang R, Stern MM, Smith L, Liu Y, Bharadwaj S, Liu G, et al. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials. 2011;32:7042–52. [DOI] [PubMed] [Google Scholar]
- 22.Kajbafzadeh A-M, Javan-Farazmand N, Monajemzadeh M, Baghayee A. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human-sized liver tissue. Tissue Eng Part C Methods. 2013;19:642–51. [DOI] [PubMed] [Google Scholar]
- 23.Mazza G, Rombouts K, Rennie Hall A, Urbani L, Vinh Luong T, al-Akkad W, et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep. 2015;5: 13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson MJ, Soibam B, O’Leary JG, Sampaio LC, Taylor DA. Recellularization of rat liver: an in vitro model for assessing human drug metabolism and liver biology. PLoS One. 2018;13:e0191892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogiso S, Yasuchika K, Fukumitsu K, Ishii T, Kojima H, Miyauchi Y, et al. Efficient recellularisation of decellularised whole-liver grafts using biliary tree and foetal hepatocytes. Sci Rep. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima H, Yasuchika K, Fukumitsu K, Ishii T, Ogiso S, Miyauchi Y, et al. Establishment of practical recellularized liver graft for blood perfusion using primary rat hepatocytes and liver sinusoidal endothelial cells. Am J Transplant. 2018;18:1351–9. [DOI] [PubMed] [Google Scholar]
- 27.•.Devalliere J, Chen Y, Dooley K, Yarmush ML, Uygun BE. Improving functional re-endothelialization of acellular liver scaffold using REDV cell-binding domain. Acta Biomater. 2018;78: 151–64 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows a novel method of functionalizing the decellularized liver grafts for higher endothelizalization efficiency, rendering such scaffolds applicable for transplantation.
- 28.••.A Mao S Sustained in vivo perfusion of a re-endothelialized tissue engineered porcine liver. Int J Transplant Res Med. 2017;3 [Google Scholar]; This study shows 72 hour-long in vivo perfusion of decellularized porcine livers after endothelialization, in the absence of anticoagulants.
- 29.Yang W, Chen Q, Xia R, Zhang Y, Shuai L, Lai J, et al. A novel bioscaffold with naturally-occurring extracellular matrix promotes hepatocyte survival and vessel patency in mouse models of heterologous transplantation. Biomaterials. 2018;177:52–66. [DOI] [PubMed] [Google Scholar]
- 30.Hussein KH, Park K-M, Kang K-S, Woo H-M. Biocompatibility evaluation oftissue-engineered decellularized scaffolds for biomedical application. Mater Sci Eng C. 2016;67:766–78. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, et al. Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Sci Rep. 2016;6(24779). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verstegen MMA, Willemse J, van den Hoek S, Kremers GJ, Luider TM, van Huizen NA, et al. Decellularization of whole human liver grafts using controlled perfusion for transplantable organ bioscaffolds. Stem Cells Dev. 2017;26:1304–15. [DOI] [PubMed] [Google Scholar]
- 33.Hannan NRF, Segeritz C-P, Touboul T, Vallier L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc. 2013;8:430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siller R, Greenhough S, Naumovska E, Sullivan GJ. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Rep. 2015;4:939–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanninen LK, Harjumäki R, Peltoniemi P, Bogacheva MS, Salmi T, Porola P, et al. Laminin-511 and laminin-521-based matrices for efficient hepatic specification of human pluripotent stem cells. Biomaterials. 2016;103:86–100. [DOI] [PubMed] [Google Scholar]
- 36.Park K-M, Hussein KH, Hong SH, Ahn C, Yang SR, Park SM, et al. Decellularized liver extracellular matrix as promising tools for transplantable bioengineered liver promotes hepatic lineage commitments of induced pluripotent stem cells. Tissue Eng Part A. 2016;22:449–60. [DOI] [PubMed] [Google Scholar]
- 37.Jaramillo M, Yeh H, Yarmush ML, Uygun BE. Decellularized human liver extracellular matrix (hDLM)-mediated hepatic differentiation of human induced pluripotent stem cells (hIPSCs). J Tissue Eng Regen Med. 2018;12:e1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palakkan AA, Nanda J, Ross JA. Pluripotent stem cells to hepatocytes, the journey so far. Biomed Rep. 2017;6:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattei G, Magliaro C, Pirone A, Ahluwalia A. Decellularized human liver is too heterogeneous for designing a generic extracellular matrix mimic hepatic scaffold. Artif Organs. 2017;41:E347–55. [DOI] [PubMed] [Google Scholar]
- 40.Tolosa L, Pareja E, Gómez-Lechón MJ. Clinical application of pluripotent stem cells: an alternative cell-based therapy for treating liver diseases? Transplantation. 2016;100:2548–57. [DOI] [PubMed] [Google Scholar]
- 41.Fox I Hepatocyte transplantation. J Hepatol. 2004;40:878–86. [DOI] [PubMed] [Google Scholar]
- 42.Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63: 559–69. [DOI] [PubMed] [Google Scholar]
- 43.Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–9. [DOI] [PubMed] [Google Scholar]
- 44.Puppi J, Strom SC, Hughes RD, Bansal S, Castell JV, Dagher I, et al. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant. 2012;21:1–10. [DOI] [PubMed] [Google Scholar]
- 45.Horner R, Kluge M, Gassner J, Nosser M, Major RD, Reutzel-Selke A, et al. Hepatocyte isolation after laparoscopic liver resection. Tissue Eng Part C Methods. 2016;22:839–46. [DOI] [PubMed] [Google Scholar]
- 46.••.Uygun BE, Izamis ML, Jaramillo M, Chen Y, Price G, Ozer S, et al. Discarded livers find a new life: engineered liver grafts using hepatocytes recovered from marginal livers. Artif Organs. 2017;41:579–85 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a method of increasing the pool of available human hepatocytes, the most thoroughly tested cells for liver function replacement.
- 47.Jitraruch S, Dhawan A, Hughes RD, Filippi C, Lehec SC, Glover L, et al. Cryopreservation of hepatocyte microbeads for clinical transplantation. Cell Transplant. 2017;26:1341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gramignoli R, Vosough M, Kannisto K, Srinivasan RC, Strom SC. Clinical hepatocyte transplantation: practical limits and possible solutions. Eur Surg Res Eur Chir Forsch Rech Chir Eur. 2015;54: 162–77. [DOI] [PubMed] [Google Scholar]
- 49.Terry C, Dhawan A, Mitry RR, Lehec SC, Hughes RD. Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2010;16:229–37. [DOI] [PubMed] [Google Scholar]
- 50.Tolosa L, Bonora-Centelles A, Donato MT, Mirabet V, Pareja E, Negro A, et al. Influence of platelet lysate on the recovery and metabolic performance of cryopreserved human hepatocytes upon thawing. Transplantation. 2011;91:1340–6. [DOI] [PubMed] [Google Scholar]
- 51.Yoshizumi Y, Yukawa H, Iwaki R, Fujinaka S, Kanou A, Kanou Y, et al. Immunomodulatory effects of adipose tissue-derived stem cells on Concanavalin A-induced acute liver injury in mice. Cell Med. 2017;9:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Najimi M, Berardis S, el-Kehdy H, Rosseels V, Evraerts J, Lombard C, et al. Human liver mesenchymal stem/progenitor cells inhibit hepatic stellate cell activation: in vitro and in vivo evaluation. Stem Cell Res Ther. 2017;8:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.••.Zhao T, Zhang Z-N, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–5 [DOI] [PubMed] [Google Scholar]; It was shown that iPSCs have immunogenic features and the immunogenicity of therapeutically of these cells should be evaluated before any clinical application.
- 54.Deng L, Kong X, Liu G, Li C, Chen H, Hong Z, et al. Transplantation of adipose-derived mesenchymal stem cells efficiently rescues thioacetamide-induced acute liver failure in mice. Transplant Proc. 2016;48:2208–15. [DOI] [PubMed] [Google Scholar]
- 55.Shi D, Zhang J, Zhou Q, Xin J, Jiang J, Jiang L, et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut. 2017;66:955–64. [DOI] [PubMed] [Google Scholar]
- 56.LIU T, MU H, SHEN Z, SONG Z, CHEN X, WANG Y. Autologous adipose tissue-derived mesenchymal stem cells are involved in rat liver regeneration following repeat partial hepatectomy. Mol Med Rep. 2016;13:2053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tautenhahn H-M, Bruckner S, Uder C, Erler S, Hempel M, von Bergen M, et al. Mesenchymal stem cells correct haemodynamic dysfunction associated with liver injury after extended resection in a pig model. Sci Rep. 2017;7:2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao N, et al. Adipose tissue-derived stem cells promote the reversion of non-alcoholic fatty liver disease: an in vivo study. Int J Mol Med. 2016;37:1389–96. [DOI] [PubMed] [Google Scholar]
- 59.Tautenhahn H-M, Brückner S, Baumann S, Winkler S, Otto W, von Bergen M, et al. Attenuation of postoperative acute liver failure by mesenchymal stem cell treatment due to metabolic implications. Ann Surg. 2016;263:546–56. [DOI] [PubMed] [Google Scholar]
- 60.Huang B, Cheng X, Wang H, Huang W, la Ga hu Z, Wang D, et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med. 2016;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilsanz C, Aller MA, Fuentes-Julian S, Prieto I, Blázquez-Martinez A, Argudo S, et al. Adipose-derived mesenchymal stem cells slow disease progression of acute-on-chronic liver failure. Biomed Pharmacother. 2017;91:776–87. [DOI] [PubMed] [Google Scholar]
- 62.Kaur S, Siddiqui H, Bhat MH. Hepatic progenitor cells in action: liver regeneration or fibrosis? Am J Pathol. 2015;185:2342–50. [DOI] [PubMed] [Google Scholar]
- 63.Asawa S, Saito T, Satoh A, Ohtake K, Tsuchiya T, Okada H, et al. Participation of bone marrow cells in biliary fibrosis after bile duct ligation. J Gastroenterol Hepatol Aust. 2007;22:2001–8. [DOI] [PubMed] [Google Scholar]
- 64.Jang YO, Jun BG, Baik SK, Kim MY, Kwon SO. Inhibition of hepatic stellate cells by bone marrow-derived mesenchymal stem cells in hepatic fibrosis. Clin Mol Hepatol. 2015;21:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu F, Ji S, Su L, Wan L, Zhang S, Dai C, et al. Adipose-derived mesenchymal stem cells inhibit activation of hepatic stellate cells in vitro and ameliorate rat liver fibrosis in vivo. J Formos Med Assoc. 2015;114:130–8. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L-T, et al. Bone marrow-derived mesenchymal stem cells inhibit the proliferation of hepatic stellate cells by inhibiting the transforming growth factor β pathway Mol Med Rep. 2015;12: 7227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang W-P, Akahoshi T, Piao JS, Narahara S, Murata M, Kawano T, et al. Basic fibroblast growth factor-treated adipose tissue-derived mesenchymal stem cell infusion to ameliorate liver cirrhosis via paracrine hepatocyte growth factor: ADSCs relieve liver cirrhosis. J Gastroenterol Hepatol. 2015;30:1065–74. [DOI] [PubMed] [Google Scholar]
- 68.Nagamoto Y, Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M, et al. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64:1068–75. [DOI] [PubMed] [Google Scholar]
- 69.Kubo K, Ohnishi S, Hosono H, Fukai M, Kameya A, Higashi R, et al. Human amnion-derived mesenchymal stem cell transplantation ameliorates liver fibrosis in rats. Transplant Direct. 2015;1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang H, Huang K, Su T, Li Z, Hu S, Dinh PU, et al. Mesenchymal stem cell/red blood cell-inspired nanoparticle therapy in mice with carbon tetrachloride-induced acute liver failure. ACS Nano. 2018;12:6536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. [DOI] [PubMed] [Google Scholar]
- 73.Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells Dayt Ohio. 2008;26:1117–27. [DOI] [PubMed] [Google Scholar]
- 74.Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7:288–98. [DOI] [PubMed] [Google Scholar]
- 75.Babaei A, Katoonizadeh A, Ranjbar A, Naderi M, Ahmadbeigi N. Directly injected native bone-marrow stem cells cannot incorporate into acetaminophen-induced liver injury. Biologicals. 2018;52:55–8. [DOI] [PubMed] [Google Scholar]
- 76.Ribes-Koninckx C, Ibars EP, Agrasot MÁC, Bonora-Centelles A, Miquel BP, Carbó JJV, et al. Clinical outcome of hepatocyte transplantation in four pediatric patients with inherited metabolic diseases. Cell Transplant. 2012;21:2267–82. [DOI] [PubMed] [Google Scholar]
- 77.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2:205–13. [DOI] [PubMed] [Google Scholar]
- 78.Nicolas CT, Hickey RD, Chen HS, Mao SA, Lopera Higuita M, Wang Y, et al. Concise review: liver regenerative medicine: from hepatocyte transplantation to bioartificial livers and bioengineered grafts: liver regenerative medicine. Stem Cells. 2017;35:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smets F, Najimi M, Sokal EM. Cell transplantation in the treatment of liver diseases. Pediatr Transplant. 2008;12:6–13. [DOI] [PubMed] [Google Scholar]
- 80.Song W, Lu YC, Frankel AS, An D, Schwartz RE, Ma M. Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation. Sci Rep. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]


