Abstract
Tetraspanins co-emerged with multi-cellular organisms during evolution are typically localized at the cell–cell interface, and form tetraspanin-enriched microdomains (TEMs) by associating with each other and other membrane molecules. Tetraspanins affect various biological functions, but how tetraspanins engage in multi-faceted functions at the cellular level is largely unknown. When cells interact, the membrane microextrusions at the cell–cell interfaces form dynamic, digit-like structures between cells, which we term digitation junctions (DJs). We found that (1) tetraspanins CD9, CD81, and CD82 and (2) TEM-associated molecules integrin α3β1, CD44, EWI2/PGRL, and PI-4P are present in DJs of epithelial, endothelial, and cancer cells. Tetraspanins and their associated molecules also regulate the formation and development of DJs. Moreover, (1) actin cytoskeleton, RhoA, and actomyosin activities and (2) growth factor receptor-Src-MAP kinase signaling, but not PI-3 kinase, regulate DJs. Finally, we showed that DJs consist of various forms in different cells. Thus, DJs are common, interactive structures between cells, and likely affect cell adhesion, migration, and communication. TEMs probably modulate various cell functions through DJs. Our findings highlight that DJ morphogenesis reflects the transition between cell–matrix adhesion and cell–cell adhesion and involves both cell–cell and cell–matrix adhesion molecules.
Electronic supplementary material
The online version of this article (10.1007/s00018-018-2803-2) contains supplementary material, which is available to authorized users.
Keywords: Cell–cell adhesion, Cell–cell communication, Microextrusion, Nanotubule, Tetraspanin, Integrin
Introduction
The superfamily of tetraspanins encompasses the type III proteins containing four transmembrane domains, a fully conserved CCG motif in the large extracellular loop, and three largely conserved membrane-embedded polar residues [1–6]. Tetraspanins are considered as molecular facilitators or scaffold proteins by modulating the activities of their associated molecules [3–6]. Tetraspanins engage various cellular functions [1–6]. Many of these functions are attributed to the interactions between cell membranes. For example, tetraspanins regulate cell–cell adhesion, cell–cell fusion, and pathogen–cell interaction [1–6], all of which involve the direct contact between cell membranes. Tetraspanins also regulate cell migration and cell proliferation, both of which can also be altered by the interactions between cell membranes. Evolutionarily, tetraspanins are found only in multi-cellular organisms [7, 8]. Moreover, tetraspanins are typically expressed at the cell surface and physically associate with cell–cell adhesion molecules, such as IgSF proteins [1–6]. Hence, tetraspanins likely modulate intercellular interactions.
In eukaryotes, cell–cell interaction leads to the formation of cell junctions, such as tight and septate junctions, adherens junctions, gap and plasmodesmata junctions, and desmosomes. Recent studies revealed that, when cells come in contact with each other, they generate membrane tubular structures (memtubs) or microextrusions to form adhesion zippers [9–15]. We demonstrated that tetraspanins regulate the morphogenesis of microextrusions [14, 15]. Adhesion zippers or digitation junctions (DJs) can form when microextrusions are positioned between cells. Earlier studies reported that tetraspanins CD9, CD81, and CD151 are localized in the adhesion zippers of NIH3T3 murine fibroblast cells, Du145 human metastatic prostate cancer cells, and immortalized human microvascular endothelial cells (HMECs) [12–14, 16]. In addition, CD151 overexpression or silencing and CD9 antibody (Ab) treatment regulate the adhesion zipper morphogenesis in epithelial, epithelial-like, and immortalized endothelial cells [12, 13, 16, 17].
Because the fundamental mechanism by which tetraspanins engage various cellular functions is unclear, we predict that modulating the memtubs or microextrusions between cells, which we designate as DJ, is an important and common cellular mechanism for tetraspanins. We herein explored the mechanistic roles of tetraspanin-enriched microdomains (TEMs), i.e., tetraspanins and tetraspanin-associated molecules, in regulating DJ morphogenesis. We concluded that TEMs modulate not only cell–cell adhesion, but also cell–cell communication, through DJs.
Results
Presence of tetraspanins and their associated cell adhesion proteins in DJs
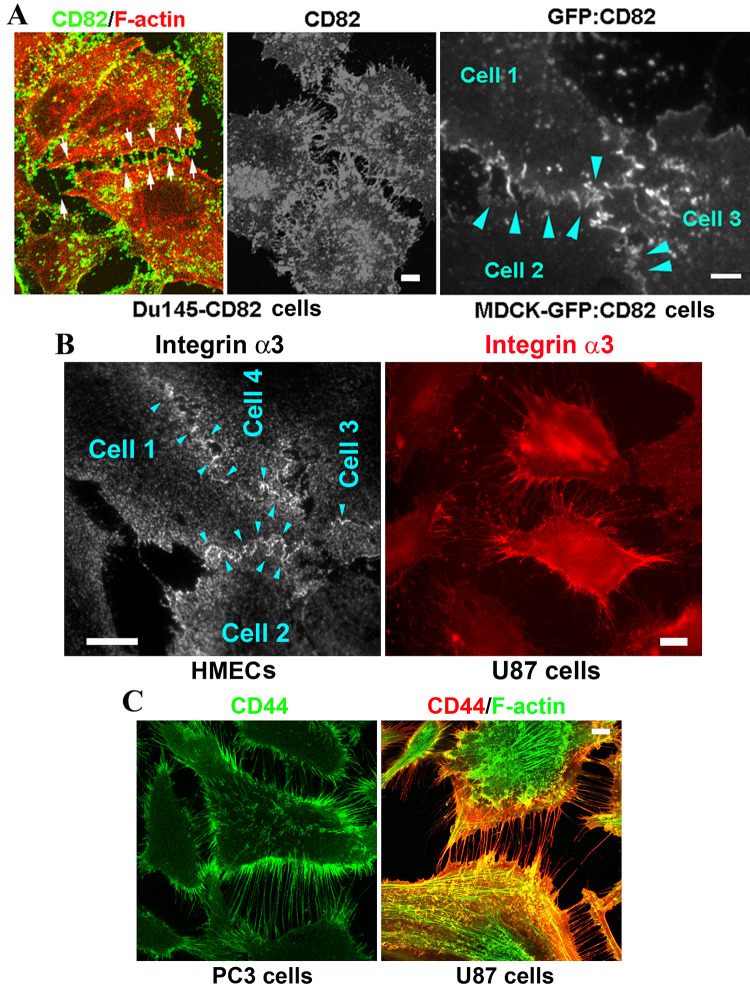
We found that tetraspanins are localized at DJs, i.e., the microextrusions formed between cells. As revealed by confocal microscopy, CD82 proteins were present in the DJs of Du145 cells with forced expression of CD82 (Fig. 1a, left panel). Du145 cells do not express endogenous CD82 [18]. Arrows indicate examples of DJs. Total internal reflection fluorescence (TIRF) microscopy revealed the presence of CD82 in the basal DJs of CD82 transfected Du145 cells (middle panel). CD82 proteins were also found in the DJs of MDCK epithelial cells that express GFP-CD82 (Fig. 1a, right panel, arrowheads). Together with our earlier observations that CD9 and CD81 are present at DJs of NIH3T3 cells and Du145 cells, respectively [14], we conclude that tetraspanin involvement in DJ morphogenesis is likely to be a general mechanism for tetraspanins to modulate cell–cell interactions. Consistent with this notion, CD9 proteins are also localized in the DJ formed between active platelets in platelet aggregates (Dr. Lisa Jennings, personal communication).
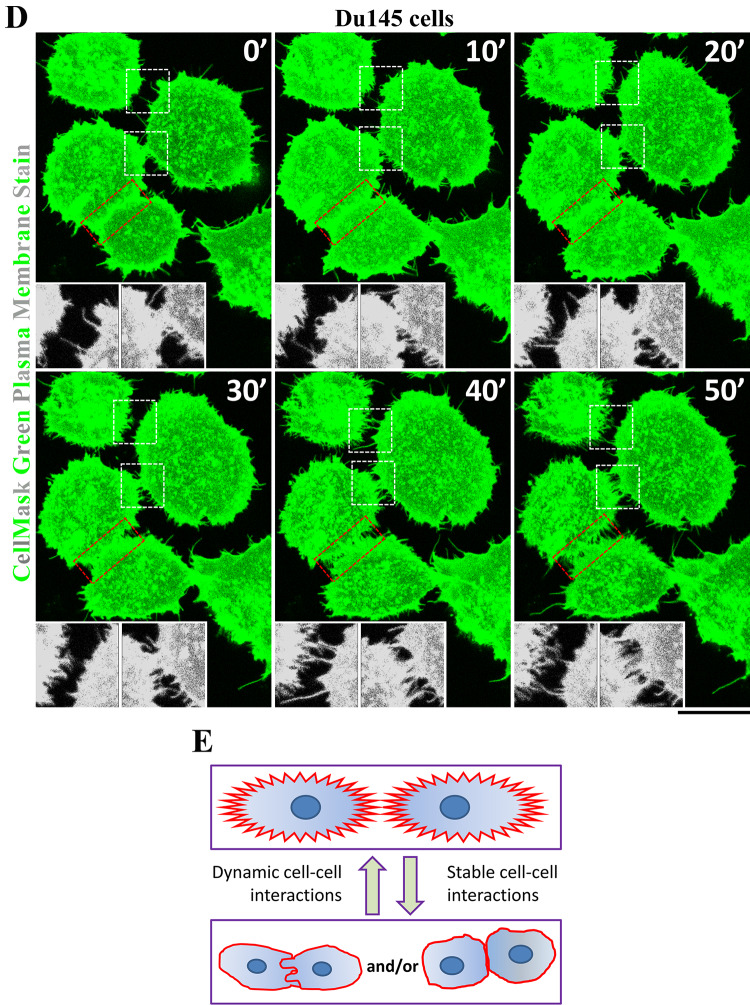
Fig. 1.
Presence of tetraspanins and their associated proteins in digitation junctions (DJs). a CD82 is present in the DJs formed between the Du145 cells overexpressing CD82 and between the MDCK cells expressing GFP-CD82. Shown are confocal microscopic images of Du145-CD82 cells and TIRF microscopic images of Du145-CD82 and MDCK-GFP-CD82 cells. Scale bars 5 μm. b Integrin α3β1 is present in the DJs formed between endothelial cells and between tumor cells. HMECs and U87 cells were stained with integrin α3 mAb, which highlights DJs, and imaged with TIRF and fluorescent microscopy, respectively. Scale bars 15 μm. c CD44 is present in the DJs formed between PC3 cells and between U87 cells. Immunofluorescence images for CD44 and actin fiber were acquired by confocal fluorescent microscopy. Scale bar 5 μm. d Time-lapse imaging analysis of DJ dynamics between Du145 cells, of which the plasma membranes were stained with CellMask green dye (see “Materials and methods” for details). Insets present the magnified views of white sketched areas. Scale bar 10 μm. e Schema of dynamic changes of DJs
We then analyzed tetraspanin-associated proteins. Integrin α3β1 is a major integrin in TEMs [5]. Using TIRF microscopy, we found that integrin α3β1 proteins were localized at the DJs formed at/near the cell–substratum interface between HMECs (example indicated by arrowheads, Fig. 1b), and the seemingly nascent DJs formed between U87 human glioblastoma cells (Fig. 1b). CD44, another tetraspanin-associated cell adhesion protein, also existed in the possibly nascent DJs formed between PC3 human metastatic prostate cancer cells and U87 cells (Fig. 1c). These observations indicate that tetraspanins and tetraspanin-associated proteins are present in DJs.
Live imaging analysis on Du145 cells revealed that (1) microextrusions between adjacent cells actively engaged with each other and dynamically formed DJs and (2) DJs constantly underwent reorganization (white sketch boxes and insets, Fig. 1d). DJs also appeared when two adjacent cells with a close body–body contact became dissociated (red sketch boxes, Fig. 1d). Based on the different morphologies of microextrusions in DJs, our observations also indicate the existence of different types of DJs and/or DJs at different morphogenic stages (Fig. 1e). It appears that DJs formed by invasive tumor cells and fibroblasts keep the cells from forming close contacts or do not undergo transformation, while DJs formed by epithelial and endothelial cells can transform or evolve to other cell–cell junctions.
Tetraspanins and their associated cell adhesion proteins regulate DJ formation and development
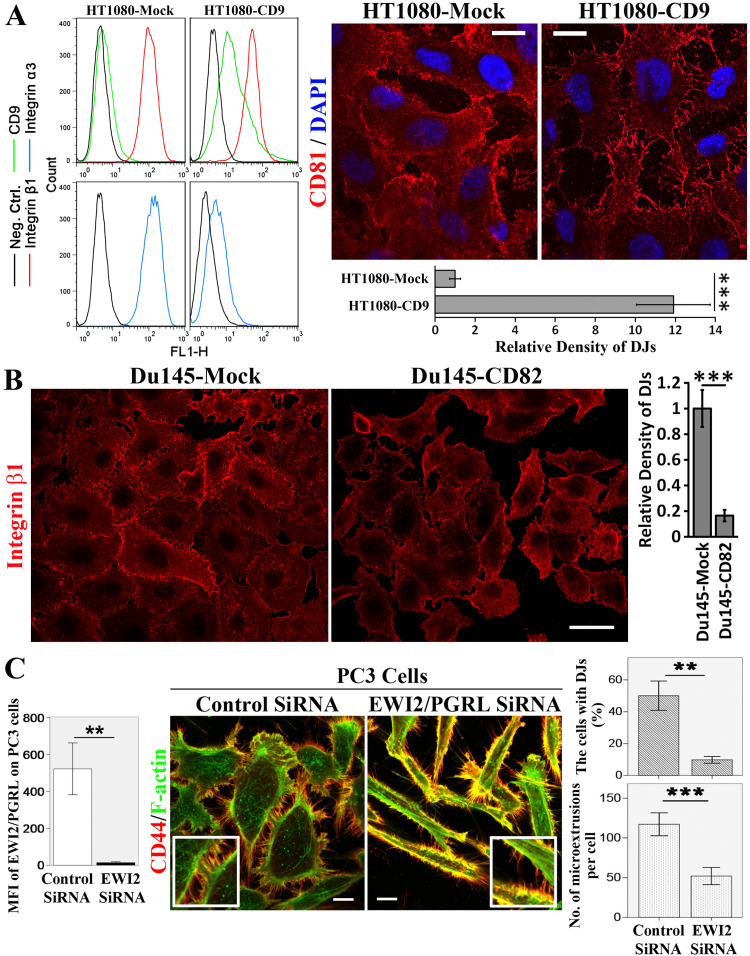
Our earlier study demonstrated that tetraspanin CD151 is needed for the proper formation and maturation of DJs in HMECs [13]. In the present study, we substantiated our previous finding by showing that CD9 overexpression in HT1080 fibrosarcoma cells promoted DJ formation (Fig. 2a), while CD82 overexpression inhibited DJ morphogenesis in Du145 cells (Fig. 2b). Notably, CD9 overexpression reduced the surface level of integrin α3β1 on HT1080 cells (Fig. 2a) and total cellular level of integrin α3β1 from HT1080 cells (our unpublished data), suggesting that integrin α3β1 is not required for DJ formation and implying that CD9 facilitates endosomal trafficking of integrin α3β1 leading to either lysosomal degradation or exosomal release of α3β1. These findings indicate that the regulation of DJ morphogenesis is not attributed to a specific tetraspanin and further suggest that the regulation of DJ morphogenesis is a general property of tetraspanins.
Fig. 2.
Tetraspanins and their associated proteins regulate the formation and/or development of DJs. a CD9 promotes DJs. HT1080-Mock and -CD9 transfectant cells were examined for the surface expression levels of CD9, integrin β1, and integrin α3 with flow cytometry as described elsewhere [14]. For immunofluorescence, the cells were cultured on glass coverslips for 2 days, fixed, stained with CD81 mAb, and imaged with fluorescence microscopy. Scale bar 10 µm. DJs were quantified as relative density of the microextrusions within DJs (mean ± SE, n = 3 individual experiments). ***P < 0.001. b CD82 overexpression inhibits the formation of DJs. Du145-Mock and -CD82 stable transfectant cells were cultured on glass coverslips for 2 days, fixed, stained with integrin β1 mAb, and imaged with immunofluorescence microscopy. Scale bar 20 µm. Quantitative analysis of the relative density of microextrusions within DJs (mean ± SE, n = 3 individual experiments). ***P < 0.001. c IgSF protein EWI2/PGRL silencing inhibits DJ formation. PC3 cells were transfected with control or EWI2/PGRL siRNA, fixed, and incubated sequentially with CD44 mAb and secondary Ab. The images were acquired with confocal microscopy. Scale bar 10 μm. The formation of DJs was quantified by counting the percentage of cells that form DJs, and the formation of microextrusions was quantified by counting the number of microextrusions per cells (mean ± SD, n = 3 independent experiments, approximately 30 cells were analyzed in each experiment). **P < 0.01; ***P < 0.001. The cell surface expression levels of EWI2/PGRL were examined with flow cytometry and presented as MFI in the histograms on the left
In addition to tetraspanins, we found that EWI2/PGRL, a tetraspanin-associated IgSF protein [19–21], also regulates DJ morphogenesis in PC3 cells. As shown in Fig. 2c, CD44 staining revealed significantly less DJ formation in the PC3 cells in which EWI2/PGRL was silenced by siRNA, than in the control cells. This observation shows that tetraspanin-associated proteins could also regulate DJ morphogenesis. Since (1) the morphology of PC3 cells underwent profound changes upon EWI2/PGRL silencing and (2) EWI2/PGRL proteins associate with cytoskeleton linker ERM proteins [22], fewer DJs in the EWI2/PGRL-silenced group could be an indirect effect, as a result of global cytoskeletal alteration.
How TEMs regulate DJ morphogenesis: the cytoskeletal and signaling factors important for DJs
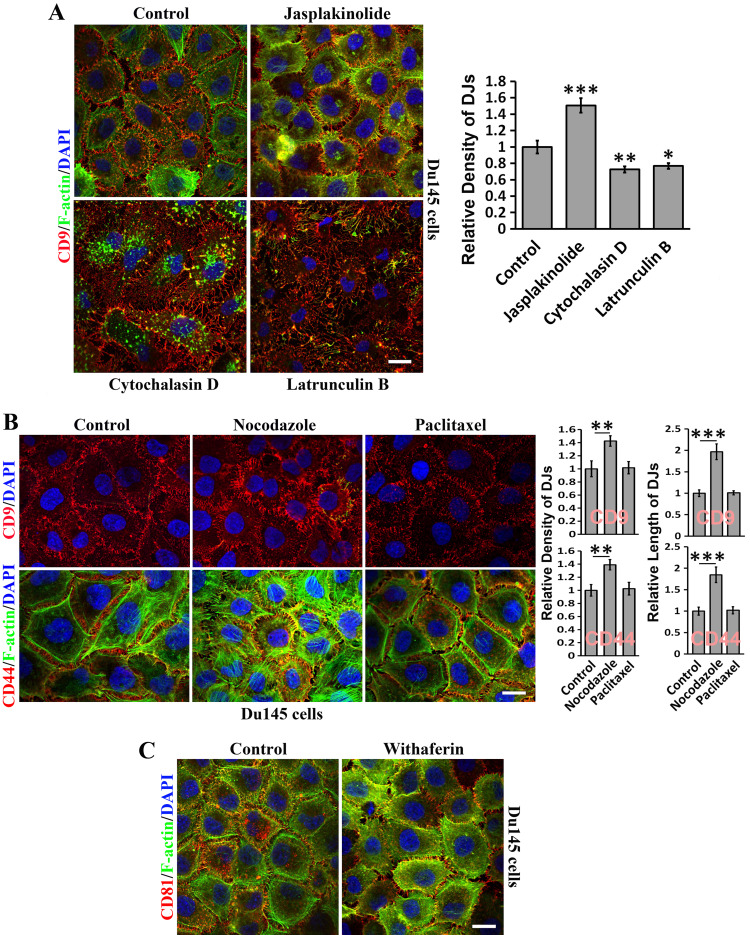
Because (1) actin cytoskeleton is consistently found in the microextrusions of DJs (Figs. 1, 2) and (2) TEMs are linked to actin cytoskeleton [6], we next systematically determined the roles of all three types of cytoskeletons in DJ morphogenesis.
Actin polymerization enhancer jasplakinolide reinforced DJ formation (Fig. 3a). Actin polymerization inhibitors cytochalasin D and latrunculin B largely disrupted the actin cytoskeleton, but memtubs at cell–cell boundaries were still present but fewer, longer, and irregular (Fig. 3a). These observations suggest that actin cytoskeleton supports but is not required for DJ formation. Because cytochalasin prevents actin polymerization and latrunculin sequesters monomeric actin, the more severe disruptive effect accompanying treatment with latrunculin B suggests the dynamic turnover of the actin cytoskeleton in DJs or microextrusions. Alternatively, the largely disrupted DJs that are observed upon latrunculin B treatment could result from cell deformation.
Fig. 3.
How TEMs regulate DJ morphogenesis: roles of cytoskeletons. Du145 cells cultured on glass coverslips were treated with the indicated reagents in serum-free media at 37 °C for 60 or 120 min before they were fixed, permeabilized, incubated with Abs, DAPI, and/or phalloidin, and examined with confocal microscopy. Quantitative analysis of microextrusion density in DJs (mean ± SE, n = 3 individual experiments). *P < 0.05; **P < 0.01; ***P < 0.001. a Role of the actin cytoskeleton. The cells were treated with actin-disrupting reagents latrunculin B (1 µM) and cytochalasin D (2 µg/ml) and actin polymerization-promoting reagent jasplakinolide (0.2 µM). b Role of microtubules. The cells were treated with microtubule-disruptive reagent nocodazole (20 µM) or microtubule-stabilizing reagent paclitaxel (10 nM). c Role of intermediate filaments. The cells were treated with desmin-disruptive reagent withaferin A (5 μM). Scale bars 10 μm
DJs formed between Du145 cells contained longer and more microextrusions upon microtubule disruption by nocodazole, but were largely unchanged after microtubule stabilization with paclitaxel (Fig. 3b). Notably, disruption of microtubules significantly reinforces the actin cytoskeleton. Given that microtubules were not found in microextrusions or DJs (data not shown), the nocodazole effect could be secondary. Disruption of desmin intermediate filaments (IFs) by withaferin A appeared not to inhibit DJs (Fig. 3c).
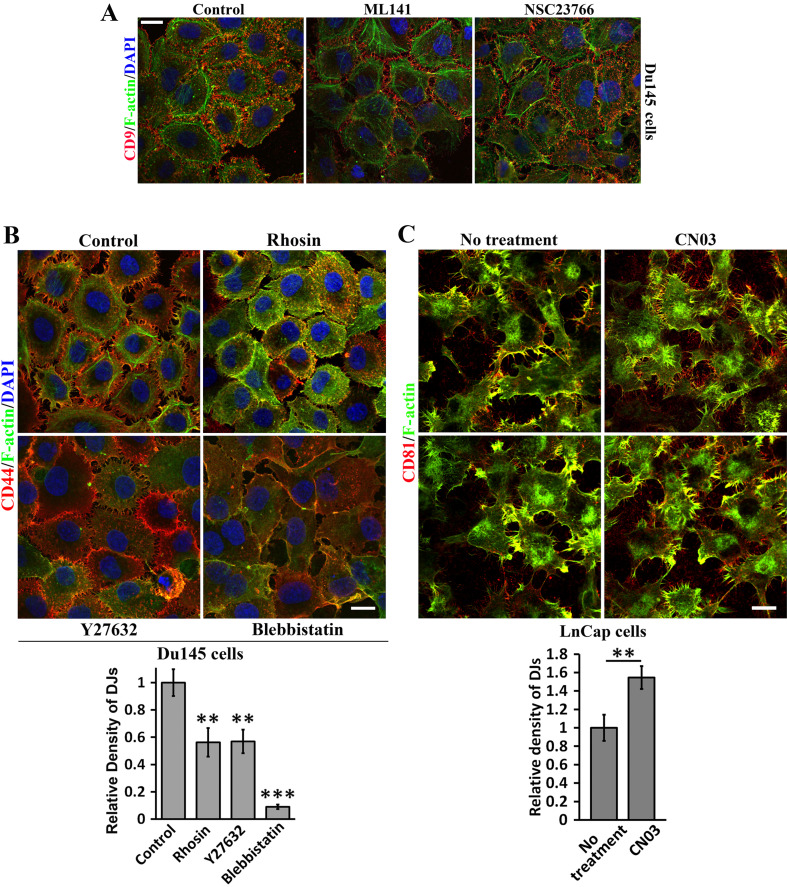
Rho small GTPases are master regulators of actin cytoskeleton. Thus, we analyzed Rho small GTPase signaling in regulating DJ formation. Cdc42 and Rac1 promote filopodia [23] and lamellipodia [24], respectively. DJs formation was not altered in the presence of Cdc42 and Rac1 inhibitors ML141 and NSC23766 (Fig. 4a), but was reduced by RhoA inhibitor Rhosin (Fig. 4b). Consistently, RhoA activator CN03 promoted DJs (Fig. 4c). We further analyzed the effect of actomyosin activity, which is downstream of RhoA signaling, on DJs. Both Rho kinase inhibitor Y27632 and myosin-II inhibitor blebbistatin markedly suppressed DJs (Fig. 4b). These observations underline essential roles of RhoA signaling and actomyosin activity in DJ morphogenesis.
Fig. 4.
How TEMs regulate DJ morphogenesis: signaling of Rho small GTPases. Du145 or LnCap cells cultured on glass coverslips were treated with the indicated reagents in serum-free medium at 37 °C for 60 or 120 min before they were fixed, incubated with Abs, DAPI, and/or phalloidin, and examined with confocal microscopy. Quantitative analysis of microextrusion density in DJs (mean ± SE, n = 3 individual experiments). **P < 0.01; ***P < 0.001. a Roles of Cdc42 and Rac signaling in DJs. Du145 cells cultured on glass coverslips were treated with ML141 (20 µM) and NSC23766 (200 µM) for 60–120 min. b Role of actomyosin signaling. Du145 cells were treated with Rhosin (30 µM), Y27632 (20 µM), or blebbistatin (30 µM) for 60–120 min. c Role of RhoA signaling in DJs. LnCap cells cultured on glass coverslips were treated with RhoA activator CN03 in serum-free medium for 2 h. Scale bars 10 μm
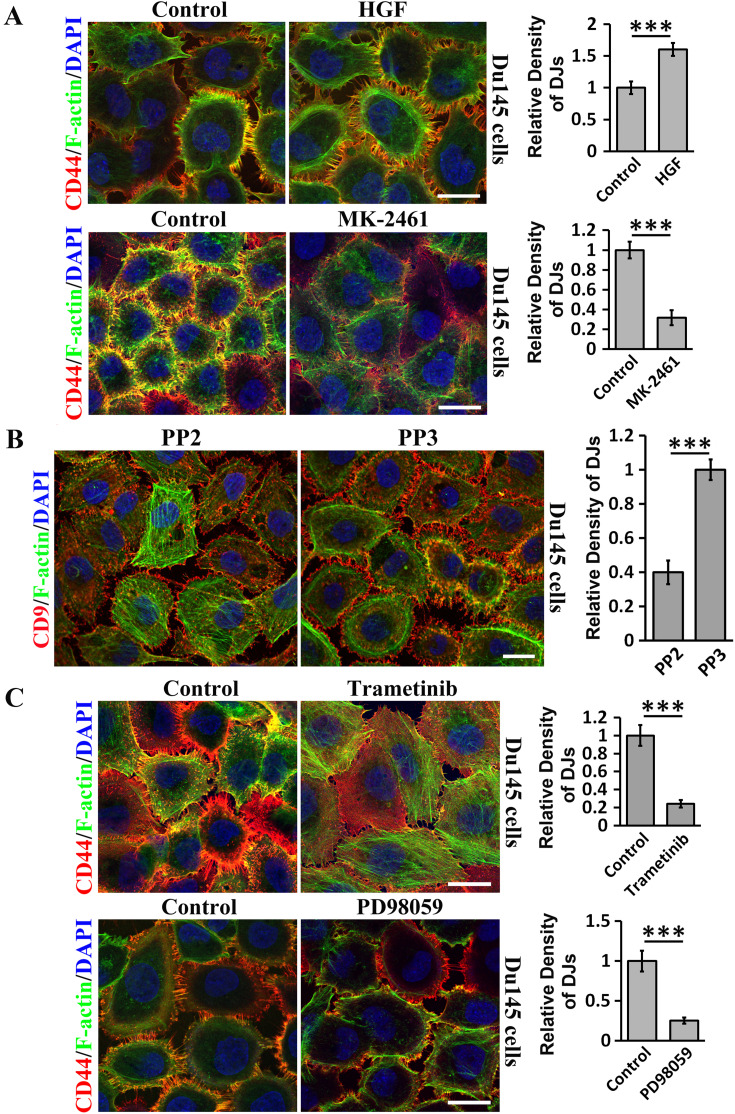
We further analyzed the signaling events controlling DJ morphogenesis. First, we examined the growth factor-receptor tyrosine kinase signaling and found that hepatocyte growth factor (HGF) promoted, while MK-2461, an inhibitor of HGF receptor c-Met, reduced DJ formation in Du145 cells (Fig. 5a). Similar effects were also observed in LnCap cells (Figure S1A). Second, given the importance of Src in growth factor-receptor tyrosine kinase signaling, we then examined Src and found that Src kinase inhibitor PP2, but not its control PP3, substantially reduced DJs (Fig. 5b). Interestingly, invadopodia-like structures were observed in PP2-treated cells. Third, we analyzed the role of MAP kinase, which situates at further downstream of receptor tyrosine kinase signaling, in DJ morphogenesis and found that MEK inhibitors Trametinib and PD98059 markedly reduced DJs in Du145 cells (Fig. 5c). We also observed similar inhibitory effects in LnCap cells (Figure S1B).
Fig. 5.
How TEMs regulate DJ morphogenesis: contributions of growth factor/receptor tyrosine kinase, and MAP kinase signaling. Du145 cells cultured on glass coverslips were treated with following activator or inhibitor in serum-free media at 37 °C for 60 min, unless otherwise indicated, before they were fixed, incubated with CD9 or CD44 mAb, DAPI and phalloidin, and then examined and imaged with confocal microscopy. a Role of HGF and c-Met signaling in DJs. The cells were treated with either HGF (100 ng/ml) after 6-h serum starvation or MK-2461 (200 nM) without serum starvation. b Role of Src family kinases in DJs. The cells were treated with PP2 (10 µM) and PP3 (10 µM). c Role of MEK signaling in DJs. The cells were treated with Trametinib (200 nM) for 3 h and PD98059 (25 µM). Quantification of microextrusion density in DJs (mean ± SE, n = 3 individual experiments). ***P < 0.001. Scale bars 10 μm
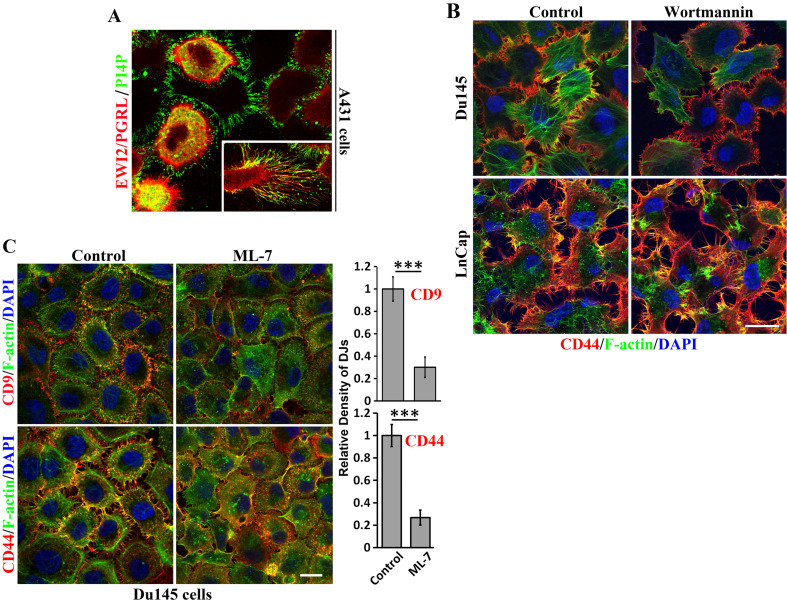
Because PI-4 kinase resides in TEMs [5], we examined PI-4 kinase product PI4P and found that PI4P molecules were localized at DJs in A431 epidermoid cancer cells and colocalized with EWI2/PGRL (Fig. 6a), which, as a TEM constituent, physically interacts with PI4P [25]. The presence of PI4P in basal DJs was observed when the cells were removed during washes in the staining process. This finding suggests that PI-4 kinase signaling is involved in DJ morphogenesis. To our surprise, PI-3 kinase inhibitor wortmannin did not significantly alter DJs in both Du145 and LnCap cells (Fig. 6b), even after 3-h prolonged treatment (data not shown). Moreover, it was previously identified that Src-induced tyrosine phosphorylations of myosin light chain kinase (MLCK) and cortactin may be important for contractility [26]. In accordance with this notion, MLCK inhibitor ML-7 treatment resulted in a significant loss of microextrusions in DJs in Du145 cells, as revealed by both CD9 and CD44 staining (Fig. 6c).
Fig. 6.
How TEMs regulate DJ morphogenesis: contributions of other kinase signaling. a Role of PI-4 kinase signaling in DJs. PI4P and EWI2/PGRL are colocalized in DJs and memtubs of A431 cells. b Role of PI-3 kinase signaling in DJs. Du145 cells cultured on glass coverslips were treated with wortmannin (100 nM) in serum-free medium at 37 °C for 60 min before they were fixed, incubated with CD9 mAb, DAPI, and phalloidin, and examined with confocal microscopy. c Role of MLCK signaling. Du145 cells cultured on glass coverslips were treated with ML-7 (30 µM) in serum-free medium at 37 °C for 60 min before they were fixed, incubated with CD9 or CD44 mAbs, DAPI, and phalloidin, and then examined with confocal microscopy. Quantification of microextrusion density in DJs (mean ± SE, n = 3 individual experiments). ***P < 0.001. Scale bars 10 μm
Then, we investigated putative subcellular events involved in DJ morphogenesis. First, we used CD81 as a marker for DJs and found that DJ formation in PC3 and Du145 cells expressing either Rab5 WT or Rab5 dominant-negative mutant S34N was not significantly altered (data not shown). Second, neither Rab7 WT nor Rab7 dominant-negative mutant T22N changed CD81-positive DJs in these cells (data not shown). These observations suggest that the trafficking of membrane proteins to Rab5 and Rab7 sorting endosomes are not essential for DJ morphogenesis.
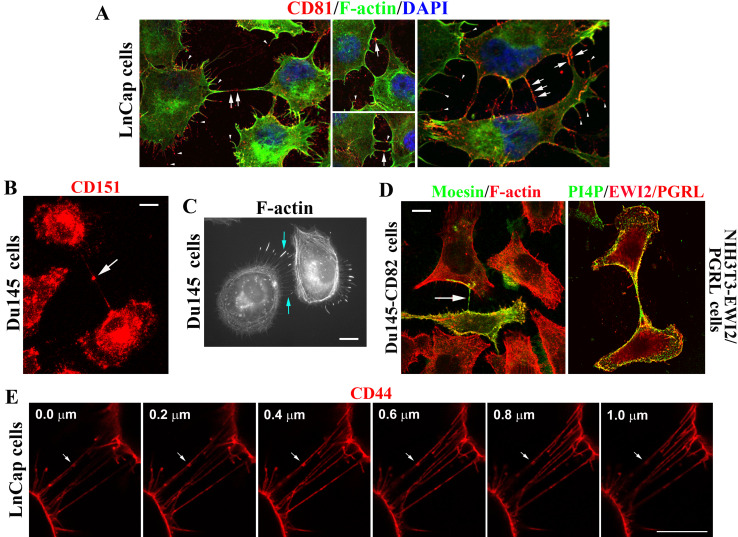
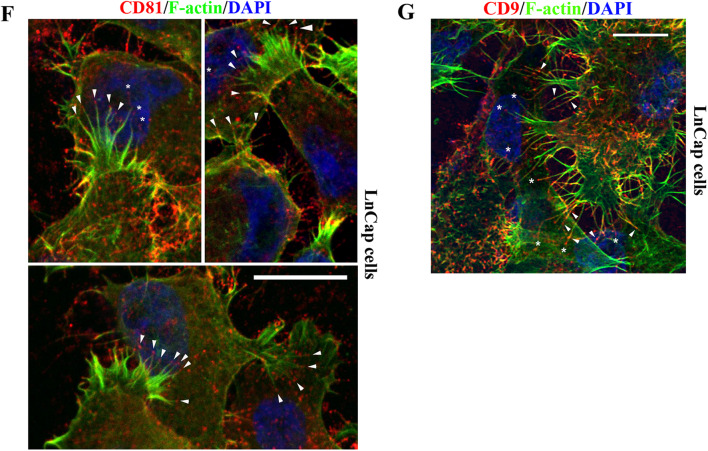
Interestingly, tetraspanins, such as CD81 (Fig. 7a, arrows) and CD151 (Fig. 7b, arrows), were enriched or became aggregated in the middle portion of DJs. Because CD81 proteins were concentrated at the tips of microextrusions (Fig. 7a, arrowheads), it is likely that tetraspanins or TEMs connect two engaged microextrusions from two adjacent cells to form DJ. Although polymerized actin is not always found in microextrusions and does not always reach the tips of microextrusions [15] (see following), F-actin is typically present in DJs and occasionally makes the end-to-end connection between two opposing microextrusions from adjacent cells (Fig. 7c, arrows). We also found that ERM protein moesin, which physically interacts with tetraspanins [22], was present in a DJ, probably transported from one Du145 cell that expressed moesin to another that did not express moesin (Fig. 7d, arrow). In this case, the DJ probably serves as (1) a tunneling nanotubule interconnecting these two cells and/or (2) a conduit for transporting membrane molecules between the cells [27, 28]. Similarly, EWI2/PGRL and PI4P were also present in this type of DJ or tunneling nanotubule, which connected two adjacent NIH3T3 cells (Fig. 7d), and CD151 proteins were present in a tunneling nanotubule between MDCK cells (Movie 1). CD44 proteins are localized in the DJs consisting of nanotubule-like structures between LnCap cells, with their aggregates or clusters along the structures (Fig. 7e). These DJs did not attach the substratum and were simply connecting the two cells (Figure S2).
Fig. 7.
TEMs are present in the joints of microextrusions and/or tunneling nanotubules and at the microextrusion tips that directly contact other cells. a Tetraspanin CD81 enrichment at the contacting points of DJs and the termini of microextrusions. b Tetraspanin CD151 enrichment at the contacting points of DJs. c F-actin enrichment in the middle of tunneling nanotubules and at the termini of microextrusions. d Presence of moesin, EWI2/PGRL, and PI4P in tunneling nanotubules between cells. The cells were fixed, fluorescent and/or immunofluorescent staining was performed, and images were captured with fluorescence microscopy. Arrows: TEM components in DJs or at the joints of two opposing microextrusions. Arrow heads: TEM components at the termini of microextrusions. e Spatial arrangement of DJs. LnCap cells cultured on glass coverslips were fixed and incubated with CD44 mAb and secondary Ab. Confocal images near DJs were acquired as z-stacks comprising sequential optical x–y sections taken at 0.2 μm z-intervals. Scale bar 10 μm. f, g Sticky fingers or unilateral DJs: enrichments of tetraspanin CD81 (f) and CD9 (g) at the microextrusion tips that directly contact other cells. LnCap cells were fixed and co-stained with CD81 or CD9 mAb and phalloidin. Images were captured with confocal microscopy. For f, arrow heads: CD81 clusters at the termini of microextrusions, and asterisks: CD81-capped microextrusions landed at the cell surface areas proximal to nuclei. For g, arrow heads: CD9 clusters at the stems of microextrusions, and asterisks: contacts of CD9-capped microextrusions with the surfaces of other cells. Scale bar 10 μm
We also observed a specific type of DJ, the finger-like microextrusions of which directly contact the bodies of other cells. For example, CD81-capped microextrusions in LnCap cells directly attached to the bodies of other cells (Fig. 7f, arrowheads). Some of this engagement occurred in the area immediately near nuclei (Fig. 7f, asterisks). CD81 proteins appear to be released from the tips of these microextrusions. Similar observations were also made for CD9 (Fig. 7g).
Presence of tetraspanins and their associated proteins in microextrusions, the building blocks of DJs
To examine whether the link between TEMs and DJs extends beyond the molecules that we experimentally analyzed, we used a bioinformatics approach to predict the TEM components that are associated with microextrusions or microvilli, the structural foundation of DJs. The approach, called Global Microarray Meta-Analysis (GAMMA), identifies highly correlated genes by processing and normalizing public microarray data sets from NCBI’s Gene Expression Omnibus (GEO) database and then identifies what the correlated genes have in common within the published literature [29, 30]. Because GAMMA analyzes genes indirectly using a set of highly correlated genes instead of the gene itself, it can be used to predict associations for genes regardless of whether there is literature available on these gene associations. GAMMA’s performance has been computationally benchmarked and experimentally validated in several studies to successfully predict phenotypic and genetic associations for uncharacterized or poorly characterized genes [31–37]. We used GAMMA to predict microvillus-associated genes in general, and found that several tetraspanins, tetraspanin relatives, and their associated proteins are linked to microextrusions/microvilli (Table 1). Although the readouts vary between these two analyses, both indicate the strong presence of TEMs and enrichment of tetraspanins in microextrusions and support the roles of TEMs in DJ formation and development.
Table 1.
Genes predicted to be associated with microvilli by GAMMA
| (a) GAMMA analysis, version 07/10/2016 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Entrez ID | Chr. | Gene_Name | Pred. association | # Shared rels | Obs/exp | Score | Lit_Str | PubMed_Freq |
| Tetraspanins and relatives | |||||||||
| TSPAN8 | 7103 | 12q21.1 | Tetraspanin 8 | Microvilli | 6 | 3.37 | 31 | 0 | 43 |
| TSPAN6 | 7105 | Xq22 | Tetraspanin 6 | Microvilli | 7 | 3.89 | 26 | 0 | 9 |
| CD9 | 928 | 12p13.3 | CD9 molecule | Microvilli | 6 | 3.24 | 25 | 16.9 | 171 |
| TSPAN1 | 10103 | 1p34.1 | Tetraspanin 1 | Microvilli | 6 | 3.26 | 18 | 0 | 19 |
| TM4SF20 | 79853 | 2q36.3 | Transmembrane 4 L six family member 20 | Microvilli | 5 | 2.83 | 14 | 0 | 2 |
| TSPAN15 | 23555 | 10q22.1 | Tetraspanin 15 | Microvilli | 5 | 2.77 | 12 | 0 | 6 |
| TM4SF5 | 9032 | 17p13.3 | Transmembrane 4 L six family member 5 | Microvilli | 5 | 2.52 | 12 | 0 | 21 |
| Tetraspanin-associated proteins | |||||||||
| CLDN1 | 9076 | 3q28 | Claudin 1 | Microvilli | 7 | 3.53 | 31 | 2 | 150 |
| EPCAM | 4072 | 2p21 | Epithelial cell adhesion molecule | Microvilli | 7 | 3.64 | 24 | 1.8 | 175 |
| MSN | 4478 | Xq11.1 | Moesin | Microvilli | 6 | 2.95 | 17 | 63.5 | 162 |
| EGFR | 1956 | 7p12 | Epidermal growth factor receptor | Microvilli | 5 | 2.73 | 17 | 9.6 | 3413 |
| MET | 4233 | 7q31 | MET proto-oncogene, receptor tyrosine kinase | Microvilli | 5 | 2.79 | 13 | 3.3 | 665 |
| CLDN7 | 1366 | 17p13.1 | Claudin 7 | Microvilli | 5 | 2.69 | 13 | 7.4 | 63 |
| ITGA6 | 3655 | 2q31.1 | Integrin subunit alpha 6 | Microvilli | 5 | 2.54 | 11 | 1.8 | 204 |
| (b) GAMMA analysis, version 11/24/2014 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | LLID | Map | Gene_Name | Object | # Shared rels | Obs/exp | Score | Lit_Str | PubMed_Freq |
| Tetraspanins and relatives | |||||||||
| CD9 | 928 | 12p13.3 | CD9 molecule | Microvilli | 9 | 3.95 | 38 | 21 | 171 |
| TSPAN8 | 7103 | 12q14.1 | Tetraspanin 8 | Microvilli | 8 | 3.85 | 31 | 0 | 43 |
| TSPAN1 | 10103 | 1p34.1 | Tetraspanin 1 | Microvilli | 8 | 3.68 | 30 | 0 | 19 |
| TM4SF1 | 4071 | 3q21 | Transmembrane 4 L six family member 1 | Microvilli | 7 | 3.55 | 24 | 5.8 | 18 |
| CD53 | 963 | 1p13 | CD53 molecule | Microvilli | 6 | 3.39 | 21 | 0.5 | 33 |
| CD82 | 3732 | 11p11.2 | CD82 molecule | Microvilli | 5 | 2.86 | 15 | 5.2 | 146 |
| Tetraspanin-associated proteins | |||||||||
| MSN | 4478 | Xq11.1 | Moesin | Microvilli | 9 | 4.57 | 41 | 81.7 | 162 |
| EPCAM | 4072 | 2p21 | Epithelial cell adhesion molecule | Microvilli | 9 | 4.63 | 40 | 4.4 | 175 |
| LIF | 3976 | 22q12.2 | Leukemia inhibitory factor | Microvilli | 9 | 4.02 | 39 | 1 | 160 |
| ITGA3 | 3675 | 17q21.33 | Integrin, alpha 3 | Microvilli | 7 | 3.54 | 30 | 3.6 | 186 |
| MET | 4233 | 7q31 | Met proto-oncogene | Microvilli | 7 | 3.56 | 24 | 6.1 | 665 |
| CLDN7 | 1366 | 17p13.1 | Claudin 7 | Microvilli | 6 | 3.43 | 21 | 7.1 | 63 |
| EGFR | 1956 | 7p12 | Epidermal growth factor receptor | Microvilli | 7 | 2.99 | 20 | 32.2 | 3413 |
| CLDN1 | 9076 | 3q28 | Claudin 1 | Microvilli | 7 | 3.03 | 20 | 2 | 150 |
| ITGA6 | 3655 | 2q31.1 | Integrin, alpha 6 | Microvilli | 6 | 2.83 | 17 | 3.6 | 204 |
| CD44 | 960 | 11p13 | CD44 molecule | Microvilli | 5 | 2.67 | 14 | 48.6 | 761 |
(a) shows the tetraspanins related genes predicted to be associated with microvilli by GAMMA in most recent database build (7/10/2016). In cases where an association is already known, the literature strength (“Lit Str”) will be greater than zero. This strength is based upon the total number of identified sentence (+ 0.8 strength) and abstract (+ 0.5 strength) co-mentions. (b) is from our first analysis (11/24/2014), which included the term “filopodia” as synonymous with “microvilli”, whereby filopodia was approximately 25% of the co-mentions identified. We later decided that filopodia, although related to microvilli, should be its own category. This is, in part, based upon the fact that Gene Ontology sees them as separate entities (microvillus is GO:0005902, filopodia is GO:0030175, and they share the same parent term of “actin-based cell projection”, GO:0098858). Thus, (b) could be considered to be microvilli- or filopodia-related. The results are similar, but not identical, primarily for this reason. The more recent version also includes more literature. With the GAMMA predictions, 7 out of 33 tetraspanin transcripts are predicted to be associated with microvilli, while 836 out of 44,236 non-tetraspanin transcripts are predicted to be associated with microvilli. Two-tailed Chi-square analysis with Yates correction gives P < 0.0001 for the significance of difference between the two groups, indicating that tetraspanins are highly enriched in microvilli or microextrusions
Discussion
DJ defines a common interaction mechanism between cells
The existence of DJs in all of the cells that we had analyzed underlines that DJ is a common, if not general, structure and mechanism for cell–cell interaction and communication, instead of a cell type- or cell line-specific device. When cells approach each other, the microextrusions between cells or DJs engage in initial, dynamic cell–cell interactions. For example, the sperm–egg interaction occurs initially at the microextrusion-enriched region of the egg membrane [38, 39]. DJ could be a transitional contact mechanism between epithelial cells before adherens junctions appear and reinforce the cell–cell contacts [9–11]. For temporary cell–cell interaction, L-selectin molecules on leukocytes are clustered at the microextrusion-enriched regions of the leukocyte plasma membrane to tether leukocytes onto endothelium [40]. Filopodia bridge/tunneling nanotubules can be considered as another type of temporary or dynamic DJ [41, 42]. In epithelial and endothelial cells, we observed that DJs or intercellular microextrusions can remain persistently interdigitated. Such stable DJs also exist between cancer cells and aggregated platelets [43]. In fibroblasts and cancer cells, we also found that DJs remain as interdigitated microextrusions. Differences in DJs between different cells may reflect the status of cellular differentiation and outcome of cell–cell contacts. Thus, DJs could be either transient cell–cell contact structures or stable junctional organizations, and may evolve to other cell–cell junctions or remain as interdigitated microextrusions.
Besides engaging the initial attachment of leukocytes onto the luminal surface of endothelial cells [44], DJs also form between stem cells and their niche [45]. Thus, DJs could mediate both homogenous and heterogeneous cell–cell contacts.
From the present study, we conclude that DJs are formed by either reciprocal engagement between microextrusions from two adjacent cells or unilateral tethering between microextrusions of one cell and the body of another cell (Figs. 7, 8), to mediate adhesion and communication between cells. In addition, our observations (Fig. 7 and Movie) underline the likelihood of tunneling nanotubules as a mechanism by which DJs mediate cell–cell communication. In this case, the tetraspanin-capped tips of two opposing microextrusions or memtubs may trigger the plasma membrane fusion, a well-reported TEM function [5, 6]. Another form of DJ-mediated cell–cell communication occurs through the extracellular vesicles released by microextrusions (our unpublished observations). Finally, DJs probably transduce bi-directional signaling between adjacent cells through direct membrane–membrane interactions between microextrusions.
Fig. 8.
Schematic presentation of DJs and DJ classification
In summary, DJs are dynamic cell–cell interactive structures or devices and exist in a variety of cells with different tissue origins. Although DJs may further develop to those well-documented cell–cell junctions (Fig. 1e), DJs are not tight junctions, adherens junctions, or desmosomes, simply because all of those cell–cell junction mechanisms require very close and extensive cell–cell interfaces and are not intermediated by membrane microextrusions.
Tetraspanins and their associated molecules play regulatory roles in DJ morphogenesis
Earlier studies showed the presence of tetraspanins CD9, CD81, and CD151 at DJs [12, 15, 20, 46]. CD9 and CD82 were previously reported to regulate cell traction and actin cytoskeleton through RhoA-ROCK pathway [47, 48]. Systematic examination from this study revealed the localizations of CD82 and tetraspanin-associated proteins, such as integrin α3β1, EWI2/PGRL, CD44, and moesin, at several types of DJs from a variety of cells. These new findings support that TEMs are closely associated with the morphogenesis and function of DJs. More importantly, we demonstrated that TEMs regulate DJs, either positively or negatively. Since tetraspanins emerged with multi-cellularity during evolution [7, 8], mediating and regulating cell–cell interactions via DJs is likely to be a primordial function of tetraspanins.
Because membrane microextrusions or memtubs are the structural units of DJs, tetraspanins likely regulate memtubs [14, 15] to affect DJ morphogenesis, probably by modulating (1) the interaction between their associated membrane proteins and cytoskeleton and/or (2) the plasma membrane curvature. For example, probably CD9 promotes, while CD82 inhibits outward curvature of the plasma membrane and, therefore, CD9 promotes, but CD82 inhibits DJ morphogenesis. Considering that DJs mark dynamic contacts between cells and that tetraspanins interact with cadherin, claudin, and IgSF protein [5, 6], DJs reflect the transition processes before the establishment or after the disassembly of stable cell–cell junctions, such as adherens and tight junctions, and, therefore, serve as a reversible cell–cell adhesion structure for apical–basolateral polarized cells.
The involvement of both cell–matrix and cell–cell adhesions highlights the uniqueness of DJ morphogenesis. Such involvement is supported by (1) the results of GAMMA analysis (Table 1) and (2) TEM compositions. Cell–matrix adhesion leads to the morphogenesis and navigation of memtubs [16], and physical contacts between these memtubs initiate their reciprocal engagement. TEMs contain integrins and IgSF proteins [5, 6], which typically mediate cell–matrix adhesion and cell–cell adhesion, respectively. Hence, TEMs as a unique platform enable and regulate DJs and memtubs to participate in both cell–matrix and cell–cell adhesions, as well as cell migration (Fig. 8). Once cells become apical–basolateral polarized by forming various stable cell–cell junctions, cell–cell adhesion molecules are segregated from cell–matrix adhesion molecules to localize at the cell–cell interface. Because migrating cells encounter cells and/or matrices during navigation, migration devices such as memtubs are required to carry both cell–cell and cell–matrix adhesion molecules to sense and respond to the constantly changing microenvironments.
Our work also revealed that DJs could serve as cell–cell communication devices and that tetraspanins affect cell–cell communication by altering DJs. DJs may mediate cell–cell communication through nanobridges/tunneling nanotubules, trogocytosis, or extracellular vesicles. Hence, we predict that tetraspanins also regulate cell–cell communication through these aspects. DJ could serve as a signaling platform that interfaces two juxtaposed cells. The signals derived from this interface could lead to cellular decisions, such as furthering cell–cell contact versus repulsing each other, continuing proliferation versus exiting the cell cycle, and stopping cell migration versus continually crawling. Thus, DJs likely have multiple functions, and may switch cells between motility and adhesiveness and from sole cell–matrix adhesiveness to integrated cell–cell and cell–matrix adhesiveness.
Our recent findings underline the regulatory roles of tetraspanins in endothelial barrier function [13] (our unpublished data). Involvement of tetraspanins in adhesion zippers [9] also suggests a regulatory role of tetraspanins in epithelial stability. Hence, DJs may serve as a cellular mechanism by which tetraspanins regulate epithelial and endothelial stability.
Molecular events that regulate DJ morphogenesis
Our studies revealed that growth factor receptor-Src-MAP kinase, RhoA-Rock, and myosin-II signaling are needed for proper formation of DJs. Src activates MLCK and MLCK in turn activates myosin-II, while RhoA-Rock signaling inactivates myosin light chain phosphatase and subsequently prevents myosin-II from inactivation [49]. Hence, either Ca++-mediated or RhoA-dependent activation of myosin-II can facilitate DJ formation and/or maintenance. Because these events are downstream of integrin signaling [50], our observations indicate that cell–matrix adhesion and actomyosin activity promote DJ morphogenesis. Our studies also demonstrate that DJs are non-filopodia and non-lamellipodia structures, as perturbations of Cdc42 and Rac activities do not affect DJs morphogenesis and maintenance in the cell lines we tested.
Cells still form microextrusions or memtubs upon disruption of actin cytoskeleton. A plausible extrapolation is that actin filament-containing microextrusions or microextrusion portions in DJs engage cell–cell adhesion, while actin filament-free microextrusions or microextrusion portions generate extracellular vesicles for cell–cell communication. Microtubules are not required for the formations of microextrusions and DJs, while the confining role of microtubules in DJ morphogenesis likely results from their antagonistic effect on actin.
Treatment with the cholesterol chelator MβCD appears not to alter the DJ formation in MDCK cells (our unpublished data), suggesting that cholesterol and cholesterol-enriched microdomains/lipid rafts are not required for DJ morphogenesis. Given the regulatory role of TEMs, such a difference underpins a distinction between cholesterol-enriched microdomains/lipid rafts and TEMs. However, cholesterol is physically and functionally linked to tetraspanins [51–53]. Because of the intricate interactions and close relationships between lipid rafts and TEMs, the roles of cholesterol and lipid rafts in DJs remain to be examined in future studies.
Materials and methods
Reagents
Cells used in this study include human microvascular endothelial cells (HMECs) (CDC, Atlanta, GA, USA), Du145 and LnCap human prostate cancer (ATCC, Manassas, VA, USA), U87 human glioblastoma (ATCC), and A431 human epidermoid cancer cell lines (ATCC), Du145-Mock and -CD82 stable transfectants [18, 48], HT1080-Mock and HT1080-CD9 stable transfectants, PC3 human prostate cancer cells (ATCC) in which EWI2/PGRL was transiently silenced with siRNA oligo against the target sequence GUUCUCCUAUGCUGUCUU of EWI2/PGRL or in which control siRNA oligo was transfected [22], MDCK-GFP:CD82 and -GFP:CD151 stable transfectants expressing the fusion proteins in which eGFP moieties were fused to the N-termini of CD82 and CD151 proteins, respectively, and NIH3T3-EWI2/PGRL transfectant [25]. The EWI2/PGRL or control siRNAs were transfected into PC3 cells with Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA, USA) in Opti-MEM medium (Invitrogen), and the cells were used for experiments at 48 or 72 h after the transfections. The MDCK stable transfectants were established by flow cytometric sorting and collection of the green fluorescent MDCK cells that are resistant to the G418 (1 mg/mL, Invitrogen) selection after Lipofectamin 2000 (Invitrogen) mediated transfection.
Du145, PC3, HT1080, MDCK, and NIH3T3 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen) supplemented with 10% fetal calf serum (FCS) (Sigma-Aldrich, St. Louis, MO), 100U/mL Penicillin, and 100 μg/mL Streptomycin (Thermo Fisher, Waltham, MA, USA). LnCap cells were cultured in RPMI-1640 (Invitrogen) with the FCS and Penicillin–Streptomycin supplements.
Antibodies (Abs) used in this study include human CD82 monoclonal Ab (mAb) TS82b (Cell Sciences, Canton, MA, USA), human CD9 mAb MAB7 [13], human CD81 mAb M38 [13], human EWI2/PGRL mAb 5E8 [20], human integrin α3 mAb A3-X8 [13], human integrin β1 mAb TS2/16 [13], human CD44 mAb IM7 (BD Biosciences, San Diego, CA), PI4P mAb (Echelon Biosciences, Salt Lake City, UT, USA), and moesin polyclonal Ab (pAb) Santa Cruz Biotechnology, Santa Cruz, CA, USA). Fluorochrome-conjugated phalloidin and secondary Abs and DAPI were from Invitrogen.
Inhibitors used in this study include latrunculin B (1 μM; Sigma), cytochalasin D (2 μg/ml; Sigma), jasplakinolide (200 nM; Sigma), nocodazole (20 μM; Sigma), paclitaxel (10 nM; Sigma), withaferin A (5 μM; Santa Cruz Biotechnology), Cdc42 inhibitor ML-141 (20 µM; Sigma), Rac1 inhibitor NSC23766 (200 µM; Sigma), RhoA inhibitor Rhosin (30 μM; Calbiochem, Gibbstown, NJ), Y27632 (20 μM; Calbiochem), ML-7 (30 μM; Santa Cruz Biotechnology), blebbistatin (30 μM; Toronto Research Chemicals, Toronto, Canada), RhoA activator II CN03 (0.5 μg/ml; Cytoskeleton Inc., Denver, CO, USA), Src family kinase inhibitor PP2 and its negative control PP3 (10 μM; Abcam, Cambridge, MA, USA), wortmannin (100 nM; Alfa Aesar, Tewksbury, MA, USA), MK-2461 (200 nM; AdipoGen, San Diego, CA, USA), PD98059 (25 µM; Sigma), and Trametinib (200 nM; Selleck Chemicals, Houston, TX, USA). Most of the inhibitors were dissolved in DMSO. DMSO concentrations were 0.1% (vol/vol) in the solutions in the control and experimental groups. The activator used in this study is recombinant human hepatocyte growth factor (HGF) (100 ng/ml; Tonbo Biosciences, San Diego, CA, USA). All listed concentrations are the final concentrations used in the experiments.
Fluorescent and confocal microscopy
After being cultured on uncoated glass coverslips (Denville Scientific, MA) in complete medium for 2–4 days, cells were fixed with 3% paraformaldehyde, permeabilized with 0.1% Brij98, blocked with 20% goat serum in PBS for 1 h at room temperature, and incubated sequentially with primary mAb and fluorochrome-conjugated secondary Ab. For F-actin staining, cells were incubated with fluorochrome-conjugated phalloidin. The images were acquired with a Zeiss Axiophot fluorescent microscope, a Zeiss 510 confocal microscope, or a Leica SP2 confocal microscope with a Plan-Apochromat 63 × 1.4 NA oil immersion objective, and were analyzed with NIH ImageJ software.
For time-lapse video microscopy, cells were spread on collagen-I (30 μg/mL)-coated glass-bottom dishes (MatTek Corp., Ashland, MA, USA) in serum-free DMEM, stained with CellMask Green Plasma Membrane Stain (Thermo Fisher) according to the manufacturer’s manual, and imaged every 10 min with a Leica SP8 microscope with a Plan-Apochromat 63 × 1.4 NA oil immersion objective in a stage-top incubator (Tokai Hit Co., Shizuoka, Japan) in 5% CO2 and at 37 °C.
Total internal reflection fluorescence (TIRF) microscopy
Cells cultured on laminin 332 (2 μg/mL)-coated glass-bottom dishes (MatTek Corp., Ashland, MA) were fixed with 3% paraformaldehyde and then incubated sequentially with primary Ab and fluorochrome-conjugated secondary Ab. For MDCK transfectants, the cells were fixed and then examined directly. Fluorescence images were acquired on a Zeiss Axiovert 200 M inverted microscope equipped with a TIRF slider under a Zeiss alpha Plan-Apochromat 100x 1.46 NA objective. A 100-nm depth or section was used for acquiring the TIRF images.
GAMMA analysis
GAMMA is an algorithm that uses public microarray data to identify genes that are highly correlated across different experimental conditions [29, 30]. Briefly, the top 40 most correlated genes are used in a kNN (k-nearest neighbors) approach to infer what a gene does via an analysis of what those genes have in common in MEDLINE abstracts (e.g., chemicals, other genes, phenotypes, diseases, ontology categories, etc.). Thus, even when there is no literature to describe a gene or ncRNA, GAMMA can infer associations. It has been used successfully in several studies to test predicted phenotypes and characterize genes for which little or no information was available before the analysis [31–37].
Data analysis
All experiments were performed at least three times. Data are presented as mean ± SD or mean ± SE. Statistical analyses were performed with Student’s t test or Pearson’s Chi-squared (χ2) test. P < 0.05 was considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video: Nanotubule was formed between two MDCK cells that were expressing GFP-CD151 proteins. Live cell imaging was captured with fluorescent light microscopy. (AVI 7444 kb)
Figure S1. How TEMs regulate DJ morphogenesis: contributions of growth factor/receptor tyrosine kinase and MAP kinase signaling. LnCap cells cultured on glass coverslips were treated with following activator or inhibitor in serum-free media at 37oC for 60 min, unless otherwise indicated, before they were fixed, incubated with CD44 mAb, DAPI and phalloidin, and then examined and imaged with confocal microscopy. A. Role of HGF and c-Met signaling in DJs. The cells were treated with either HGF (100 ng/ml) after 6-hour serum starvation or MK-2461 (200 nM) without serum starvation. B. Role of MEK signaling in DJs. The cells were treated with Trametinib (200 nM) for 3 h and PD98059 (25 µM). Quantification of microextrusion density in DJs (mean ± S.E., n = 3 individual experiments). ***: P < 0.001. Scale bars: 10 μm. (TIFF 9584 kb)
Figure S2. Spatial arrangement of DJs. LnCap cells cultured on glass coverslips were fixed and incubated with CD44 mAb and Alexa594-conjugated secondary Ab. Confocal images were acquired as z-stacks comprising sequential optical x–y sections of 46.18 µm x 46.18 µm taken at 1.0 μm z-intervals. Images with maximum cell spreading were selected to represent basal membrane at 0 µm z position. Scale bar: 10 μm. (TIFF 3414 kb)
Acknowledgements
This work was supported by National Institutes of Health Research Grants CA096991, HL132553, and HL137819, American Heart Association Grant-in-Aid 13GRNT17040028, Oklahoma Center for Advanced Science and Technology Grant, and Chapman Foundation (to X. A. Z.). We thank Ms. Kathy Kyler for English editing.
Abbreviations
- DJ
Digitation junction
- DMEM
Dulbecco’s modified eagle medium
- FCS
Fetal calf serum
- GAMMA
Global microarray meta-analysis
- GEO
Gene expression omnibus
- HGF
Hepatocyte growth factor
- HMEC
Human microvascular endothelial cell
- IF
Intermediate filament
- mAb
Monoclonal antibody
- MβCD
Methyl beta cyclodextrin
- Memtubs
Membrane tubular structures
- MLCK
Myosin light chain kinase
- pAb
Polyclonal antibody
- TEMs
Tetraspanin-enriched microdomains
- TIRF
Total internal reflection fluorescence
Author contributions
Conceived and designed the experiments: XAZ. Performed the experiments: CH, CF, XW, JDW, FZ, YHZ, SAC, and XAZ. Analyzed the data: CH, JDW, and XAZ. Contributed reagents/materials/analysis tools: TC. Wrote the paper: XAZ.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Horejsi V, Vlcek C. Novel structurally distinct family of leucocyte surface glycoproteins including CD9, CD37, CD53 and CD63. FEBS Lett. 1991;288:1–4. doi: 10.1016/0014-5793(91)80988-F. [DOI] [PubMed] [Google Scholar]
- 2.Wright MD, Tomlinson MG. The ins and outs of the transmembrane 4 superfamily. Immunol Today. 1994;15:588–594. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 3.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. doi: 10.1096/fasebj.11.6.9194523. [DOI] [PubMed] [Google Scholar]
- 4.Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001;58:1189–1205. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 6.Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Espana A, et al. Appearance of new tetraspanin genes during vertebrate evolution. Genomics. 2008;91:326–334. doi: 10.1016/j.ygeno.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Huang S, et al. The phylogenetic analysis of tetraspanins projects the evolution of cell–cell interactions from unicellular to multicellular organisms. Genomics. 2005;86:674–684. doi: 10.1016/j.ygeno.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/S0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 10.Jacinto A, et al. Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol. 2000;10:1420–1426. doi: 10.1016/S0960-9822(00)00796-X. [DOI] [PubMed] [Google Scholar]
- 11.Raich WB, Agbunag C, Hardin J. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr Biol. 1999;9:1139–1146. doi: 10.1016/S0960-9822(00)80015-9. [DOI] [PubMed] [Google Scholar]
- 12.Singethan K, et al. CD9 clustering and formation of microvilli zippers between contacting cells regulates virus-induced cell fusion. Traffic. 2008;9:924–935. doi: 10.1111/j.1600-0854.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, et al. Tetraspanin CD151 maintains vascular stability by balancing the forces of cell adhesion and cytoskeletal tension. Blood. 2011;118:4274–4284. doi: 10.1182/blood-2011-03-339531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bari R, et al. Tetraspanins regulate the protrusive activities of cell membrane. Biochem Biophys Res Commun. 2011;415:619–626. doi: 10.1016/j.bbrc.2011.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang XA, Huang C. Tetraspanins and cell membrane tubular structures. Cell Mol Life Sci. 2012;69:2843–2852. doi: 10.1007/s00018-012-0954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shigeta M, et al. CD151 regulates epithelial cell–cell adhesion through PKC- and Cdc42-dependent actin cytoskeletal reorganization. J Cell Biol. 2003;163:165–176. doi: 10.1083/jcb.200301075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zevian SC, et al. CD151 promotes alpha3beta1 integrin-dependent organization of carcinoma cell junctions and restrains collective cell invasion. Cancer Biol Ther. 2015;16:1626–1640. doi: 10.1080/15384047.2015.1095396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang XA, He B, Zhou B, Liu L. Requirement of the p130CAS-Crk coupling for metastasis suppressor KAI1/CD82-mediated inhibition of cell migration. J Biol Chem. 2003;278:27319–27328. doi: 10.1074/jbc.M303039200. [DOI] [PubMed] [Google Scholar]
- 19.Stipp CS, Kolesnikova TV, Hemler ME. EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J Biol Chem. 2001;276:40545–40554. doi: 10.1074/jbc.M107338200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XA, Lane WS, Charrin S, Rubinstein E, Liu L. EWI2/PGRL associates with the metastasis suppressor KAI1/CD82 and inhibits the migration of prostate cancer cells. Cancer Res. 2003;63:2665–2674. [PubMed] [Google Scholar]
- 21.Charrin S, et al. EWI-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells. Biochem J. 2003;373:409–421. doi: 10.1042/bj20030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sala-Valdes M, et al. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J Biol Chem. 2006;281:19665–19675. doi: 10.1074/jbc.M602116200. [DOI] [PubMed] [Google Scholar]
- 23.Krugmann S, et al. Cdc42 induces filopodia by promoting the formation of an IRSp53: mena complex. Curr Biol. 2001;11:1645–1655. doi: 10.1016/S0960-9822(01)00506-1. [DOI] [PubMed] [Google Scholar]
- 24.Ballestrem C, Wehrle-Haller B, Hinz B, Imhof BA. Actin-dependent lamellipodia formation and microtubule-dependent tail retraction control-directed cell migration. Mol Biol Cell. 2000;11:2999–3012. doi: 10.1091/mbc.11.9.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He B, et al. Differential functions of phospholipid binding and palmitoylation of tumour suppressor EWI2/PGRL. Biochem J. 2011;437:399–411. doi: 10.1042/BJ20101381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia JG, et al. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60(src) Am J Physiol. 1999;276:L989–L998. doi: 10.1152/ajplung.1999.276.6.L989. [DOI] [PubMed] [Google Scholar]
- 27.Barraud-Lange V, et al. Membrane transfer from oocyte to sperm occurs in two CD9-independent ways that do not supply the fertilising ability of Cd9-deleted oocytes. Reproduction. 2012;144:53–66. doi: 10.1530/REP-12-0040. [DOI] [PubMed] [Google Scholar]
- 28.Barraud-Lange V, Naud-Barriant N, Bomsel M, Wolf JP, Ziyyat A. Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J. 2007;21:3446–3449. doi: 10.1096/fj.06-8035hyp. [DOI] [PubMed] [Google Scholar]
- 29.Wren JD. A global meta-analysis of microarray expression data to predict unknown gene functions and estimate the literature-data divide. Bioinformatics. 2009;25:1694–1701. doi: 10.1093/bioinformatics/btp290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dozmorov MG, Giles CB, Wren JD. Predicting gene ontology from a global meta-analysis of 1-color microarray experiments. BMC Bioinfo. 2011;12(Suppl 10):S14. doi: 10.1186/1471-2105-12-S10-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisch AS, et al. Genetic variation in the platelet endothelial aggregation receptor 1 gene results in endothelial dysfunction. PLoS ONE. 2015;10:e0138795. doi: 10.1371/journal.pone.0138795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gandhapudi SK, et al. IL-18 acts in synergy with IL-7 to promote ex vivo expansion of T lymphoid progenitor cells. J Immunol. 2015;194:3820–3828. doi: 10.4049/jimmunol.1301542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towner RA, et al. Experimental validation of 5 in silico predicted glioma biomarkers. Neuro Oncol. 2013;15:1625–1634. doi: 10.1093/neuonc/not124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Towner RA, et al. ELTD1, a potential new biomarker for gliomas. Neurosurgery. 2013;72:77–90. doi: 10.1227/NEU.0b013e318276b29d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clemmensen SN, et al. Olfactomedin 4 defines a subset of human neutrophils. J Leukoc Biol. 2012;91:495–500. doi: 10.1189/jlb.0811417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lupu C, Zhu H, Popescu NI, Wren JD, Lupu F. Novel protein ADTRP regulates TFPI expression and function in human endothelial cells in normal conditions and in response to androgen. Blood. 2011;118:4463–4471. doi: 10.1182/blood-2011-05-355370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daum JR, et al. Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr Biol. 2009;19:1467–1472. doi: 10.1016/j.cub.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanagimachi R. Sperm-egg association in animals. Curr Top Dev Biol. 1978;12:83–105. doi: 10.1016/S0070-2153(08)60594-3. [DOI] [PubMed] [Google Scholar]
- 39.Rubinstein E, Ziyyat A, Wolf JP, Le Naour F, Boucheix C. The molecular players of sperm-egg fusion in mammals. Semin Cell Dev Biol. 2006;17:254–263. doi: 10.1016/j.semcdb.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Ivetic A, et al. Mutagenesis of the ezrin-radixin-moesin binding domain of L-selectin tail affects shedding, microvillar positioning, and leukocyte tethering. J Biol Chem. 2004;279:33263–33272. doi: 10.1074/jbc.M312212200. [DOI] [PubMed] [Google Scholar]
- 41.Gerdes HH, Bukoreshtliev NV, Barroso JF. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 2007;581:2194–2201. doi: 10.1016/j.febslet.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 42.Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell–cell communication and viral pathogenesis. Trends Cell Biol. 2008;18:414–420. doi: 10.1016/j.tcb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menter DG, et al. Tumor cell-platelet interactions in vitro and their relationship to in vivo arrest of hematogenously circulating tumor cells. Clin Exp Metastasis. 1987;5:65–78. doi: 10.1007/BF00116627. [DOI] [PubMed] [Google Scholar]
- 44.Barreiro O, et al. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol. 2008;183:527–542. doi: 10.1083/jcb.200805076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillette JM, Larochelle A, Dunbar CE, Lippincott-Schwartz J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat Cell Biol. 2009;11:303–311. doi: 10.1038/ncb1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang HX, Kolesnikova TV, Denison C, Gygi SP, Hemler ME. The C-terminal tail of tetraspanin protein CD9 contributes to its function and molecular organization. J Cell Sci. 2011;124:2702–2710. doi: 10.1242/jcs.085449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herr MJ, Mabry SE, Jennings LK. Tetraspanin CD9 regulates cell contraction and actin arrangement via RhoA in human vascular smooth muscle cells. PLoS ONE. 2014;9:e106999. doi: 10.1371/journal.pone.0106999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu WM, et al. Tetraspanin CD82 inhibits protrusion and retraction in cell movement by attenuating the plasma membrane-dependent actin organization. PLoS ONE. 2012;7:e51797. doi: 10.1371/journal.pone.0051797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res. 2010;87:272–280. doi: 10.1093/cvr/cvq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huveneers S, Danen EH. Adhesion signaling—crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 51.Charrin S, et al. A physical and functional link between cholesterol and tetraspanins. Eur J Immunol. 2003;33:2479–2489. doi: 10.1002/eji.200323884. [DOI] [PubMed] [Google Scholar]
- 52.Wei Q, et al. CD82 restrains pathological angiogenesis by altering lipid raft clustering and CD44 trafficking in endothelial cells. Circulation. 2014;130:1493-U1162. doi: 10.1161/CIRCULATIONAHA.114.011096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmerman B, et al. Crystal structure of a full-length human tetraspanin reveals a cholesterol-binding pocket. Cell. 2016;167:1041. doi: 10.1016/j.cell.2016.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video: Nanotubule was formed between two MDCK cells that were expressing GFP-CD151 proteins. Live cell imaging was captured with fluorescent light microscopy. (AVI 7444 kb)
Figure S1. How TEMs regulate DJ morphogenesis: contributions of growth factor/receptor tyrosine kinase and MAP kinase signaling. LnCap cells cultured on glass coverslips were treated with following activator or inhibitor in serum-free media at 37oC for 60 min, unless otherwise indicated, before they were fixed, incubated with CD44 mAb, DAPI and phalloidin, and then examined and imaged with confocal microscopy. A. Role of HGF and c-Met signaling in DJs. The cells were treated with either HGF (100 ng/ml) after 6-hour serum starvation or MK-2461 (200 nM) without serum starvation. B. Role of MEK signaling in DJs. The cells were treated with Trametinib (200 nM) for 3 h and PD98059 (25 µM). Quantification of microextrusion density in DJs (mean ± S.E., n = 3 individual experiments). ***: P < 0.001. Scale bars: 10 μm. (TIFF 9584 kb)
Figure S2. Spatial arrangement of DJs. LnCap cells cultured on glass coverslips were fixed and incubated with CD44 mAb and Alexa594-conjugated secondary Ab. Confocal images were acquired as z-stacks comprising sequential optical x–y sections of 46.18 µm x 46.18 µm taken at 1.0 μm z-intervals. Images with maximum cell spreading were selected to represent basal membrane at 0 µm z position. Scale bar: 10 μm. (TIFF 3414 kb)