Abstract
Background
How to safely treat pregnant women with epilepsy is a question for which there are guidelines, but variations in practice exist.
Methods
To better characterize how clinicians address this difficult clinical question, we distributed an anonymous survey to neurology practitioners across subspecialties and different levels of training via the Neurology®: Clinical Practice website. The survey was conducted from May 31 to December 3, 2017. We received responses from 642 participants representing 81 countries. We performed both descriptive and inferential analyses. For the inferential analysis, a multiple logistic regression model was used to analyze the effect of provider characteristics on the constructed binary outcome variables of interest.
Results
The results of this survey demonstrate a wide range in the amount of folic acid recommended and the frequency of checking levels of anti-epileptic drugs. Choice of first-line agent varied by the economic development status of the respondent's country, suggesting that access to medications plays an important role in clinical decision making in many parts of the world.
Conclusion
This survey highlights several areas where further research would be helpful in guiding practice.
Epilepsy is amongst the most common neurologic conditions with a worldwide prevalence of 1.2%. Most women with epilepsy who become pregnant or are planning to become pregnant are recommended to continue taking antiepileptic drugs (AEDs) to minimize seizure recurrence during pregnancy, adverse effects to the fetus, and unintended ill consequences during labor and/or delivery.1,2 Given the propensity of enzyme-inducing AEDs to interfere with hormonal contraceptive methods, among other factors, unplanned pregnancies occur at a higher than expected rate of 65% in women with epilepsy.3 To minimize risk of major congenital malformations (MCMs), guidelines advise considering proactive use of folic acid in women with epilepsy in the childbearing age.4 Though guidelines are clear regarding recommendation of continuing AEDs in women with epilepsy, there is paucity of evidence-based data to suggest the choice of AED in this unique population. Current practices tend to reflect data gleaned from observational practices and may lack unanimity across various settings. Similarly, rigorous scientific evidence regarding exact dose and duration of folic acid for preconception counseling is lacking.
To get more in-depth information regarding AED prescribing practices in different geographical settings, an electronic case-based survey was designed. The intent was to capture select information regarding dose and duration of folic acid for women with epilepsy of childbearing age, choice of AED in this patient population (older generation enzyme-inducing vs newer generation non-enzyme-inducing AEDs), and clinical inclination to monitor AED levels during pregnancy. The intent of this survey was to identify similarities and differences in prescribing practices based on geographic location of the neurologist/trainee/provider, level of training, and presence/absence of epilepsy specialty training.
Methods
Survey collection
Using an anonymous electronic survey, we asked participants for their answers to 9 clinical questions pertaining to 2 hypothetical cases.5 Case 1 featured a 21-year-old woman with focal epilepsy. She had no plans on becoming pregnant at the time of diagnosis, and participants were asked whether or not they would supplement folate, what dosage and what anti-epileptic they would start in a young woman of childbearing age. In the second half of the case, the patient was now planning a pregnancy. Participants were asked to describe their primary concern regarding AEDs in pregnancy and how frequently (if at all) they monitor AED levels in pregnant patients. In case 2, a 24-year-old woman with juvenile myoclonic epilepsy on high doses of valproic acid and lamotrigine presents to the clinic in her first trimester of pregnancy. The questions in this case addressed medication management.
The survey was launched by the Practice Current section of Neurology: Clinical Practice and was freely available on the journal's website6 from May 31 to December 3, 2017. We analyzed completed questionnaires from 642 respondents out of 764 participants (122 provided partial answers and were excluded from the analysis). Participants were asked to voluntarily provide answers to the several demographic and practice-related questions including years in practice, level of training, number of pregnant women treated for epilepsy in the past year and location (see appendix e-1 for full survey links.lww.com/CPJ/A86).
Participants could access the survey via links provided in the Neurology journals webpages, print versions of the journals, online ads, and via social media outreach. Participants were not compensated for participating and access to the survey did not require AAN membership.
Individual Internet Protocol addresses were automatically collected to ensure authenticity of responses and to utilize a geolocation feature.
Standard protocol approvals, registrations, and patient consents
The study was certified as exempt from review by Children's National Medical Center Institutional Review Board.
Data extraction
We categorized survey responses into non-overlapping categories. We classified neurologist and neurology provider characteristics by experience, specialization status, and training into 6 mutually exclusive groupings. We created practice variables incorporating geography, setting, and patient population. We used geographic neurologist/neurology provider data to determine if the physician/provider practiced in a developed economy using the 2012 World Economic Situation and Prospects Report prepared for the United Nations Department of Economic and Social Affairs.7 Practice setting (inpatient or outpatient) and population age (if the physician/provider treated children) were separate variables. Responses to hypothetical treatment scenarios were similarly arranged into categorical outcome variables. Where responses were contingent on a prior question, we incorporated affirmative responses into the variable of interest (i.e., folate supplementation and dosing). Where questions were open-ended or had many possible responses, we constructed binary variables to capture the top 3 responses representing >90% of responses (e.g., AED choice and monitoring). Alternately, we grouped multiple responses to single questions into categorical variables using a clinically relevant cut point (e.g., AED monitoring frequency by trimester).
Data analysis
We performed both descriptive and inferential analyses using the data variables defined above. Physician/provider characteristics analyzed by counts and percentage of total respondents. Hypothetical survey question responses were stratified by provider characteristics and positive responses presented as percentages of provider characteristics groupings. For the inferential analysis, we used a multiple logistic regression model to analyze the effect of provider characteristics on the constructed binary outcome variables of interest. Results are reported as odds ratios (ORs) with significance set at p = 0.05. All analyses were performed using STATA 14.0 (StataCorp, College Park, TX).
Data availability
Data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Results
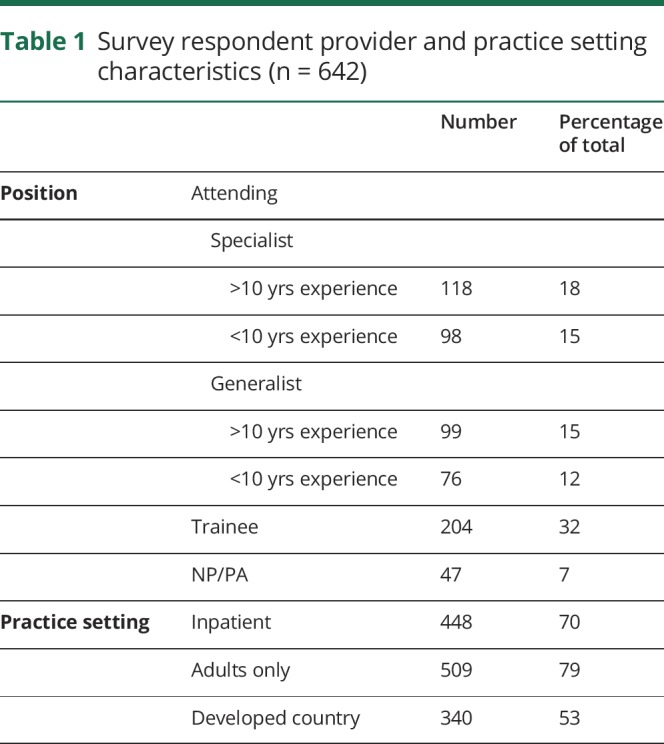
We collected anonymous complete survey responses from 642 respondents in 81 countries. Seventy percent or 448 respondents reported working primarily in a hospital-based setting (table 1). A majority (79% or 509 respondents) treated only adults. Roughly half of all respondents were based in a country with a developed economy.7 When separated into “specialist” (those who identified as specializing in neurophysiology/epilepsy) and “generalist” (those who did not), there were 118 people reporting greater than 10 years of experience who identified as specialists (18%) and 98 people in that group who had less than 10 years' experience (15%). Generalists with greater than 10 years' experience were 15% (98 respondents) and those with less than 10 years' experience 12% (76 respondents). Trainees made up 32% of survey-takers and mid-level providers 7%. Respondents were asked how many pregnant women with epilepsy they had seen in the past 12 months. Most reported 10 or less (66%, 425 respondents), with 94 respondents (15%) reporting having seen no pregnant patients with epilepsy in the past year, 91 (14%) having seen between 11 and 49, and 22 (3.5%) with 50 or more patients; 8 individuals did not answer this survey question.
Table 1.
Survey respondent provider and practice setting characteristics (n = 642)
Case 1
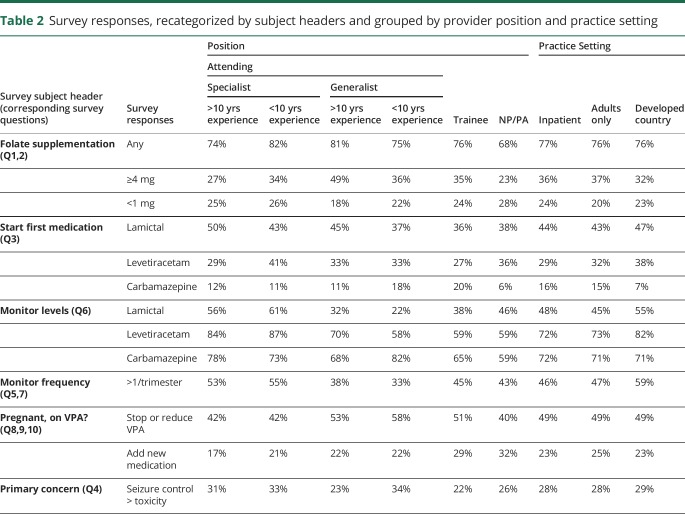
The majority of all categories of respondents would start folate supplementation for the patient described in case 1 (tables 2 and 3), however, the amount of supplementation varied. Of specialists with greater than 10 years' experience, 27% would supplement with at least 4 mg of folate, while 25% would use less than 1 mg. This is compared to 34% and 26%, respectively, of specialists with less than 10 years' experience, 49% and 18%, respectively, of generalists with greater than 10 years' experience and 36% and 22%, respectively, of generalists with less than 10 years' experience.
Table 2.
Survey responses, recategorized by subject headers and grouped by provider position and practice setting
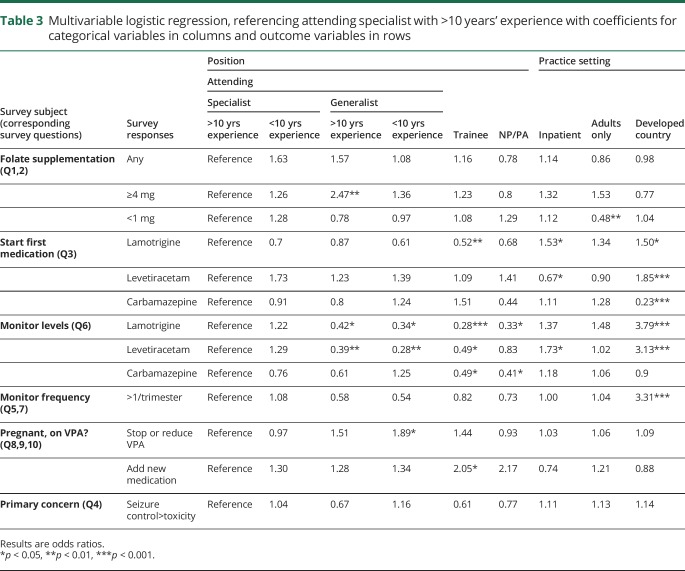
Table 3.
Multivariable logistic regression, referencing attending specialist with >10 years' experience with coefficients for categorical variables in columns and outcome variables in rows
Using specialists with greater than 10 years' experience as a reference, we found that generalists with greater than 10 years' experience had 2.47 greater OR (OR 2.47, p < 0.001) of recommending 4 mg or more of daily folate supplementation. Respondents who treat mostly adults had lower OR (OR 0.48, p < 0.01) of recommending less than 1 mg of folate daily. The remainder of the findings did not reach statistical significance.
Regarding the question of what medication to start in a WWE of childbearing age, specialists with greater than 10 years' experience were most likely to start lamotrigine (50%) and this percentage decreased with fewer years of experience. Levetiracetam was the first choice for 41% of specialists with less than 10 years' experience; however, roughly 30% of respondents at the other levels of training would start this medication first.
Respondents from countries with developed economies had greater OR of starting lamotrigine (OR 1.5, p < 0.05) and levetiracetam (OR 1.85, p < 0.001) and lower OR of starting carbamazepine (OR 0.23, p < 0.001).
When monitoring AED levels in pregnancy, all levels of training aside from epilepsy specialists had significantly lower OR of monitoring levels of both lamotrigine and levetiracetam (mid-level providers also did not show a significant OR compared to specialists with >10 years of experience in terms of checking levetiracetam levels). Trainees and mid-levels had significantly lower OR of monitoring carbamazepine levels (OR 0.49 for trainees, OR 0.41 for mid-levels, p < 0.05 for both categories). Respondents from countries with developed economies had at least 3 times greater OR of monitoring lamotrigine and levetiracetam levels than respondents from countries with developing economies (OR 3.79, OR 3.13, p < 0.001 for both). Similarly, respondents from countries with developed economies were 3 times more likely to check levels more than once a trimester (OR 3.31, p < 0.001).
Case 2
Our second case, featuring a young woman with juvenile myoclonic epilepsy on high doses of valproic acid and lamotrigine, had fewer questions and the answers were more varied. Respondents across the board reported they would choose to decrease dosage or stop valproic acid, with responses in the 40%–50% range (lowest percentage was 40% in mid-levels, highest percentage was 58% in generalists with >10 years of experience). The only statistically significant difference in respondents were generalists with >10 years' experience who had greater OR of stopping or reducing this medication, compared to specialists with >10 years' experience (OR 1.89, p < 0.05). Trainees and mid-levels had slightly greater OR of starting an additional medication in this case, although this (OR 2.05, p < 0.05) was only significant in trainees.
Discussion
Our goal in creating this survey was to engage practitioners who treat women with epilepsy throughout the world to see how clinicians approach challenging clinical questions. The variations we found reflect the range of practice norms in different clinical settings. Our results also highlight a few areas where further research is needed to help refine clinical guidelines.
Notably, although a majority of respondents would start folic acid supplementation in a patient of childbearing age, there were a range of responses as to the appropriate dosing. The AAN's Practice Parameters recommend at least 0.4 mg daily4; higher doses are generally recommended when there is a personal or family history of neural tube defects.4 The rate of MCMs in the children of women on AEDs is increased compared to the general population, particularly if these medications are prescribed during the first trimester of pregnancy.8 However, based on data amassed by several pregnancy in epilepsy registries as well as other large databases, there is no evidence that folic acid supplementation decreases the risk of major fetal malformations in women on AEDs,8–11 suggesting the mechanism of MCM formation, especially neural tube defects, may not be related to folate metabolism in this population.10,12 One study looking specifically at high dose (5 mg daily) folic acid supplementation also failed to find a significant difference, although the authors acknowledged that many of their participants did not begin supplementation until the second month of pregnancy.8
Although MCM rates may not be affected by folic acid supplementation, there is a growing body of evidence demonstrating neurocognitive benefits. Bjørk et al.13 found that in the Norwegian population, folic acid supplementation early in pregnancy decreased the risk for autistic traits at 18 and 36 months of age in the offspring of WWE exposed to AEDs, regardless of the AED. Other studies have demonstrated that fetal valproic acid exposure has an adverse effect on cognitive outcomes in school-aged children14–16 and pre-teens,17 with evidence that folic acid supplementation can mitigate these negative effects.16 The negative effects on fetal and childhood outcomes may be dose dependent for valproic acid,9 which makes using the minimum effective dose in patients on this medication an important goal, as is avoiding polytherapy when possible.
Barring case reports reporting decreased levels of some AEDs, primarily phenytoin, in (non-pregnant) patients on high-dose folic acid, there seem to be few drawbacks to folic acid supplementation18 and many potential benefits, including a decreased rate of spontaneous abortion.19
Perhaps unsurprisingly, the most common first agents prescribed for the young woman with focal epilepsy in our survey were lamotrigine and levetiracetam, both of which appear to be relatively safe in pregnancy. A 2016 Cochrane review of lamotrigine vs carbamazepine in focal epilepsy concluded that carbamazepine might provide better seizure control, but lamotrigine had fewer adverse events leading to drug discontinuation.20 A potential advantage of carbamazepine compared to lamotrigine is that it generally requires less frequent monitoring of levels, because its clearance is not significantly affected by pregnancy.21 Our survey findings are reflected in the literature: Several studies examining first-time AED prescriptions have demonstrated a significant decrease in overall prescribing rates of carbamazepine and increases in lamotrigine and levetiracetam.22
One highly significant result in our data was a difference in choice of first AED when comparing respondents from countries with developed vs developing economies. Respondents from countries with developed economies were significantly more likely to prescribe levetiracetam and less likely to prescribe carbamazepine, which may reflect limited availability and prohibitive pricing of newer AEDs in the developing world, but may not necessarily reflect better efficacy or safety of levetiracetam.
Similarly, our survey demonstrated there are wide practice variations in checking AED levels in pregnancy. This also was split along economic lines, where respondents who checked levels more than once a trimester were far more likely to be from economically developed countries; the same results were seen for respondents checking levels for lamotrigine and levetiracetam.
Outside of the question of how best to treat pregnant women with epilepsy, there is the broader issue of the vast treatment gap in epilepsy. It is estimated that 80% of patients with epilepsy live in countries with developing economies where access to physicians, diagnostic tools and medications is limited.23 The treatment gap, or the number of people with active epilepsy who are untreated, has an inverse relationship to the income level of the country and rural areas are particularly affected.24
For pregnant patients well-controlled on valproic acid, there is a balancing act between the risks from continuing this medication vs loss of seizure control. A study using data from EURAP (International Registry of Antiepileptic Drugs and Pregnancy) found that women maintained on valproic acid had lower probability of generalized tonic-clonic seizure compared to the groups that were either taken off this medication or switched to another medication in the first trimester.25 However, remaining on valproic acid early in pregnancy carries risks for offspring, including congenital malformations, particularly neural tube defects, cognitive or learning impairment and risk of autism spectrum disorders.13–17,25 There is evidence of a dose-dependent relationship between valproic acid administration and risk of MCMs, where doses higher than 1500 mg per day were associated with much greater OR compared with 700 mg or less per day,9 suggesting that decreasing the dosage of valproic acid in patients who require this medication might mitigate risk. Per the AAN guidelines, fetal exposure to valproic acid, and fetal exposure to AED polytherapy, should be limited as much as possible to minimize risk of MCMs.26 In addition, cognitive deficits after in utero valproic acid exposure are also dose-dependent,16 and these effects may extend into the third trimester. Thus, in general, valproate should be avoided in women of childbearing potential if at all possible.
Participation in this study was voluntary and the pool of respondents was limited to those AAN members and readers of Neurology and its spoke journals who chose to participate by following the links provided to the survey, whether online or in print. These results are only a sample of the possible respondents. In addition, although based on not uncommon clinical scenarios, our survey cases were intentionally concise to minimize survey fatigue and dropout rate, and therefore lacked the detail inherent in a patient encounter. On data review, we also chose to include 2 responses, which may have been duplicates of prior responses based on identical geographic information, but had separate survey login attempts. Although this could give additional weight (2) to single respondents, it was determined that these observations were not influential or led to bias in overall results. Lastly, we acknowledge that there is some loss of information accompanying the construction of dichotomous responses from the raw data. These decisions were made for ease of interpretation of otherwise unwieldy results.
Conclusions
The range of responses across practice types suggests there are opportunities to refine the guidelines for the treatment of epilepsy in pregnant women. Our survey data suggests several areas of potential future study, particularly identifying the optimum amount of folic acid supplementation to prevent neurocognitive impairments in children exposed to AEDs in utero. Another relevant clinical question is how aggressively to manage focal seizures in pregnant women, especially in patients with a history of secondary generalization vs those with preserved awareness.
Pregnancy in epilepsy registries have provided valuable large-scale outcomes information on women with epilepsy and their children. With several new AEDs on the market, in future, research will be needed to identify the risks, if any, for malformations, deleterious cognitive or behavioral effects, and other long-term outcomes in children with in utero exposure.
Appendix. Authors
Footnotes
Explore this topic: NPub.org/NCP/pc5
Interactive world map: NPub.org/NCP/map05
More Practice Current: NPub.org/NCP/practicecurrent
Study funding
No targeted funding reported.
Disclosure
I.C. George was previously a member of the Residents and Fellows Section of Neurology. She has received fellowship funding from the National MS Society. L. Bartolini is Section Editor for Neurology: Clinical Practice. Dr. Bartolini is an employee of the federal government. This manuscript was not a term of his employment, nor did he receive any compensation for the manuscript. J. Ney is an employee of the federal government. This manuscript was not a term of his employment, nor did he receive any compensation for the manuscript. Dr. Ney is a consultant for Ceribell, SpecialtyCare, and JEM Research Institute; has served on the Scientific Merit Review Board, HSR1 Section, VA; has received a speaker honorarium from the AAN; has received travel funding from the AAN Medical Economics and Management Committee; and serves on the editorial board of Neurology: Clinical Practice. D. Singhal reports no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Bollig KJ, Jackson DL. Seizures in pregnancy. Obstet Gynecol Clin North Am 2018;45:349–367. [DOI] [PubMed] [Google Scholar]
- 2.Meador KJ, Pennell PB, May RC, et al. Changes in antiepileptic drug-prescribing patterns in pregnant women with epilepsy. Epilepsy Behav 2018;84:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzog AG, Mandle HB, Cahill KE, Fowler KM, Hauser WA. Predictors of unintended pregnancy in women with epilepsy. Neurology 2017;88:728–733. [DOI] [PubMed] [Google Scholar]
- 4.Harden CL, Pennell PB, Koppel BS, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): III. Vitamin K, folic acid, blood levels, and breast-feeding: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia 2009;50:1247–1255. [DOI] [PubMed] [Google Scholar]
- 5.George IC. How do you treat epilepsy in pregnancy? Neurol Clin Pract 2017;7:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Practice current. Available at: neurology.org/collection/practice_current. Accessed June 4, 2018.
- 7.United Nations Department of Economic and Social Affairs. World Economic Situation and Prospects 2012. New York: United Nations; 2012. [Google Scholar]
- 8.Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One 2015;10:e0131130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 2011;10:609–617. [DOI] [PubMed] [Google Scholar]
- 10.Morrow JI, Hunt SJ, Russell AJ, et al. Folic acid use and major congenital malformations in offspring of women with epilepsy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatr 2009;80:506–511. [DOI] [PubMed] [Google Scholar]
- 11.Campbell E, Kennedy F, Russell A, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatr 2014;85:1029–1034. [DOI] [PubMed] [Google Scholar]
- 12.Harden CL. Pregnancy and epilepsy. Continuum (Minneap Minn) 2014;20:60–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjørk M, Riedel B, Spigset O, et al. Association of folic acid supplementation during pregnancy with the risk of autistic traits in children exposed to antiepileptic drugs in utero. JAMA Neurol 2018;75:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bromley R, Weston J, Adab N, et al. Treatment for epilepsy in pregnancy: neurodevelopmental outcomes in the child. Cochrane Database Syst Rev 2014:CD010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker GA, Bromley RL, Briggs M, et al. IQ at 6 years after in utero exposure to antiepileptic drugs: a controlled cohort study. Neurology 2015;84:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 2013;12:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkjær LS, Bech BH, Sun Y, Laursen TM, Christensen J. Association between prenatal valproate exposure and performance on standardized language and mathematics tests in school-aged children. JAMA Neurol 2018;75:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asadi-Pooya AA. High dose folic acid supplementation in women with epilepsy: are we sure it is safe? Seizure 2015;27:51–53. [DOI] [PubMed] [Google Scholar]
- 19.Pittschieler S, Brezinka C, Jahn B, et al. Spontaneous abortion and the prophylactic effect of folic acid supplementation in epileptic women undergoing antiepileptic therapy. J Neurol 2008;255:1926Y1931. [DOI] [PubMed] [Google Scholar]
- 20.Nolan SJ, Tudur Smith C, Weston J, Marson AG. Lamotrigine versus carbamazepine monotherapy for epilepsy: an individual participant data review. Cochrane Database Syst Rev 2016:CD001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson EL, Stowe ZN, Ritchie JC, et al. Carbamazepine clearance and seizure stability during pregnancy. Epilepsy Behav 2014;33:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickrell WO, Lacey AS, Thomas RH, Lyons RA, Smith PE, Rees MI. Trends in the first antiepileptic drug prescribed for epilepsy between 2000 and 2010. Seizure 2014;23:77–80. [DOI] [PubMed] [Google Scholar]
- 23.Birbeck GL. Epilepsy care in developing countries: part I of II. Epilepsy Curr 2010;10:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer AC, Dua T, Ma J, Saxena S, Birbeck G. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ 2010;88:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomson T, Battino D, Bonizzoni E, et al. Withdrawal of valproic acid treatment during pregnancy and seizure outcome: observations from EURAP. Epilepsia 2016;57:e173–7. [DOI] [PubMed] [Google Scholar]
- 26.Harden CL, Meador KJ, Pennell PB, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009;73:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.