Abstract
Background
Patients with biopsy-proven systemic sarcoidosis who develop a chronic CNS disorder are often presumed to have neurosarcoidosis (NS), however, the possibility of comorbid neurologic disease, such as MS, must be considered if presentation and course are not typical for NS.
Methods
Retrospective chart review across 4 academic MS centers was undertaken to identify patients with diagnosis of MS (2017 McDonald criteria) and biopsy-confirmed extraneural sarcoidosis. Data were abstracted from each chart using a case report form that systematically queried for demographic, clinical, and paraclinical characteristics relevant to NS and MS.
Results
Ten patients met our inclusion criteria (mean age 47.7 [±5.9] years; 80% female). Noncaseating granulomas consistent with sarcoidosis were found on biopsy in all cases (lung 7/10, mediastinum 2/10, liver 1/10, spleen 1/10, and skin 1/10). Diagnosis of MS was based on clinical history of MS-like relapses and MRI findings characteristic of demyelination and typical disease evolution during follow-up (average of 7 years). No patient developed features of NS that could be considered a “red flag” against the diagnosis of MS (such as meningeal enhancement, hydrocephalus, and pituitary involvement). All patients were treated with disease-modifying therapy for MS.
Conclusions
We propose a rational diagnostic approach to patients with sarcoidosis who may have comorbid MS. When the clinical picture is equivocal, the presence of multiple “MS-typical lesions” and the absence of any “NS-typical lesions” on MRI favor diagnosis of MS. Close follow-up is required to ascertain whether clinical and radiologic disease evolution and response to MS therapies conform to the proposed diagnosis of MS.
Sarcoidosis is a rare multisystem inflammatory disease characterized by noncaseating granulomas in affected tissues.1 Neurologic organ system manifestations of sarcoidosis—neurosarcoidosis (NS)—are reported in 5%–34% of patients in clinical series and 14%–27% in postmortem studies.2–4 Sarcoidosis may occasionally present with as NS5 and may be confined to the brain or spinal cord without any systemic involvement in up to 20% of patients.6,7 Diagnostic criteria for NS include typical clinical features and supportive evidence of sarcoidosis on histopathology and exclusion of alternative diagnoses.8–10
MS is a much more prevalent disease than NS (208 cases of MS per 100,000 in the United states vs less than 5 per 100,000 for NS2,11). However, in patients with preexisting sarcoidosis who develop chronic neurologic illness, there is a tendency to assume that NS is the more likely possibility.12 Both MS and NS can follow a relapsing or progressive course; however, several distinctive clinical syndromes of NS are not seen in MS, e.g., multiple cranial neuropathies, myeloradiculopathy with spinal root involvement, endocrinopathy due to lesions along the hypothalamic-pituitary axis (although hypothalamic involvement could be seen in both conditions13), aseptic meningitis, and hydrocephalus. On the other hand, some NS presentations—e.g., optic neuritis (ON), myelitis or myelopathy, and ataxic paraparesis—can be seen in MS as well.
For patients with visual symptoms, distinguishing the 2 etiologies can be particularly challenging. Uveitis that is confined to the anterior segment of the eye is the most frequent ocular manifestation of sarcoidosis and much less common in MS, which typically manifests as unilateral retrobulbar ON.14–16 Acute bilateral ON, on the other hand, is seen in 30% of patients with NS, but in less than 1% with MS.17,18 Myelopathy may also be seen in both conditions manifesting more as a chronic progressive paraparesis; spinal MRI in NS often demonstrates meningeal and nerve root involvement,19 whereas in MS, meningeal enhancement has not been reported.
As features of NS and MS overlap, diagnostic certainty may not be always possible without recourse to brain or cord biopsy. However, pathologic confirmation is often not feasible or desirable given the risk, and the treating physician must make a judgment—based on clinical and paraclinical features—as to whether the patient has 1 or 2 diseases and treat accordingly. The correct diagnosis is essential because the misdiagnosis of NS would not only deprive the patient of highly effective MS therapies but may also expose them to NS-specific therapies that may worsen MS, such as tumor necrosis factor alpha antagonists.20,21
In this article, we report a series of 10 patients followed in specialized MS centers with biopsy-proven systemic sarcoidosis who were diagnosed and treated for comorbid MS. These patients were followed in our centers for more than 7 years (on average) following the diagnosis of MS, and their clinical and radiographic course was deemed consistent with MS by treating neurologists. We outline the rationale that led us to propose dual diagnoses of sarcoidosis and MS and suggest an approach for differentiating MS from NS in patients with sarcoidosis for whom brain or cord biopsy is not a practical option.
Methods
After local institutional review board approval, retrospective chart review was conducted in 4 academic Multiple Sclerosis Centers (NYU Langone Medical Center, New York, NY; Vanderbilt University Medical Center, Nashville, TN; Brigham and Woman's Hospital, Boston, MA; and University of California San Francisco, San Francisco, CA). We included all patients meeting McDonald criteria for MS (with the proviso that brain/cord biopsy was not performed to confirm the diagnosis of MS and refute diagnosis of NS). All patients had biopsy-confirmed sarcoidosis outside of the CNS. Data were abstracted from the chart by the onsite investigator using a case report form that allowed for systematic collection of demographic and disease-related characteristics. The form included a comprehensive checklist of clinical, laboratory, and radiologic findings that have been integrated into outside diagnostic criteria and described in the literature as relevant to the diagnosis of NS and MS.8,9,22 Categorical variables were summarized using frequencies and percentages. Continuous variables were summarized using mean and SDs or median and interquartile range, as appropriate.
Standard protocol approvals, registrations, and patient consents
Approval from the local institutional review board was obtained at each site before chart review.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
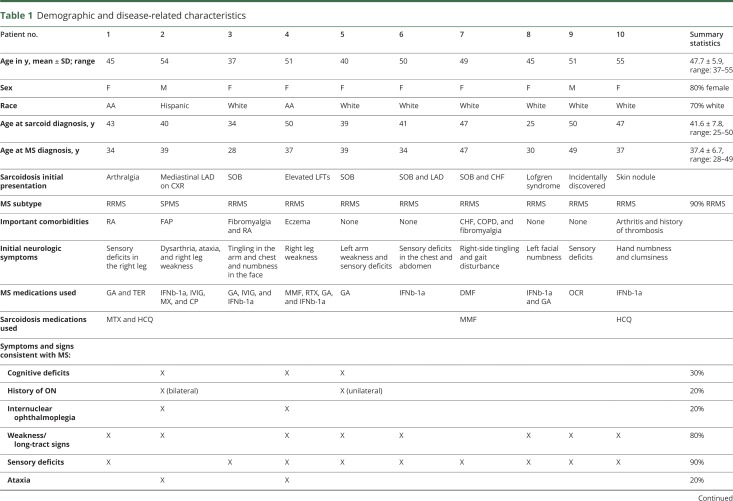
Ten patients (3 at NYU; 3 at Vanderbilt; 3 at Brigham and Women's; and 1 at UCSF) met our inclusion criteria. Baseline demographic characteristics are presented in table 1.
Table 1.
Demographic and disease-related characteristics
Sarcoidosis history
Mean age at diagnosis of sarcoidosis was 41.6 years (SD 7.8). Presenting sarcoidosis symptoms were pulmonary (N = 4), rheumatologic (N = 1), dermatologic (N = 1), and Löfgren syndrome (N = 1); 2 patients were found to have mediastinal lymphadenopathy on chest x-ray, but were asymptomatic at presentation, and 1 patient had elevated liver enzymes at presentation. Findings consistent with sarcoidosis were seen on chest x-ray or CT of the chest in 9/10 patients. Whole-body fluorodeoxyglucose-PET scan results were recorded for 2 patients and were consistent with systemic sarcoidosis in both. Biopsy was performed in all patients, with 2 patients requiring biopsy in more than 1 site. Location of biopsy was as follows: lung in 7/10, mediastinum in 2/10, liver in 1/10, spleen in 1/10, and skin in 1/10. Noncaseating granulomas consistent with sarcoidosis were seen in all patients. Only 3 patients were prescribed sarcoidosis-specific immunotherapy, and 2 of them remained on this therapy at final follow-up. The average sarcoidosis duration at final follow-up was 6.1 years (range 1–20 years).
Neurologic history
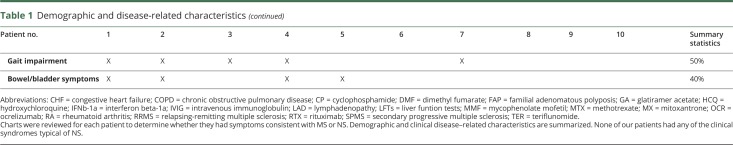
Age at onset of neurologic symptoms was 36.3 years (range 28–47 years). Neurologic symptoms predated systemic sarcoidosis diagnosis in 7 of 10 patients. Patients were followed by 1 or more MS specialists at the respective centers for an average of 7.4 years (range 1–17 years). All patients met 2017 criteria for MS22 at final follow-up. Disease subtype was relapsing-remitting in 9 patients and secondary progressive in 1 patient at final follow-up. Neurologic symptoms on presentation and at final follow-up are shown in table 1. Most common findings were sensory deficits (90%), weakness/long tract signs (80%), and gait impairment (50%). All patients were started on disease-modifying therapy (DMT) for MS, and 7 patients were receiving DMT at the time of final follow-up. During follow-up, 4/10 patients had experienced clinical relapses consistent with MS. None of the patients experienced clinical syndromes characteristic of NS—listed in table 3—at any point in their disease course.
Table 3.
Clinical syndrome and neuroradiologic findings reported in neurosarcoidosis
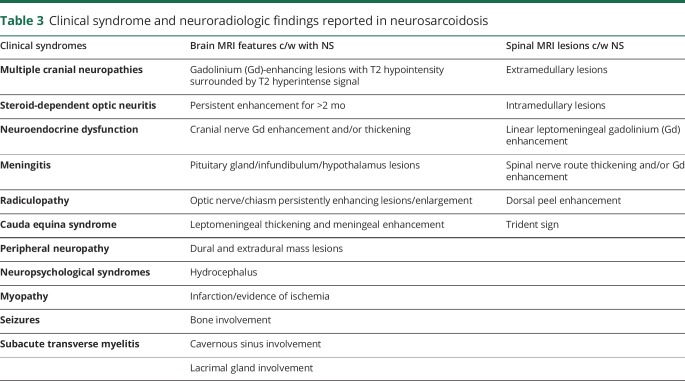
One or more MRIs of the brain were available for review for all 10 patients, MRI of the cervical spine for 9 patients, and MRI of the thoracic spine for 4 patients. All MRIs were reviewed, and findings documented by the onsite investigators using a checklist of MS- and NS-typical features listed in table 2. Each patient had radiographic findings in the brain and spinal cord typical for MS (table 4), and all fulfilled the Barkhof criteria for space dissemination, with the exception of 1 patient with predominantly spinal MS. The most common lesion type was “periventricular lesion/Dawson finger” (in 9/10 patients) and “short, peripheral cord lesion” (in 9/10 patients). Other common lesion types were cortical/juxtocortical lesions (5/10 patients) and corpus callosum lesions (5/10 patients). Examples of MS-typical MRI findings in patient 2 and patient 4 are shown in figure 1. Importantly, other than findings of radiographic ON, which are common to both MS and NS, none of the patients had brain or spinal MRI features typical of NS. A list of NS-typical brain and cord lesions is provided in table 3, and illustrative examples of brain and cord lesions from patients diagnosed with NS our practices are shown in figure 2. During follow-up, 7/10 patients developed new T2 and/or gadolinium-enhancing lesions; all were typical for MS.
Table 2.
Brain and spinal cord MRI findings characteristic of MS
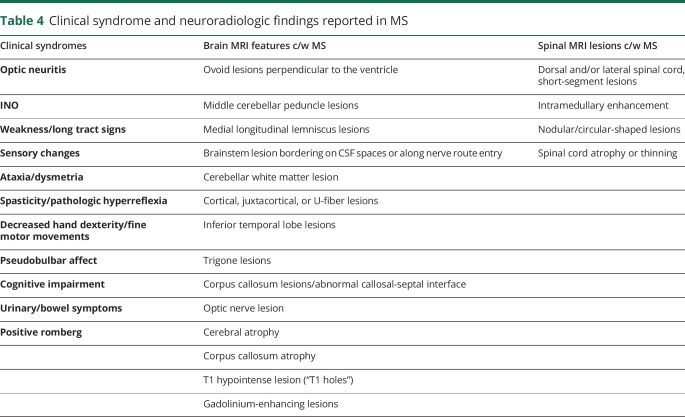
Table 4.
Clinical syndrome and neuroradiologic findings reported in MS
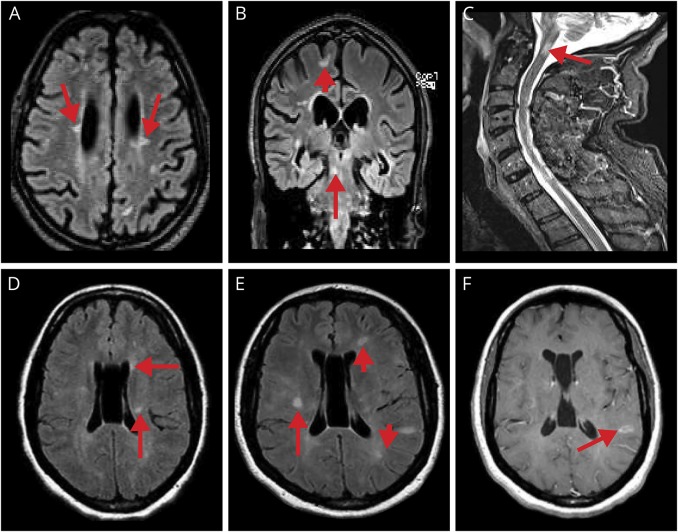
Figure 1. Examples of typical MS MRI findings.
A (axial FLAIR), B (coronal FLAIR), and C (sagittal T2W) show images from patient 2. The arrows in A point to “Dawson fingers”; the arrowhead in B points to a U-fiber lesion, and the longer arrow points to a peripheral pontine lesion; the long arrow in C points to a short, intramedullary C2 lesion. D (axial FLAIR), E (axial FLAIR), and F (axial T1 postgadolinium) show images from patient 4. (D) Arrows point to a few T2 hyperintense periventricular lesions. (E) MRI 1 year later demonstrates the development of several new T2 hyperintense lesions including a right subcortical lesion (long arrow), left frontal periventricular Dawson finger (short arrow), and a left temporoparietal juxtacortical lesion (arrowhead). (F) The arrow points to enhancement of the left temporoparietal juxtacortical lesion seen in E. FLAIR = fluid-attenuated inversion recovery.
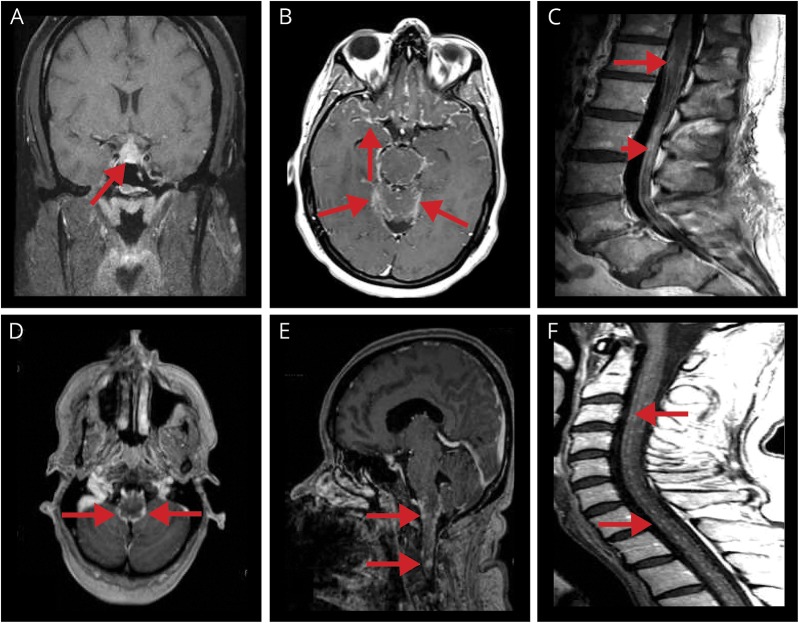
Figure 2. Examples of typical NS MRI findings.
(A) Coronal T1 postgadolinium pituitary and pituitary stalk lesion with enhancement (arrow). (B) Axial T1 postgadolinium leptomeningeal contrast enhancement primarily about the base of the brain (in a patient with probable NS, known to have biopsy-confirmed pulmonary sarcoidosis; arrows). (C) Midsagittal T1 postgadolinium of lumbar spine thickening and smooth/nodular leptomeningeal enhancing lesions surrounding the conus medullaris (arrow) and cauda equina (arrowhead). (D) Axial T1 postgadolinium leptomeningeal enhancement of the midbrain (arrows). (E) Sagittal postgadolinium nodular leptomeningeal enhancement of the lower brainstem (arrows). (F) Midsagittal T1 postgadolinium of cervical spine multiple nodular leptomeningeal enhancing lesions (arrows; in a patient with probable NS, known to have biopsy-confirmed pulmonary sarcoidosis). NS = neurosarcoidosis.
CSF analysis was performed for all 10 patients. White cell counts were mildly elevated in 10%, and protein and glucose were normal in all patients; multiple unmatched oligoclonal bands in CSF were found in 6 patients and elevated immunoglobulin G index in 4 patients. Although, both MS and NS can have similar findings in CSF (e.g., unmatched oligoclonal bands), features that would be typical for NS and atypical for MS (e.g., low glucose, very high protein, and white blood cell count >50) were absent in all our patients. CSF angiotensin converting enzyme (ACE) was measured in 2 patients and was within normal limits in both cases.
Discussion
NS is considered an MS “mimicker” and is included in the differential diagnosis of a patient with T2/fluid-attenuated inversion recovery hyperintense lesions on brain or spinal MRI. Patients with biopsy-proven systemic sarcoidosis are sometimes presumed to have NS if they develop a neurologic illness. However, the possibility of a co-occurring neurologic disease, such as MS, should not be discounted.
Clinical and radiographic features of MS and NS are sufficiently distinctive to allow for these 2 disorders to be differentiated with a reasonable degree of certainty in many cases. Therefore, a patient with systemic sarcoidosis and clinical and radiologic features consistent with MS and not NS should—in our view—be diagnosed with MS and systemic sarcoidosis, rather than NS.
This conclusion is contrary to the prevailing tendency to doubt the coexistence of MS and systemic sarcoidosis if a patient is found to have sarcoidosis outside of the nervous system.12 An obvious objection to the “dual diagnosis” paradigm is that it appears to contradict the “law of parsimony” (“Occam's razor”) whereby it is preferable to invoke a single disease etiology to explain diverse clinical manifestations rather than to invoke multiple etiologies. However, we believe that disease definitions should not be stretched beyond their established boundaries in the name of parsimony. If a patient with sarcoidosis develops symptoms or MRI lesions inconsistent with NS, an alternative diagnosis should be pursued. Radiologic features of both diseases are well described (as summarized in tables 3 and 4). By systematically applying these checklists to individual patients, clinicians can improve diagnostic confidence. Additional support for the idea that MS and systemic sarcoidosis can coexist comes from recent experience with immunomodulatory DMT for MS, daclizumab and alemtuzumab, which have been associated with the emergence of systemic sarcoidosis.23–26 However, the possibility that these cases represent drug-induced sarcoidosis cannot be excluded.
The 10 patients with sarcoidosis in our series presented with clinical relapses that were not specifically typical for NS, but some presentations—e.g., progressive spastic paraparesis—could be seen in NS as well. Thus, differentiating MS or NS on clinical grounds alone is not always possible. Analyses of lesions on MRI are of great value in separating MS from NS, despite some degree of radiographic overlap between the 2 conditions. Applying the MRI Lesion Checklist approach, we have shown that none of the patients have exhibited any of the radiographic stigmata of NS, and all of them had multiple typical radiographic stigmata of MS. The radiologic considerations were decisive in proposing a diagnosis of comorbid MS and treating them accordingly. During the mean follow-up of 7.4 years, all patients experienced either clinical or MRI disease activity that was characteristic of MS and, none of the patients had developed features typical of NS (table 3), furthering the confidence in dual diagnoses in these patients. The MRI findings in our series stand in contrast to a series of 32 patients diagnosed with NS who exhibited MRI findings that were inconsistent with MS (multiple cranial nerve enhancement, leptomeningeal disease, and dural involvement), which continued to evolve in sync with clinical changes.3
Adjuvant laboratory and radiographic studies have been described in the literature to identify markers for disease differentiation. Elevated serum ACE has a well-known association with sarcoidosis and is also elevated in a small proportion of NS cases even in the absence of systemic disease. However, serum ACE lacks sensitivity and specificity for sarcoidosis diagnosis and is not useful for monitoring response to therapy.27 CSF findings, which are sometimes associated with NS, such as elevated CD4:CD8 ratio >5, and abnormal CSF/serum albumin quotient28 are not always measured in clinical practice. Elevated ACE in CSF is neither sufficiency sensitive nor specific for NS.27 Of the radiologic studies, PET/CT has been used for identifying active NS29 it was found helpful for differentiating spinal NS from spinal malignancies.30 To our knowledge, this technique has not yet been applied for discriminating NS from other inflammatory diseases of CNS.
Although the idea of co-occurring MS and sarcoidosis has been proposed before,31,32 it appears to be epidemiologically rare, given that our larger denominator series found only 10 proposed cases in clinical records of 4 large referral MS centers that have evaluated over 15,000 patients with MS. Whether this may relate in part to divergent susceptibility to MS and sarcoidosis based on genetic, environmental, or other factors remains unknown. It is also notable that sarcoidosis disease activity in these patients tended to be relatively benign and quiescent over time. Although this could relate in part to MS immunomodulatory therapy benefiting sarcoidosis—and all our patients were receiving MS DMT—it is also possible that the immunologic processes that drive sarcoidosis and MS may somehow be antagonistic. The pathophysiologic mechanisms that underlie co-occurrence and its relative rarity thus remain an interesting area for future study.
A limitation of our study is the lack of neuropathologic confirmation of MS. Previous studies have also highlighted the frequency of nonspecific white matter lesions in NS and the difficulty with which they are differentiated from MS.1,33,34 Researchers have also pointed to cases in which MRI abnormalities that were potentially consistent with MS were later biopsied and found to be granulomatous.12,28,33 Scott et al.12 report a case of a patient who was thought to have MS, with biopsy of a deep white matter lesion revealing NS; the details of this case, however, are not presented. Miller et al.33 also report a case of a large parenchymal lesion thought to be a tumefactive demyelinating lesion that was subsequently shown to be NS on biopsy; however, the radiologic features of this latter case—“diffuse, patchy, cortical, meningeal enhancement” on CT of the head—are highly uncharacteristic of MS.33 Application of the MS Lesion Checklist35 may help to differentiate MRI with un-MS-like lesions from the more typical demyelinating lesions of MS, but this approach still requires validation.
Conclusions
We have presented patients with typical clinical and radiologic features of MS and no features characteristic of recognized NS syndromes. We were able to observe evolution of our patients' disease over an extended period—7 years on average—which afforded us the opportunity to observe additional features typical of MS and document the absence of “red flags” for MS diagnosis, thereby increasing confidence in dual diagnoses. Patients with biopsy-confirmed systemic sarcoidosis who develop a neurologic syndrome with clinical and MRI findings typical for NS (table 3) can be diagnosed and treated as probable NS after the relevant mimics are excluded (e.g., infectious causes in a patient with meningitis). However, in patients without typical NS clinical or radiologic findings, the possibility of a comorbid neurologic disorder must be carefully considered. Clinicians should be alert to the possibility of a dual diagnosis and not assume that a CNS disease in patients with sarcoidosis represents NS. The presence of multiple MS-typical lesions and the absence of NS-typical lesions19 in a clinical context consistent with MS, in our opinion, favor diagnosis of MS. Patients should continue to be followed closely to determine whether clinical and radiologic disease evolution and response to MS therapies conform with MS diagnosis and the emergence of any MS-atypical features should lead to reassessment. Correct diagnosis is essential as treatments for MS and NS differ, and some therapies that may benefit NS could exacerbate MS. In patients with sarcoidosis and chronic CNS inflammatory disorder of unclear etiology, one could consider treatment with therapies that are felt to be safe and possibly effective for both NS and MS, such as methotrexate, mycophenolate, and pulse cyclophosphamide.
Author contributions
C. Tyshkov: drafting/revising the manuscript, data acquisition, study concept or design, and analysis or interpretation of data. S. Pawate, M. J. Bradshaw, and D. J. Kimbrough: drafting/revising the manuscript, data acquisition, and analysis or interpretation of data. T. Chitnis: data acquisition and study supervision. J. M. Gelfand: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, and acquisition of data. L. Zhovtis Ryerson and I. Kister: drafting/revising the manuscript, data acquisition, study concept or design, analysis or interpretation of data, and study supervision.
Study funding
No targeted funding reported.
Disclosure
C. Tyshkov has received a clinical MS Fellowship supported and funded by Biogen. S. Pawate has attended advisory board meetings for Biogen. M. J. Bradshaw serves on the editorial board of Continuum: Lifelong Learning in Neurology. D. J. Kimbrough reports no disclosures. T. Chitnis has served on the scientific advisory boards of Biogen, Celgene, Novartis, and Sanofi-Genzyme and has received research grants for her institution from Octave, Serono, and Verily. J. M. Gelfand has received research support from Genentech, MedDay, and Quest Diagnostics, personal fees for consulting from Biogen, and personal fees for medical legal consulting. L. Zhovtis Ryerson has served on the speakers' bureaus of Teva, Genzyme, and Biogen and on the advisory boards of Biogen and Celgene and has received research support from Biogen. I. Kister has served on the scientific advisory boards of Biogen Idec and Genentech and received research support for his institution from the Guthy-Jackson Charitable Foundation, National Multiple Sclerosis Society, Biogen Idec, Serono, Genzyme, and Novartis. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Patients with biopsy-proven systemic sarcoidosis who develop a chronic CNS disorder are usually presumed to have neurosarcoidosis (NS); however, the possibility of a comorbid neurologic disease, such as multiple sclerosis (MS), must be considered if presentation and course are not typical for NS.
→ Clinical and radiographic features of MS and NS are sufficiently distinctive to allow for these 2 disorders to be differentiated from 1 another with a reasonable degree of certainty in most cases.
→ Where clinical picture is equivocal, the presence of multiple “MS-typical lesions” and the absence of any “NS-typical lesions” on MRI favor diagnosis of MS.
→ Close follow-up is required to ascertain whether clinical and radiologic disease evolution and response to MS therapies conform to the proposed diagnosis of MS.
References
- 1.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007;357:2153–2165. [DOI] [PubMed] [Google Scholar]
- 2.Joseph FG, Scolding NJ. Sarcoidosis of the nervous system. Pract Neurol 2007;7:234–244. [DOI] [PubMed] [Google Scholar]
- 3.Shah R, Roberson GH, Curé JK. Correlation of MR imaging findings and clinical manifestations in neurosarcoidosis. AJNR Am J Neuroradiol 2009;30:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joubert B, Chapelon-Abric C, Biard L, et al. . Association of prognostic factors and immunosuppressive treatment with longterm outcomes in neurosarcoidosis. JAMA Neurol 2017;74:1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zajicek JP, Scolding NJ, Foster O, et al. . Central nervous system sarcoidosis—diagnosis and management. QJM 1999;92:103–117. [DOI] [PubMed] [Google Scholar]
- 6.Pawate S, Moses H, Sriram S. Presentations and outcomes of neurosarcoidosis: a study of 54 cases. QJM 2009;102:449–460. [DOI] [PubMed] [Google Scholar]
- 7.Gelfand JM, Bradshaw MJ, Stern BJ, et al. . Infliximab for the treatment of CNS sarcoidosis: a multi-institutional series. Neurology 2017;89:2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zajicek JP. Neurosarcoidosis. Curr Opin Neurol 2000;13:323–325. [DOI] [PubMed] [Google Scholar]
- 9.Marangoni S, Argentiero V, Tavolato B. Neurosarcoidosis: clinical description of 7 cases with a proposal for a new diagnostic strategy. J Neurol 2006;253:488–495. [DOI] [PubMed] [Google Scholar]
- 10.Stern BJ, Royal W III, Gelfand JM, et al. . Definition and consensus diagnostic criteria for neurosarcoidosis: from the neurosarcoidosis consortium consensus group. JAMA Neurol 2018;75:1546–1553. [DOI] [PubMed] [Google Scholar]
- 11.Wallin M. The prevalence of MS in the United States: A population-based estimate using health claims data. US Multiple Sclerosis Prevalence Workgroup. Neurology 2019;92:e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott TF, Yandora K, Kunschner LJ, Schramke C. Neurosarcoidosis mimicry of multiple sclerosis: clinical, laboratory, and imaging characteristics. Neurologist 2010;16:386–389. [DOI] [PubMed] [Google Scholar]
- 13.Burfeind KG, Yadav V, Marks DL. Hypothalamic dysfunction and multiple sclerosis: implications for fatigue and weight dysregulation. Curr Neurol Neurosci Rep 2016;16:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabs DA, Johns CJ. Ocular involvement in chronic sarcoidosis. Am J Ophthalmol1986;102:297–301. [DOI] [PubMed] [Google Scholar]
- 15.Bachman OM, Rosenthal AR, Beckingsale AB. Granulomatous uveitis in neurological disease. Br J Ophthalmol 1985;69:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rush JA. Retrobulbar optic neuropathy in sarcoidosis. Ann Ophthalmol 1980;12:390–394. [PubMed] [Google Scholar]
- 17.Kidd DP, Burton BJ, Graham EM, Plant GT. Optic neuropathy associated with systemic sarcoidosis. Neurol Neuroimmunol Neuroinflamm 2016;3:e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burman J, Raininko R, Fagius J. Bilateral and recurrent optic neuritis in multiple sclerosis. Acta Neurol Scand 2011;123:207–210. [DOI] [PubMed] [Google Scholar]
- 19.Ginat DT, Dhillon G, Almast J. Magnetic resonance imaging of neurosarcoidosis. J Clin Imaging Sci 2011;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study: the lenercept multiple sclerosis study group and the university of British columbia MS/MRI analysis group. Neurology 1999;53:457–465. [PubMed] [Google Scholar]
- 21.van Oosten BW, Barkhof F, Truyen L, et al. . Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 1996;47:1531–1534. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AJ, Banwell BL, Barkhof F, et al. . Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–173. [DOI] [PubMed] [Google Scholar]
- 23.Rhone EE, Cho PSP, Birring SS, Galloway J, Silber E. Pulmonary sarcoidosis in a patient with multiple sclerosis on daclizumab monotherapy. Mult Scler Relat Disord 2018;20:2527. [DOI] [PubMed] [Google Scholar]
- 24.Willis MD, Hope-Gill B, Flood-Page P, et al. . Sarcoidosis following alemtuzumab treatment for multiple sclerosis. Mult Scler 2018;24:1779–1782. [DOI] [PubMed] [Google Scholar]
- 25.Viana de Andrade AC, Brito EA, Harris OM, Viana de Andrade AP, Leite MF, Pithon MM. Development of systemic sarcoidosis and xanthoma planum during multiple sclerosis treatment with interferon-beta 1a: case report. Int J Dermatol 2015;54:e140–e145. [DOI] [PubMed] [Google Scholar]
- 26.Carbonelli C., Montepietra S., Caruso A, et al. . Sarcoidosis and multiple sclerosis: systemic toxicity associated with the use of interferon-beta therapy. Monaldi Arch Chest Dis 2012;77:29–31. [DOI] [PubMed] [Google Scholar]
- 27.Hoyle JC, Jablonski C, Newton HB. Neurosarcoidosis: clinical review of a disorder with challenging inpatient presentations and diagnostic considerations. Neurohospitalist 2014;4:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wengert O, Rothenfusser-Korber E, Vollrath B, et al. . Neurosarcoidosis: correlation of cerebrospinal fluid findings with diffuse leptomeningeal gadolinium enhancement on MRI and clinical disease activity. J Neurol Sci 2013;335:124–130. [DOI] [PubMed] [Google Scholar]
- 29.Khodarahmi I, Turbin RE, Frohman LP, Ghesani N. 18F-FDG uptake in neurosarcoid dural plaque on PET/CT. Clin Nucl Med 2016;41:e410–e411. [DOI] [PubMed] [Google Scholar]
- 30.Flanagan EP, Hunt CH, Lowe V, et al. . [(18)F]fluorodeoxyglucose-positron emission tomography in patients with active myelopathy. Mayo Clin Proc 2013;88:1204–1212. [DOI] [PubMed] [Google Scholar]
- 31.Kimbrough D, Reyes-Mantilla M, Arango JJ, Pardo-Villamizar C. Identifying multiple sclerosis in patients with systemic sarcoidosis. Neurology 2013;80(7 suppl):P06. 214. [Google Scholar]
- 32.Reess J, Mauch E, Kornhuber HH. Simultaneous occurrence of sarcoidosis and MS or sarcoidosis within the clinical picture of multiple sclerosis? Nervenarzt 1992;63:503–505. [PubMed] [Google Scholar]
- 33.Miller DH, Kendall BE, Barter S, et al. . Magnetic resonance imaging in central nervous system sarcoidosis. Neurology 1988;38:378–383. [DOI] [PubMed] [Google Scholar]
- 34.Metyas S, Tawadrous M, Yeter KC, Arkfeld DG. Neurosarcoidosis mimicking multiple sclerosis successfully treated with methotrexate and adalimumab. Int J Rheum Dis 2014;17:214–216. [DOI] [PubMed] [Google Scholar]
- 35.Kister I. The MS Lesion Checklist: clinician's guide for evaluating brain MRI in “improbable MS”. Practical Neurology. 2018;68–73.30097553 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.