Abstract
Purpose of review
In September 2018, the Centers for Disease Control and Prevention (CDC) published an evidence-based guideline on the diagnosis and management of mild traumatic brain injury (mTBI) among children.
Recent findings
Based on a systematic review of the evidence that covers research published over a 25-year span (1990–2015), the CDC Pediatric mTBI Guideline strives to optimize the care of pediatric patients with mTBI. The guideline was developed using a rigorous methodology developed by the American Academy of Neurology.
Summary
Clinical practice recommendations in the CDC Pediatric mTBI Guideline can help guide neurologists with critical diagnostic and management decisions and to implement evidence-based strategies for the recovery of their young patients with this injury.
In its recently published Guideline on the Diagnosis and Management of Mild Traumatic Brain Injury (mTBI) Among Children, the Centers for Disease Control and Prevention (CDC) defines mTBI to be inclusive of patients, with “Glasgow Coma Scale scores of 13–15 with or without the complication of intracranial injury (ICI) on neuroimaging, and regardless of potentially requiring a hospital admission and/or neurosurgical intervention”.1 Caused by a force or impact to the head or body that causes the brain to accelerate and decelerate with translational, rotational, and/or angular forces, an mTBI is associated with a complex cascade of ionic, metabolic, and physiologic events2–6 (figure 1).
Figure 1. Mechanism of mild traumatic brain injury (mTBI).
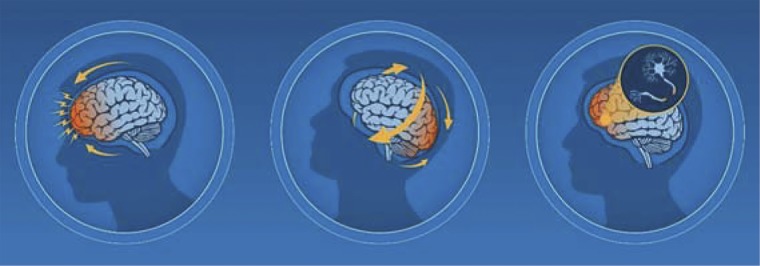
mTBI results from an impact to the head or body, which leads to a complex cascade of ionic, metabolic, and physiologic events. Source: Used with permission from the Centers for Disease Control and Prevention, cdc.gov/HEADSUP.
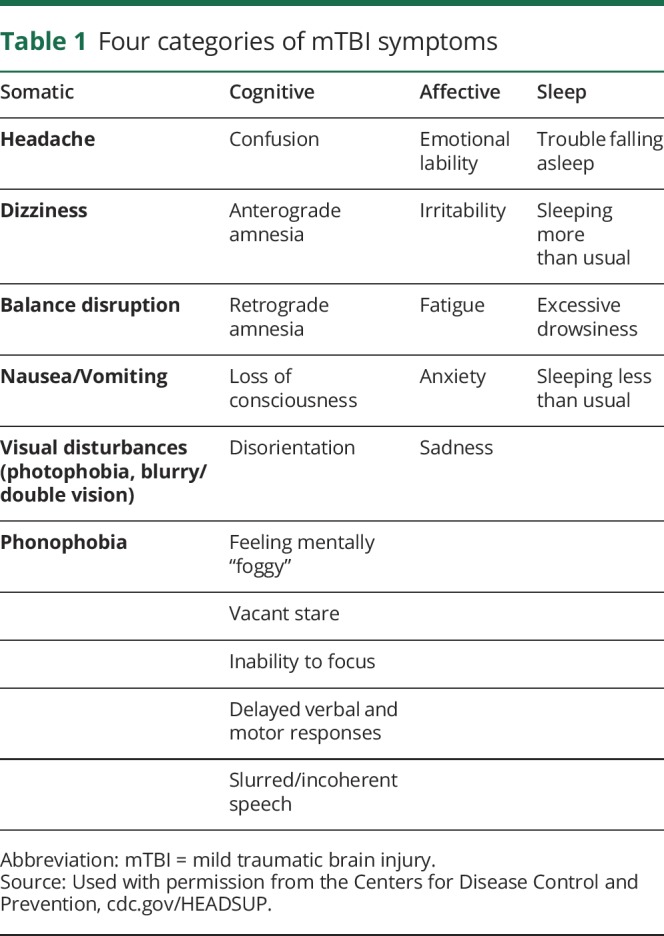
An individual with mTBI generally presents with clinical symptoms that fall into 4 categories: somatic, cognitive, affective, and sleep (table 1).7 Most pediatric patients with mTBI will no longer experience symptoms within a couple of weeks8,9; 70%–80% return to baseline within 3 months.10–12 The factors associated with a prolonged recovery include the following: Hispanic ethnicity, age (particularly adolescents), neurologic or mental health disorders, learning difficulties, and family and social stressors.1 For pediatric patients whose symptoms are ongoing, an mTBI can affect their ability to participate in school and other activities of daily living, such as social activities with friends and physical exercise.
Table 1.
Four categories of mTBI symptoms
To help optimize the care and support the recovery of pediatric patients with mTBI, CDC's Pediatric mTBI Guideline includes 19 sets of clinical practice recommendations. These recommendations cover diagnosis, prognosis, and management/treatment. This review provides an overview of the process used by CDC to develop the guideline, as well as the practice recommendations most relevant to neurologists, such as those that support diagnostic and management decisions for this injury.
Development of the CDC pediatric mTBI guideline
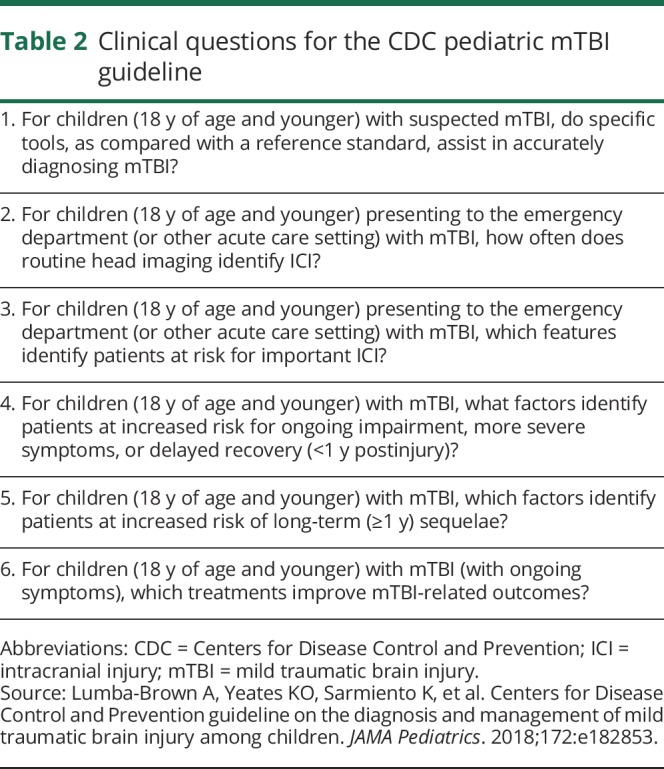
Consistent with other guidelines developed using the American Academy of Neurology (AAN) methodology, the CDC Pediatric mTBI Guideline is based on a comprehensive review and analysis of peer-reviewed literature, public comment, and feedback from experts in the field. The authors of the guideline developed it by independently nominating pertinent clinical questions for consideration, according to an analytic framework utilizing the Patient-Intervention-Comparator-Outcome format.13 Collated questions were presented to the authors for ranking using a modified Delphi process over 3 rounds of voting. Through this process, the authors ultimately selected 6 clinical questions for evaluation via systematic review (table 2).
Table 2.
Clinical questions for the CDC pediatric mTBI guideline
An extensive literature search, spanning 1990–2015, was conducted to identify evidence for each question. Data from each selected full-text article were extracted by at least 2 authors working independently of each other using a standardized form. Disagreement regarding the extracted elements, classification of evidence, or assessment of effect size was resolved by a discussion to reach consensus among the authors.
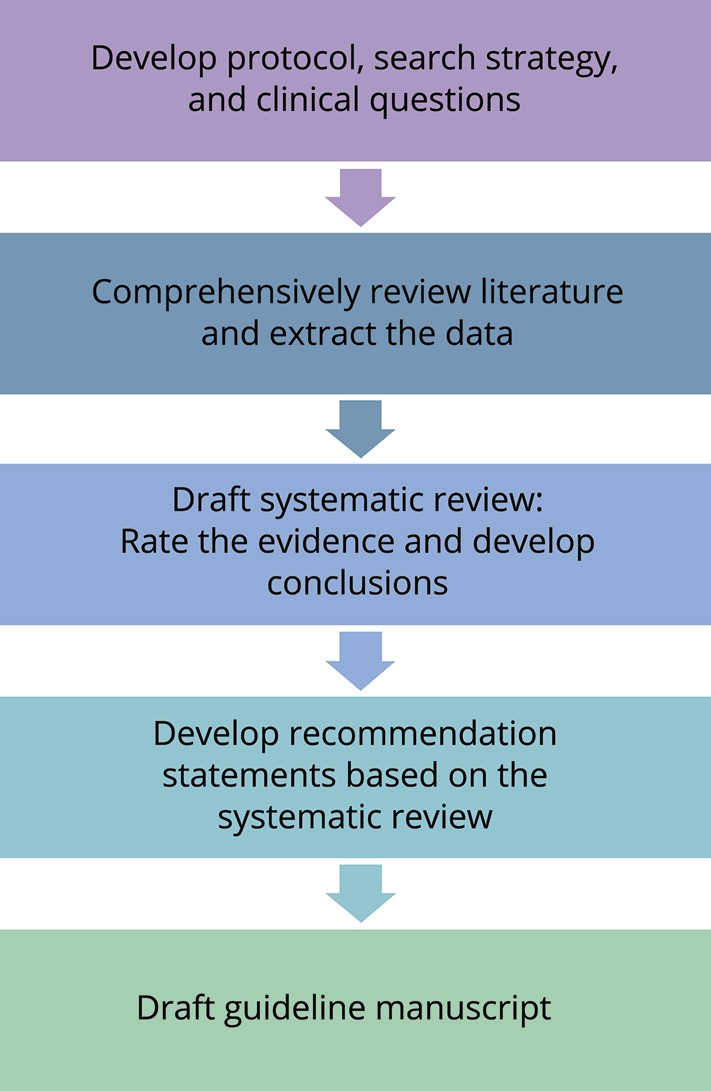
Findings from the literature review and data abstraction were compiled into evidence tables. To judge overall confidence in the evidence, the authors used a modified Grading of Recommendations, Assessment, Development and Evaluations methodology. This process explicitly considered the risk of bias in individual studies (class of evidence), consistency between studies, precision, directness, and magnitude of effect relative to the risk of bias, presence of an expected dose-response relationship, and the direction of bias.14 This method has been designed to be compliant with the 2010 National Academy of Science standards. A summary of the guideline development process is shown in figure 2.
Figure 2. Centers for Disease Control and Prevention pediatric mild traumatic brain injury guideline development process.
CDC pediatric mTBI guideline clinical practice recommendations
In 2013, AAN published the “Summary of evidence-based guideline update: Evaluation and management of concussion in sport” in the journal Neurology®.15 Expanding from this effort, the CDC Pediatric mTBI Guideline is inclusive of all causes of mTBI and focuses solely on children age 18 and under. The CDC Pediatric mTBI Guideline provides practice recommendations relevant to neurologists and other healthcare providers that are consistent with the AAN Concussion in Sports guideline. Below is a snapshot of recommendations contained in the CDC Pediatric mTBI Guideline most relevant for neurologists related to imaging, assessment, posttraumatic headache management/treatment, return to activity, and patient and family education (table 3).
Table 3.
Examples of clinical practice recommendations on the diagnosis and management of mTBI
Imaging
Head CT plays a critical role in distinguishing patients presenting with a suspected traumatic brain injury who are at risk for ICI. However, head CT should not be routinely used to diagnose patients with mTBI.1 The CDC Pediatric mTBI Guideline recommends that, neurologists and other healthcare providers, “use validated clinical decision rules to identify children with mTBI at low risk for ICI, in whom head CT is not indicated, as well as children who may be at higher risk for clinically important ICI.”1 The use of validated tools, such as the Pediatric Emergency Care Applied Research Network (PECARN) decision rules,16 serves to avoid unnecessary pediatric patient's exposure to radiation, while also ensuring that children at risk for clinically important ICI receive the needed imaging and interventions. For children found to have a risk of ICI, but not at the level to justify an immediate imaging study, healthcare providers should counsel their parents and provide information regarding monitoring children for any changes that indicate a more severe injury.
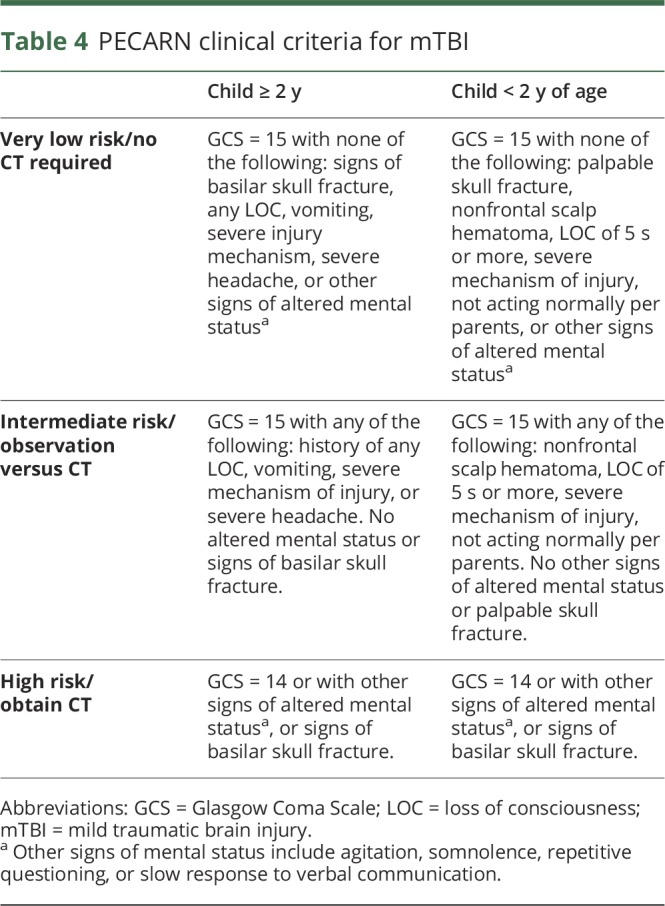
PECARN conducted the largest prospective dedicated pediatric trial for mTBI, which included more than 40,000 children, in 2009.16 The initial PECARN study investigated both children <2 years of age and those 2–17 years of age and identified clinical criteria to stratify those with very low, intermediate, and relatively high risk for significant ICI in the setting of minor head trauma (table 4). This algorithm determined children at very low risk for clinically significant ICI with a 99.9% negative predictive value (NPV) and a 96.8% sensitivity in those ≥2 years of age and a 100% NPV and sensitivity in those <2 years of age. Of note, decisions to image by healthcare providers are also dependent on other clinical factors including multiple vs isolated findings, worsening symptoms or signs over time, age younger than 3 months, and parental preference. In addition, subsequent studies have independently validated the PECARN algorithm, one of which is a large prospective trial in Australia and New Zealand which demonstrated the PECARN criteria for very low risk of clinically significant brain injury to have a 100% NPV and 99% sensitivity in children ≥2 years of age and a 100% NPV and sensitivity in children <2 years of age.17 While the PECARN decision rules are well known by most healthcare providers, the implementation of these rules when evaluating children with mTBI is not universal. Challenges and barriers to the implementation of these rules should be explored and addressed. In addition, while the PECARN decision rules are valuable, the final decision regarding the need for brain imaging rests with the clinical judgment of the healthcare provider treating the patient.
Table 4.
PECARN clinical criteria for mTBI
Assessment
Neurologists should perform a thorough neurologic history and examination on all children presenting with an mTBI. As part of this evaluation, CDC recommends the use of “an age-appropriate, validated symptom rating scale.”1 Examples of validated scales include, but are not limited to, the following: Post-Concussion Symptom Inventory,18 Health and Behavior Inventory,19 Post-Concussion Symptom Scale,20 and Acute Concussion Evaluation.21 Based on the available evidence, the guideline concluded that computerized cognitive testing may also be used as a component of assessment for mTBI.1 However, computerized tools, as well as symptom scales, should not be used in isolation.
Several paper-and-pencil diagnostic/neurocognitive tools exist for older teens and young adults, including the Standardized Assessment of Concussion (SAC) as part of the Sport Concussion Assessment Tool (SCAT). At the time of this paper, there was no good validation of the Child-SCAT; however, more recently several studies have explored baseline normative values of the Child-SCAT in pre-teens and younger children.22,23 These include values for cognitive (SAC-Child) and balance testing (Balance Error Scoring System). Further validation of these tools holds promise for healthcare providers without access to computerized testing.
Posttraumatic headache management/treatment
Early treatment for headache should focus on nonopioid analgesics. However, neurologists are often called on to care for patients with chronic headaches. Because chronic headaches may have multiple contributing factors, neurologists should consider a multidisciplinary evaluation and treatment plan. For patients who present with severe or worsening acute headache, neurologists should consider the use of head CT to assess for more serious injury.
Return to activity
Neurologists should discuss the expected recovery trajectory for their patients with mTBI if management recommendations are followed. This may include counseling patients to refrain from activities with a high risk of fall or other activities that place a child at risk for head or brain injury. Neurologists should review management of cognitive and physical activity and levels of rest with the patient and their families. For most patients, this will entail a gradual resumption of a patient's regular, nonsports activities within a few days at intensity levels that do not exacerbate symptoms.
Healthcare providers should assist children with mTBI to progress through a graduated return to activity plan (figure 3). According to the CDC Pediatric mTBI Guideline, “Following the first several days, healthcare providers should counsel patients and families to resume a gradual schedule of activity that does not exacerbate symptoms, with close monitoring of symptom expression (number, severity).”1 Prior guideline recommendations were relatively regimented regarding return to regular, nonsports activity advice.24 To provide more clarity to healthcare providers, CDC Pediatric mTBI Guideline emphasizes that return to regular, nonsports activity should be individualized approach—recognizing that each concussion and each patient is unique. Certain patients have risk factors that may prolong symptoms and may affect the return to regular, nonsports activity. These factors include a premorbid history of mTBI, lower cognitive ability, presence of intracranial lesion, neurologic or psychiatric disorder, learning difficulties, increased preinjury symptoms, and family and/or social stressors. Most children can return to school within 2–3 days after the injury. However, the return to contact sports process (which includes a separate stepwise process outlined in the AAN Concussion in Sports guideline15) should only be initiated once the child is returning to their regular activities.
Figure 3. Return to non-sports activity plan for children following mild traumatic brain injury.
Source: Used with permission from the Centers for Disease Control and Prevention, cdc.gov/HEADSUP.
Patient and family education
Patient and family education about mTBI, symptom monitoring, graded return to activity, and modified school activities are associated with improved health outcomes for patients with mTBI.1 The CDC Pediatric mTBI Guideline recommends that healthcare providers provide assurance and instructions to the family that is inclusive of warning signs for more serve injury, symptom monitoring tips, the return to activity (such as return to school and play) process, and when to follow up for additional care. Both verbal and written instructions may be beneficial.
Conclusion
This commentary provides a snapshot of the clinical practice recommendations contained in the CDC Pediatric mTBI Guideline that are most relevant to neurologists. To review the full CDC Pediatric mTBI Guideline, Systematic Review, and all 19 recommendation sets, visit: cdc.gov/HEADSUP. There, you can also download educational tools developed by CDC to help support implementation of these evidence-based recommendations.
Our understanding of pediatric-specific differences in injury response, including treatment and recovery from mTBI, is expanding rapidly. Children with mTBI have distinct epidemiology, assessment, management, and recovery trajectories compared with adults. The areas covered in the CDC Pediatric mTBI Guideline are evolving. The development of the CDC Pediatric mTBI Guideline was constrained by the lack of data and quality studies available on pediatric mTBI. Contributions to mTBI research are needed that provide further information on age-appropriate assessments, objective markers, well-controlled management and outcome studies, and optimal management for recovery. It is increasingly recognized that children with mTBI may initially encounter a wide range of healthcare providers and that those with complicated recoveries may benefit from a multidisciplinary approach.
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
B. Weissman reports no disclosures. M. Joseph serves on the editorial board of Pediatric Emergency Medicine Practice. G. Gronseth serves on the editorial advisory board of Brain & Life and as an Associate Editor for Neurology and receives research support from the American Academy of Neurology. K. Sarmiento reports no disclosures. C. C. Giza serves on the data safety monitoring board for LA BioMed Institute at Harbor-UCLA Medical Center; has received funding for travel and speaker honoraria for invited lectures on traumatic brain injury/concussion; has received funding for travel to attend meetings from Major League Soccer and United States Soccer Federation; receives publishing royalties for Neurological Differential Diagnosis: A Prioritized Approach (Blackwell Publishing/Wiley Publishing, 2005-present); serves as a consultant for Neural Analytics, Inc., Highmark Interactive, NFL-Neurological Care Program, NHL Players' Association, and the Los Angeles Lakers; serves on the Medical Education Speakers’ Network; serves on advisory boards/steering committees relating to concussion/TBI for the Centers for Disease Control and Prevention Pediatric Mild Traumatic Brain Injury Guideline Workgroup, Major League Soccer, National Basketball Association, and US Soccer Federation; receives research support from NIH/NINDS, US Department of Defense, NCAA-DOD CARE consortium, UCLA, and Richie's Fund; received stock options from Highmark Interactive; and has served as an expert on a maximum of 1 or 2 medico-legal cases annually. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
The CDC Pediatric mTBI Guideline outlines 5 key recommendations for neurologists and other healthcare providers:
→ Do not routinely image pediatric patients to diagnose mTBI.
→ Use validated, age-appropriate symptom scales to diagnose mTBI.
→ Assess for risk factors for prolonged recovery, including history of mTBI or other brain injury, severe symptom presentation immediately after the injury, and personal characteristics and family history (such as learning difficulties and family and social stressors).
→ Provide patients with instructions on returning to activity customized to their symptoms.
→ Counsel patients to return gradually to nonsports activities after no more than a 2–3 days of rest.
References
- 1.Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr 2018;172:e182853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blennow K, Brody DL, Kochanek PM, et al. Traumatic brain injuries. Nat Rev Dis Primers 2016;2:16084. [DOI] [PubMed] [Google Scholar]
- 3.McAllister TW, Sparling MB, Flashman LA, Saykin AJ. Neuroimaging findings in mild traumatic brain injury. J Clin Exp Neuropsychol 2001;23:775–791. [DOI] [PubMed] [Google Scholar]
- 4.Institute of M, National Research C, Committee on Sports-Related Concussions in Y, Board on Children Y, Families. The National Academies Collection: Reports funded by National Institutes of Health. In: Graham R, Rivara FP, Ford MA, Spicer CM, editors. Sports-Related Concussions in Youth: Improving the Science, Changing the Culture . Washington, DC: National Academies Press (US). Copyright 2014 by the National Academy of Sciences. All rights reserved; 2014. [Google Scholar]
- 5.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery 2014;75(suppl 4):S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Concussion signs and symptoms. Available at: cdc.gov/traumaticbraininjury/symptoms.html. Accessed August 28, 2018.
- 7.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med 2017;51:838–847. [DOI] [PubMed] [Google Scholar]
- 8.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 2003;290:2556–2563. [DOI] [PubMed] [Google Scholar]
- 9.Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics 2010;126:e374–e381. [DOI] [PubMed] [Google Scholar]
- 10.Yeates KO, Taylor HG, Rusin J, et al. Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics 2009;123:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babikian T, Satz P, Zaucha K, Light R, Lewis RS, Asarnow RF. The UCLA longitudinal study of neurocognitive outcomes following mild pediatric traumatic brain injury. J Int Neuropsychol Soc 2011;17:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gronseth GS, Woodroffe LM, Getchius TSD. Clinical Practice Guideline Process Manual. St. Paul: American Academy of Neurology; 2011. [Google Scholar]
- 13.Eder M, Feightner A, Webber E, Guirguis-Blake J, Whitlock EP. Developing and selecting Topic Nominations for systematic reviews. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 14.Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013;80:2250–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet 2009;374:1160–1170. [DOI] [PubMed] [Google Scholar]
- 16.Babl FE, Lyttle MD, Bressan S, et al. A prospective observational study to assess the diagnostic accuracy of clinical decision rules for children presenting to emergency departments after head injuries (protocol): the Australasian Paediatric Head Injury Rules Study (APHIRST). BMC Pediatr 2014;14:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of M, National Research C, Committee on Sports-Related Concussions in Y, Board on Children Y, Families. The National Academies Collection: Reports funded by National Institutes of Health. In: Graham R, Rivara FP, Ford MA, Spicer CM, editors. Sports-Related Concussions in Youth: Improving the Science, Changing the Culture . Washington: National Academies Press (US); 2014. [PubMed] [Google Scholar]
- 18.Gioia GA, Schneider JC, Vaughan CG, Isquith PK. Which symptom assessments and approaches are uniquely appropriate for paediatric concussion? Br J Sports Medicine 2009;43(suppl 1):i13–i22. [DOI] [PubMed] [Google Scholar]
- 19.Schatz P, Pardini JE, Lovell MR, Collins MW, Podell K. Sensitivity and specificity of the ImPACT test battery for concussion in athletes. Arch Clin Neuropsychol 2006;21:91–99. [DOI] [PubMed] [Google Scholar]
- 20.Zuckerbraun NS, Atabaki S, Collins MW, Thomas D, Gioia GA. Use of modified acute concussion evaluation tools in the emergency department. Pediatrics 2014;133:635–642. [DOI] [PubMed] [Google Scholar]
- 21.Nelson LD, Loman MM, LaRoche AA, Furger RE, McCrea MA. Baseline performance and psychometric properties of the child sport concussion assessment tool 3 (Child-SCAT3) in 5- to 13-year-old athletes. Clin J Sport Med 2017;27:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks MA, Snedden TR, Mixis B, Hetzel S, McGuine TA. Establishing baseline normative values for the child sport concussion assessment tool. JAMA Pediatr 2017;171:670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly JP, Rosenberg JH. Diagnosis and management of concussion in sports. Neurology 1997;48:575–580. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. HEADS UP campaign. Available at: cdc.gov/HEADSUP. Accessed September 4, 2018.