Abstract
Objective
We tested the hypothesis that use of mechanical ventilator support in children hospitalized with influenza during the 2009 H1N1 influenza A(H1N1) pandemic (pH1N1) was higher than would be expected in children hospitalized for seasonal influenza after adjusting for patient risk.
Design
Retrospective cohort study.
Setting
43 U.S. pediatric hospitals.
Patients
Children <18 years old with a discharge diagnosis of influenza admitted July 2006 through March 2009 (seasonal influenza) and June through December 2009 (pH1N1).
Interventions
None.
Measurements and Main Results
We included 10,173 children hospitalized with seasonal influenza and 9,837 with presumed pH1N1. The pH1N1 cohort was older (median 5.0 vs. 1.9 years), more likely to have asthma (30% vs. 18%), and less likely to receive mechanical ventilation (7.1% [n=701]) versus 9.2% [n=940]). Using logistic regression, we created a multivariable model of risk factors associated with endotracheal mechanical ventilator support in the seasonal influenza cohort and used this model to predict the number of expected mechanical ventilation cases in children with presumed pH1N1. Adjusted for underlying health conditions, race, age and a co-diagnosis of bacterial pneumonia, the observed/expected rate of mechanical ventilation in the presumed pH1N1 cohort was 0.74 (95% CI 0.68–0.79). Early hospital treatment with influenza antiviral medications was associated with decreased initiation of mechanical ventilation on hospital day ≥3 in the seasonal influenza (OR 0.66; 95% CI, 0.45–0.97) and pH1N1 (OR 0.23; 95% CI, 0.16–0.34) periods; influenza antiviral use in the pH1N1 period was much higher (70% versus 19%, P<0.001).
Conclusions
Although the number of children with a hospital discharge diagnosis of influenza almost tripled during the 2009 pandemic H1N1 period, the risk-adjusted proportion of children receiving mechanical ventilation was lower than we would have predicted in a seasonal influenza cohort. Early hospital use of influenza antiviral medications was associated with a decrease in late-onset mechanical ventilation.
Keywords: Influenza, Human; Pandemics; Child; Infant; Hospitals, Pediatric; Respiration, Artificial
Introduction
The emergence of a transmissible novel influenza A virus can cause widespread illness, and often results in higher rates of influenza-related complications than observed with seasonal influenza virus infections. 2009 pandemic influenza A (H1N1) [pH1N1] virus infection was associated with school closures (1, 2), high hospitalization rates (3), and concerns that higher than expected rates of respiratory failure could lead to a shortage of intensive care unit beds and mechanical ventilators (4, 5). Early reports of profound refractory hypoxia and death in predominantly young adult patients hospitalized in Mexico (6) heightened concerns that infection with the pH1N1 virus had a high probability of leading to life-threatening complications.
Risk factors have been established for seasonal influenza-related complications such as hospitalization in children (7–11). Mechanical ventilation is a resource intensive life-saving technology managed mostly by critical care subspecialists in the intensive care unit. Using a large dataset of discharges from U.S. pediatric hospitals, we created a multivariable model of risk factors for use of mechanical ventilation among children hospitalized with a diagnosis of influenza prior to the pandemic, and used this model to predict mechanical ventilator use among children hospitalized with presumed pH1N1. Because pH1N1 virus can cause severe illness, we hypothesized that after adjustment for patient risk factors, the proportion of children with an influenza diagnosis receiving mechanical ventilator support would be higher during the pH1N1 period than would be predicted from the cohort of patients discharged with a diagnosis of seasonal influenza.
Materials and Methods
Data sources
We obtained data from the Pediatric Health Information System (PHIS), an administrative database of inpatient admissions from 43 not-for-profit, tertiary care pediatric hospitals in the United States representing 17/20 major metropolitan U.S. areas. These hospitals are affiliated with the Child Health Corporation of America (Shawnee Mission, KS), a business alliance of children’s hospitals. In 2009, discharges from PHIS hospitals represented 15% of all U.S. discharges among patients <21 years old (excluding postnatal care of normal newborns), and 46% of all discharges from U.S. children’s hospitals (Matthew Hall, Child Health Corporation of America, personal communication, March 27, 2012). Data quality and reliability are evaluated through a joint effort between the Child Health Corporation of America and participating hospitals. The data warehouse function for the PHIS database is managed by Thomson Reuters (Ann Arbor, MI). Participating hospitals provide detailed information for each discharged patient, including demographic characteristics and primary diagnoses and procedures. Each hospital chooses whether to also provide detailed billing information for each admission, including medications, bed charges, and laboratory tests. Data are de-identified at the time of submission, and then subjected to numerous reliability and validity checks before being processed into data quality reports. Data from hospitals failing PHIS data quality standards are excluded. The Children’s Hospital Boston institutional review board waived the need for informed consent.
Patients and Variable Definitions
We included children (<18 years old on hospital admission) discharged with a diagnosis of influenza (International Classification of Disease, Ninth Revision, Clinical Modification [ICD-9-CM] code 487 or 488.1) as indicated in any of the up to 21 discharge diagnoses recorded (see Supplemental Table 1 for list of ICD-9-CM codes). We used Centers for Disease Control and Prevention (CDC) influenza surveillance data (3) to determine cutoff dates for seasonal and presumed pH1N1 virus infections. We assigned patients admitted from July 1, 2006 through March 31, 2009 to the seasonal influenza cohort. We excluded patients admitted between April 1 and June 6, 2009 to minimize overlap. Starting the week of June 7, 2009, pH1N1 virus represented 93% of all influenza-positive clinical specimens reported to CDC laboratories (3); we therefore assigned patients admitted from June 7 through December 31, 2009 with a discharge diagnosis of influenza to the pH1N1 cohort. To increase the likelihood that influenza was the primary admission diagnosis, we excluded newborns (patients <2 days old at admission), patients with primary discharge diagnoses unrelated to influenza (e.g., poisoning, injury, appendicitis, cellulitis, constipation), and patients admitted primarily to burn, trauma, psychiatric, or neurosurgical units. Readmissions within 90 days of the original discharge were excluded unless they appeared after case review to be a new diagnosis of pH1N1 after a prior admission with seasonal influenza.
Demographic information, including gender, age at admission, race, and principal payer, were captured for each admission, as was inpatient mortality. We determined the presence of a health condition known to put the patient at risk of influenza-related complications as listed by the Advisory Committee on Immunization Practices (ACIP) in 2009 by mapping these conditions to ICD-9-CM diagnosis codes for chronic lung (asthma vs. other), cardiovascular, renal, hepatic, non-malignant hematologic, metabolic, or neurological (including neuromuscular) disease, as well as immunosuppression and long-term aspirin use (12). Patients without an ACIP-defined high-risk condition were either classified as previously healthy or considered to have a non-ACIP condition if they had an ICD-9-CM diagnosis code indicating another chronic illness. We also used ICD-9-CM discharge diagnosis codes for pneumonia with bacterial organisms to indicate a secondary discharge diagnosis of bacteria pneumonia, which has been linked to severe influenza outcomes (13–16). Billing codes were used to identify charges for use of influenza antiviral medications (including amantadine, rimantadine, oseltamivir, and zanamivir) and complications including pediatric intensive care unit admission and use of high-frequency oscillatory ventilation, vasopressors, and extracorporeal membrane oxygenation. The primary outcome variable was mechanical ventilation via the trachea in the pediatric intensive care unit, which was identified using billing codes.
Data analysis
We performed unadjusted between-group comparisons using Chi Square and Fisher’s exact tests for dichotomous outcomes, the Wilcoxon Rank Sum test for continuous variables, and simple logistic regression for associations between predictors and outcome variables. We used multiple logistic regression to test the association of risk factors with mechanical ventilator use; in addition to gender and age category, factors possibly associated (P≤0.20) were considered for inclusion in the multivariable model containing all independent predictors of respiratory failure (P<0.05). Missing data were not imputed. We performed K-fold cross-validation to develop and validate multivariable predictive models in the seasonal influenza cohort (17, 18). Briefly, we divided the cohort into 10 sub-samples of approximately equal size by sampling randomly without replacement. We performed logistic regression on 9 sub-samples of the data (training set) and applied the training model to the remaining sub-sample (validation set). This was performed a total of 10 times, with each sub-sample used once as the validation set; the same covariates were included in each of the 10 logistic regression training models. For each of the 10 models, we measured pseudo-r2 values in the training set. We used area under the receiver operating characteristic curve and standardized complication ratio (SCR; ratio of observed to expected complications) to measure goodness of fit and assess the predictive capability of the training model in the validation set. The average of the estimated logistic regression model coefficients across the 10 folds served as the seasonal influenza predictive model.
To test whether illness with pH1N1 virus infection was associated with higher rates of mechanical ventilation than would be expected based on experience with seasonal influenza A or B virus infections, we applied the seasonal influenza predictive model to patients admitted with presumed pH1N1, using SCR with 95% confidence intervals to compare observed to expected events. All analyses were performed using the SAS System version 9.2 (SAS Institute Inc.; Cary, NC).
Results
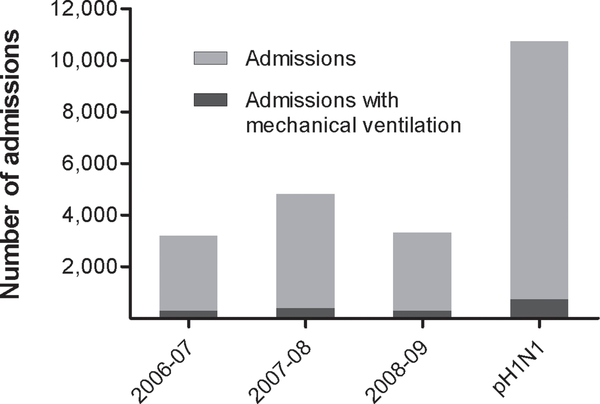
We identified 22,514 patient admissions with a reported diagnosis of influenza at 43 hospitals during 2006–2009. We excluded 580 admissions (2.6%) due to age or evidence that influenza was not the primary diagnosis (primary discharge diagnosis unrelated to influenza or admission to burn, trauma, psychiatric, or neurosurgical units), 1,549 admissions (6.9%) for which billing data was not submitted to PHIS, and 375 admissions (1.7%) within 90 days of a prior discharge. We included 20,010 admissions (89% of the study population) in final analyses. Of these, 9,837 (49%) admissions had presumed pH1N1 and represented 43% of admissions requiring mechanical ventilation (Figure 1).
Figure 1.
Influenza-related hospital admissions and mechanical ventilation during three seasonal influenza epidemics compared to 2009 pandemic H1N1 (pH1N1). There were increased numbers of admissions and cases requiring mechanical ventilation during the pH1N1 pandemic (P<0.001).
Patients discharged with an influenza diagnosis during the pH1N1 period were older than patients discharged with a seasonal influenza diagnosis (Table 1), were more likely to have asthma and more likely to have a co-diagnosis of bacterial pneumonia but less likely to receive mechanical ventilator support. Influenza antiviral medication use increased during the pandemic period (70% vs. 19%, P<0.001); oseltamivir represented 99% of all influenza antiviral medication use. There were a similar proportion of deaths in both cohorts. Combining both cohorts, overall reported mortality was higher (odds ratio [OR] 38.67, 95% confidence interval [CI], 27.87–53.67, P<0.001) in children receiving mechanical ventilator support (9.8%) compared to children who did not receive mechanical ventilation (0.3%).
Table 1.
Characteristics of patients admitted to 43 U.S. children’s hospitals with seasonal influenza and presumed 2009 pandemic H1N1 (pH1N1).
| Seasonal influenza | Presumed pH1N1 | ||
|---|---|---|---|
| n=10,173 (%) | n=9,837 (%) | P | |
| Gender | 0.28 | ||
| Male | 5,760 (57%) | 5,644 (57%) | |
| Female | 4,413 (43%) | 4,193 (43%) | |
| Age median (interquartile range) | 1.9 (0.5, 6.3) | 5.0 (1.4, 9.5) | <0.001 |
| Race | <0.001 | ||
| White | 6,393 (63%) | 4,899 (50%) | |
| Black | 2,573 (25%) | 2,438 (25%) | |
| Non-White/non-Black | 1,207 (12%) | 2,500 (25%) | |
| Primary payer | <0.001 | ||
| Government | 5,516 (54%) | 3,638 (37%) | |
| Private | 2,620 (26%) | 3,230 (33%) | |
| Self-pay | 214 (2.1%) | 166 (1.7%) | |
| Other/missing | 1,823 (18%) | 2,803 (28%) | |
| Influenza season | |||
| 2006 – 07 | 2,870 (28%) | ||
| 2007 – 08 | 4,336 (43%) | ||
| 2008 – 09 | 2,967 (29%) | ||
| Health status | <0.001 | ||
| Previously healthy | 4,415 (43%) | 3,427 (35%) | |
| Non-ACIP condition | 713 (7.0%) | 547 (5.6%) | |
| ACIP-defined condition | 5,045 (50%) | 5,863 (60%) | |
| Asthma | 1,861 (18%) | 2,985 (30%) | <0.001 |
| Non-asthma chronic lung disease | 899 (8.8%) | 898 (9.1%) | 0.47 |
| Cardiovascular disease | 650 (6.4%) | 563 (5.7%) | 0.05 |
| Immunosuppression | 1,008 (9.9%) | 886 (9.0%) | 0.03 |
| Hematologic disease | 410 (4.0%) | 463 (4.7%) | 0.02 |
| Renal disease | 109 (1.1%) | 123 (1.2%) | 0.24 |
| Metabolic disease | 343 (3.4%) | 447 (4.5%) | <0.001 |
| Neurological disease | 1,425 (14%) | 1,385 (14%) | 0.88 |
| Hepatic disease | 82 (0.8%) | 68 (0.7%) | 0.35 |
| Chronic aspirin use | 22 (0.2%) | 25 (0.2%) | 0.58 |
| Treatment and complications | |||
| Influenza antiviral medication | 2,052 (20%) | 6,905 (70%) | <0.001 |
| Bacterial pneumonia | 567 (5.6%) | 829 (8.4%) | <0.001 |
| PICU admission | 2,075 (20%) | 1,859 (19%) | 0.008 |
| Mechanical ventilation | 940 (9.2%) | 701 (7.1%) | <0.001 |
| High-frequency ventilation | 130 (1.3%) | 98 (1.0%) | 0.06 |
| Shock requiring vasopressors | 502 (4.9%) | 445 (4.5%) | 0.17 |
| Extracorporeal life support | 50 (0.5%) | 43 (0.4%) | 0.57 |
| Mortality a | 111 (1.1%) | 95 (1.0%) | 0.55 |
Due to a change in PHIS disposition variable format in 2009, mortality data is missing or unreliable for 1,012 patients. These patients were excluded from mortality analyses.
Abbreviations: ACIP: Advisory Committee on Immunization Practices. PICU: Pediatric intensive care unit.
Factors associated with mechanical ventilation
In univariate analyses of risk factors for mechanical ventilation among patients with a seasonal influenza discharge diagnosis (Table 2), factors potentially associated with respiratory failure (P≤0.20) included age ≥2 months, non-White/non-Black race, cardiovascular disease, neurological disease, non-asthma chronic lung disease, metabolic disease, hepatic disease, non-ACIP defined underlying health conditions, and a diagnosis of bacterial pneumonia. Asthma and hematologic disease were associated with lower risk of mechanical ventilation; there was no effect of gender, primary payer, or influenza season.
Table 2.
Univariate analyses of predictors of mechanical ventilator use among 10,173 U.S. children hospitalized with seasonal influenza.
| n (%) | OR (95% CI) | P | |
|---|---|---|---|
| Gender | 0.72 | ||
| Male (N=5,760) | 527 (9.2) | Reference | |
| Female (N=4,413) | 413 (9.4) | 1.02 (0.90 – 1.17) | |
| Age | <0.001 | ||
| <2 months (N=1,412) | 97 (6.9) | Reference | |
| 2–11 months (N=2,317) | 264 (11) | 1.74 (1.37 – 2.22) | |
| 1–4 years (N=3,252) | 287 (8.8) | 1.31 (1.03 – 1.67) | |
| 5–11 years (N=2,133) | 184 (8.6) | 1.28 (0.99 – 1.65) | |
| 12–17 years (N=1,059) | 108 (10) | 1.54 (1.16 – 2.05) | |
| Race | <0.001 | ||
| White (N=6,393) | 585 (9.2) | Reference | |
| Black (N=2,573) | 191 (7.4) | 0.80 (0.67 – 0.94) | |
| Non-White/non-Black (N=1,207) | 164 (14) | 1.56 (1.30 – 1.88) | |
| Primary payer | 0.70 | ||
| Private (N=2,620) | 247 (9.4) | Reference | |
| Non-Private (N=7,553) | 693 (9.2) | 0.97 (0.83 – 1.13) | |
| Season | 0.28 | ||
| 2006 – 07 (N=2,870) | 280 (9.8) | Reference | |
| 2007 – 08 (N=4,336) | 378 (8.7) | 0.88 (0.75 – 1.04) | |
| 2008 – 09 (N=2,967) | 282 (9.5) | 0.97 (0.82 – 1.16) | |
| Health status | |||
| Previously healthy (N=4,415) | 205 (4.6) | Reference | |
| Non-ACIP condition a (N=713) | 92 (13) | 3.04 (2.35 – 3.94) | <0.001 |
| ACIP-defined condition b | |||
| Cardiovascular disease (N=650) | 158 (24) | 3.59 (2.96 – 4.36) | <0.001 |
| Neurological disease (N=1,425) | 334 (23) | 4.11 (3.55 – 4.77) | <0.001 |
| Chronic lung disease (N=899) | 195 (22) | 3.17 (2.66 – 3.78) | <0.001 |
| Immunosuppression (N=1,008) | 97 (9.6) | 1.05 (0.84 – 1.31) | 0.66 |
| Metabolic disease (N=343) | 48 (14) | 1.63 (1.19 – 2.23) | 0.002 |
| Renal disease (N=109) | 13 (12) | 1.34 (0.74 – 2.39) | 0.33 |
| Hepatic disease (N=82) | 21 (26) | 3.44 (2.09 – 5.67) | <0.001 |
| Hematologic disease (N=410) | 3 (0.7) | 0.07 (0.02 – 0.22) | <0.001 |
| Asthma (N=1,861) | 135 (7.2) | 0.73 (0.60 – 0.88) | 0.001 |
| Chronic aspirin therapy (N=23) | 0 | ||
| Bacterial pneumonia (N=567) | 199 (35) | 6.47 (5.36 – 7.81) | <0.001 |
Compared to previously healthy children.
Compared to children without each risk factor.
Using the average of the coefficients of the 10 training models, we developed a multivariable logistic regression model predicting mechanical ventilation for seasonal influenza illness; we included gender, age, race, medical conditions associated with either high or low risk of mechanical ventilator use, and bacterial pneumonia (Table 3). The median of the pseudo-r2 values generated for each training set was 0.18 (range, 0.17–0.19); in the validation sets, median area under the receiver operating characteristic curve was 0.76 (range, 0.72–0.79) and median SCR (observed to expected) was 0.97 (range, 0.88–1.16), indicating good fit. Bacterial pneumonia, neurological disease, hepatic disease, cardiovascular disease, non-ACIP conditions, and chronic lung disease were most strongly associated with increased use of mechanical ventilation. Hematologic disease was most strongly associated with decreased risk.
Table 3.
Multivariable logistic regression model of factors associated with use of mechanical ventilation among 10,173 U.S. children hospitalized with seasonal influenza.
| OR (95% CI) | P | |
|---|---|---|
| Gender | ||
| Male | Reference | |
| Female | 1.01 (0.96–1.06) | 0.84 |
| Age | ||
| 0 – 2m | Reference | |
| 2 – 11m | 1.36 (1.24–1.50) | <0.001 |
| 1 – 4y | 0.91 (0.83–0.99) | 0.03 |
| 5 – 11y | 0.86 (0.79–0.94) | <0.001 |
| 12 −17y | 0.84 (0.77–0.92) | <0.001 |
| Race | ||
| White | Reference | |
| Black | 1.02 (0.98–1.06) | 0.42 |
| Non-White, non-Black | 1.56 (1.43–1.71) | <0.001 |
| Health status | ||
| Cardiovascular disease | 2.78 (2.59–3.00) | <0.001 |
| Neurological disease | 4.05 (3.79–4.33) | <0.001 |
| Chronic lung disease | 2.06 (1.91–2.22) | <0.001 |
| Metabolic disease | 1.45 (1.27–1.65) | <0.001 |
| Hepatic disease | 3.34 (2.78–3.99) | <0.001 |
| Hematologic disease | 0.13 (0.08–0.19) | <0.001 |
| Asthma | 0.78 (0.74–0.83) | <0.001 |
| Non-ACIP condition | 2.33 (2.12–2.57) | <0.001 |
| Bacterial pneumonia | 5.81 (5.36–6.29) | <0.001 |
Odds ratios are based on mean coefficients from 10 regression models, each created using 90% of the cohort and validated in the remaining 10%. Model intercept is −3.077.
Using the mean coefficients from the regression models to predict expected rates of mechanical ventilator use in the pH1N1 cohort, the area under the receiver operating characteristic curve was 0.77 (95% CI, 0.75–0.79). The SCR for pH1N1 was 0.74 (95% CI, 0.68–0.79), indicating that after risk-adjustment, children hospitalized with presumed pH1N1 had a lower risk of mechanical ventilation than children hospitalized with seasonal influenza.
To determine if increased influenza antiviral use during the pandemic period could partly explain the finding of decreased risk-adjusted mechanical ventilation among children hospitalized with presumed pH1N1 by decreasing late-onset mechanical ventilation (hospital day ≥3), we analyzed the subgroup of patients who did not require mechanical ventilation on hospital day 1 or 2 or chronic ventilation through a tracheostomy. We excluded 1,353 admissions from this analysis, resulting in a subgroup of 18,657 admissions. In the late-onset mechanical ventilation group, we included only early use of influenza antiviral medications beginning prior to mechanical ventilation initiation. We first examined characteristics of these patients, comparing patients admitted with seasonal influenza and presumed pH1N1 (Supplemental Table 2); compared to the base population, this group of patients who did not receive early mechanical ventilation had a similar demographic distribution and similar percentage of patients receiving influenza antiviral medications, fewer patients with certain chronic illness (neurological, cardiovascular, and chronic lung disease), fewer bacterial pneumonia diagnoses, and fewer complications (PICU admission, mechanical ventilation, high-frequency ventilation, shock requiring vasopressors, extracorporeal membrane oxygenation, and mortality). Late-onset mechanical ventilation represented 21% of all cases receiving mechanical ventilation. Combining the seasonal and pandemic cohorts to ensure adequate statistical power, we identified univariate risk factors for late-onset mechanical ventilation. Including factors in the univariate analysis with P≤0.20 and controlling for seasonal vs. presumed pH1N1, we created a multivariable logistic regression model of factors associated with late-onset mechanical ventilation to determine the adjusted effect of influenza antiviral medication (Table 4). As expected, there was a significant interaction between influenza cohort (seasonal vs. presumed pH1N1) and influenza antiviral medication use. However, the adjusted frequency of late-onset mechanical ventilation was lower in patients treated with influenza antiviral medications during both the seasonal influenza (OR 0.66; 95% CI, 0.45–0.97, P=0.04) and pH1N1 (OR 0.23; 95% CI, 0.16–0.34, P<0.001) periods.
Table 4.
Multivariable logistic regression model of factors associated with late-onset need for mechanical ventilation among children who did not need mechanical ventilation on the first two days of hospitalization (N=18,657).
| OR (95% CI) | P | |
|---|---|---|
| Gender | ||
| Male | Reference | |
| Female | 1.15 (0.92–1.44) | 0.23 |
| Age | ||
| 0 – 2m | Reference | |
| 2 – 11m | 1.59 (0.98–2.58) | 0.06 |
| 1 – 4y | 0.69 (0.42–1.14) | 0.15 |
| 5 – 11y | 0.69 (0.41–1.17) | 0.18 |
| 12 −17y | 1.04 (0.60–1.79) | 0.90 |
| Race | ||
| White | Reference | |
| Black | 1.29 (0.96–1.72) | 0.09 |
| Non-White, non-Black | 1.83 (1.38–2.43) | <0.001 |
| Health status | ||
| Cardiovascular disease | 4.09 (3.06–5.46) | <0.001 |
| Neurological disease | 3.97 (3.07–5.13) | <0.001 |
| Chronic lung disease | 3.04 (2.32–4.00) | <0.001 |
| Immunosuppression | 3.12 (2.28–4.26) | <0.001 |
| Metabolic disease | 2.15 (1.42–3.25) | <0.001 |
| Renal disease | 2.24 (1.11–4.54) | 0.02 |
| Hepatic disease | 5.12 (2.69–9.75) | <0.001 |
| Hematologic disease | 0.36 (0.11–1.14) | 0.08 |
| Asthma | 1.28 (0.96–1.72) | 0.10 |
| Non-ACIP condition | 2.34 (1.45–3.77) | <0.001 |
| Bacterial pneumonia | 5.62 (4.22–7.48) | <0.001 |
| Influenza period a | ||
| Seasonal influenza | Reference | |
| Presumed pH1N1 | 1.13 (0.84–1.53) | 0.41 |
| Influenza antiviral treatment b | ||
| Seasonal influenza | 0.66 (0.45–0.97) | 0.04 |
| Presumed pH1N1 | 0.23 (0.16–0.34) | <0.001 |
Model intercept is −5.031.
Among patients who did not receive influenza antiviral treatment.
We created a variable for the interaction between influenza period and early antiviral treatment, which was significant at P<0.001, indicating that the effect of influenza antiviral treatment was different during the seasonal and presumed pH1N1 influenza periods.
In the subgroup of patients with a discharge diagnosis of bacterial pneumonia who received mechanical ventilation, Staphylococcus aureus was the most frequently identified organism reported for admissions with both seasonal and presumed pH1N1 influenza (Supplemental Table 3). As our study utilized administrative data, systematic testing for bacterial pathogens was not performed, reporting of specific bacterial organisms may be variable, and we are unable to determine which bacterial coinfections were present on admission.
Discussion
Across 43 tertiary pediatric hospitals in the United States, there were almost as many hospitalizations with presumed influenza during the 2009 pH1N1 pandemic as during the three preceding seasonal influenza epidemics combined. We created and validated a multivariable model predicting use of mechanical ventilation in children discharged with a diagnosis of influenza prior to the 2009 pandemic. Contrary to our hypothesis that patients discharged with influenza during the pH1N1 period would have higher risk adjusted use of mechanical ventilation than would be expected in seasonal influenza virus infection, we found a lower than expected use of mechanical ventilation in this cohort of children hospitalized with presumed pH1N1. We also found early hospital use of antiviral medications to be associated with decreased need for late-onset mechanical ventilation at hospital day ≥3 during both the seasonal and pandemic periods.
Although Belongia and colleagues reported that hospitalization rates among influenza-positive patients with fever or cough were not higher during the 2009 H1N1 pandemic (19), we found a markedly higher number of discharges with an influenza diagnosis during the pH1N1 period compared to the 3 prior seasonal influenza periods. Previous smaller studies of U.S. and Canadian children hospitalized with pH1N1 have shown unadjusted rates of mechanical ventilation similar to the 7.1% in our cohort (20–22), and 3 Canadian studies containing few patients with severe outcomes found similar unadjusted rates of ICU admission and mechanical ventilation between children hospitalized with seasonal and pH1N1 influenza (21, 23, 24).
There are several potential explanations for our finding that the adjusted use of mechanical ventilation among children hospitalized during the 2009 H1N1 pandemic was not higher than for seasonal influenza. A higher proportion of patients with presumed pH1N1 were admitted with asthma exacerbations, and we found that hospitalized children with an underlying diagnosis of asthma were less likely to require mechanical ventilation than children without asthma. Although we adjusted for asthma in our prediction model, it still may have influenced the frequency of respiratory failure. Heightened concern of severe complications related to pH1N1 may have led to a lower threshold for hospitalization in children with less severe illness and it is possible that earlier hospitalization prevented the most severe complications. It is also possible that more widespread testing for influenza during the pH1N1 pandemic may have identified more influenza virus infections in hospitalized patients who were not severely ill and who might otherwise not have been tested for influenza prior to the pandemic.
In addition, we found that influenza antiviral treatment decreased the requirement for mechanical ventilation beginning on the third hospital day or later, and it is possible that increased use of influenza antiviral medications for presumed pH1N1 in children may have been partly responsible for the decrease in risk-adjusted mechanical ventilation during the pandemic period. Previous observational studies of hospitalized children have shown early oseltamivir treatment was associated with decreased hospital length of stay (25), ICU admission (26), and mortality (27). As the PHIS database does not include events prior to emergency department or inpatient admission, we could not fully assess whether outpatient use of influenza antiviral medication decreased mechanical ventilator use for acute respiratory failure. A national study of U.S. children admitted to the pediatric intensive care unit with pH1N1 virus infection reported that only 6% received influenza antiviral medication prior to PICU admission (28), making it unlikely that the majority of patients in our cohort received these medications as outpatients.
Infants, young children, and children with chronic health conditions are at increased risk of severe complications from seasonal influenza virus infection (7–11). We found that children 2–11 months old had higher use of mechanical ventilation than children <2 months old, which may be due to a lower threshold for hospitalizing young infants or the presence of protective maternal antibodies in those patients. Among ACIP-defined conditions prioritized for influenza vaccination, we found that cardiovascular disease, neurological disease, and hepatic disease were highly associated with mechanical ventilation. Previous studies of children hospitalized with seasonal influenza (11, 29, 30) or pH1N1 (22, 28, 31–34) have also highlighted the contribution of cardiovascular and neurological disease to worse influenza outcomes.
Recent studies have found increased risk of ICU admission and mortality among children hospitalized with bacterial and seasonal influenza virus coinfection compared to influenza virus infection alone (13, 35), and a recent study of fatal cases of seasonal influenza and pH1N1 in U.S. children reported that approximately 50% had evidence of bacterial coinfection (36). We found that children with a discharge co-diagnosis of bacterial pneumonia had high risk of respiratory failure and when a bacterial organism was specified, and Staphylococcus aureus bacterial pneumonia was most common. This is consistent with a recent study which found that S. aureus was the most commonly identified respiratory bacterial pathogen among children admitted to the pediatric intensive care unit with pH1H1, and that lung infection with methicillin-resistant S. aureus was independently associated with mortality (28). Other studies have highlighted the importance of S. aureus in fatal or otherwise severe cases of influenza virus infection during seasonal outbreaks as well as pandemics (14–16, 37–40).
This study has several strengths. This is a large study of children hospitalized with a diagnosis of influenza during the 2009 pandemic, and the first to compare risk-adjusted use of mechanical ventilation between seasonal influenza and pH1N1 among hospitalized children. Using a large administrative database allowed us to create multivariable models of respiratory failure based on a large number of children hospitalized with influenza. Instead of evaluating pH1N1 as a risk factor and combining the seasonal influenza and pH1N1 cohorts, we developed a prediction model in the seasonal influenza cohort and tested its ability to predict respiratory failure in the cohort of patients with presumed pH1N1. We used K-fold cross-validation to internally validate our multivariable training models, decreasing the likelihood that our final model overfits our data. We believe that our multivariable model predicting respiratory failure in children with seasonal influenza may be useful for evaluating the severity of future influenza pandemics and their likely impact on use of mechanical ventilators.
There are several limitations of this study. Though PHIS data are subjected to numerous quality checks, administrative data are limited by the possibility of miscoded or incomplete information. Diagnostic coding is based on clinical diagnosis and practice patterns rather than a systematic method of diagnosis, which may result in different frequencies of diagnosis of influenza, chronic diseases, or bacterial coinfection among institutions or over time. We are unable to determine which cases had laboratory-confirmed influenza virus infection. Also, more children without laboratory-confirmed influenza may have been assigned a discharge diagnosis of influenza during the pH1N1 period due to heightened awareness of pandemic influenza. Increased testing for influenza during the pH1N1 period and increased testing for bacterial coinfection in sicker patients may have contributed to the high number of admissions identified in our pH1N1 cohort, and to the relationship between bacterial pneumonia and severe outcomes. It is also possible that influenza testing was more likely to be performed in sicker patients, and that our population includes a higher proportion of children with more severe influenza-related illness than would have been present if rigorous and highly sensitive screening of all patients had been implemented. Unfortunately, due to a PHIS data format change in 2009 affecting reporting of mortality and delays in hospitals transitioning to the new coding, we were unable to use mortality as the outcome variable. Use of mechanical ventilation is a strong indicator of disease severity and in our dataset was strongly associated with hospital mortality.
The PHIS database, despite its broad demographic and geographic distribution of patients, only includes tertiary care children’s hospitals in the U.S., and generalizing to other types of institutions or countries may not be appropriate. Although we cannot determine which cases of influenza infection or bacterial pneumonia were present on admission and which cases were nosocomially-acquired, nosocomial pneumonia in the patients admitted to the PICU whose length of stay is sufficiently long to put them at risk is reportedly low (28). Microbiologic testing and the diagnostic accuracy of the bacterial pneumonia diagnosis are also uncertain. Our dataset for seasonal influenza hospitalizations was limited to 2006–2009. Because we did not include cases from more severe seasonal influenza epidemics, such as the 2003–04 season (29), this may limit generalizability of our findings because seasonal influenza epidemics have variable severity (20). Although the vast majority of influenza virus infections in the U.S. after June 6, 2009 were due to pH1N1 virus, it is possible that we misclassified a small number of seasonal influenza A and B admissions as presumed pH1N1 admissions early in the pandemic. Any contamination of groups would be expected to bias results toward the null.
Conclusions
During periods when pH1N1 virus was circulating, there were higher numbers of hospitalized children with a discharge diagnosis of influenza than during periods when seasonal influenza viruses were circulating. Despite this, we found that a lower proportion of these hospitalized children required mechanical ventilation than we would have predicted after accounting for associated risk factors. Our findings are consistent with the possibility that early antiviral treatment of hospitalized children with influenza reduced the risk of late-onset respiratory failure after hospital admission. A heightened focus on influenza prevention and early antiviral treatment of influenza in infants, young children, and those with underlying cardiovascular, neurological, or hepatic disease may reduce the risk of influenza-related acute respiratory failure requiring mechanical ventilator support.
Supplementary Material
Table Supplemental Table 1. International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes.
Supplemental Table 2. Characteristics of patients who did not receive early mechanical ventilation.
Supplemental Table 3. Specific bacterial pneumonia organisms among children hospitalized with influenza who required mechanical ventilation.
Acknowledgments
Financial support: Children’s Hospital Anesthesia Foundation and the National Institutes of Health R01 AI084011 (Randolph).
Abbreviations
- pH1N1
2009 pandemic influenza A (H1N1)
- ACIP
Advisory Committee on Immunization Practices
- ICD-9-CM
International Classification of Disease, Ninth Revision, Clinical Modification
- PHIS
Pediatric Health Information System
- SCR
Standardized complication ratio
Footnotes
The authors have not disclosed any potential conflict of interest.
Disclosure: This work represents the findings of the authors and not necessarily the views of the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller JC, Danon L, O’Hagan JJ, et al. Student behavior during a school closure caused by pandemic influenza A/H1N1. PLoS One 2010;5(5):e10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Education. H1N1 Flu and U.S. Schools: Answers to Frequently Asked Questions. May 5, 2009. [cited April 15, 2011]Available from: http://www2.ed.gov/admins/lead/safety/emergencyplan/pandemic/guidance/flu-faqs.pdf
- 3.U.S. Centers for Disease Control and Prevention. CDC - Seasonal Influenza (Flu) - Weekly Report: Influenza Summary Update. [cited 2010 June 23]Available from: http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/data/whoAllregt39.htm
- 4.Smetanin P, Stiff D, Kumar A, et al. Potential intensive care unit ventilator demand/capacity mismatch due to novel swine-origin H1N1 in Canada. Can J Infect Dis Med Microbiol 2009;20(4):e115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggertson L Alberta obtains standby ventilators from federal stockpile. CMAJ 2009;182(1):E25–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 2009;361(7):680–689. [DOI] [PubMed] [Google Scholar]
- 7.Mullooly JP, Barker WH. Impact of type A influenza on children: a retrospective study. Am J Public Health 1982;72(9):1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuzil KM, Mellen BG, Wright PF, et al. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000;342(4):225–231. [DOI] [PubMed] [Google Scholar]
- 9.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000;342(4):232–239. [DOI] [PubMed] [Google Scholar]
- 10.Neuzil KM, Wright PF, Mitchel EF Jr., et al. The burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr 2000;137(6):856–864. [DOI] [PubMed] [Google Scholar]
- 11.Keren R, Zaoutis TE, Bridges CB, et al. Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection. JAMA 2005;294(17):2188–2194. [DOI] [PubMed] [Google Scholar]
- 12.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009;58(RR-8):1–52. [PubMed] [Google Scholar]
- 13.Reed C, Kallen AJ, Patton M, et al. Infection with community-onset Staphylococcus aureus and influenza virus in hospitalized children. Pediatr Infect Dis J 2009;28(7):572–576. [DOI] [PubMed] [Google Scholar]
- 14.Finelli L, Fiore A, Dhara R, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics 2008;122(4):805–811. [DOI] [PubMed] [Google Scholar]
- 15.Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-August 2009. MMWR Morb Mortal Wkly Rep 2009;58(38):1071–1074. [PubMed] [Google Scholar]
- 16.Shieh WJ, Blau DM, Denison AM, et al. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol 2010;177(1):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 18.Gould MK, Ananth L, Barnett PG. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131(2):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belongia EA, Irving SA, Waring SC, et al. Clinical characteristics and 30-day outcomes for influenza A 2009 (H1N1), 2008–2009 (H1N1), and 2007–2008 (H3N2) infections. JAMA 2010;304(10):1091–1098. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Havens PL, Chusid MJ, et al. Clinical and epidemiologic characteristics of children hospitalized with 2009 pandemic H1N1 influenza A infection. Pediatr Infect Dis J 2010;29(7):591–594. [DOI] [PubMed] [Google Scholar]
- 21.Bettinger JA, Sauve LJ, Scheifele DW, et al. Pandemic influenza in Canadian children: a summary of hospitalized pediatric cases. Vaccine 2010;28(18):3180–3184. [DOI] [PubMed] [Google Scholar]
- 22.Louie JK, Gavali S, Acosta M, et al. Children Hospitalized With 2009 Novel Influenza A(H1N1) in California. Arch Pediatr Adolesc Med 2010;164(11):1023–1031. [DOI] [PubMed] [Google Scholar]
- 23.O’Riordan S, Barton M, Yau Y, et al. Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ 2009;182(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jouvet P, Hutchison J, Pinto R, et al. Critical illness in children with influenza A/pH1N1 2009 infection in Canada. Pediatr Crit Care Med 2010;11(5):603–609. [DOI] [PubMed] [Google Scholar]
- 25.Coffin SE, Leckerman K, Keren R, et al. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J 2011;30(11):962–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Launes C, Garcia-Garcia JJ, Jordan I, et al. 2009. Influenza A H1N1 infections: delays in starting treatment with oseltamivir were associated with a more severe disease. Pediatr Infect Dis J;30(7):622–625. [DOI] [PubMed] [Google Scholar]
- 27.Farias JA, Fernandez A, Monteverde E, et al. Critically ill infants and children with influenza A (H1N1) in pediatric intensive care units in Argentina. Intensive Care Med 2010;36(6):1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randolph AG, Vaughn F, Sullivan R, et al. Critically Ill Children During the 2009–2010 Influenza Pandemic in the United States. Pediatrics 2011;128(6):e1450–e1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med 2005;353(24):2559–2567. [DOI] [PubMed] [Google Scholar]
- 30.Coffin SE, Zaoutis TE, Rosenquist AB, et al. Incidence, complications, and risk factors for prolonged stay in children hospitalized with community-acquired influenza. Pediatrics 2007;119(4):740–748. [DOI] [PubMed] [Google Scholar]
- 31.Stein M, Tasher D, Glikman D, et al. Hospitalization of Children With Influenza A(H1N1) Virus in Israel During the 2009 Outbreak in Israel: A Multicenter Survey. Arch Pediatr Adolesc Med 2010;164(11):1015–1022. [DOI] [PubMed] [Google Scholar]
- 32.Campbell A, Rodin R, Kropp R, et al. Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ 2010;182(4):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.del Rosal T, Baquero-Artigao F, Calvo C, et al. Pandemic H1N1 influenza-associated hospitalizations in children in Madrid, Spain. Influenza Other Respi Viruses 2011;5(6):e544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yung M, Slater A, Festa M, et al. Pandemic H1N1 in children requiring intensive care in Australia and New Zealand during winter 2009. Pediatrics 2011;127(1):e156–163. [DOI] [PubMed] [Google Scholar]
- 35.Schrag SJ, Shay DK, Gershman K, et al. Multistate surveillance for laboratory-confirmed, influenza-associated hospitalizations in children: 2003–2004. Pediatr Infect Dis J 2006;25(5):395–400. [DOI] [PubMed] [Google Scholar]
- 36.Cox CM, Blanton L, Dhara R, et al. 2009 Pandemic Influenza A (H1N1) deaths among children - United States, 2009–2010. Clin Infect Dis 2011;52(Suppl 1):S69–74. [DOI] [PubMed] [Google Scholar]
- 37.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008;198(7):962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis 2006;12(6):894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schertzer JD, Antonescu CN, Bilan PJ, et al. A transgenic mouse model to study glucose transporter 4myc regulation in skeletal muscle. Endocrinology 2009;150(4):1935–1940. [DOI] [PubMed] [Google Scholar]
- 40.Williams DJ, Hall M, Brogan TV, et al. Influenza coinfection and outcomes in children with complicated pneumonia. Arch Pediatr Adolesc Med 2011;165(6):506–512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table Supplemental Table 1. International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes.
Supplemental Table 2. Characteristics of patients who did not receive early mechanical ventilation.
Supplemental Table 3. Specific bacterial pneumonia organisms among children hospitalized with influenza who required mechanical ventilation.