Abstract
This preliminary study examined the effects of tobacco-free snuff (intervention, n=52) compared with no snuff (control, n=54) for reducing tobacco use among smokeless tobacco (ST) users not interested in quitting. Both groups received behavioral instructions, and intervention subjects received tobacco-free snuff for 8 weeks. Participants were required to reduce their intake by 50% during the first 4 weeks and by 75% during the subsequent 4 weeks. Follow-up occurred at 12 weeks. Significant reductions were observed from baseline to week 8 (end of treatment) for both treatment groups in the amount of ST use (tins/week and dips/day, p<.001); mean urinary cotinine (p<.001); and mean urinary total NNAL, a carcinogen biomarker (p<.001). At week 8 the intervention resulted in a lower mean total NNAL (p=.048). Compared with the control condition, the intervention resulted in a higher percentage of subjects achieving at least a 50% reduction in cotinine (p=.046) and total NNAL (p=.002) at the end of treatment, more quit attempts (p=.030), and a longer mean duration of abstinence (p=.013) through follow-up. An ST reduction intervention incorporating tobacco-free snuff could potentially reduce risk for ST-related disease beyond that achieved with no snuff by increasing the number of patients who achieve significant reductions in carcinogen exposure and, more important, by facilitating tobacco abstinence by increasing quit attempts and abstinence duration.
Introduction
Tobacco abstinence has been the primary goal for the vast majority of disease prevention efforts. Unfortunately, many individuals trying to quit tobacco are unsuccessful in achieving abstinence. In clinical trials among smokeless tobacco (ST) users, tobacco abstinence rates are 25%–35% at 1 year (Hatsukami & Severson, 1999). Abstinence rates are likely to be lower among ST users quitting on their own.
Although tobacco abstinence is the goal for eliminating health risks associated with tobacco use, reduction might serve as a stepping stone toward abstinence or as a means to reduce morbidity and mortality associated with tobacco use among individuals not interested in quitting completely (Henningfield & Slade, 1998; Hughes, 1995; Shiffman, Mason, & Henningfield, 1998; Stratton, Shetty, Wallace, & Bondurant, 2001). Previous studies have observed a dose-response relationship between the amount of ST consumption and adverse health consequences (Tomar, Winn, Swango, Giovino, & Kleinman, 1997). A reduction in the incidence of adverse health consequences associated with ST use could theoretically be achieved through a reduction in ST toxicant exposure. In a previous preliminary ST reduction study, we observed a significant reduction in amount of tobacco use and biomarkers for tobacco toxicant exposure over an 8-week treatment period and 12-week follow-up (Hatsukami et al., 2003). This finding suggests the potential efficacy of a tobacco reduction strategy for reducing disease incidence associated with ST use.
One method to reduce ST exposure is the use of tobacco-free snuff as an ST substitute. This product retains the sensory aspects and frequency of dips while reducing exposure to nicotine and other toxicants. In cigarette smokers, the provision of smoking-related sensory stimuli has been observed to reduce craving and facilitate abstinence (Behm, Schur, Levin, Tashkin, & Rose, 1993; Rose, Behm, & Levin, 1993; Westman, Behm, & Rose, 1995). In a previous cessation trial conducted among ST users, we observed that tobacco-free snuff reduced craving and withdrawal symptoms (Hatsukami et al., 2000). The use of a product that provides ST sensory stimuli may facilitate ST reduction.
The goal of this preliminary study was to compare a tobacco reduction intervention using tobacco-free snuff with a no-snuff control condition. We hypothesized that compared with the no-snuff condition, the use of tobacco-free snuff would lead to greater reduction in ST use; less tobacco toxicant exposure; a greater percentage of ST users achieving at least 50% and 75% reduction in ST use; and as secondary outcomes a greater number of quit attempts, percentage of subjects achieving 7-day tobacco abstinence, and longer duration of abstinence. We also hypothesized that a relationship would exist between amount of ST use and biomarkers of exposure. The results of this study would indicate whether tobacco-free snuff could be a potential intervention to reduce ST use.
Method
Subject recruitment
Subjects were recruited from the Minneapolis, Minnesota, metropolitan area through newspapers and television advertisements. Subjects were screened over the telephone to determine interest and eligibility. During this screening, subjects were informed that the study compared two different interventions for ST use reduction. Interested participants were asked to attend a meeting for orientation and screening and to provide informed consent. At the orientation, subjects were required to complete a ST use questionnaire, medical history form, and baseline measurements. Potential subjects were eligible for enrollment if they were (a) aged 18–70 years, (b) interested in reducing ST use but not quitting (i.e., having an established quit date within the next 90 days), (c) using ST daily (⩾6 dips/day) for the past 6 months, (d) in good physical health (i.e., absence of an unstable medical condition or use of a medication that might affect tobacco use or be affected by tobacco use reduction), and (e) in good mental health (i.e., not taking psychotropic medications or manifesting a psychiatric comorbidity within the past 6 months). The rate of tobacco use was used as an inclusion criterion to target heavy ST users so that a reduction in toxicant exposure could be observed. This number also was based on prior studies demonstrating that the mean number of daily dips for ST users seeking cessation treatment is 6–10 (Hatsukami & Severson, 1999). Potential subjects were excluded if they were using other tobacco or nicotine products or were pregnant or lactating.
Products
Tobacco-free snuff contains no pharmacologically active ingredients and consists of organic material such as mint leaf, red clover, or alfalfa. A variety of tobacco-free snuff such as Smokey Mountain Wintergreen Herbal Snuff and Smokey Mountain Classic Herbal Snuff (Smokey Mountain Chew, Inc., Dallas, Texas) was made available to subjects.
Experimental procedures
After a 2-week period of baseline measurements, subjects were randomly assigned to tobacco-free snuff (intervention) or no snuff (control). Subjects assigned to the tobacco-free snuff group were asked to alternate the use of their usual brand of ST with tobacco-free snuff to achieve the targeted reduction. The number of ST dips required to achieve this reduction was determined from baseline measurements. The percentage reduction in intake was 50% for the first 4 weeks and 75% for the subsequent 4 weeks. If more tobacco-free snuff was necessary to reduce ST use for the targeted goal, the subjects were encouraged to use more product. Subjects recorded the time and type (regular snuff or tobacco-free snuff) of each dip. To maximize reporting accuracy, subjects were informed they would not be penalized for not following use instructions.
The control condition was assigned the same reduction schedule as the tobacco-free snuff group without the use of the tobacco-free substitute. They were instructed on behavioral methods for reduction such as extending the interval between dips, eliminating use in certain situations, and delaying use in the morning for as long as possible. To ensure that the behavioral treatment procedures were similar between the treatment conditions, these instructions also were provided to the tobacco-free snuff group. Subjects in the control condition were asked to record the time of their dips. All subjects were counseled that they might increase the duration of use or amount per dip to compensate for nicotine reduction and to be vigilant against this behavior.
Clinic visits and measurements occurred weekly during the 2-week baseline and the 8-week treatment period. At the end of the 8 weeks, subjects were asked whether they would like to quit ST use and encouraged to do so. If so, they set a quit date and follow-up calls were made at 1 week and 1 month after their quit date. If not, they were encouraged to maintain ST use reduction or reduce even further. Subjects in the tobacco-free snuff condition were offered more of this product if they chose to continue its use. A 2-week supply of the product was dispensed. If more tobacco-free snuff was needed, subjects were required to come in at week 10 and were given another 2-week supply. No more product was dispensed after week 12.
Follow-up
A follow-up session occurred at week 12 from the start of the treatment period. Every attempt was made to follow all subjects who completed the study, even if they did not meet the goal of at least a 50% ST reduction. All dependent measures were assessed at this follow-up. Subjects were asked to monitor the amount of ST and other tobacco use for 1 week prior to the follow-up clinic visit using daily diary cards and to provide a first-morning urine on the clinic visit day. The cards and urine cup were dispensed at visit 8. A reminder call was provided 1 week before the follow-up session.
Counseling
Subjects met with a counselor during the eight weekly clinic visits for individual sessions lasting no more than 10 min. During these sessions, a specific format was followed including (a) asking about tobacco use status, (b) discussing motivations for tobacco reduction, (c) discussing encountered problems, (d) problem solving difficult situations or compliance issues, and (e) providing support. For individuals wanting to quit after the 8-week period, counseling involved (a) discussing reasons for wanting to quit and potential obstacles, (b) discussing the content of the treatment manual, Tough Enough to Quit Snuff, including preparation for the quit day, identification of high-risk situations, and strategies to deal with these situations, and (c) identifying sources of support.
Compliance
Compliance with attending sessions was maximized by paying subjects US$20 for each visit and a $50 bonus at the end of treatment if they attended all sessions. In addition, subjects were paid $25 for the follow-up clinic visit.
Measures
At each clinic visit, ST and, if applicable, tobacco-free snuff use were determined by averaging self-recorded data captured on daily diary cards. Carbon monoxide levels using a Bedfont Micro Smokerlyzer also were evaluated at each visit. Urine samples were collected once during baseline and at the end of weeks 4, 8, and 12 to assess for cotinine, a metabolite of nicotine and biomarker of nicotine exposure, and for metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-l-(3-pyridyl)-l-butanone (NNK). NNK metabolite measurements were reported as total NNAL, consisting of 4-(methylnitrosamino)-l-(3 pyridyl) l-butanol (NNAL) and its glucuronides (NNAL-Glucs). Total urinary cotinine concentrations were determined by gas chromatography/mass spectrometry, as described previously (Hecht et al., 1999). Analysis for total NNAL was conducted as described previously (Hatsukami et al., 2003).
Data analyses
Analysis of the self-reported and quantitative ST use and exposure measures (i.e., number of tins/week and dips/day and urinary concentrations of cotinine and total NNAL) used repeated-measures linear models to investigate whether the two interventions had different effects on ST use reduction (Cnaan, Laird, & Slasor, 1997; Littell, Pendergast, & Natarajan, 2000; Schluchter, 1988). The analysis modeled, as a function of treatment group, the absolute change from baseline in reported ST use and concentrations of total cotinine and NNAL at each visit the assessments were collected. The models included main effects for time and treatment group as well as interactions between treatment group and time. These models were fitted using restricted maximum likelihood (REML) to estimate the covariance structure of the responses within a subject. REML has the added benefit of effectively handling the presence of unbalanced and missing data. The primary hypothesis tested was whether the treatment group had different effects on level of ST reduction at the end of 4 and 8 weeks and at the 12-week follow-up.
The analysis of the percentage in each group that achieved the targeted reduction level at the end of 4 (⩾50%), 8 (⩾50% and ⩾75%), and 12 (⩾50%) weeks compared the success rates for the different treatment groups using a logistic regression model for categorical data (Agresti, 1990). We examined both reduction levels (⩾50% and ⩾75%) at 8 weeks because, although the targeted reduction was 75%, 50% is the typical outcome of reduction studies. This analysis modeled the probability of success as a function of treatment group. Because of the repeated nature of the data, generalized estimating equations were used to estimate parameters, and the Z statistic was used to test whether an association existed between treatment group and achievement of the targeted level of ST reduction. In this analysis, the percentage reduction for a subject who did not complete a particular visit was assumed to be less than the targeted reduction and coded as unsuccessful.
The two treatment groups also were compared on (a) 7-day point-prevalence tobacco abstinence with biochemical verification using urinary cotinine concentrations (<100 ng/ml), (b) number of quit attempts lasting at least 24 hr in the previous 4 weeks, and (c) mean duration of abstinence. For all analyses, p values of .05 or less were considered statistically significant.
Results
Subject characteristics
Of the 227 telephone-screened potential subjects, 182 were considered eligible to attend the orientation meeting. Potential subjects were considered ineligible because of insufficient ST use (n=18), significant or recent health or psychiatric problems (n=9), excessive use of alcohol or other tobacco products (n=5), interested in quitting (n=3), or multiple combinations of the listed exclusions (n=10). Of the eligible subjects, 132 attended the orientation meeting, 132 signed informed consent, 26 subjects dropped out prior to or during the baseline period and before randomization, and 106 were randomized to treatment (52 in the tobacco-free snuff group and 54 in the control group). Among randomized subjects, 34 dropped out of the study, 14 from the tobacco-free snuff group and 20 from the control group. No statistically significant differences in baseline variables were observed between these dropouts and completers, or between the two treatment groups (Table 1).
Table 1.
Demographics and tobacco use history among smokeless tobacco (ST) users enrolled in a smokeless tobacco reduction study.
| Variable | Tobacco-free snuff |
Control |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | % | Number of subjects |
Mean (SD) | % | Number of subjects |
|
| Age, years | 33.7 (7.3) | 52 | 31.5 (6.6) | 54 | ||
| Total cotinine, ng/ml urine | 10,602 (6,340) | 51 | 10,250 (5,750) | 53 | ||
| Age at daily ST use, years | 20.2 (6.1) | 52 | 18.6 (5.1) | 54 | ||
| Age at first ST use, years | 17.1 (6.6) | 52 | 15.5 (3.5) | 54 | ||
| Tins/week | 4.2 (1.7) | 52 | 4.2 (1.8) | 54 | ||
| Total NNAL, pmol/ml urine | 3.0 (2.3) | 40 | 2.6 (1.50) | 42 | ||
| Brand of smokeless | ||||||
| Copenhagen | 28.8 | 15 | 35.2 | 19 | ||
| Skoal | 19.2 | 10 | 14.8 | 8 | ||
| Kodiak | 42.3 | 22 | 33.3 | 18 | ||
| Red Man | 7.7 | 4 | 14.8 | 8 | ||
Note. SD, standard deviation.
Tobacco-free snuff use
Throughout the 8 weeks of treatment with tobacco-free snuff, the mean number of dips per day of tobacco-free snuff ranged from 3.0 to 3.9, with standard deviations ranging from 1.8 to 3.4. Based on 31 subjects for whom we had daily diary records of dips per day of tobacco-free snuff for at least 6 weeks of the 8-week treatment period, a repeated-measures analysis to estimate individual slopes was conducted. Individual slopes were classified subsequently as positive (n=14) or negative (n=17) based on valence, and this classification was used to determine mean use. Mean use for the positive slope group was 4.1 (SD=2.1) at week 1, 4.3 (SD=2.1) at week 4, and 6.5 (SD=2.4) at week 8. Mean use for the negative slope group was 2.9 (SD=1.5) at week 1, 1.7 (SD=1.6) at week 4, and 1.4 (SD=2.0) at week 8.
Primary outcome measures: Smokeless tobacco and biomarker reduction
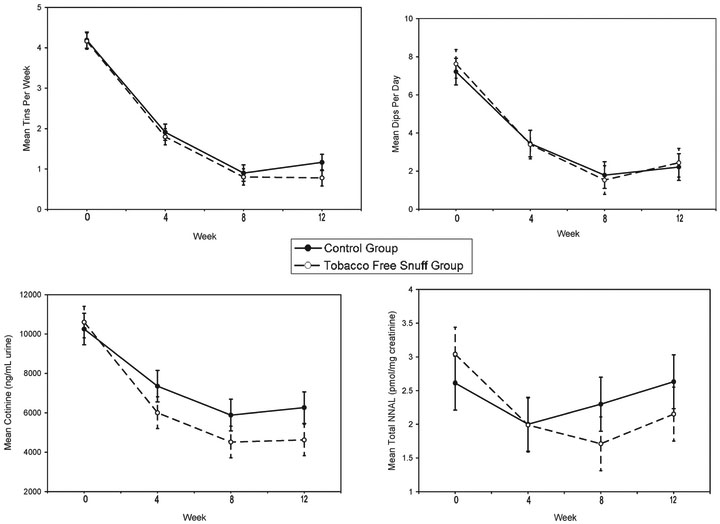
The primary analysis was conducted on data obtained during the treatment phase (baseline to week 8). Figure 1 shows the results for amount of tobacco use and biomarkers of exposure. For both groups, significant time effects were observed for tins/week (F2,254=162.06, p<.001) and dips/day (F2,253=164.54, p<.001). Significant differences were observed between baseline and weeks 4 and 8 and between week 4 and week 8 for both measures (all p values <.001). No significant treatment or treatment×time effects were observed for tins/week or dips/day.
Figure 1.
The effects of tobacco-free snuff and a control condition on amount and frequency of smokeless tobacco use and biomarkers for exposure (total NNAL and cotinine) during the treatment period (baseline to week 8) and at the 12-week follow-up.
Significant time effects were observed for cotinine concentration (ng/ml creatinine, F2,143=42.26, p<.001). Significant differences were observed between baseline and weeks 4 and 8 (p<.001) and between week 4 and week 8 (p=.019). No significant treatment effects or treatment × time effect were observed. A significant time effect was observed for total NNAL concentration (pmol/mg creatinine; F2,139= 16.60, p<.001). No significant treatment effect was observed. However, a significant time×treatment interaction was observed when taking into consideration baseline values (F2,139=3.36, p=.038).
Because reduction patterns differed between treatment conditions for total NNAL concentration, time effect and contrasts between weeks were examined within each condition. For the tobacco-free snuff condition, significant reductions occurred over time (F2,74= 15.02, p<.001), with significant reductions found between baseline and weeks 4 and 8 (p<.001, respectively), but no further reductions were observed between week 4 and week 8. For the control condition, a significant time effect (F2,67=3.73, p=.029) was observed. A significant reduction was observed between baseline and week 4 (p=.009) but not between baseline and week 8. Greater reductions were observed in mean total NNAL with the tobacco-free snuff condition compared with the control condition (p=0.048), particularly at week 8.
At 12 weeks, significant reductions compared with baseline continued to be observed for tins/week (p<.001), dips/day (p<.001), and cotinine (p<.001) but not for total NNAL. No significant differences were observed for any of these variables from week 8 to week 12.
Table 2 shows the mean percentage reduction in tins/week, dips/day, cotinine, and total NNAL among study participants. Consistent with the analyses involving change from baseline, significant time effects were observed for the percentage reduction in tins/week (p<.001), dips/day (p<.001), and cotinine (p=.048) but not for total NNAL. Significant treatment effects were observed for percentage reduction in cotinine (p=.026) and total NNAL (p<.001). Greater reductions were observed in the tobacco-free snuff group than in the control group.
Table 2.
Mean percentage reduction and proportion of subjects achieving at least a 50% reduction in baseline smokeless tobacco (ST) use by self-reported tobacco use and biomarkers of exposure.
| Variable | Mean percentage reduction, % (SD)a |
Percentage achieving at least 50% reduction at week 8 |
Percentage achieving at least 75% reduction at week 8 |
|||
|---|---|---|---|---|---|---|
| Tobacco-free snuff | Control | Tobacco-free snuff (n=52) |
Control (n=54) | Tobacco-free snuff (n=52) |
Control (n=54) | |
| Tins/week | ||||||
| Week 4 | 55.9 (15.0) | 52.9 (12.7) | 48.1 | 50.0 | ||
| Week 8 | 80.2 (15.4) | 77.8 (17.0) | 71.2 | 57.4 | 23.1 | 18.5 |
| Week 12 | 78.0 (26.1) | 70.2 (23.4) | 34.6 | 25.9 | ||
| Dips/day | ||||||
| Week 4 | 56.4 (15.2) | 51.1 (16.3) | 44.2 | 42.6 | ||
| Week 8 | 80.2 (17.8) | 72.5 (18.7) | 71.2 | 53.7 | 15.4 | 9.3 |
| Week 12 | 71.5 (46.8) | 66.6 (27.4) | 23.1 | 24.1 | ||
| Cotinineb,d | ||||||
| Week 4 | 37.9 (37.8) | 23.7 (37.7) | 30.8 | 16.7 | ||
| Week 8 | 55.8 (41.1) | 39.2 (51.9) | 42.3 | 24.1e | 25.0 | 13.0 |
| Week 12 | 54.1 (59.5) | 39.4 (47.5) | 44.2 | 16.7f | ||
| Total NNALc,d | ||||||
| Week 4 | 29.3 (32.1) | 18.5 (30.9) | 15.4 | 7.4 | ||
| Week 8 | 42.6 (46.9) | 4.0 (66.4) | 36.5 | 11.1f | 23.1 | 9.3e |
| Week 12 | 35.5 (56.6) | −1.6 (111.8) | 26.9 | 14.8 | ||
Note. SD, standard deviation.
Exclusive of dropouts; subject sample size varies from 42 to 26, with higher numbers of subjects earlier in treatment.
Treatment effect for mean percentage reduction in cotinine concentrations, p=.026.
Treatment effect for mean percentage reduction in total NNAL concentrations, p<.001.
When analysis was conducted on intent-to-treat basis with missing data reset as baseline values, the results were similar to the analysis exclusive of dropouts, except significant time effects were not observed for cotinine and tins/day. Additional treatment effects were observed for tins/week (p=.014) and dips/day (p=.020), most likely due to the higher dropout rates in the control group.
Treatment effect for percentage who achieved at least 50% or at least 75% ST reduction, p⩽.05.
Treatment effect for percentage who achieved at least 50% ST reduction, p<.01.
Table 2 also shows the proportion of subjects who reduced ST by at least 50% of baseline levels by time with missing values counted as not having achieved 50%. At week 8, significant differences between the treatment conditions were observed for the percentage of subjects who achieved at least a 50% reduction on measures of cotinine (p=.046) and total NNAL (p = .002), and near significant differences were observed for dips/day (p=.064). Near significant differences were observed in subjects who achieved at least a 75% reduction at week 8 for total NNAL (p=.053). For both 50% and 75% reductions, higher percentages were found in the tobacco-free snuff group than in the control group.
Secondary outcome measures
Correlation between measures.
We observed a significant correlation between tins/week and dips/day at weeks 4 (r=.65, p<.001), 8 (r=.73, p<.001), and 12 (r=0.87, p<.001). Significant correlations also were observed at each time point between cotinine concentrations and tins/week and dips/day, with the greatest variation in cotinine accounted for by tins/week and dips/day occurring at week 8 (R2=.30 and .29, respectively; p<.001) and the lowest variation occurring at week 4 (R2=.07 and .13, respectively; p=.022 to p=.002). Although at week 4, dips/day (p=.032) but not tins/week was a significant contributor to the variation in cotinine, at weeks 8 and 12, tins/week (p=.037 and p=.021, respectively) but not dips/day was a significant contributor. Significant correlations also were observed at each of the time points between total NNAL with tins/week and dips/day, with the greatest or similar variation of total NNAL accounted for by tins/week and dips/day occurring at week 12 (R2=.21 and .15, respectively; p<.001) and the lowest variation occurring at week 4 (R2=.15 and .10, respectively; p<.001 to p=.0087). For total NNAL, tins/week (p=.025) but not dips/day contributed significantly to the variation in total NNAL at week 4, and trends for tins/week were found at weeks 8 (p=.067) and 12 (p=.069). Significant correlation also was observed between cotinine and total NNAL at weeks 8 and 12 (r=.44, respectively; p<.001).
Quit attempts and abstinence.
In an analysis excluding individuals who already quit and in which missing values were considered as no attempts at quitting, a larger percentage of subjects made quit attempts lasting at least 24 hr in the previous 4 weeks in the tobacco-free snuff condition than in the control group, but significance was achieved only at week 12 (34.2% vs. 14.6%, respectively; χ2=4.69, p=.030). Using an intention-to-treat analysis in which missing values were considered to be 0, we observed significantly longer mean durations of abstinence for the tobacco-free snuff group compared with the control group (F1,104=6.33, p=.013).
The mean duration of abstinence assessed from baseline to week 12 was 14.9 days (SD=23.3) for the tobacco-free snuff group and 5.53 days (SD=12.9) for the control group. Among study completers, the mean duration of abstinence was 22.6 days (SD=25.2) versus 10.7 days (SD=16.4), respectively, with a significant treatment effect (F1,85=5.63, p=.026). No significant differences were observed in biochemically verified (urinary cotinine <100 ng/ ml) 7-day point-prevalence tobacco abstinence between the tobacco-free snuff and control groups at week 4 (1.9% vs. 0.0%), week 8 (13.5% vs. 9.3%), or week 12 (19.2% vs. 11.1%).
Discussion
We observed that, among tobacco users not interested in quitting, ST use reduction interventions with and without tobacco-free snuff are feasible and result in significant reductions in ST use and nicotine and carcinogen exposure. Compared with no snuff at end of treatment, the use of tobacco-free snuff resulted in significantly lower mean carcinogen exposure and significantly larger percentages of ST users achieving at least a 50% reduction in exposure to nicotine and carcinogens. Individual differences in tobacco-free snuff use were observed, with participants who increased their use of tobacco-free snuff during treatment demonstrating an increased amount of use at the onset of treatment compared with those who had decreased their use of tobacco-free snuff during treatment. In a post-hoc analyses, we found a modest but positive relationship between amount of tobacco-free snuff use and reduction in use of usual brand at each of the treatment weeks (r=.49−.59, all p values <.01). Whether greater use of tobacco-free snuff led to reduced use of usual ST brand or whether those who used greater amounts were more motivated to cut down is unknown. The use of tobacco-free snuff also was associated with more quit attempts and a longer duration of abstinence.
The amount of tobacco reduction achieved by ST users in the present study is similar to that found in studies of reduction interventions with cigarette smokers. When both treatment groups were combined, about 60% were able to achieve at least a 50% reduction in dips/day at week 8; the mean percentage reduction was 77% at week 8. By week 12, a little less than 24% reported at least a 50% reduction in ST use with a mean reduction of 69%. Among cigarette smokers required to reduce their smoking by 75%, mean reduction at the end of treatment at 6 weeks was 71% and at 12 weeks was 62% (Hatsukami et al., 2005).
In addition to the similarity in the amount of tobacco reduction in cigarette smokers and ST users, the extent of compensatory tobacco use was also almost identical. In a similar protocol in which smokers were required to reduce their smoking in 25% increments every 2 weeks over 6 weeks and sustain reduction over 26 weeks, mean reductions of 66% and 63% in cigarette use were observed at 8 and 12 weeks, which was associated with mean reductions of 35% and 28% in total NNAL levels (Hecht et al., 2004) or compensation indices of .47 and .56, respectively (1–percentage reduction in biomarker vs. percentage reduction in tobacco use, where 1 would be full compensation and 0 would no compensation; Scherer, 1999). These compensation indices are similar to those found in cigarettes smokers using carbon monoxide as the biomarker (Hughes & Carpenter, 2005).
In the present study, ST users in the tobacco-free snuff condition achieved mean reductions of 80% and 78% in tins/week of ST at weeks 8 and 12, respectively, and mean reductions of 43% and 36% in total NNAL, or compensation indices of .46 and .54, respectively. The extent of compensation may be even less if the ST users are given nicotine replacement therapy to replace decreasing levels of nicotine, as in the study with smokers. Despite this compensatory behavior in ST users, a significant relationship continued to exist between frequency and amount of ST use and biomarkers of exposure. We observed that the variation in cotinine concentrations was accounted for predominantly by dips/day initially and by tins/week later in the study. We conclude that as ST users reduce use, the quantity of use is more predictive of biomarker exposure than is frequency of use. Therefore, reduction strategies aimed at reducing the quantity of ST use have the greatest potential for reducing exposure among ST users.
In the present study, in general, we did not observe significant differences in self-reported ST use between treatment groups; however, the tobacco-free snuff group had a significantly higher proportion of subjects achieving at least a 50% reduction in exposure to nicotine and carcinogens. In a previous study, we observed that the greatest correlation with total cotinine and NNAL was with dip duration (Lemmonds et al., 2005). One possible explanation for greater biomarker exposure in the control group is that ST users were compensating for reduced nicotine intake by increasing dip duration. An indirect method to examine group differences in ST use topography such as dip duration, given that dips/ day and tins/week were similar across groups, is to calculate total cotinine and total NNAL per dip over time. In a post hoc analysis, we observed that subjects in the tobacco-free snuff group had less biomarker exposure per dip than the control group, but differences were not statistically significant. However, we may have been underpowered for this analysis, and additional studies are needed to precisely define the impact of tobacco-free snuff on dip duration.
We also observed a higher number of quit attempts and longer duration of abstinence in the tobacco-free snuff group compared with the control group, although we found no difference between the groups in 7-day point-prevalence tobacco abstinence rates. Previous studies investigating the effect of the provision of tobacco-free snuff (mint) to ST users in the dental setting found that the amount of tobacco-free snuff was correlated significantly with the number of tobacco-free days (Zavela, Harrison, Smith, Smith, & Manske, 1995). Another large clinical trial observed no difference in abstinence rates between subjects assigned to the tobacco-free snuff or control condition, although the mint snuff condition resulted in significantly reduced symptoms of withdrawal and craving (Hatsukami et al., 2000).
Our overall observed mean 7-day point-prevalence tobacco abstinence rate of 14.0% at week 12 is consistent with if not slightly higher than results of smoking reduction studies, which have ranged from 6.0% to 8.0% in cigarette smokers receiving medicinal nicotine and 0.5% to 6.0% in smokers receiving placebo (Bolliger et al., 2000; Hatsukami et al., 2005; Wennike, Danielsson, Landfeldt, Westin, & Tonnesen, 2003). Although observed abstinence rates did not differ significantly by treatment group, we observed that ST users in the tobacco-free snuff group had a significantly longer mean duration of abstinence. Clinical studies among cigarette smokers have consistently observed that duration of abstinence is a major predictor of long-term success (Gilpin, Pierce, & Farkas, 1997), although no such observations have been made among ST users. Our study suggests that ST reduction interventions could potentially mediate long-term abstinence by increasing the number and duration of quit attempts. Future studies are needed to assess if ST reduction increases ST abstinence rates compared with other ST treatment strategies.
Whether or not ST users would derive health benefits from sustaining reduced carcinogen exposure remains to be determined. We observed that the tobacco-free snuff condition reduced the mean total NNAL from 3.0 pmol/mg creatinine at baseline to 1.7 pmol/mg creatinine at the end of treatment (44% reduction). In a previous study, we observed that Copenhagen and Kodiak users who switched to General snus, a Swedish snuff product, reduced their mean total NNAL concentrations of 3.2 pmol/mg creatinine (95% CI=2.3–4.2) to 1.4 pmol/mg creatinine (95% CI=0.9–2.0), a 48% reduction (Hatsukami, Lemmonds, Zhang et al., 2004). In Sweden, the risk of oral cancer associated with ST use is low, and some studies show that the risks are no different for ST users than for nonusers, although ST users have an increased risk for pancreatic cancer (Hatsukami, Lemmonds, & Tomar, 2004; International Agency for Research on Cancer, in press). One explanation for this lower oral cancer risk is that the manufacturing process of U.S. ST products is fundamentally different from that of snus in Sweden, resulting in lower product carcinogen concentrations in the latter (Foulds, Ramstrom, Burke, & Fagerström, 2003). To achieve a reduction in carcinogen biomarker exposure similar to that achieved by switching to snus, users of U.S. ST products would have to achieve a mean reduction of 75%–80%. The ability of ST users to maintain this degree of reduction is uncertain.
The major limitation of the present study is that it was a preliminary study; therefore, the sample size was small for a clinical trial. Also, a significant number of participants dropped out of the study—about a third, a rate similar to that observed in studies of cigarette reduction interventions (e.g., Bolliger et al., 2000; Hatsukami et al., 2005; Wennike et al., 2003) but higher than in other studies (e.g., Etter, Laszlo, Zellweger, Perrot, & Perneger, 2002).
The results from the present study show that a gradual reduction in ST use is achievable both with and without tobacco-free snuff. Although the use of tobacco-free snuff does not affect the frequency or amount of ST reduction, it may result in greater reductions in exposure to nicotine and carcinogens by decreasing the extent of compensatory tobacco use resulting from reductions in nicotine levels. Tobacco-free snuff can potentially facilitate longterm abstinence by possibly increasing the number of quit attempts and duration of abstinence. Thus, ST reduction intervention programs appear to hold promise for decreasing disease risk associated with ST use by reducing carcinogen exposure and eliminating risk by facilitating tobacco abstinence.
Acknowledgments
This study was funded by National Institute on Drug Abuse grants DA14404 and DA013333. The authors thank Herb Severson for his valuable consultation on the design of this study and Marc Mooney for his statistical assistance.
Contributor Information
Dorothy K. Hatsukami, University of Minnesota, Tobacco Use Research Center and the Cancer Center, Minneapolis, MN
Jon O. Ebbert, Mayo Clinic College of Medicine, Minneapolis, MN.
Amanda Edmonds, University of Minnesota, Tobacco Use Research Center and the Cancer Center, Minneapolis, MN.
Casey Li, University of Minnesota, Tobacco Use Research Center and the Cancer Center, Minneapolis, MN.
Haiying Lin, University of Minnesota, Tobacco Use Research Center and the Cancer Center, Minneapolis, MN.
Chap Le, University of Minnesota, Tobacco Use Research Center and the Cancer Center, Minneapolis, MN.
Stephen S. Hecht, University of Minnesota, Tobacco Use Research Center and the Cancer Center, Minneapolis, MN
References
- Agresti A (1990). A categorial data analysis. New York: Wiley. [Google Scholar]
- Behm FM, Schur C, Levin ED, Tashkin DP, & Rose JE (1993). Clinical evaluation of a citric acid inhaler for smoking cessation. Drug and Alcohol Dependence, 31, 131–138. [DOI] [PubMed] [Google Scholar]
- Bolliger CT, Zellweger J-P, Danielsson T, van Biljon X, Robidou A, Westin A, Perruchoud A, & Sawe U (2000). Smoking reduction with oral nicotine inhalers: Double blind, randomised clinical trial of efficacy and safety. British Medical Journal, 321, 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnaan A, Laird N, & Slasor P (1997). Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Statistics in Medicine, 16, 2349–2380. [DOI] [PubMed] [Google Scholar]
- Etter J, Laszlo E, Zellweger JP, Perrot C, & Perneger TV (2002). Nicotine replacement to reduce cigarette consumption in smokers who are unwilling to quit: A randomized trial. Journal of Clinical Psychopharmacology, 22, 487–495. [DOI] [PubMed] [Google Scholar]
- Foulds J, Ramstrom L, Burke M, & Fagerström K (2003). Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco Control, 12, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin EA, Pierce JP, & Farkas AJ (1997). Duration of smoking abstinence and success in quitting. Journal of the National Cancer Institute, 89, 572–576. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Edmonds A, Schulte S, Jensen J, Le C, Losey L, Carmella S, & Hecht S (2003). Preliminary study on reducing oral moist snuff use. Drug and Alcohol Dependence, 70, 215–220. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Grillo M, Boyle R, Allen S, Jensen J, Bliss R, & Brown S (2000). Treatment of spit tobacco users with transdermal nicotine system and mint snuff. Journal of Consulting and Clinical Psychology, 68, 241–249. [PubMed] [Google Scholar]
- Hatsukami D, Kotlyar M, Allen S, Jensen J, Li S, Le C, & Murphy S (2005). Effects of cigarette reduction on cardiovascular risk factors and subjective measures. Chest, 128, 2528–2537. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Lemmonds C, & Tomar S (2004). Smokeless tobacco use: Harm reduction or induction approach? Preventive Medicine, 38, 309–317. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Lemmonds CA, Zhang Y, Murphy SE, Le C, Carmella SG, & Hecht SS (2004). Evaluation of carcinogen exposure in people who used “reduced exposure” tobacco products. Journal of the National Cancer Institute, 96, 844–852. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, & Severson H (1999). Oral spit tobacco: Addiction, prevention and treatment. Nicotine & Tobacco Research, 1, 21–44. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Chen M, Koch JD, Miller A, Murphy SE, Jensen JA, Zimmerman CL, & Hatsukami DK (1999). Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Research, 59, 590–596. [PubMed] [Google Scholar]
- Hecht SS, Murphy SE, Carmella SG, Zimmerman CL, Losey L, Kramarczuk I, Roe MR, Puumala SS, Li YS, Le CT, Jensen J, & Hatsukami DK (2004). Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. Journal of the National Cancer Institute, 96, 107–115. [DOI] [PubMed] [Google Scholar]
- Henningfield J, & Slade J (1998). Tobacco-dependence medications: Public health and regulatory issues. Food and Drug Law Journal, 53(Suppl.), 75–114. [PubMed] [Google Scholar]
- Hughes JR (1995). Applying harm reduction to smoking. Tobacco Control, 4(Suppl. 2), 533–538. [Google Scholar]
- Hughes JR, & Carpenter MJ (2005). The feasibility of smoking reduction: An update. Addiction, 100, 1074–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. (in press). Smokeless tobacco and tobacco-specific nitrosamines. (Vol. 89). Lyon, France: Author. [Google Scholar]
- Lemmonds CA, Hecht SS, Jensen JA, Murphy SE, Carmella SG, Zhang Y, & Hatsukami DK (2005). Smokeless tobacco topography and toxin exposure. Nicotine & Tobacco Research, 7, 469–474. [DOI] [PubMed] [Google Scholar]
- Littell R, Pendergast J, & Natarajan R (2000). Modelling covariance structure in the analysis of repeated measures data. Statistics in Medicine, 19, 1793–1819. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, & Levin ED (1993). Role of nicotine dose and sensory cues in the regulation of smoke intake. Pharmacology, Biochemistry, and Behavior, 44, 891–900. [DOI] [PubMed] [Google Scholar]
- Scherer G (1999). Smoking behaviour and compensation: A review of the literature. Psychopharmacology, 145, 1–20. [DOI] [PubMed] [Google Scholar]
- Schluchter M (1988). Analysis of incomplete multivariate data using linear models with structured covariance matrices. Statistics in Medicine, 7, 317–324. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Mason KM, & Henningfield JE (1998). Tobacco dependence treatments: Review and prospectus. Annual Review of Public Health, 19, 335–358. [DOI] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, & Bondurant S (Eds.). (2001). Clearing the smoke: Assessing the science base for tobacco harm reduction. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Tomar S, Winn D, Swango G, Giovino G, & Kleinman D (1997). Oral mucosa smokeless tobacco lesions among adolescents in the United States. Journal of Dental Research, 76, 1277–1286. [DOI] [PubMed] [Google Scholar]
- Wennike P, Danielsson T, Landfeldt B, Westin A, & Tonnesen P (2003). Smoking reduction promotes smoking cessation: Results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction, 98, 1395–1402. [DOI] [PubMed] [Google Scholar]
- Westman EC, Behm FM, & Rose JE (1995). Airway sensory replacement combined with nicotine replacement for smoking cessation: A randomized, placebo-controlled trial using a citric acid inhaler. Chest, 107, 1358–1364. [DOI] [PubMed] [Google Scholar]
- Zavela K, Harrison L, Smith C, Smith M, & Manske K (1995). The effectiveness of mint snuff as an oral substitute in smokeless tobacco cessation. Journal of the Canadian Dental Association, 73, 26–27. [PubMed] [Google Scholar]