Abstract
We present the case of a 45-year-old healthy man who successfully completed three stages of the Bruce protocol but developed inferolateral ST segment elevation in the recovery phase. The ECG change was associated with a marked drop in blood pressure. He underwent emergency coronary angiography which revealed normal coronary arteries. It is likely that post-exercise hypotension triggered coronary spasm which caused the ST segment elevation. Alternatively, coronary spasm may have been the primary event, inducing sufficient myocardial ischaemia to cause a marked drop in blood pressure. Exercise tolerance testing is often a reliable test to rule out reversible myocardial ischaemia. While the physician is focused on ischaemic changes or rhythm abnormalities developing during the exercise phase, the recovery period is just as important and requires as much vigilance. Coronary vasospasm can result in significant ST changes and haemodynamic compromise at any point during the test, and the ECG traces can be indistinguishable from a classic ST elevation myocardial infarction, as in the present case.
Keywords: interventional cardiology, ischaemic heart disease
Background
The treadmill test is used for detection of reversible myocardial ischaemia. It is cheap, simple to perform and readily available. Interpretation of the results is often straightforward. In the context of coronary disease, the physician is interested in symptoms and ECG changes suggestive of coronary insufficiency, both of which can be atypical/subtle. ST segment depression and T wave changes are the most common abnormalities. We present the case of a 45-year-old man who developed ST segment elevation during the recovery phase of an exercise test performed as part of a routine health check.
Case presentation
A 45-year-old man attended a treadmill test as part of a routine medical check-up. He was medically fit and a keen athlete who had previously completed three marathons. His prior medical history was unremarkable. He was a non-smoker and had no significant family history of note.
He successfully completed three stages of a standard BRUCE protocol, achieving a peak heart rate of 176 bmp, without developing any cardiac symptoms, arrhythmias or repolarisation changes on the ECG to suggest reversible myocardial ischaemia (table 1 and figure 1). He had an appropriate BP response to exercise.
Table 1.
HR and BP according to BRUCE protocol stage and in the recovery phase
| BRUCE stage | BP (mm Hg) | HR (bpm) |
| Baseline | 120/90 | 109 |
| I | 130/85 | 118 |
| II | 151/75 | 133 |
| III | 155/74 | 160 |
| Recovery (1 min) | 121/65 | 158 |
| Recovery (3 min) | 121/65 | 110 |
| Recovery (3.5 min) | 79/56 | 88 |
| Recovery (5 min) | 100/60 | 78 |
Figure 1.
ECG during exercise at 110 bpm (BRUCE stage I).
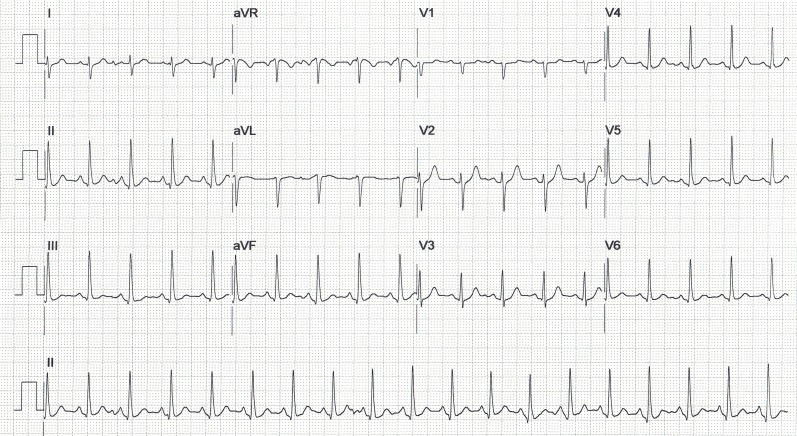
Three-and-a-half minutes into recovery, there was a significant drop in systolic BP from a peak of 155 mm Hg during exercise to 79 mm Hg (baseline BP was 120 mm Hg). This was associated with dizziness and gross ST segment elevation in the inferolateral leads (figure 2), without any chest pain. There was reciprocal ST depression in leads I, aVL and (minimally) V1– V3.
Figure 2.
ECG at 4 min into recovery period.
He was promptly transferred to the nearest heart attack centre for primary percutaneous coronary intervention. While awaiting transfer, his symptoms resolved and his ECG returned to baseline. The ECG changes lasted for 2 min and settled without any medications (eg, glyceryl trinitrate or antiplatelet therapy).
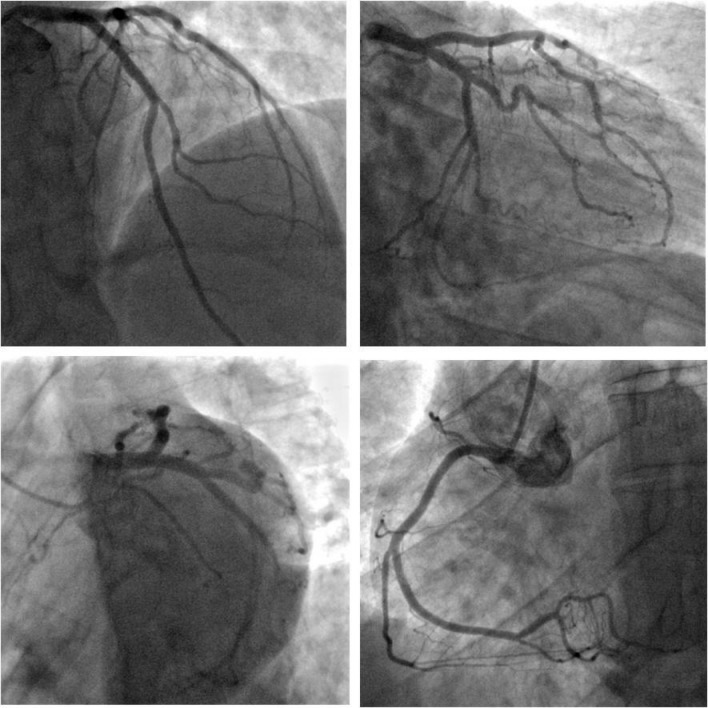
Coronary angiography revealed a dominant right coronary artery (RCA) and no significant coronary artery disease (figure 3). Left ventricular (LV) angiography revealed normal LV systolic function with no wall motion abnormalities. High-sensitivity Troponin T levels were within normal limits but his low-density lipoprotein cholesterol was raised at 4.8 mmol/L.
Figure 3.
Coronary angiography revealed normal coronaries.
Echocardiography revealed normal LV size, wall thickness and systolic and diastolic function. No regional wall motion abnormalities were detected. A subsequent cardiac magnetic resonance scan reported an LV ejection fraction of 58% with no structural abnormalities or evidence of previous MI, infiltration or fibrosis.
Given the likelihood of coronary spasm as a cause for his presentation, he was discharged home on Diltiazem and remained well and asymptomatic at clinical review 3 months later.
Differential diagnosis
It is assumed that his ST elevation was secondary to severe coronary spasm triggered by marked hypotension. We believe this is the most likely course of events given the evidence for hypotension just before the development of ECG changes as documented, and that the patient would likely have exhibited significant haemodynamic instability and symptoms if the coronary vasospasm had been the initial trigger for the marked drop in BP as this would suggest a significant drop in cardiac output (CO) as a consequence of cardiac ischaemia. Alternatively, coronary spasm may have been the primary event causing severe myocardial ischaemia and a subsequent fall in blood pressure. We cannot definitively rule this out without an invasive blood pressure reading (eg, arterial line) during the event in order to record the exact timing of the BP drop in relation to the ECG changes. It is highly likely that a combination of both the peripheral vasodilation and coronary vasospasm resulted in the profound hypotension to some degree.
Treatment
The patient was discharged home on a calcium channel blocker (Diltiazem) as recommended for the treatment of angina induced by coronary vasospasm. Coronary vasospasm can occur without symptoms, and it has been suggested that follow-up with a Holter monitor could help diagnose ‘silent’ vasospasm, but there is no evidence for any long-term benefit in diagnosing silent vasospasm in coronaries free from atherosclerotic disease on angiography.
Outcome and follow-up
Telephone consultation a year later confirmed that he remains asymptomatic and well and continues to engage in running activities following an initial cautious interval where he gradually intensified his workout regimen.
Discussion
Although ischaemic changes appearing only during the recovery phase post exercise have been reported, this remains a rare occurrence.1 2 It is described as a specific finding in patients with Brugada syndrome and is a predictor of poor outcome in this subset of cardiac patients,3 but is a less specific finding in healthy and asymptomatic individuals.
For decades, exercise tolerance testing (ETT), or the treadmill test, has been a useful tool at the disposal of the physician to assess the likelihood of coronary artery disease. More recently, tests such as CT coronary angiography and CT calcium scoring have become more readily available and are gradually replacing ETT. The latter, however, remains a useful test, given its non-invasive nature and non-reliance on X-rays or contrast medium, making it ideal for inclusion in a routine medical check-up.
In healthy individuals, heart rate and blood pressure increase during exercise and fall during the recovery phase. An inadequate rise, or indeed a fall, in BP during exercise is a sign of underlying coronary artery disease4; however, it has been suggested that the maximal systolic blood pressure achieved during exercise is a better predictor of 5-year survival.5 Small-scale studies have suggested that hypotension during exercise does not necessarily indicate coronary disease in females, but does so in males. Males with hypotension have a higher incidence of coronary disease, but the extent and distribution of the disease is similar to that seen in patients with a normal BP response to exercise.6 Hypotension during exercise is thought to be a predictor of cardiac events only in the subset of patients with a previous history of MI and those who exhibit signs or symptoms of ischaemia during the exercise test.7 This, however, requires further large-scale studies with longer-term follow-up.
A certain degree of post-exercise hypotension can be explained using basic physiology. In the recovery phase, in non-endurance trained males, there is a drop of up to 10 mm Hg in systolic BP compared with baseline. This is thought to be a result of peripheral vasodilatation in the muscle groups, not offset by the increase in CO. The stroke volume is generally maintained and heart rate remains elevated following exercise, leading to an increased CO. In endurance-trained men, there is a reduction in CO when compared with pre-exercise levels, which, interestingly, is not seen in female athletes.8 9 There are two types of post-exercise vasodilatation: an immediate hyperaemia which lasts up to 20 min, and a sustained post-exercise vasodilatation which can last in excess of 2 hours. The latter is thought to be a result of Histamine H1 and H2 receptor activation, but the mechanisms behind the immediate vasodilatation are less clearly defined and more likely to be multifactorial.10
Our patient exhibited a fall in BP of 41 mm Hg compared with baseline measurements. To examine how this compares with other test results, we undertook an analysis of 100 consecutive negative treadmill tests (mean age 51, median 55 years) performed at our department over the last 18 months. The results show a mean increase of 7 mm Hg in recovery BP when compared with baseline BP (range −38 to +57 mm Hg, SD 17). As such, our patient exhibited a post-exercise BP drop which is at the extreme end of the range seen in clinical practice. His heart rate of 83 bpm does not suggest excessive vagal activity (although it is clear that vagal activity was increasing to some degree at that stage—see table 1), and does not account for the fall in blood pressure. We must assume, therefore, that the mechanism of the blood pressure fall in this case was due to (1) profound peripheral vasodilatation, (2) a substantial fall in CO or (3) a combination of the two.
The ECG recorded at 4 min of recovery is grossly abnormal. The rhythm is sinus, the PR interval is unchanged and normal in duration and there is no evidence of ventricular pre-excitation. ST elevation is confined to leads II, III, aVF and in V4– V6, with reciprocal ST depression in leads I, aVL, aVR and (minimally) V1– V3. These changes point to acute ischaemia in a single coronary artery territory, most likely a dominant RCA. Yet, no significant coronary artery disease was found on coronary angiography, including the dominant RCA, suggesting the possibility of intense, but reversible, RCA spasm during the recovery period. Our patient was a trained amateur athlete and therefore intense peripheral vasodilation, with a subsequent fall in CO and blood pressure, may have triggered selective intense spasm in his dominant RCA with subsequent ischaemia and ST segment elevation in the inferior and lateral leads.
Alternatively, intense spasm in the RCA during the recovery phase may have been the primary event, triggering ischaemia of the right ventricle and inferoposterior LV wall, and a subsequent fall in CO and blood pressure.
Coronary vasospasm usually presents with chest pain and ECG changes; the absence of chest pain, despite gross ECG changes, is unusual. There is one case report of an incidental finding of ST elevation without any symptoms, although that patient was older and had a prior medical history of hypertension and hypercholesterolaemia.11 Asymptomatic ST elevation has been reported in patients with coronary vasospasm. A study of 26 patients with variant angina has reported episodes of asymptomatic ST elevation on 24 hours monitoring. However, these patients were selected for the study based on their initial presentation of chest pain at rest, and angiography was reported as normal/minimal lesion in only 5 of the 26 patients (19.2%).12 Another study of 138 patients with transient ST elevation has reported normal coronaries in 8 (5.8%). Again, all patients were included in the study due to their initial reports of chest pain despite episodes of asymptomatic ST elevation on further investigation.13 The novelty of our case is that the patient is a fit, asymptomatic and active man who performed the treadmill test without any symptoms and only developed ST elevation and dizziness during the recovery phase, while hypotensive relative to baseline. This is highly suggestive of hypotension-triggered vasospasm. Since we have ruled out all pathologies using invasive and non-invasive investigations, the most likely remaining cause of the ECG changes is presumed to be coronary spasm, and the patient was discharged on calcium channel blockers.
Provocative testing using intracoronary ergonovine, acetylcholine, dopamine and neuropeptide Y has been described for diagnosing coronary vasospasm, but is not routinely performed for a number of reasons, including (1) a significant risk of triggering severe and uncontrollable vasospasm, leading to infarction or arrhythmias; (2) triggering vasospasm in healthy vessels, rendering the test non-specific and (3) provocative testing does not necessarily change the management plan as, regardless of the result, the patient is often discharged on nitrates, calcium channel blockers or both. We accept that a definitive diagnosis of vasospasm using provocative testing would have helped to justify the need for long-term drug therapy and its burden on the patient, but in the absence of other identifiable triggers, in this case, we believe the patient would benefit from medication to prevent future events. A major adverse cardiac event rate of 5.5% has been reported in the 32-month period following provocative testing.14 15 Further large-scale studies are required to formulate definitive guidelines based on a diverse population of patients. Hyperventilation or a cold pressor test can also be used to provoke coronary spasm, but in our lab, none of these provocative tests is routinely used, which may be a limitation of this case report.
Repeat exercise-testing on medication has been suggested as a means to assess symptoms and ECG evidence of ischaemia during the follow-up period,16 but the long-term prognostic benefit of this in a patient without any symptoms and no atherosclerotic disease on angiography remains uncertain. The mechanisms behind hypotension-triggered coronary vasospasm at the cellular level are beyond the scope of this article. However, this clinical phenomenon has been described previously in situations where there is coronary underfilling such as in the postoperative hypotensive patient.17 In our case, the patient is active and back to running marathons without any further indications for cardiac investigations.
Learning points.
The recovery phase of the exercise tolerance test is just as important as the exercise stage, and the physician must remain vigilant for ECG changes and BP variations during both.
Despite very significant ST deviations, coronary angiography can reveal normal coronary arteries, suggesting coronary spasm as an aetiology.
Provocative testing may be considered during coronary angiography to support a diagnosis of coronary vasospasm.
Footnotes
Contributors: The contributions to the manuscript preparation are as follows: RA and ME-O: planning; ME-O: conduct; RA, DR and ME-O: conception and design; RA, SME-O, DR and ME-O: reporting, acquisition of data, analysis and interpretation of data and manuscript writing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Sunbul M, Erdogan O, Sari I. Asymptomatic ST segment elevation in the recovery phase of the exercise stress test due to slow coronary flow. Postepy Kardiol Interwencyjnej 2014;10:53–6. 10.5114/pwki.2014.41471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiner DA, Schick EC, Hood WB, et al. ST-segment elevation during recovery from exercise. A new manifestation of Prinzmetal’s variant angina. Chest 1978;74:133–8. 10.1378/chest.74.2.133 [DOI] [PubMed] [Google Scholar]
- 3. Makimoto H, Nakagawa E, Takaki H, et al. Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J Am Coll Cardiol 2010;56:1576–84. 10.1016/j.jacc.2010.06.033 [DOI] [PubMed] [Google Scholar]
- 4. Watson G, Mechling E, Ewy GA. Clinical significance of early vs late hypotensive blood pressure response to treadmill exercise. Arch Intern Med 1992;152:1005–8. 10.1001/archinte.1992.00400170089017 [DOI] [PubMed] [Google Scholar]
- 5. Hammermeister KE, DeRouen TA, Dodge HT, et al. Prognostic and predictive value of exertional hypotension in suspected coronary heart disease. Am J Cardiol 1983;51:1261–6. 10.1016/0002-9149(83)90296-5 [DOI] [PubMed] [Google Scholar]
- 6. Levites R, Baker T, Anderson GJ. The significance of hypotension developing during treadmill exercise testing. Am Heart J 1978;95:747–53. 10.1016/0002-8703(78)90505-7 [DOI] [PubMed] [Google Scholar]
- 7. Dubach P, Froelicher VF, Klein J, et al. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 1988;78:1380–7. 10.1161/01.CIR.78.6.1380 [DOI] [PubMed] [Google Scholar]
- 8. Senitko AN, Charkoudian N, Halliwill JR. Influence of endurance exercise training status and gender on postexercise hypotension. J Appl Physiol 2002;92:2368–74. 10.1152/japplphysiol.00020.2002 [DOI] [PubMed] [Google Scholar]
- 9. Meade RD, Crandall CG, Gagnon D, et al. Greater fluid loss does not fully explain the divergent hemodynamic balance mediating postexercise hypotension in endurance-trained men. J Appl Physiol 2018;124:1264–73. 10.1152/japplphysiol.00988.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halliwill JR, Buck TM, Lacewell AN, et al. Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp Physiol 2013;98:7–18. 10.1113/expphysiol.2011.058065 [DOI] [PubMed] [Google Scholar]
- 11. Mohammed I, Zaatari MS, Tyrogalas N, et al. Asymptomatic coronary artery spasm with acute pathological ST elevation on routine ECG: is it common? BMJ Case Rep 2014;2014:bcr2013202586 10.1136/bcr-2013-202586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Araki H, Koiwaya Y, Nakagaki O, et al. Diurnal distribution of ST-segment elevation and related arrhythmias in patients with variant angina: a study by ambulatory ECG monitoring. Circulation 1983;67:995–1000. 10.1161/01.CIR.67.5.995 [DOI] [PubMed] [Google Scholar]
- 13. Maseri A, Severi S, Nes MD, et al. "Variant" angina: one aspect of a continuous spectrum of vasospastic myocardial ischemia. Pathogenetic mechanisms, estimated incidence and clinical and coronary arteriographic findings in 138 patients. Am J Cardiol 1978;42:1019–35. [DOI] [PubMed] [Google Scholar]
- 14. Davies O, Ajayeoba O, Kurian D. Coronary artery spasm: An often overlooked diagnosis. Niger Med J 2014;55:356–8. 10.4103/0300-1652.137231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaya M, Mehta PK, Merz CN. Provocative testing for coronary reactivity and spasm. J Am Coll Cardiol 2014;63:103–9. 10.1016/j.jacc.2013.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tamura A, Nagao K, Inada T, et al. Exercise-induced vasospastic angina with prominent ST elevation: a case report. Eur Heart J Case Rep 2018;2:yty141 10.1093/ehjcr/yty141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hadi H, D’souza S, El-Omar M. Hypovolemia-induced severe coronary spasm leading to acute myocardial infarction. Exp Clin Cardiol 2012;17:74–6. [PMC free article] [PubMed] [Google Scholar]