Abstract
A 56-year-old man was referred with left-sided hip pain. MRI scans demonstrated an undisplaced stress fracture in the femoral neck and subchondral oedema within the femoral head. Bone densitometry showed T-scores of −2.0 at the spine, −3.5 at the femoral neck and −2.4 for the total hip. Laboratory tests revealed 25-hydroxyvitamin D <10 nmol/L. He was prescribed a 10-day course of calciferol 1.25 mg (50 000 IU)/day and started on calcium carbonate 1.25 g twice daily. Following the correction of vitamin D deficiency, his symptoms resolved. A striking feature of this patient was the complete reversal of ‘osteoporosis’ within 14 months with vitamin D and calcium supplementation. Bone mineral densities (BMDs) increased by 19.5% and 33.4% at the spine and hip, respectively. Such changes are never seen with conventional pharmacological management of osteoporosis. Vitamin D deficiency should be considered as a cause for reduced BMD in people with risk factors.
Keywords: calcium and bone, endocrine system, musculoskeletal and joint disorders
Background
Osteomalacia is an important metabolic bone disease caused by severe vitamin D deficiency and characterised by defective bone mineralisation. Osteomalacia can lead to reduced bone mineral density (BMD) and an increased risk of fractures.
We present a patient with typical clinical and biochemical features of osteomalacia. The patient also had markedly low BMDs at the spine and hip. Correction of the vitamin D deficiency led to a rapid improvement in clinical symptoms and normalisation of BMDs without the need for antiresorptive therapy. The case demonstrated the importance of considering vitamin D deficiency as a cause for low BMDs and that anti-resorptive therapy may not be necessary in such instances.
Case presentation
A 56-year-old man of Indian descent was referred to us with left-sided hip pain and reduced bone density. Nine months prior to this consult, he developed severe pain in his left hip following a fall down several steps. An MRI scan of the patient’s left hip at that time demonstrated a small undisplaced stress fracture in the inferomedial femoral neck. His symptoms gradually eased over the following months, but he still experienced pain on walking, and his mobility was greatly reduced. A repeat MRI scan 6 months after the initial scan showed partial healing of the original stress fracture, but some new subchondral oedema within the left femoral head, suggesting further bony injury. A week prior to his consultation with us, he had bone densitometry, which showed BMD T-scores of −2.0 at the spine (L1–L4), −3.5 at the femoral neck and −2.4 for the total hip.
His past medical history included psoriasis, for which he was on calcipotriol ointment, and asthma. He occasionally used topical steroids for psoriasis and had several short courses of oral prednisone for asthma. He was otherwise well with no other symptoms. He did not drink alcohol or smoke. He consumed a vegetarian diet with intake of yoghurt two to three times weekly and milk in his tea twice daily. He worked as a train conductor on the local suburban railway, which involved regular outdoor exposure at each train stop. Examination demonstrated pain on weight-bearing but was otherwise unremarkable.
Investigations
Laboratory tests revealed normal full blood count, electrolytes, albumin, renal function, liver function, thyroid function, erythrocyte sedimentation rate, coeliac screen, prolactin, testosterone and prostate-specific antigen. 25-hydroxyvitamin D was <10 nmol/L (reference range 50–150 nmol/L), parathyroid hormone (PTH) 35 pmol/L (1.7–7.3 pmol/L), calcium (albumin adjusted) 1.92 mmol/L (2.10–2.55 mmol/L), alkaline phosphatase (ALP) 132 U/L (40–120 U/L) and procollagen-1 N-terminal propeptide (P1NP) 118 µg/L (20–85 µg/L).
Diagnosis
A diagnosis of vitamin D deficiency osteomalacia was made.
Treatment
He was prescribed a 10-day course of calciferol 1.25 mg (50 000 IU)/day and started on calcium carbonate 1.25 g twice daily.
Outcome and follow-up
A month later, he was pain-free and walking normally. His laboratory tests showed normalisation of calcium, ALP and 25-hydroxyvitamin D (89 nmol/L), a reduction of PTH (17.9 pmol/L) and a further increase in P1NP (223 µg/L). He was advised to continue with calcium carbonate and incorporate calcium into his diet. He was continued on a monthly dose of calciferol 1.25 mg. Repeat bone densitometry 2 months following treatment demonstrated BMD T-scores of −0.5 at the spine, −3.0 at the femoral neck and −1.8 for the total hip.
Six months following the start of treatment, he was back to full activities with no symptoms. Further bone densitometry revealed T-scores of −0.7 at the spine, −2.1 at the femoral neck and −1.2 for the total hip. His PTH (9.1 pmol/L) and P1NP (117 µg/L) were still raised, and he was advised to continue calcium carbonate.
Fourteen months following treatment, T-scores were −0.5 at the spine, −1.3 at the femoral neck and −0.4 for the total hip. His BMDs were now in the middle of the age-appropriate normal range (figure 1). His PTH and P1NP normalised, and he was advised to discontinue calcium carbonate therapy and continue on monthly calciferol supplementation.
Figure 1.
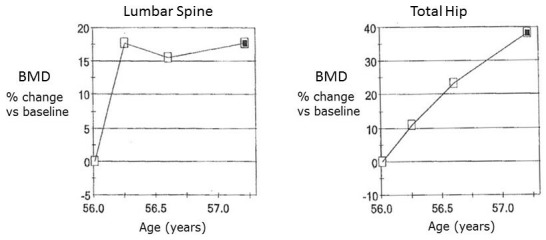
Response of bone mineral densities (BMDs) to treatment with Vitamin D and calcium at the lumbar spine and total hip.
Discussion
Vitamin D deficiency is the most common cause of osteomalacia worldwide and results in reduced calcium and phosphate absorption in the gut. This in turn leads to secondary hyperparathyroidism with a subsequent increase in bone turnover and bone loss and failure of mineralisation of organic osteoid tissue. Our patient presented with the typical clinical and biochemical features of osteomalacia but also had markedly reduced BMD. Following correction of the vitamin D deficiency, his symptoms resolved, and bone biochemistry and BMD normalised. Characteristic radiological features of osteomalacia include Looser zones (visualised on plain radiography as narrow radiolucent lines with sclerotic borders that lie perpendicular to the cortical margins of bones), which represent insufficiency-type fractures, and were seen on the MRI of our patient. The cause of his severe vitamin D deficiency is uncertain. Low 25-hydroxyvitamin D levels are common in people of Indian ethnicity.1 He consumed a vegetarian diet, and in New Zealand, mainstream dairy products are not fortified with vitamin D, so it is unlikely he was consuming significant vitamin D via dietary intake. His mobility was greatly reduced following his initial fall and likely led to a reduction in outdoor sun exposure. Therefore, it is likely his initial vitamin D deficiency became more profound during that period, resulting in osteomalacia. It is unlikely his infrequent courses of topical and oral steroid therapy contributed to his bone disease.
To monitor treatment response, we used the bone turnover marker, P1NP. This marker was raised even following normalisation of calcium, ALP, vitamin D and PTH. This persistent increase mirrored the ongoing improvements in BMD, which continued for over 12 months and demonstrates the value of P1NP as a non-invasive marker of osteoblast activity.
A striking feature of this patient was the presence of osteoporosis at presentation and the complete reversal of this with vitamin D and calcium supplementation. Absolute BMD increased by 19.5% and 33.4% at the spine and hip, respectively, within 12 months. Such changes are never seen with conventional pharmacological management of osteoporosis. In patients with low bone density, consideration of their risk of severe vitamin D deficiency is important so that the differential diagnosis of osteomalacia is not overlooked. Treatment of this patient with antiresorptive agents, as simple consideration of the bone density might have indicated, would probably have caused severe and sustained hypocalcaemia2 and failed to address the underlying bone pathology. Data on the BMD response to treatment of osteomalacia are limited, but other case series have reported similar findings.3–6 Bhambri et al reported 26 patients who had osteomalacia, almost all due to vitamin D deficiency.4 All patients demonstrated large (50%–60%) increases in BMD at the spine, total hip and femoral neck following treatment initiation, particularly in the first few months. In some patients, further increases in BMD were noted even after 2 years of follow-up.
Learning points.
Osteomalacia can dramatically lower bone mineral density.
Treatment of vitamin D deficiency can rapidly reverse musculoskeletal morbidity.
Consider vitamin D deficiency prior to initiation of antiresorptive therapy.
Serial procollagen-1 N-terminal propeptide measurements are useful in monitoring osteomalacia treatment response.
Footnotes
Contributors: IR reviewed the patient and was the lead clinician in managing the patient. RKN drafted the case report, and IR revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Beloyartseva M, Mithal A, Kalra S, et al. Widespread Vitamin D Deficiency among Indian Health Professionals. Osteoporosis Int 2011;22:S587–S8. [DOI] [PubMed] [Google Scholar]
- 2. Rosen CJ, Brown S. Severe hypocalcemia after intravenous bisphosphonate therapy in occult vitamin D deficiency. N Engl J Med 2003;348:1503–4. 10.1056/NEJM200304103481521 [DOI] [PubMed] [Google Scholar]
- 3. Al-Ali H, Fuleihan GE. Nutritional osteomalacia: substantial clinical improvement and gain in bone density posttherapy. J Clin Densitom 2000;3:97–101. [DOI] [PubMed] [Google Scholar]
- 4. Bhambri R, Naik V, Malhotra N, et al. Changes in bone mineral density following treatment of osteomalacia. J Clin Densitom 2006;9:120–7. 10.1016/j.jocd.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 5. El-Desouki M, Al-Jurayyan N. Bone mineral density and bone scintigraphy in children and adolescents with osteomalacia. Eur J Nucl Med 1997;24:202–5. 10.1007/BF02439554 [DOI] [PubMed] [Google Scholar]
- 6. El-Desouki MI, Othman SM, Fouda MA. Bone mineral density and bone scintigraphy in adult Saudi female patients with osteomalacia. Saudi Med J 2004;25:355–8. [PubMed] [Google Scholar]


