Abstract
The major concern of pancreatic islet transplantation is that the implanted islets are exposed to the immune system of the recipient. To overcome this challenge, the peptide amphiphile (PA) nanomatrix gel was used for immunoisolation of islets through microencapsulation. The PA can self-assemble to form a nanomatrix gel with an extracellular matrix-mimicking, islet nurturing microenvironment and a semipermeable immune barrier. In this study, the islet protective effect of the PA nanomatrix gel was evaluated by coculture of PA-encapsulated human islets with differentiated U937 cells (human monocyte cell-line) for 3 and 7 days. The coculture of the bare islets with the differentiated U937 cells stimulated proinflammatory cytokine (IL-1β and TNF-α) secretion and caused islet death after 7 days, which simulated an early inflammatory response environment after islet transplantation. The PA-encapsulated islets, however, did not stimulate proinflammatory cytokine secretion and maintained islet viability up to 7 days. More insulin-producing β cells were observed when islets were PA-encapsulated than control islets with the differentiated U937 cells for 7 days compared to the bare islets. This result was also confirmed by dithizone staining analysis. Further evaluation of islet functionality was assessed by a glucose-stimulated insulin secretion test. The PA-encapsulated islets showed greater insulin secretion response to glucose stimulation than the bare islets with the differentiated U937 cells after 3 and 7 days. These results demonstrated that islet encapsulation with the PA nanomatrix gel was able to improve islet survival and function in the presence of inflammatory responses, which will increase the success rate of islet engraftment and the efficacy of islet transplantation.
Keywords: peptide amphiphile nanomatrix gel, islet encapsulation, semipermeable immune barrier, islet nurturing microenvironment, inflammatory responses
Graphical Abstract
INTRODUCTION
Type 1 diabetes is a widespread disease wherein the patients cannot produce insulin for proper blood glucose control because the body’s immune system has destroyed the insulin-producing beta cells.1 Insulin therapy has been used for treatment of type 1 diabetes, but it cannot continuously and accurately meet the demands associated with variations in the level of food intake, stress, and physical activity for each individual patient.2 Pancreatic islet transplantation has been validated as an effective, sustainable treatment for the reversal of type 1 diabetes. After successful pancreatic islet transplantation, the recipients do not require rigorous blood glucose monitoring; moreover, the progress of diabetic complications is also prevented.3 However, one of the major challenges facing routine clinical application of pancreatic islet transplantation is the body’s immune response to the foreign islets. When the implanted islets are exposed to the recipient immune system, a rapid immune response occurs and destroys the islets.4,5 As a traditional approach, immune suppressive drugs are utilized during and after islet transplantation, but this has been shown to generate many negative side effects, such as mouth sores, anemia, fatigue, peripheral edema, neutropenia, and diarrhea.4,6 Thus, approaches to protect the implanted islets without the use of immune suppressive drugs are strongly desired. To address this challenge, several studies aimed to develop a bioartificial pancreas to protect islets.7–10 A bioartificial pancreas encapsulates the pancreatic islets within a semipermeable membrane and protects islets from exposure to the body’s immune responses while allowing diffusion of insulin, oxygen, nutrients, and waste products through the membrane.9 Prevention of islet exposure to immunocompetent cells can decrease the cell-mediated immune response from the recipients. Various types of bioartificial pancreases have been studied by utilizing macro- and microencapsulation, as well as islet surface modification.7,9
Macroencapsulation is the encapsulation of a large number of islets within an implantable device. A diffusion chamber is a type of macrocapsule in which islets are contained in a semipermeable immunoisolation membrane with a pore size of approximately one micrometer.11,12 However, the islets tend to clump together inside the chamber and often become necrotic due to oxygen deficiency, which leads to a significant decrease in insulin production.12,13 Microencapsulation is based on the isolation and containment of one or several islets inside a hydrogel capsule, which is typically several hundred micrometers in diameter. Various materials have been used for islet microencapsulation. Alginate microcapsules are widely used to encapsulate islets through emulsification using a microdroplet generator.14,15 The size of alginate microcapsules can be modified by changing the physical and chemical parameters of the microdroplet generator.15 The alginate is typically obtained from brown seaweed, and thus its molecular composition varies greatly depending on the source. This variation may result in different biocompatibility when using alginate, and various impurities such as endotoxins, polyphenolic compounds, heavy metals, and proteins might also be present.16 In addition, slight variations in alginate composition can cause different degrees of permeability to immune cells, insulin, and nutrients.17 Another approach for microencapsulation utilizes polyethylene glycol (PEG)-based microcapsules which have been developed to enclose islets by interfacial photopolymerization.18 The thickness of the PEG microcapsules can be controlled by using different photoinitiators, concentrations, and changing the irradiation parameters. However, islet encapsulation with photopolymerized biomaterials may result in several potential problems after implantation, such as the formation of fibrotic processes, poor degradation of the capsule, and local or systemic toxicity.19 Surface modification is also another immunoisolation strategy which alters the islet surface to generate an immune barrier. For example, a PEG complex was covalently attached to the islet surface to create a thin barrier which could block the effects of splenocytes.20 Surface modification approaches may block a very specific immune cell or molecule, but it is difficult to fully immunoisolate the islets from all of the immune cells and harmful cytokines with such specific surface molecules.21 In addition, covalent conjugation may change the physiology of the islet clusters, which can significantly reduce the secreted insulin output.22
Pancreatic islets may also be microencapsulated within a peptide amphiphile (PA)-based nanomatrix gel as an immunoisolation strategy. The PA is composed of a hydrophilic peptide attached to a hydrophobic alkyl chain. The hydrophobic alkyl tails self-assemble into a cylindrical micelle forming a nanoscale PA fiber.23,24 By the addition of calcium ions at a particular pH, PA nanofibers form a nanomatrix gel which can encapsulate pancreatic islets.23,25–28 This self-assembly property of PAs without any chemical treatment reduces the risk of cytotoxicity derived from toxic chemicals or cross-linking agents used in the microencapsulation process. Also, the PA is a peptide-based and less immunogenic molecule and has less variation in material composition compared to other natural polymers, such as alginate and collagen.27,28 Importantly, the PA nanomatrix gel provides a semipermeable barrier with a pore size of 100–300 nm,23,29 which can protect islets from cellular immune responses while allowing transfer of oxygen, nutrients, and insulin through the nanomatrix. In addition, various bioactive peptide sequences, such as cell adhesive ligands and enzyme-mediated degradation sites, can be incorporated into the PA, which enables mimicry of essential properties of the natural extracellular matrix (ECM), a critical component in regulation of cell behaviors.23,25,30,31 Our previous study successfully demonstrated that islet encapsulation in the RGD peptide (cell adhesive ligand: arginine, glycine, and aspartic acid)-incorporated PA nanomatrix gel improved islet viability and function in vitro.25 For in vivo application of the PA nanomatrix gel to the omentum, an alternative extrahepatic islet transplantation site, our group also developed a hybrid nanosack by combination of a PA nanomatrix gel and an electrospun poly(ε-caprolactone) (ePCL) nanofiber sheet with craterlike structures.32,33 In the omentum, the ePCL nanofiber sheet maintained the mechanical stability of the PA nanomatrix gel. In addition, angiogenic growth factor delivery (FGF-2) along with craterlike structures of the ePCL nanofiber sheet promoted blood vessel generation surrounding the hybrid nanosack in the omentum for sufficient supply of oxygen and nutrients.32 Therefore, the PA nanomatrix gel has great potential to serve as an ideal bioartificial pancreas which provides an ECM-mimicking, islet nurturing environment with a semipermeable immunoisolation barrier to enhance islet survival and function after islet transplantation.
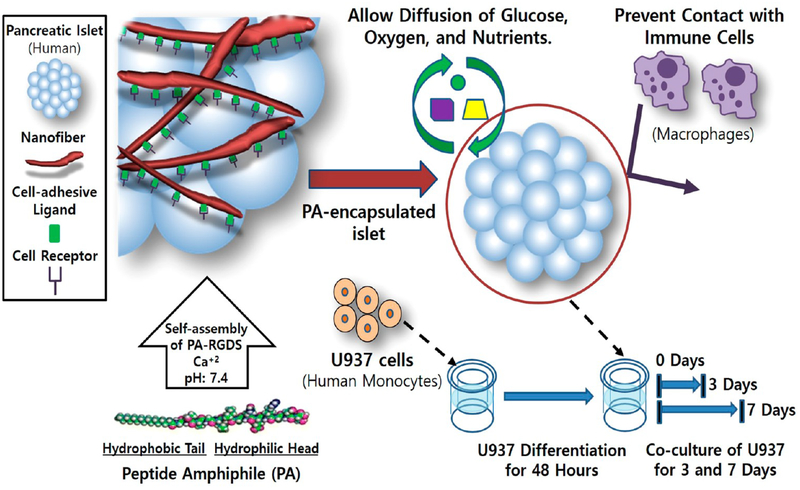
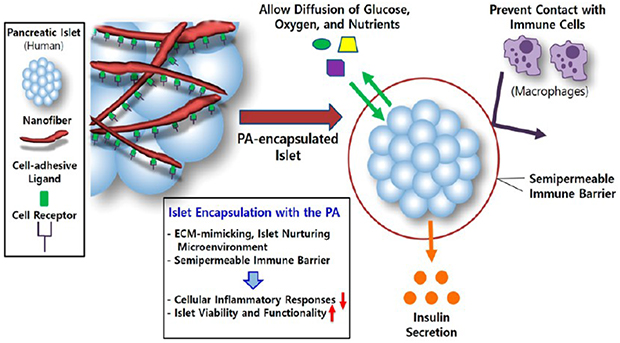
In the present study, we evaluated the islet protective effects of the PA nanomatrix gel against inflammatory cells. Human islets were encapsulated within the PA nanomatrix gel and cocultured with differentiated human monocytes. Secretion of inflammatory cytokines from the differentiated human monocytes was measured to test whether the PA-encapsulated islets stimulated inflammatory activity of the differentiated monocytes. Islet viability, insulin-producing beta cell staining, and glucose-stimulated insulin secretion function were also evaluated to confirm the islet protective effects of the PA nanomatrix gel. Thus, this study aimed to test the feasibility of using PA nanomatrix gel as a platform for microencapsulation in order to produce a bioartificial pancreas for islet transplantation (Scheme 1).
Scheme 1.
Microencapsulation of Human Islets Using the PA Nanomatrix Gel with a Semipermeable Immune Barrier and an ECM-Mimicking, Islet Nurturing Microenvironment
MATERIALS AND METHODS
Synthesis of Peptide Amphiphile.
Advanced Chemtech Apex 396 peptide synthesizer was used to synthesize the peptide sequence GTAGLIGQRGDS using Fmoc-chemistry as described previously.26,34,35 Palmitic acid was used to alkylate the synthesized peptide at the N-terminus using a manual coupling reaction. The alkylation process was conducted in a mixture of o-benzotriazole-N,N,N’,N’- tetramethyluronium hexafluorophosphate (HBTU; Novabiochem, MA, US), N,N-diisopropylethylamine (DIEA; Sigma, MO, US), and dimethylformamide (DMF; Fisher Scientific, MA, US) for 24 h at room temperature. Then, the alkylated peptide (PA) was cleaved in a mixture of trifluoroacetic acid (TFA; Fisher Scientific, MA, US), deionized water, triisopropylsilane (TIPS; Sigma, MO, US), and anisole (40:1:1:1) for 2 h at room temperature. The excess TFA was removed from the PA solution by rotary evaporation. Then, the PA product was precipitated in cold ether and lyophilized. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) was used to characterize the PA product.
Islet Encapsulation Using the PA Nanomatrix Gel and Coculture with the Differentiated U937 Cells.
Human pancreatic islets were obtained by donation from the Clinical Islet Laboratory at the University of Alberta (Alberta, Canada). The synthesized PA stock solution (2% weight/volume, pH 7) was prepared.25,27 For islet encapsulation, self-assembly of the PA (55 μL) was induced by adding 40μL of CMRL-1066 medium (10% fetal bovine serum (FBS) and 1% penicillin/streptomycin/amphotericin B (P/S/A); Gibco, MA, US) with 50 hand-picked human pancreatic islets and 17 μL of 0.1 M CaCl2.25 Fifty islets were selected based on our previously published in vitro studies using 50 rat islets for encapsulation, which demonstrated successful characterization of islet viability and function.25 U937 cells (human monocyte cell line; ATCC, VA, US) were cultured with RPMI 1640 medium (10% FBS and 1% P/S/A; Gibco, MA, US) to create an inflammatory environment; the monocytes were differentiated into macrophages by treatment with phorbol 12-myristate 13-acetate (PMA, 1 μg/mL; Sigma, MO, US) for 48 h.36,37 Differentiation was completed after 48 h and observed through imaging of the cells attached to the bottom of the culture well. Human islets (50 hand-picked) encapsulated within the PA nanomatrix gel and bare islets without encapsulation were cocultured with the differentiated U937 cells (3 × 105 cells) on the cell culture insert (24 well, 3.0 μm pore size membrane, Corning, NC, US) in CMRL-1066/RPMI 1640 (1:1 ratio mixture) medium (1.5 mL per each insert well) for 3 and 7 days. The culture medium was replaced every 3 days without loss of any coculture cells due to using the cell culture insert. For this study, 4 experimental conditions were designed: (a) bare islets (BI), (b) bare islets with differentiated U937 cells (BD) (c) PA-encapsulated islets (PI), and (d) PA-encapsulated islets with differentiated U937 cells (PD).
Evaluation of Inflammatory Cytokine Secretion.
To measure the amount of secreted intereukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) from the differentiated U937 cells, enzyme-linked immunosorbent assay (ELISA; R&D system, MN, USA) was conducted. After 3 and 7 days of coculture, the supernatant was collected from the cell culture insert, and the secreted IL-1β and TNF- α were analyzed by sandwich ELISA method and detection of substrate conversion.
Islet Viability Assessment.
Islet viability was analyzed by staining with fluorescein diacetate/propidium iodide (FDA/PI; Molecular Probes, OR, USA) after 3 and 7 days of islet culture. FDA stock solution (5 mg of FDA dissolved in 1 mL of acetone) was prepared and diluted with phosphate buffered saline (PBS; 1:100 dilution). PI (1 mg/mL in water solution) was diluted with PBS (1:10 dilution). For islet viability staining, each sample was treated with the mixture of 5 μL of diluted FDA, 10 μL of diluted PI, and 1 mL of PBS.25 Images of stained cells were taken using a Nikon TE2000-S fluorescence microscope and NIS Elements imaging software. The deacetylation of FDA by nonspecific esterases in the cytoplasm of living cells was detected under a fluorescent blue filter; conversely, PI was used to stain the nucleic acids of dead cells and was viewed under a fluorescent green filter.
Evaluation of Insulin-Producing β-Cells.
To detect insulin-producing pancreatic islet β-cells, dithizone (DTZ) staining was performed after 3 and 7 days of islet culture. DTZ is a zinc chelating agent and forms a red-colored complex by binding with zinc in insulin producing β-cells.38 DTZ solution (50 mg of DTZ (Sigma, MO, USA) dissolved in 5 mL of dimethyl sulfoxide (DMSO)) was prepared and diluted with 30 mL of PBS.25 The diluted DTZ solution was filtered with a 0.2 μm filter. The filtered DTZ solution was treated to each group, and DTZ-stained insulin-producing β-cells were examined by microscope.
Evaluation of Glucose-Stimulated Insulin Secretion.
Glucose-stimulated insulin release test was performed at 3 and 7 days for all groups. For this study, 50 islets with similar sizes and morphology were selected to normalize insulin secretion by each group. Low-glucose Krebs—Ringer bicarbonate buffer (low-glucose KRB) (25 mM HEPES, 115 mM NaCl, 24 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, and 0.1% bovine serum albumin, and 3 mM D-glucose, pH 7.4) and high-glucose Krebs—Ringer bicarbonate buffer (high-glucose KRB) (25 mM HEPES, 115 mM NaCl, 24 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, and 0.1% bovine serum albumin, and 30 mM D-glucose, pH 7.4) were prepared.25 The medium from the samples was drained, and the samples were incubated in 1 mL of low-glucose KRB (3 mM D-glucose) for 1 h to remove residual glucose. The buffer was replaced, the samples were incubated in 1 mL of low-glucose KRB for another hour, and the supernatant was collected to obtain the data for low glucose-stimulated insulin secretion. Then, the samples were incubated in a high-glucose KRB (30 mM D-glucose; 1 mL per sample) for 1 h, and the supernatant was also collected for analysis of high glucose-stimulated insulin secretion.25 Human insulin ELISA (Abcam, U.K.) was performed on the collected samples to measure the insulin concentration in the samples tested.
Statistical Analysis.
All experiments were conducted three independent times in quadruplicate. Results for the experiments are expressed as a mean ± standard deviation. Statistical comparisons were performed using ANOVA for multiple comparisons, with Tukey post hoc analysis for parametric data using SPSS 15.0 software. Variance was determined under one-way analysis which was used for statistical comparison. Statistical significance was given to a value of p < 0.05.
RESULTS AND DISCUSSION
To overcome the challenges of the body’s immune responses after pancreatic islet transplantation, our studies investigated the PA nanomatrix gel as a bioartificial pancreas that protects the islets from exposure to immune-related cells. Several immunoisolation approaches with a semipermeable membrane have been developed with various materials, but they still have some limitations, such as islet clumping, variation in material composition, toxicity generated from the encapsulation process, and changes in islet physiology.10,12,16,19,22 The PA can microencapsulate pancreatic islets by self-assembly without any chemical treatment, and it is also a highly biocompatible peptide-based molecule with less variation in composition. Our previous study already demonstrated that the microencapsulation of rat islets using the PA nanomatrix gel containing ECM-mimicking components enhanced islet survival and function.25 In this study, we evaluated the islet protective effects of the PA nanomatrix gel against cell-mediated inflammatory responses by coculture of PA-encapsulated human islets and differentiated human monocytes.
Rodent islets are most frequently used as an experimental model due to the readily available source and relatively low expense compared to other animal models. However, there is still a difference between the architecture of human islets and rodent islets. The β-cell fraction in humans is smaller than that of rodents and other species.39,40 Also, the distribution of the endocrine cells in a human islet is random, which does not match the distribution in rodent islets, which display β-cells in a core and α, δ, and pancreatic polypeptide (PP) cells in a mantle.39,41 These features generate differences in islet physiology and glucose sensitivity between human and rodent islets, as they are affected by interactions among endocrine cells and their fraction.42,43 Thus, it is important to perform experiments using human islets rather than rodent islets for potential clinical application of the PA nanomatrix gel. In addition, differentiated human monocytes were used as active inflammatory cells in this study. When monocytes encounter pathogens or foreign materials, they differentiate and convert into macrophages. These macrophages generate more inflammatory cytokines and increase the phagocytic activity.36,37 When pancreatic islets are transplanted to the recipient, initially the host inflammatory cells (mostly tissue macrophages) invade and cause severe cellular injury to the implanted islets.44,45 Within the first several days after implantation, islet apoptosis was observed in syngeneically implanted islets, which accounts for about 60% of total implanted islets;46,47 it is also highly associated with the early inflammatory response.44,45,48 Thus, the coculture of islets and differentiated monocytes may simulate an early inflammatory environment after islet transplantation, which is one of the major contributing factors to destruction of the implanted islets.46,47 Thus, in this study, we focused to test whether PA encapsulation can protect islets against inflammatory cells at early time points (3 and 7 days) by coculture of islets with differentiated monocytes.
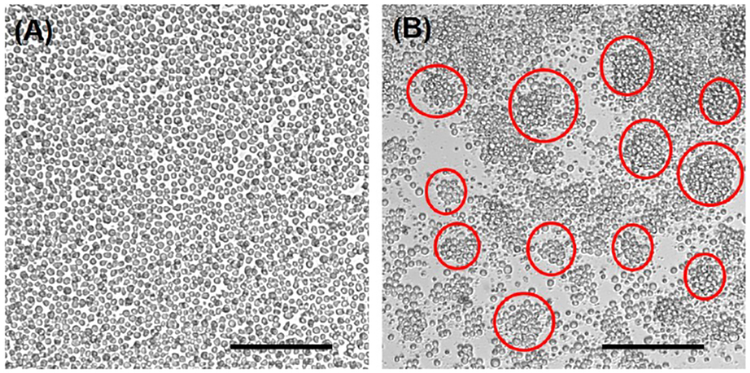
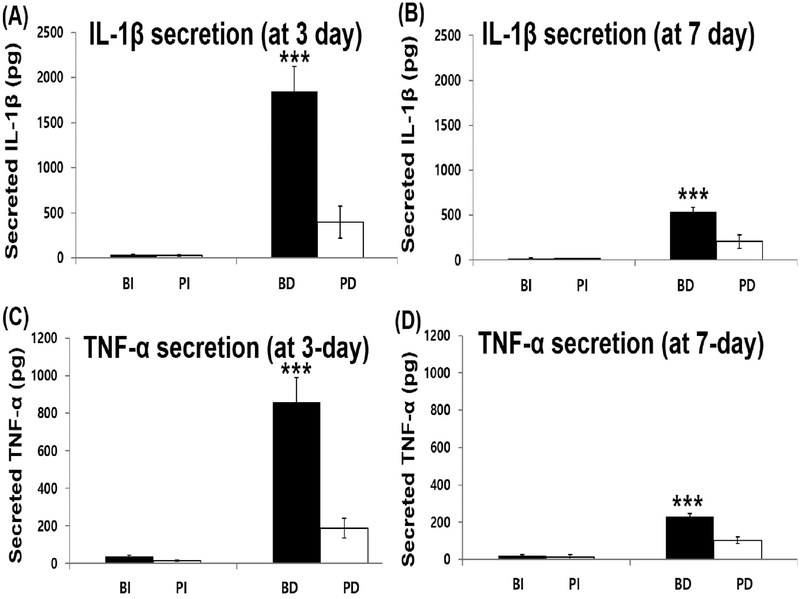
To differentiate monocytes, U937 cells (human monocyte cell-line) were cultured on a cell culture insert and differentiated by PMA (phorbol 12-myristate 13-acetate) treat-ment.36,37 After 48 h of differentiation, most U937 cells became slightly adherent to the bottom of the cell culture insert and also formed clusters (Figure 1B). In addition, proliferation of the differentiated U937 cells became slower than that of the undifferentiated U937 cells. Then, human islets were encapsulated with a PA nanomatrix gel and cocultured with the differentiated U937 cells. Four experimental groups were prepared for this study: (a) bare islets (BI), (b) bare islets with differentiated U937 cells (BD), (c) PA-encapsulated islets (PI), and (d) PA-encapsulated islets with differentiated U937 cells (PD). Each experimental group was cultured for 3 and 7 days. After pancreatic islet transplantation, the levels of proin-flammatory cytokines, such as interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ), are elevated and are mostly produced by activated macro-phages.48,49 IL-1β in particular is known to be one of the most critical cytokine mediators causing islet injury.47,50 IL-1β reduces glucose-stimulated insulin synthesis and secretion through intracellular signaling cascades;51 high doses of IL-1β lead to apoptosis of islets.52 In addition, TNF-α is involved in islet destruction;53 it stimulates apoptosis of islets in combination with other inflammatory cytokines, such as IL-1β and IFN-γ.54,55 Thus, it is important to evaluate the levels of secreted inflammatory cytokines after coculture of the PA-encapsulated islets with the differentiated U937 cells to determine whether the encapsulated islets promote inflammatory activities of the differentiated U937 cells. To measure the secreted inflammatory cytokines IL-1β and TNF-α, the coculture supernatant was collected in all groups at 3 and 7 days and analyzed by ELISA. In addition, the secreted inflammatory cytokines from undifferentiated U937 cells (Un U937) and differentiated U937 cells (Diff U937) were also evaluated as control groups. IL-1β was barely secreted from the undifferentiated U937 cells (33.2 ± 8.4 pg), but its secretion was significantly increased (464.3 ± 68.4 pg) after 48 h of differentiation (Un U937 vs Diff U937). This data indicates that differentiation could stimulate the inflammatory activity of U937 cells by increasing the secretion of inflammatory cytokines. After 3 days of coculture, IL-1β secretion was barely detected in both groups without U937 cells (Figure 2A; BI and PI groups). The BD group showed a drastic increase in IL-1β secretion (Figure 2A; 1846 ± 275.8 pg). However, the PD group only secreted 397.1 ± 178.3 pg of IL-1β, which had no significant difference compared to the initial secretion of IL-1β after U937 cell differentiation (Diff U937: 464.3 ± 68.4 pg). This result clearly demonstrates that the differentiated U937 cells were able to sense the bare islets and then boost the secretion of IL-1β. On the contrary, islet encapsulation with the PA nanomatrix gel provided an immune barrier to the islets and prevented the differentiated U937 cells from recognizing the islets, and thus did not further stimulate IL-1β secretion. After 7 days of coculture, IL-1β secretion was decreased in all groups compared to 3 days of coculture (Figure 2B). However, the trend of IL-1β secretion was still the same as the results of 3 days. This may be attributed to the fact that the U937 cells at 7 days after differentiation might become less active than those at 3 days after differentiation.
Figure 1.
Differentiation of U937 cells (human monocyte cell-line). (A) Undifferentiated U937 cells. (B) Differentiated U937 cells after 48 h of PMA (1 μg/mL) treatment. (Red circles indicate U937 cell clusters. Scale bar: 200 μm.)
Figure 2.
Inflammatory cytokine secretion during coculture. (A) IL-1β secretion after 3 days of coculture. (B) IL-1β secretion after 7 days of coculture. (C) TNF-α secretion after 3 days of coculture. (D) TNF-α secretion after 7 days of coculture. BI: bare islets. PI: PA-encapsulated islets. BD: bare islets with differentiated U937 cells. PD: PA-encapsulated islets with differentiated U937 cells. (***p < 0.001 vs PD group. n = 4.)
TNF-α secretion was also evaluated in all groups using ELISA. The differentiated U937 cells (48 h of differentiation) secreted much higher TNF-α (282.6 ± 21.6 pg) than the undifferentiated U937 cells (16.1 ± 1.8 pg) (Un U937 vs Diff U937). Overall trends of TNF-α secretion after 3 and 7 days of coculture were the same as the IL-1β secretion results. After 3 days, TNF-α secretion of the BD group was drastically increased up to 860.5 ± 129.6 pg. However, the PD group only secreted 186.7 ± 52.4 pg of TNF-α (Figure 2C; BD vs PD). After 7 days, TNF-α secretion was also reduced in all groups compared to its secretion after 3 days (Figure 2D). Based on these results, we were able to confirm that the U937 cells increased the secretion of inflammatory cytokines (IL-1β and TNF-6α) after differentiation; moreover, the secretion was further highly stimulated when the differentiated U937 cells were cocultured with the bare islets. However, the PA-encapsulated islets did not further stimulate the secretion of IL-1β and TNF-α from the differentiated U937 cells, which strongly supports that the PA nanomatrix gel served as an immune barrier and the gel itself did not stimulate the secretion of inflammatory cytokines. This experimental setup evaluating inflammatory cytokine secretion provides a simple and effective method to test the effects of PA encapsulation on stimulation of the inflammatory response. This protocol can also be used to provide clear results to confirm the inflammatory response for various encapsulation approaches, and is not just limited to PA encapsulation.
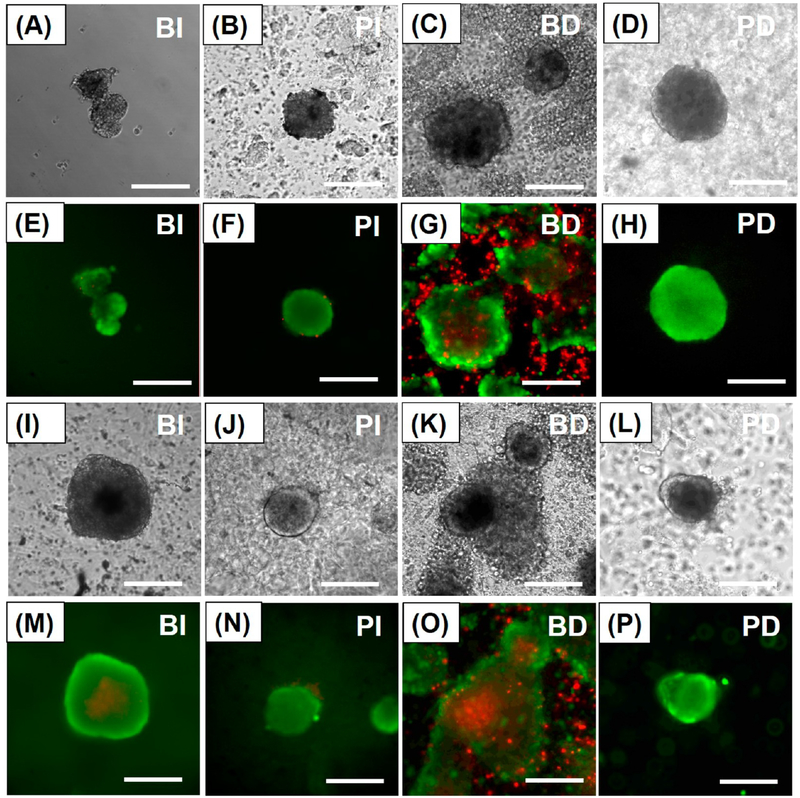
To test the islet protective effects of PA nanomatrix gel encapsulation, islet viability in all groups was analyzed by staining the islets with fluorescein diacetate/propidium iodide (FDA/PI) after 3 and 7 days of coculture. After 3 days, the BI group showed good islet viability with distinct islet morphology (Figures 3A and 3E). The BD group started to present some death in the center of islets, but overall the islets still maintained good islet viability (Figures 3C and 3G). Both the PA-encapsulated islet groups (PI and PD groups) also showed very good islet viability with distinct islet morphology (Figures 3B, 3F and 3D, 3H). After 7 days, the BI group still showed good islet viability, but some islets presented mild central necrosis (Figures 3I and 3M). There was significant islet death in the BD group (Figures 3K and 3O). The main cause of islet death in the BD group could be the increased inflammatory activities of the differentiated U937 cells on the bare islets. As shown in our results, the secretion of inflammatory cytokines, such as IL-1β and TNF-α, was highly stimulated from the differentiated U937 cells during coculture with the bare islets, which might have significant effects on islet death. However, we may also still need to further characterize the cell-mediated inflammatory activities of the differentiated U937 cells. Both the PA-encapsulated islet groups (PI and PD groups) still showed very good islet viability and maintained islet integrity (Figures 3J, 3N and 3L, 3P). Therefore, these results demonstrate that PA nanomatrix gel encapsulation provided an immune barrier from cellular inflammatory responses and an islet nurturing microenvironment, which enhanced the maintenance of islet viability.
Figure 3.
Islet morphology and viability during coculture. Bright field images of (A) BI, (B) PI, (C) BD, and (D) PD after 3 days of coculture. Fluorescein diacetate/propidium iodide (FDA/PI) stained images of (E) BI, (F) PI, (G) BD, and (H) PD after 3 days of coculture. Bright field images of (I) BI, (J) PI, (K) BD, and (L) PD after 7 days of coculture. FDA/PI stained images of (M) BI, (N) PI, (O) BD, and (P) PD after 7 days of coculture. (Green staining: live cells. Red staining: dead cells.) BI: bare islets. PI: PA-encapsulated islets. BD: bare islets with differentiated U937 cells. PD: PA-encapsulated islets with differentiated U937 cells. (Scale bar: 200 μm.)
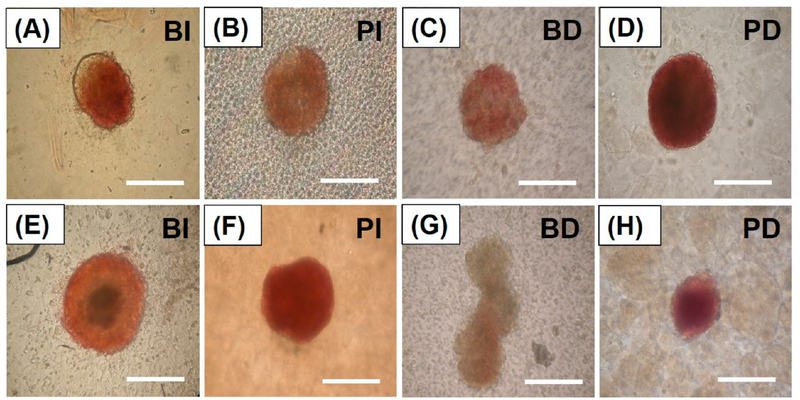
Insulin-producing pancreatic β-cells were also confirmed using dithizone (DTZ) staining in all experimental groups. DTZ is a zinc chelating molecule which selectively stains insulin-producing β-cells with a crimson red color due to their high zinc content.38,56 After 3 days of coculture, all experimental groups were stained with DTZ, which indicated that insulin-producing β-cells were identified in every group of islets (Figures 4A—4D). After 7 days of coculture, the BI group still presented well-stained islets with DTZ (Figure 4E). However, the BD group was barely stained with DTZ, and also showed less stain than any other group (Figure 4G). Thus, the coculture of the bare islets and the differentiated U937 cells significantly reduced the identification of insulin-producing β-cells, which could be the result of β-cell death from the increased inflammatory activities of the differentiated U937 cells. Both the PI and PD groups also showed well-stained islets with DTZ (Figures 4F and 4H). Importantly, we clearly identified insulin-producing β-cells in the PD group, which demonstrated that islet encapsulation with the PA nanomatrix gel protected the islets and maintained the characteristics of insulin-producing β-cells even in coculture with the differentiated U937 cells. Both the results of islet viability and DTZ staining were consistent with each other; more insulin-producing β-cells were identified in the healthy islets.
Figure 4.
Observation of insulin-producing β-cells using dithizone staining during coculture. Dithizone (DTZ) staining images of (A) BI, (B) PI, (C) BD, and (D) PD after 3 days of coculture. DTZ staining images of (E) BI, (F) PI, (G) BD, and (H) PD after 7 days of coculture. (DTZ staining: crimson red color.) BI: bare islets. PI: PA-encapsulated islets. BD: bare islets with differentiated U937 cells. PD: PA-encapsulated islets with differentiated U937 cells. (Scale bar: 200 μm.)
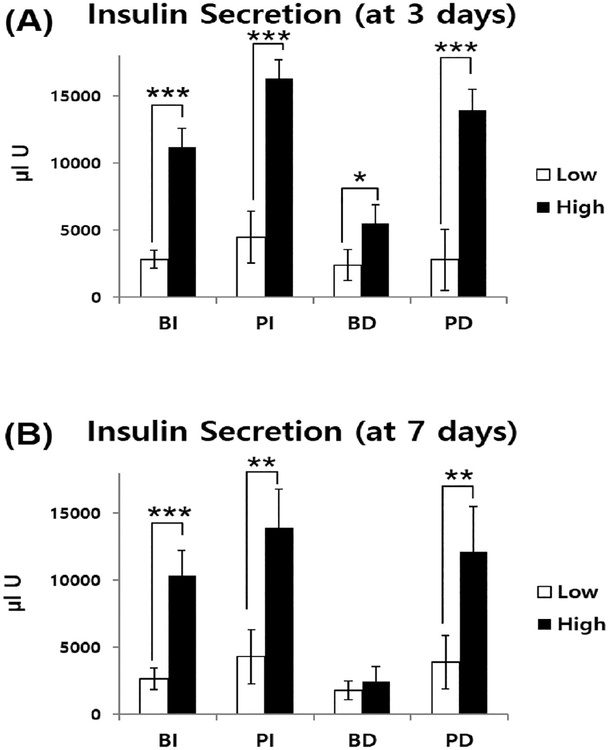
Glucose-stimulated insulin secretion test was also conducted on all groups to evaluate whether the PA-encapsulated islets could maintain their function with coculture with the differentiated U937 cells. The radius of the PA nanomatrix gel was about 1.5 mm, and this gel had 100—300 nm size pores,23,29 which did not disturb diffusion of secreted insulin through the nanomatrix membrane.25 After 3 days of coculture, there was a significant difference in glucose-stimulated insulin secretion between the low and high glucose responses in all groups (Figure 5A). The low glucose response values showed no significant difference among all groups (BI, PI, BD, and PD groups: 2828.48 ± 670.2, 4465.52 ± 1936.16, 2395.15 ± 1147.15, and 2797 ± 2284.72 μIU, respectively). However, the high glucose response values of the BD group (5476.63 ± 1421.74) were significantly reduced compared to all other groups (BI, PI, and PD groups: 11198.85 ± 1368.02, 16324.78 ± 1390.25, and 13954.41 ± 1519.98 μIU, respectively) (Figure 5A). These insulin secretion values were also validated by the stimulation index (SI) to compare glucose secretion between groups. The average SI was obtained by dividing average high glucose response value with average low glucose value of each group. The BD group showed the lowest average SI value (2.28) when compared to any other group (BI, PI, and PD groups: 3.95, 3.66, and 4.98, respectively). Thus, the glucose-stimulated insulin secretion function of the BD group was significantly reduced after 3 days of coculture. However, the PA-encapsulated islet groups (PI and PD groups) were able to maintain the insulin secretion function regardless of coculture with the differentiated U937 cells. After 7 days of coculture, the insulin secretion values of the BD group had no significant difference between the low and high glucose responses (Figure 5B). The low glucose response values showed no significant difference among all groups (BI, PI, BD, and PD groups: 2368.67 ± 808.52, 4311.81 ± 2017.86, 1791.44 ± 719.86, and 3902.56 ± 1986.94 μIU, respectively). However, the high glucose response values of the BD group (2449.78 ± 1112.90 μIU) were significantly reduced compared to all other groups (BI, PI, and PD groups: 10317.37 ± 1899.46, 13871.44 ± 2916.63, and 12121.07 ± 3403.77 μIU, respectively) (Figure 5B). The insulin secretion results of the high glucose response value for the 7 day coculture showed greater reduction in the BD group (2449.78 ± 1112.90 μIU) when compared with that of the 3 day coculture results (5476.63 ± 1421.74 μIU). The average SI value of the BD group (1.36) was also the lowest among all other groups (BI, PI, and PD groups: 3.91, 3.21, and 3.10, respectively). Thus, we confirmed that the BD group did not maintain insulin secretion function when exposed to the increased glucose concentration after 7 days of coculture. However, the PA-encapsulated islet groups (PI and PD groups) still successfully maintained insulin secretion function after glucose stimulation. Upon comparison of the PA-encapsulated islet groups (PI and PD groups) and the bare islet (BI) group, a significant statistical difference in insulin secretion was observed between their respective high glucose response values during the 3 day study (Figure 5A). The significant increase in insulin secretion observed in the PI and PD groups may be attributed to RGD peptide-incorporated PA encapsulation providing an ECM-mimicking, islet nurturing microenvironment; it allows for more efficient insulin secretion as already well demonstrated in our previous study of rat islet encapsulation using the PA nanomatrix gel.25 During the 7 day study, although there was no statistically significant difference in the high glucose response values between the PA-encapsulated islet groups (PI and PD groups) and the bare islet (BI) groups, the PI and PD groups on average showed greater high glucose response values than that of the BI group (Figure 5B). Our glucose-stimulated insulin secretion results were consistent with other experimental results; the PA-encapsulated islets did not further stimulate inflammatory responses during coculture with the differentiated U937 cells and also showed increased islet survival, insulin-producing β-cell population, and maintenance of insulin secretory function. Thus, these results verified the capability of islet encapsulation with the PA nanomatrix gel for creation of a bioartificial pancreas.
Figure 5.
Glucose-stimulated insulin secretion during coculture. Secreted insulin after (A) 3 days and (B) 7 days of coculture. (* Significant differences in insulin secretion between low-glucose incubation (3 mM) and high-glucose incubation (30 mM), *p < 0.05, **p < 0.01, and ***p < 0.001) (n = 4).
CONCLUSION
In this study, we successfully demonstrated the protective effects of PA encapsulation on the human islets from the differentiated U937 cells (human monocytes). The coculture of the bare islets with the differentiated U937 cells drastically stimulated the secretion of proinflammatory cytokines (IL-1ft and TNF-α), which promotes the early inflammatory environment after islet transplantation. This coculture with the differentiated U937 cells also significantly reduced islet viability and function. Islet encapsulation with the PA nanomatrix gel was used to prevent the detrimental events. PAs that include bioactive peptide sequences can self-assemble into a nanomatrix gel without any organic solvents or chemicals, which creates an ECM-mimicking, islet nurturing microenvironment with a semipermeable immune barrier. Thus, islet encapsulation with the PA nanomatrix gel was able to protect the islets against cellular inflammatory responses and maintain islet viability and function for 7 days of coculture with the differentiated U937 cells. Further research is necessary to fully characterize the progression of immune responses by coculture of the islets with various immune-related cells under conditions mimicking in vivo application.
The PA nanomatrix gel is a highly biocompatible peptide-based material used to minimize inflammatory responses,27,28 which can be an ideal islet encapsulation scaffold for in vivo application. The number of encapsulated islets is expected to be increased in in vivo studies. Each PA nanomatrix gel is able to encapsulate up to 1,500—2,000 islets, and multiple islet encapsulated PA nanomatrix gels can also be administered to the recipients if they require higher number of islets for transplant. For future in vivo application, we will conduct islet transplantation using the PA nanomatrix gel in the omentum area. The omentum is an alternative extrahepatic islet transplantation site, and it provides a large implantation volume and ease of surgical manipulation and also has some immune privilege.57,58 For the application of the PA nanomatrix gel at the omentum, our group already developed a hybrid nanosack by combination of a PA nanomatrix gel and an ePCL nanofiber sheet with craterlike structures.32,33 The hybrid nanosack enhanced blood vessel generation surrounding the hybrid nanosack while maintaining the PA gel stability in the rat omentum. We expect that PA encapsulation of the islets will significantly enhance islet survival and function after transplantation by providing an islet-protective and nurturing microenvironment, which will lead to improved islet engraft-ment in the omentum. Therefore, islet encapsulation using the PA nanomatrix gel could be a promising bioartificial pancreas, which will increase the efficacy of islet transplantation for type 1 diabetes treatment.
ACKNOWLEDGMENTS
We gratefully acknowledge the Clinical Islet Laboratory at the University of Alberta for human pancreatic islet donation. This research was supported by NIH NIBIB (1R03EB017344–01), NSF career award (CBET-0952974), NHLBI (1R01HL125391–01), NHLBI (1RO1HL128695 to J.K.), Alabama EPSCoR Graduate Scholar fellowship funded by NSF, and the University of Alabama at Birmingham Bioengineering—Surgery Collaborative Program 2017 Pilot Funding.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Daneman D Type 1 diabetes. Lancet 2006, 367 (9513), 847—58. [DOI] [PubMed] [Google Scholar]
- (2).Kim KW Islet transplantation: a realistic alternative for the treatment of insulin deficient diabetes mellitus. Diabetes Res. Clin. Pract 2004, 66 (Suppl. 1), S11—S17. [DOI] [PubMed] [Google Scholar]
- (3).Robertson RP Islet transplantation as a treatment for diabetes - a work in progress. N. Engl J. Med 2004, 350 (7), 694—705. [DOI] [PubMed] [Google Scholar]
- (4).Gibly RF; Graham JG; Luo X; Lowe WL Jr.; Hering BJ; Shea LD Advancing islet transplantation: from engraftment to the immune response. Diabetologia 2011, 54 (10), 2494—505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kanak MA; Takita M; Kunnathodi F; Lawrence MC; Levy MF; Naziruddin B Inflammatory response in islet transplantation. Int. J. Endocrinol 2014, 2014, 451035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hirshberg B; Rother KI; Digon BJ; Lee J; Gaglia JL; Hines K; Read EJ; Chang R; Wood BJ; Harlan DM Benefits and Risks of Solitary Islet Transplantation for Type 1 Diabetes Using Steroid-Sparing Immunosuppression. Diabetes Care 2003, 26 (12), 3288—3295. [DOI] [PubMed] [Google Scholar]
- (7).Teramura Y; Iwata H Bioartificial pancreas microencapsulation and conformal coating of islet of Langerhans. Adv. Drug Delivery Rev 2010, 62 (7—8), 827—40. [DOI] [PubMed] [Google Scholar]
- (8).Kizilel S; Garfinkel M; Opara E The bioartificial pancreas: progress and challenges. Diabetes Technol. Ther 2005, 7 (6), 968—85. [DOI] [PubMed] [Google Scholar]
- (9).Sumi S Regenerative medicine for insulin deficiency: creation of pancreatic islets and bioartificial pancreas. J. Hepatobiliary Pancreat. Sci 2010. 18 (1), 6—12. [DOI] [PubMed] [Google Scholar]
- (10).Hwang PT; Shah DK; Garcia JA; Bae CY; Lim DJ; Huiszoon RC; Alexander GC; Jun HW Progress and challenges of the bioartificial pancreas. Nano Converg 2016, 3 (1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hirotani S; Eda R; Kawabata T; Fuchinoue S; Teraoka S; Agishi T; Ohgawara H Bioartificial endocrine pancreas (Bio-AEP) for treatment of diabetes: effect of implantation of Bio-AEP on the pancreas. Cell Transplant 1999, 8 (4), 399—404. [DOI] [PubMed] [Google Scholar]
- (12).Lee SH; Hao E; Savinov AY; Geron I; Strongin AY; Itkin-Ansari P Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation 2009, 87 (7), 983—91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Schweicher J; Nyitray C; Desai TA Membranes to achieve immunoprotection of transplanted islets. Front. Biosci., Landmark Ed 2014, 19, 49—76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Poncelet D Production of alginate beads by emulsification/internal gelation. Ann. N. Y. Acad. Sci 2001, 944, 74—82. [DOI] [PubMed] [Google Scholar]
- (15).Calafiore R; Basta G; Luca G; Boselli C; Bufalari A; Bufalari A; Cassarani MP; Giustozzi GM; Brunetti P Transplantation of pancreatic islets contained in minimal volume microcapsules in diabetic high mammalians. Ann. N. Y. Acad. Sci 1999, 875, 219—32. [DOI] [PubMed] [Google Scholar]
- (16).Lee KY; Mooney DJ Alginate: properties and biomedical applications. Prog. Polym. Sci 2012, 37 (1), 106—126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).de Vos P; Faas MM; Strand B; Calafiore R Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27 (32), 5603—17. [DOI] [PubMed] [Google Scholar]
- (18).Desmangles AI; Jordan O; Marquis-Weible F Interfacial photopolymerization of beta-cell clusters: approaches to reduce coating thickness using ionic and lipophilic dyes. Biotechnol. Bioeng 2001, 72 (6), 634—41. [PubMed] [Google Scholar]
- (19).Baroli B Photopolymerization of biomaterials: issues and potentialities in drug delivery, tissue engineering, and cell encapsulation applications. J. Chem. Technol. Biotechnol 2006, 81 (4), 491—499. [Google Scholar]
- (20).Lee DY; Nam JH; Byun Y Effect of polyethylene glycol grafted onto islet capsules on prevention of splenocyte and cytokine attacks. J. Biomater. Sci. Polym. Ed 2004, 15 (6), 753—66. [DOI] [PubMed] [Google Scholar]
- (21).Lee DY; Nam JH; Byun Y Effect of polyethylene glycol grafted onto islet capsules on prevention of splenocyte and cytokine attacks. J. Biomater. Sci. Polym. Ed 2004, 15 (6), 753—66. [DOI] [PubMed] [Google Scholar]
- (22).Kellam B; De Bank PA; Shakesheff KM Chemical modification of mammalian cell surfaces. Chem. Soc. Rev 2003, 32 (6), 327—337. [DOI] [PubMed] [Google Scholar]
- (23).Jun HW; Yuwono V; Paramonov SE; Hartgerink JD Enzyme-Mediated Degradation of Peptide-Amphiphile Nanofiber Networks. Adv. Mater 2005, 17 (21), 2612—2617. [Google Scholar]
- (24).Hartgerink JD; Beniash E; Stupp SI Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294 (5547), 1684—1688. [DOI] [PubMed] [Google Scholar]
- (25).Lim DJ; Antipenko SV; Anderson JM; Jaimes KF; Viera L; Stephen BR; Bryant SM; Yancey BD; Hughes KJ; Cui W; Thompson JA; Corbett JA; Jun HW Enhanced rat islet function and survival in vitro using a biomimetic self-assembled nanomatrix gel. Tissue Eng. Part A 2011, 17 (3—4), 399—406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Anderson JM; Andukuri A; Lim D; Jun HW Modulating the gelation properties of self-assembling peptide amphiphiles. ACS Nano 2009, 3, 3447—3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Anderson J; Patterson J; Vines J; Javed A; Gilbert S; Jun HW Biphasic Peptide Amphiphile Nanomatrix Embedded with Hydroxyapatite Nanoparticles for Stimulated Osteoinductive Response. ACS Nano 2011, 5, 9463—9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Ban K; Park HJ; Kim S; Andukuri A; Cho KW; Hwang JW; Cha HJ; Kim SY; Kim WS; Jun HW; Yoon YS Cell therapy with embryonic stem cell-derived cardiomyocytes encapsulated in injectable nanomatrix gel enhances cell engraftment and promotes cardiac repair. ACS Nano 2014, 8 (10), 10815—25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Paramonov SE; Jun HW; Hartgerink JD Self-assembly of peptide-amphiphile nanofibers: the roles of hydrogen bonding and amphiphilic packing. J. Am. Chem. Soc 2006, 128 (22), 7291—8. [DOI] [PubMed] [Google Scholar]
- (30).Lim DJ; Antipenko SV; Vines JB; Andukuri A; Hwang PT; Hadley NT; Rahman SM; Corbett JA; Jun HW Improved MIN6 beta-cell function on self-assembled peptide amphiphile nanomatrix inscribed with extracellular matrix-derived cell adhesive ligands. Macromol Biosci. 2013, 13 (10), 1404—12. [DOI] [PubMed] [Google Scholar]
- (31).Tambralli A; Blakeney B; Anderson J; Kushwaha M; Andukuri A; Dean D; Jun HW A hybrid biomimetic scaffold composed of electrospun polycaprolactone nanofibers and selfassembled peptide amphiphile nanofibers. Biofabrication 2009, 1 (2), 025001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hwang PT; Lim DJ; Fee T; Alexander GC; Tambralli A; Andukuri A; Tian L; Cui W; Berry J; Gilbert SR; Jun HW A bio-inspired hybrid nanosack for graft vascularization at the omentum. Acta Biomater. 2016, 41, 224—34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Hwang PT; Murdock K; Alexander GC; Salaam AD; Ng JI; Lim DJ; Dean D; Jun HW Poly(varepsilon-caprolactone)/gelatin composite electrospun scaffolds with porous crater-like structures for tissue engineering. J. Biomed. Mater. Res. Part A 2016, 104 (4), 1017—29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Anderson JM; Kushwaha M; Tambralli A; Bellis SL; Camata RP; Jun HW Osteogenic differentiation of human mesenchymal stem cells directed by extracellular matrix-mimicking ligands in a biomimetic self-assembled peptide amphiphile nanomatrix. Biomacromolecules 2009, 10 (10), 2935—44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kushwaha M; Anderson JM; Bosworth CA; Andukuri A; Minor WP; Lancaster JR Jr.; Anderson PG; Brott BC; Jun HW A nitric oxide releasing, self assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices. Biomaterials 2010, 31 (7), 1502—8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Whyte J; Roberts AD; Morley KA; Sharp R J.; Marsh, P. D. Phagocytosis of mycobacteria by U937 cells: a rapid method for monitoring uptake and separating phagocytosed and free bacteria by magnetic beads. Lett. Appl. Microbiol 2000, 30 (1), 90—4. [DOI] [PubMed] [Google Scholar]
- (37).Caron E; Liautard JP; Kohler S Differentiated U937 cells exhibit increased bactericidal activity upon LPS activation and discriminate between virulent and avirulent Listeria and Brucella species. J. Leukocyte Biol 1994, 56 (2), 174—181. [DOI] [PubMed] [Google Scholar]
- (38).Latif ZA; Noel J; Alejandro R A simple method of staining fresh and cultured islets. Transplantation 1988, 45 (4), 827—830. [PubMed] [Google Scholar]
- (39).Cabrera O; Berman DM; Kenyon NS; Ricordi C; Berggren PO; Caicedo A The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. U. S. A 2006, 103 (7), 2334—9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Kim A; Miller K; Jo J; Kilimnik G; Wojcik P; Hara M Islet irchitecture: A comparative study. Islets 2009, 1 (2), 129—36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Steiner DJ; Kim A; Miller K; Hara M Pancreatic islet lasticity: interspecies comparison of islet architecture and composion. Islets 2010, 2 (3), 135—45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Dolensek J; Rupnik MS; Stozer A Structural similarities and lifferences between the human and the mouse pancreas. Islets 2015, 7;(1), e1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Huypens P; Ling Z; Pipeleers D; Schuit F Glucagon eceptors on human islet cells contribute to glucose competence of nsulin release. Diabetologia 2000, 43 (8), 1012—9. [DOI] [PubMed] [Google Scholar]
- (44).Kaufman DB; Gores PF; Field MJ; Farney AC; Gruber SA; Stephanian E; Sutherland DE Effect of 15-deoxyspergualin on mmediate function and long-term survival of transplanted islets in nurine recipients of a marginal islet mass. Diabetes 1994, 43 (6), 778—3. [DOI] [PubMed] [Google Scholar]
- (45).Kaufman DB; Platt JL; Rabe FL; Dunn DL; Bach F 3.; Sutherland, D. E. Differential roles of Mac-1+ cells, and CD4+ and 3D8+ T lymphocytes in primary nonfunction and classic rejection of slet allografts. J. Exp. Med 1990, 172 (1), 291—302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Biarnes M; Montolio M; Nacher V; Raurell M; Soler J; ontanya E Beta-cell death and mass in syngeneically transplantedslets exposed to short- and long-term hyperglycemia. Diabetes 2002, 51 (1), 66—72. [DOI] [PubMed] [Google Scholar]
- (47).Barshes NR; Wyllie S; Goss JA Inflammation-mediated ysfunction and apoptosis in pancreatic islet transplantation: mplications for intrahepatic grafts. J. Leukocyte Biol 2005, 77 (5),;87—597. [DOI] [PubMed] [Google Scholar]
- (48).Bottino R; Fernandez LA; Ricordi C; Lehmann R; Tsan MF; Oliver R; Inverardi L Transplantation of allogeneic islets of angerhans in the rat liver: effects of macrophage depletion on graft; urvival and microenvironment activation. Diabetes 1998, 47 (3), 316—3. [DOI] [PubMed] [Google Scholar]
- (49).Nathan CF Secretory products of macrophages. J. Clin. Invest 1987, 79 (2), 319—26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Mandrup-Poulsen T The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 1996, 39 (9), 1005—29. [DOI] [PubMed] [Google Scholar]
- (51).Xenos ES; Stevens RB; Sutherland DE; Lokeh A; Ansite D; Casanova D; Gores PF; Platt JL The role of nitric oxide in L-1 beta-mediated dysfunction of rodent islets of Langerhans. mplications for the function of intrahepatic islet grafts. Trans-plantation 1994, 57 (8), 1208—12. [DOI] [PubMed] [Google Scholar]
- (52).Bendtzen K; Mandrup-Poulsen T; Nerup J; Nielsen JH; 3inarello, C. A.; Svenson, M. Cytotoxicity of human pI 7 interleukin-1 or pancreatic islets of Langerhans. Science 1986, 232 (4757), 1545—7. [DOI] [PubMed] [Google Scholar]
- (53).Dahlen E; Dawe K; Ohlsson L; Hedlund G Dendritic cells nd macrophages are the first and major producers of TNF-alpha in ancreatic islets in the nonobese diabetic mouse. J. Immunol 1998, 160 (7), 3585—3593. [PubMed] [Google Scholar]
- (54).Rabinovitch A; Sumoski W; Rajotte R V.; Warnock, G. L. ytotoxic effects of cytokines on human pancreatic islet cells in nonolayer culture. J. Clin. Endocrinol. Metab 1990, 71 (1), 152—6. [DOI] [PubMed] [Google Scholar]
- (55).Delaney CA; Pavlovic D; Hoorens A; Pipeleers DG; izirik DL Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology 1997, 138 (6), 2610—4. [DOI] [PubMed] [Google Scholar]
- (56).Clark SA; Borland KM; Sherman SD; Rusack TC; hick WL Staining and in vitro toxicity of dithizone with canine, porcine, and bovine islets. Cell Transplant 1994, 3 (4), 299—306. [DOI] [PubMed] [Google Scholar]
- (57).Merani S; Toso C; Emamaullee J; Shapiro AM Optimal mplantation site for pancreatic islet transplantation. Br. J. Surg 2008, 95 (12), 1449—61. [DOI] [PubMed] [Google Scholar]
- (58).Contreras JL Extrahepatic transplant sites for islet xenotransplantation. Xenotransplantation 2008, 15 (2), 99—101. [DOI] [PubMed] [Google Scholar]