Abstract
Background
We recently reported interrelated digestive, cognitive, and hedonic responses to a meal. The aim of this study was to identify brain networks related to the hedonic response to eating.
Methods
Thirty-eight healthy subjects (20-38 age range) were evaluated after a 5-hour fast and after ingestion of a test meal (juice and warm ham and cheese sandwich, 300 mL, 425 kcal). Perceptual and affective responses (satiety, abdominal fullness, digestive well-being, and positive mood), and resting scans of the brain using functional MRI (3T Trio, Siemens, Germany) were evaluated immediately before and after the test meal. A high-order group independent component analysis was performed to investigate ingestion-related changes in the intrinsic connectivity of brain networks, with a focus on thalamic and insular networks.
Key Results
Ingestion induced satiation (3.3±0.4 score increase; P<.001) and abdominal fullness (2.4±0.3 score increase; P<.001). These sensations included an affective dimension involving digestive well-being (2.8±0.3 score increase; P<.001) and positive mood (1.8±0.2 score increase; P<.001). In general, thalamo-cortical connectivity increased with meal ingestion while insular-cortical connectivity mainly decreased. Furthermore, larger meal-induced changes (increase/decrease) in specific thalamic connections were associated with smaller changes in satiety/fullness. In contrast, a larger meal-induced decrease in insular-anterior cingulate cortex connectivity was associated with increased satiety, fullness, and digestive well-being.
Conclusions and Inferences
Perceptual and emotional responses to food intake are related to brain connectivity in defined functional networks. Brain imaging may provide objective biomarkers of subjective effects of meal ingestion.
1. Introduction
Meal ingestion has been well established to induce symptoms in some patient populations1–3 and similar sensations can be induced experimentally using aversive stimuli in the laboratory.4–7 However, the physiological mechanisms involved in the generation of various subjective postprandial sensations remain incompletely understood.4, 8–10 The subjective response to a meal involves a hedonic dimension in the form of pleasure, satisfaction, and wellness, which occurs when the characteristics of the meal (appearance, smell, taste, quantity) and the digestive response are appropriate.11, 12 By manipulating the experimental conditions, we have previously demonstrated that the postprandial sensation (satiation, fullness) and the valence of this sensation (pleasure, digestive well-being vs unpleasant sensation, dissatisfaction) are dissociable,12 ie depending on the conditions, equal degree of satiation/fullness may have a pleasant or unpleasant connotation. To further understand the central mechanisms underlying the experience of postprandial sensations, we used resting state MRI (rsMRI) to identify brain networks engaged by the ingestion of a pleasurable meal. We examined changes in intrinsic brain oscillations before and after a test meal which induced postprandial satiation/fullness associated with satisfaction in healthy male subjects. In rsMRI, information from the intrinsic fluctuations in regional blood oxygenation is used to identify brain networks without the need of an external stimulus and is thus well suited to the study of physiological states. We specifically focused on networks involving the thalamus and anterior insula which are involved in sensory processing and interoceptive awareness.13, 14
By analyzing postprandial intrinsic connectivity changes within these networks in healthy males following the ingestion of a pleasant meal, we tested the following hypotheses: (i) meal ingestion is associated with changes in the intrinsic connectivity of thalamic and insular networks; (ii) subjective sensations with positive or negative valence are correlated with distinct thalamic and insular connectivity changes.
2 Material and Methods
2.1 Participants
Thirty-eight healthy non-obese male subjects (median age 27.16 years; age range, 20-38 years, body mass index range, 19.6-30.7 kg/m2), right-handed, and without history of gastrointestinal symptoms were recruited by public advertising to participate in the study. Absence of current digestive symptoms was verified using a standard abdominal symptom questionnaire (no symptom ≤2 on a 0-10 scale). Psychological and eating disorders were excluded using the following tests: Hospital Anxiety and Depression scale (HAD), Dutch Eating Behaviour Questionnaire (DEBQ—Emotional eating, External eating, Restrained eating), and Physical Anhedonia Scale (PAS). Handedness was determined using the Edinburgh test (laterality index).
The protocol for the study was approved by the Institutional Review Board of the University Hospital Vall d’Hebron and was registered with Clinical Trials.gov as part of the study NCT02592239. All participants gave written informed consent.
2.2 Experimental design
The responses to a probe meal were studied in the afternoon after a 5-hour fast on two separate days (Figure 1). Participants were instructed to refrain from strenuous physical activity the day before and to have their usual breakfast after the overnight fast. On both days, a series of perception measurements (see below) were performed before and at different intervals after the probe meal. (i) On the first day, participants were studied in a quiet, isolated room sitting on a chair; perception was measured at 5-minute intervals immediately before and up to 30 minutes after ingestion of the probe meal. (ii) On the second day, brain imaging was performed (see below) immediately before (fasting scans) and after the ingestion of the probe meal (postprandial scan); the probe meal was ingested sitting in an isolated room adjacent to the brain imaging room. To examine the effect of time, two fasting resting scans were acquired before meal ingestion. Perception was measured at three time points: after the fasting scan and before and after the postprandial scan (20 minutes after the ingestion of the meal). For this pilot study no formal sample size calculations were performed.
Figure 1.
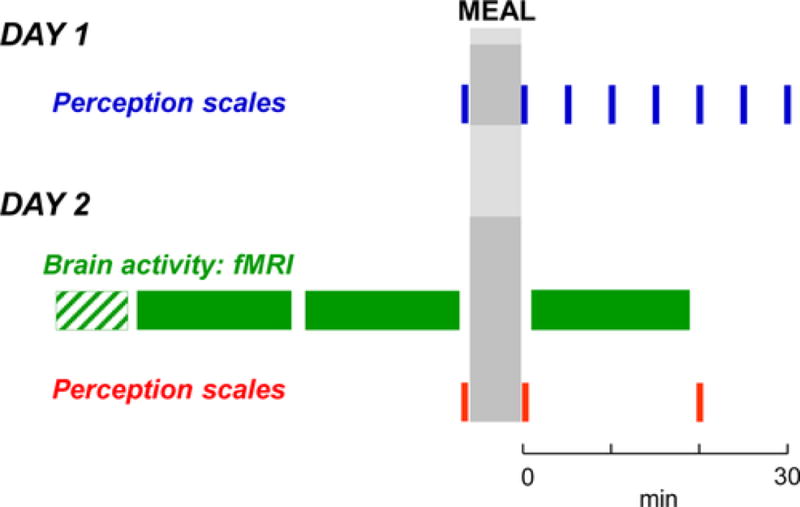
Experimental design. Perception in response to a probe meal was studied without (Day 1) and with brain imaging (Day 2: structural scan; functional scan)
2.3 Probe meal
A probe meal that induced a consistent cognitive response (satiation) with a pleasurable hedonic dimension was developed by a systematic series of preliminary studies performed in healthy subjects. The probe meal consisted of a warm sandwich (58 g bread with 12 g butter, 38 g ham and 38 g cheese) and an orange juice [(200 mL) 300 mL total volume (total caloric content of 425 kcal; 47 g carbohydrates, 17 g lipids, 18 g proteins)]. The sandwich was cooked in a hot plate (Sandwich Maxi 20, Fagor, Olite, Spain) for 3 minutes and administered at standard temperature (allowed to cool down covered by a napkin for 3 minutes at room temperature). Subjects were instructed to eat at their rate of choice.
2.4 Assessment of subjective responses
Four 10-cm scales graded from −5 to +5 were used to measure: (i) palatability (very bad/disagreeable to very good/delicious), (ii) hunger/satiety-satiation, (iii) digestive well-being (unpleasant sensation/dissatisfaction to pleasant sensation/satisfaction), (iv) desire of eating a food of choice (impossible/eagerly); and (v) mood (negative/positive). Two additional 10-cm scales graded from 0 (not at all) to 10 (very much) were used to measure: (vi) abdominal bloating/fullness; and (vii) discomfort/pain. Subjects received standard instructions on how to complete the scales. The palatability scale was only scored once immediately after meal ingestion on the first study day. The other scales were completed both before and after meal ingestion on both study days. These scales have been previously used and were shown to be sensitive to detect the effect of dietary interventions under different conditions.12,15
2.5 Brain imaging
2.5.1 fMRI acquisition
One structural scan and two sequential resting (fast-1 and fast-2) scans (total duration, 40 minutes) were performed immediately before the probe meal and one resting scan (duration, 20 minutes) was performed immediately after finishing the meal (postprandial scan) (Figure 1). During the resting scans, subjects rested with eyes closed. Images were acquired on a 3.0 T whole-body MR scanner with a 12-channel phased-array head coil and a whole-body transmit coil (Trio, Siemens, Germany). The protocol parameters were chosen based on the ADNI initiative (http://www.adni-info.org/Scientists/ADNIStudyProcedures.html) and included: (i) fast dual echo T2-weighted transverse sequence (TR=3080 msec, TE1=21 msec, TE2=91 msec, voxel size=0.78 × 0.78 × 3.0 mm3); (ii) transverse T2-FLAIR sequence (TR=9000 msec, TE=87 000 msec, TI=2500 msec, voxel size=0.49 × 0.49 × 3.0 mm3); (iii) axial 3D T1-weighted gradient-echo (MPRAGE) sequence (TR=2200 msec, TE=3.26 msec, voxel size=1.0 × 1.0 × 1.0 mm3); and (iv) resting-state BOLD sequence (TR=2000 msec, TE=28 msec, voxel size=3.4 × 3.4 × 4.0 mm3).
2.5.2 fMRI pre-processing
Resting scan images were preprocessed using SPM8 software (Welcome Department of Cognitive Neurology, London, UK). Data were slice-time and motion corrected, spatially normalized to the Montreal Neurological Institute standard template using their structural image, spatially smoothed with a 5 mm Gaussian kernel, and resampled to a voxel size=2 × 2 × 2 mm3. The first two volumes were discarded to allow for stabilization of the magnetic field.
2.5.3 Brain network connectivity
Group independent component analysis (ICA) was conducted to quantify resting scan network (RSN) connectivity pre- and post-meal ingestion. High-model-order ICA approaches of 70 components yield refined independent component networks (ICNs) which correspond to known anatomical and functional segmentations.16–19 The pre- and post-meal ingestion scans from all subjects were simultaneously entered into the ICA which was implemented in GIFT v4.0a (http://icatb.sourceforge.net). Seventy independent components were extracted by independent component decomposition using the infomax algorithm.20 Multiple runs (20 iterations) were performed using ICASSO to increase robustness of the results.21 Individual subject maps were back-reconstructed and converted into z-score maps representing the degree of correlation between the voxel signal and the group-averaged time course of the network (ie intra-network functional connectivity). Higher z values indicate greater connectivity strength or influence of that voxel on the network.22
Components of interest were identified by spatial correlation with published ICN templates.23 The anterior insula and the thalamus were selected as the primary networks of interest and cortical networks associated with sensorimotor, emotion-interoceptive, and emotion-cognitive functions were selected as secondary networks of interest. The thalamus was represented in two components, one comprising a more dorsal aspect, and one comprising a more ventral aspect, extending into the midbrain. In addition, the anterior insula was represented in two components, one comprising the left insula and one comprising the right insula.
2.6 Statistical analysis
2.6.1 Perception measurements
Mean values (±SE) of the parameters measured were calculated in each group of subjects. Normality of data distribution was evaluated by the Kolmogorov-Smirnov test. Comparisons of parametric, normally distributed data were made by Student’s t-test, paired tests for intragroup comparisons and unpaired tests for intergroup comparisons; otherwise, the Wilcoxon signed rank test was used for paired data within groups, and the Mann-Whitney Utest for unpaired data between groups. Correlations between parameters were evaluated by Pearson’s test. Differences were considered significant at a P value <.05.
2.6.2 Brain imaging and correlations with perception
The impact of meal ingestion on intra-network and inter-network functional connectivity was evaluated using the MANCOVAN toolbox in GIFT v4.0a. For the selected networks of interest, changes in intra-network connectivity were evaluated with a paired t-test (postprandial vs fast-2) using the subjects’ reconstructed spatial maps. To evaluate changes in inter-network connectivity between the primary and secondary networks of interest, subject-specific ICN time courses were first detrended, despiked, and filtered using a fifth-order Butterworth low-pass filter with a high frequency cutoff of 0.15 Hz. Pairwise correlations between the preprocessed ICN time courses were calculated and transformed to z-scores using Fisher’s z-transformation for each scan. The transformed correlation coefficients were then entered into a paired t-test (postprandial vs fast-2 scores). A corrected P<.05 using a false discovery rate (FDR) was considered significant.24 The relationship between changes in cognitive and hedonic perceptions and changes in inter-ICN connectivity was evaluated by Pearson correlation. A P<.05 was considered significant.
3. Results
3.1 Perceptual/hedonic responses to the meal
On the first experimental day (without brain imaging) before the meal (baseline fasting period), subjects reported a sensation of hunger and a desire to eat which was accompanied by a positive mood (Figure 2). Hunger correlated with the desire of choice eating (R=0.72; P<.01), but not with digestive well-being or mood. Subjects did not report any baseline symptoms of bloating, fullness, pain, or discomfort. Participants ingested the test meal in 203±12 seconds (at the rate of their choice) and reported a palatability score ≥2 (3.8±0.1 score). Ingestion of the test meal induced satiation, mild fullness, reduced the desire of eating, induced a sensation of digestive well-being (P<.01; 0 minute postprandial vs fast for all), and slightly improved the positive mood state (P<.01; 0 minute postprandial vs fast), but did not induce discomfort/pain. These sensations developed immediately after ingestion and persisted over the 30-minute postprandial period: 30 minutes after ingestion all sensations were still significantly higher than before ingestion (P<.01 for all); satiation and fullness decreased slightly but significantly over the postprandial period as compared to immediately after the meal (P<.01 vs 0 minute postprandial); however, no significant changes were detected in digestive well-being, mood, or desire for eating a food of choice during the postprandial observation period (P>.05 vs 0 minute postprandial for the three) (Figure 2).
Figure 2.
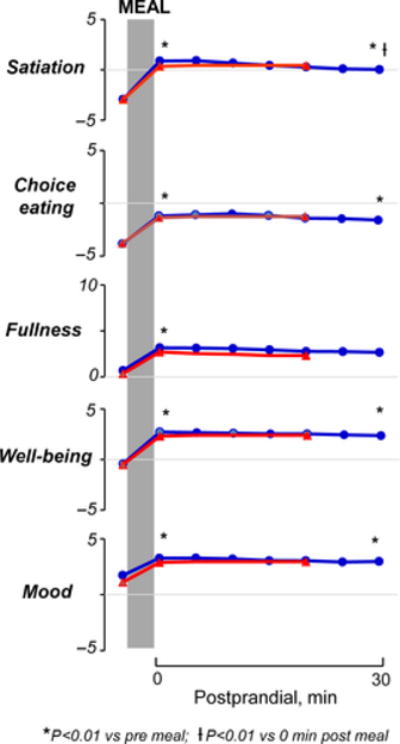
Cognitive and hedonic responses to probe meal in separate experiments with and without brain imaging. No statistically significant differences between study days were detected
3.2 Brain network connectivity
No differences were found between the two baseline fasting scans (data not shown); thus, the second scan acquired immediately before ingestion was chosen to represent the pre-meal brain activity.
3.2.1 Intra-ICN connectivity
A significant meal-induced reduction in connectivity within several component networks was observed, including the ventral thalamus ICN and sensorimotor cortical ICNs (left and right sensorimotor ICNs, bilateral secondary somatosensory ICN, bilateral SMA/paracentral lobule ICN) (Figure 3).
Figure 3.
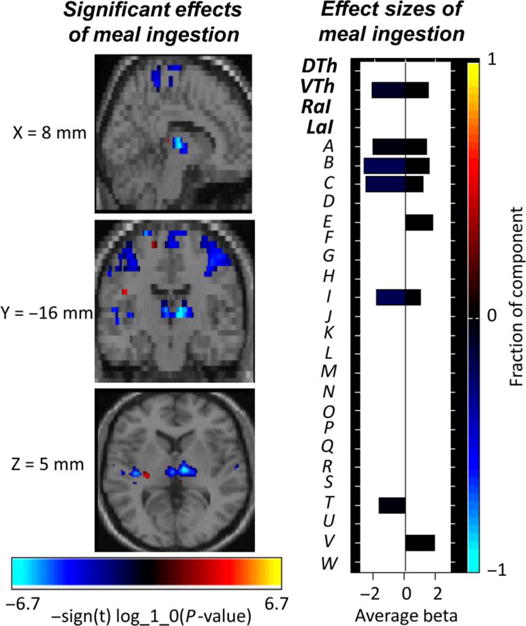
Composite image of the significant effects of meal ingestion on intra-network connectivity. The effect size for each component is shown to the right; primary networks of interest: dorsal thalamus (DTh), ventral thalamus (VTh), right anterior insula (RaI), left anterior insula (LaI); secondary networks of interest: bilateral secondary somatosensory cortex (A), right sensorimotor cortex (B), left sensorimotor cortex (C), bilateral subgenual anterior cingulate cortex (D), bilateral caudate (E), bilateral pallidum/putamen (F), bilateral hippocampus/parahippocampal gyrus/amygdala (G), bilateral anterior cingulate cortex/medial orbitofrontal cortex (H), bilateral paracentral lobule (I), bilateral primary somatosensory cortex (J), bilateral superior parietal (K), bilateral supramarginal (L), left temporoparietal junction (M), right superior/inferior parietal (N), bilateral ventrolateral prefrontal cortex (O), right left temporoparietal junction (P), bilateral medial cingulate cortex (Q), right inferior frontal operculum (R), bilateral precuneus (S), bilateral superior temporal (T), bilateral dorsal anterior cingulate cortex/medial cingulate cortex (U), bilateral medial prefrontal cortex (V), left dorsolateral prefrontal cortex (W)
3.2.2 Inter-ICN connectivity
Meal ingestion significantly changed the inter-network connectivity of the anterior insula with several other brain components, primarily resulting in decreases in functional connectivity (Figure 4). Significant meal-induced decreases were observed between the right insula ICN and ICNs representing: (i) bilateral anterior cingulate cortex/medial orbitofrontal cortex (ACC/mOFC), (ii) right temporoparietal junction (TPJ), and (iii) left dorsolateral prefrontal cortex (dlPFC). Significant meal-induced decreases were also observed between the left insula ICN and ICNs representing: (i) bilateral subgenual ACC, (ii) bilateral ACC/mOFC, (iii) left and right TPJ, (iv) right inferior frontal operculum, and (v) bilateral medial PFC. Significant meal-induced increases were observed between the left insula ICN and ICNs representing: (i) bilateral midcingulate cortex and (ii) dorsal thalamus.
Figure 4.
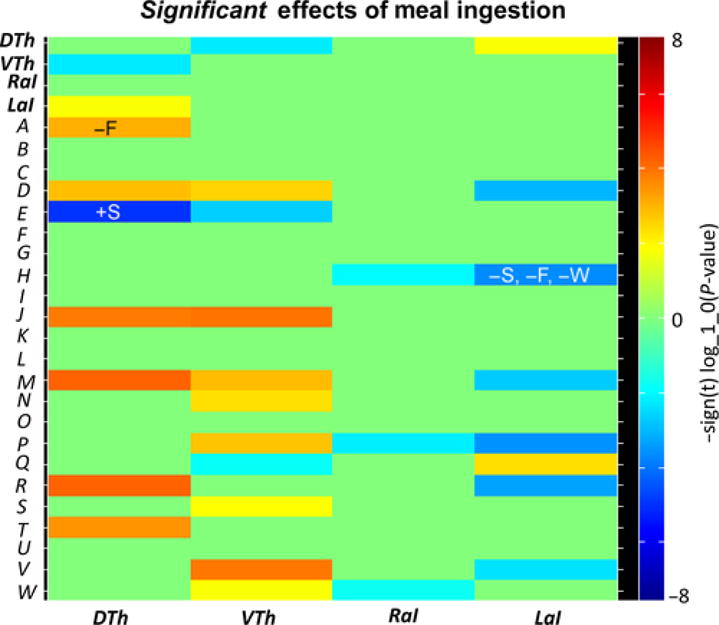
Effects of meal ingestion on inter-ICN connectivity. Heatmap depicting significant effects between the primary networks of interest (thalamus and anterior insula) and the secondary networks of interest. The thalamus and anterior insula ICNs displayed significant changes in connectivity with sensorimotor, emotion/cognitive, and interoceptive ICNs. Significant correlations, positive (+) and negative (−) correlation, between meal-induced connectivity changes and meal-induced perceptual changes are also indicated: Δsatiety (S), Δfullness (F), Δwell-being (W). Considering the direction of meal-induced changes, a greater decrease in insula-ACC/mOFC connectivity is associated with a greater increase in satiety, fullness, and digestive well-being. In contrast, a greater meal-induced decrease in thalamus-caudate connectivity is associated with a smaller increase in satiety, and a greater meal-induced increase in thalamus-S2 connectivity is associated with a smaller increase in fullness. Primary networks of interest: dorsal thalamus (DTh), ventral thalamus (VTh), right anterior insula (RaI), left anterior insula (LaI); secondary networks of interest: bilateral secondary somatosensory cortex (A), right sensorimotor cortex (B), left sensorimotor cortex (C), bilateral subgenual anterior cingulate cortex (D), bilateral caudate (E), bilateral pallidum/putamen (F), bilateral hippocampus/parahippocampal gyrus/amygdala (G), bilateral anterior cingulate cortex/medial orbitofrontal cortex (H), bilateral paracentral lobule (I), bilateral primary somatosensory cortex (J), bilateral superior parietal (K), bilateral supramarginal (L), left temporoparietal junction (M), right superior/inferior parietal (N), bilateral ventrolateral prefrontal cortex (O), right left temporoparietal junction (P), bilateral medial cingulate cortex (Q), right inferior frontal operculum (R), bilateral precuneus (S), bilateral superior temporal (T), bilateral dorsal anterior cingulate cortex/medial cingulate cortex (U), bilateral medial prefrontal cortex (V), left dorsolateral prefrontal cortex (W)
Meal ingestion also significantly changed the inter-network connectivity of the thalamus with several other brain components, primarily resulting in increases in functional connectivity (Figure 4). Significant meal-induced increases were observed between the dorsal thalamus ICN and ICNs representing: (i) primary somatosensory (S1) cortex, (ii) secondary somatosensory (S2) cortex, (iii) subgenual ACC, (iv) superior temporal cortex, (v) left TPJ, and (vi) right inferior frontal operculum. Significant meal-induced increases were observed between the ventral thalamus ICN and ICNs representing: (i) S1 cortex, (ii) subgenual ACC, (iii) right and left TPJ, (iv) precuneus, (v) mPFC, (vi) dlPFC, and (vii) right superior/inferior parietal cortex. In addition, significant meal-induced decreases were observed: (i) between the dorsal thalamus ICN and ventral thalamus ICN, (ii) between the caudate ICN and dorsal/ventral thalamic ICNs, and (iii) between the ventral thalamus ICN and the MCC ICN.
3.3 Relationship between perceptual and hedonic perceptions and changes in inter-network connectivity
All significant meal ingestion-related changes in inter-ICN connectivity were examined for relationships with changes in subjective perceptions and hedonic responses (post-meal-1 minus pre-meal; ΔSatiety, ΔFullness, ΔWell-being, ΔMood) (Figure 4). A significant negative correlation was found between meal ingestion-related changes in left anterior insula-ACC/mOFC connectivity and meal ingestion-related changes in satiety, fullness, and digestive well-being ratings. In addition, a significant negative correlation was found between meal ingestion-related changes in dorsal thalamus-S2 connectivity and ΔFullness. Finally, a significant positive correlation was found between meal ingestion-related changes in dorsal thalamus-caudate connectivity and ΔSatiety. Considering the direction of meal-induced changes as stated in the previous sections, these significant correlations reflect: A greater meal-induced decrease in insula-ACC/mOFC connectivity was associated with a greater increase in satiety, fullness, and digestive well-being. In contrast, a greater meal-induced decrease in thalamus-caudate connectivity was associated with a smaller increase in satiety and a greater meal-induced increase in thalamus-S2 connectivity was associated with a smaller increase in fullness.
4. Discussion
The main findings of the study were: (i) Meal ingestion changed the predominant baseline sensations and motivation from hunger and a desire to eat to one of satiation and mild fullness. These postprandial changes were associated with a feeling of digestive well-being. (ii) Subjective postprandial responses were associated with significant changes both in the connectivity within and between several component resting state networks of the brain. (iii) Functional network changes were correlated with changes in subjective responses to meal ingestion. To our knowledge, this is the first demonstration of distinct food-related changes in resting state networks in the brain following the ingestion of a palatable meal which are associated with food-related sensations and digestive well-being.
4.1 Subjective responses to the meal
The ingestion of the test meal changed the sensation of hunger and motivation to eat into prolonged sensations of fullness and satiation, and a feeling of digestive wellness. These changes can be assumed to be mediated by the effects of endocrine and vagal afferent inputs to the hypothalamus and thalamic nuclei.25 Postprandial hypothalamic and thalamic activity signals the insular cortex and associated regions of the extended central reward network, including the prefrontal/orbitofrontal cortex, anterior cingulate cortex, hippocampus and amygdala and basal ganglia.26
4.2 Meal-related changes in intrinsic connectivity
Consistent with the known effects of meal-induced neuroendocrine signaling to the brain, we observed major, distinct changes in the brain’s resting state activity and architecture.
4.2.1 Thalamic connectivity
The thalamus is a relay station for a wide range of sensory information from the body. In particular, the ventral medial nucleus receives direct input from the vagal nucleus of the solitary tract, which conveys visceral and gustatory afferent activity, and projects to the anterior insula.14, 27, 28 The thalamus and anterior insula then project to a wide range of cortical regions. Ingestion of the test meal was associated with extensive connectivity increases of both ventral and dorsal thalamic ICNs with sensory and affective brain regions, and with reduced connectivity between the two thalamic subregions and with the caudate nucleus, a brain region involved in reward and motivation. These changes may reflect increased transmission of visceral and gustatory signals from the thalamus to other brain regions, and a reduction in communication with the caudate nucleus, a major component of the brain’s reward region.
4.2.2 Insula connectivity
While the posterior aspects of the insular cortex represent the primary interoceptive cortex, anterior insula activity is associated with the awareness of sensations, including hunger and satiation, and this brain region is concerned with the integration of interoceptive, affective, attentional, and motivational signals.13 Furthermore, through its connections with several other brain networks, the anterior insula is a key node in other overlapping brain networks involved in ingestive behavior and food-related sensations. These networks include the salience network, which in close association with the anterior cingulate cortex functions to identify and respond to salient, homeostatically relevant events among both internal and external stimuli,28 and the extended reward network.28, 29
Despite minor differences between left and right insula, meal-induced reductions in connectivity were observed between the bilateral insula and resting state networks representing bilateral anterior cingulate and medial orbitofrontal cortex (ACC/mOFC), right temporoparietal junction (TPJ), and medial and lateral prefrontal regions. The ACC and its subregions have close connections with anterior insula and their co-activation plays an important role in the generation of sensory and motivational aspects of emotional feelings.14 The medial OFC is part of the prefrontal cortex and receives projections from thalamic subregions. It plays an important role in reward-related decision-making.30 The TPJ is an association area which integrates information from both the external and the internal environment. It incorporates information from the thalamus and emotion-related brain regions, and from the visual, auditory, and somatosensory systems. When viewed together, the ingestion of the test meal was associated with extensive reductions in connectivity of the bilateral anterior insula with brain regions involved in sensory integration, salience assessment, and reward. This may reflect a reduction in communication within key regions of the salience network following meal ingestion.
4.3 Correlation of functional network changes with subjective meal responses
Postprandial subjective sensations (satiety, fullness) and valenced feeling states (digestive well-being) depend on multiple factors. While the underlying neural circuitry responsible for these subjective responses is incompletely understood, we demonstrate significant correlations between these subjective responses and particular alterations in brain intrinsic connectivity. A greater meal-induced decrease in insula-ACC/mOFC connectivity was associated with a greater increase in satiety, fullness, and digestive well-being. Thus, a reduction in communication within key regions of the salience network following meal ingestion appears to be important for overall success of the meal in producing a homeostatically favorable state. In contrast, a greater meal-induced decrease in thalamus-caudate connectivity was associated with a smaller increase in satiety, and a greater meal-induced increase in connectivity between the thalamus and the sensory cortex was associated with a smaller increase in fullness. Thus, changes in thalamic connectivity appear to be more related to a state of unfulfillment following meal ingestion.
4.4 Limitations of study
We acknowledge several limitations of our study. Firstly, to prevent potential gender-related variability in the responses to food ingestion, only males were included in this proof-of-concept study. Furthermore, no formal sample size calculations were performed, and given the large number of variables analyzed, some effects may have been missed.
4.5 Conclusions and clinical implications
Our findings demonstrate extensive meal induced changes in the connectivity of key brain networks and regions which are involved in the sensory, affective and motivational aspects of food intake. The general pattern suggests a reduction in connectivity within major sensory brain networks, and between key regions of the extended reward network. While the interpretation of all network changes will require additional research, our findings suggest that postprandial sensations of satiety and well-being are a reflection of specific connectivity changes within the extended reward network (including anterior insula, thalamus, medial orbital frontal cortex, and caudate nucleus) and in sensory brain networks. Future studies in patients with functional dyspepsia who experience greater satiation and primarily negative-valence postprandial sensations will be able to identify disease-related differences in these central network changes.
Key Points.
Meal ingestion induces cognitive and hedonic sensations, and our aim was to identify brain networks related to these sensations
Perceptual and emotional responses to food intake are related to brain connectivity in defined functional networks
Brain imaging may provide objective biomarkers of subjective effects of meal ingestion
Acknowledgments
The authors thank Gloria Santaliestra for secretarial assistance.
Footnotes
Conflict of Interest
No competing interests declared by all investigators.
Author Contributions
Teodora Pribic, Lisa Kilpatrick and Barbara Ciccantelli contributed equally as co-first authors. EM and FA contributed equally as co-principal investigators in the design and interpretation of the study. TP was involved in study management, conduction of experiments and data analysis; LK was involved in analysis and interpretation of brain imaging studies and manuscript preparation; BC contributed to study management and conduction of experiments; CM contributed to study management and data analysis; AA was involved in study design and supervision of studies; AR was involved in analysis of brain imaging studies; DP contributed to study design, analysis of brain imaging studies and manuscript revision; EM contributed to study design, data interpretation, and manuscript preparation (brain imaging aspects); FA contributed to study design, data interpretation, and manuscript preparation (physiological aspects).
References
- 1.Azpiroz F, Bouin M, Camilleri M, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Mot. 2007;19:62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 2.Kellow JE, Azpiroz F, Delvaux M, et al. Applied principles of neurogastroenterology: physiology/motility sensation. Gastroenterology. 2006;130:1412–1420. doi: 10.1053/j.gastro.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 3.Azpiroz F, Feinle C, Grundy D, Tack J. Gastric sensitivirty and reflexes: basic mechanism underlying clinical problems. J Gastroenterol. 2014;49:206–218. doi: 10.1007/s00535-013-0917-8. [DOI] [PubMed] [Google Scholar]
- 4.Tack J, Caenepeel P, Piessevaux H, Cuomo R, Janssens J. Assessment of meal induced gastric accommodation by a satiety drinking test in health and in severe functional dyspepsia. Gut. 2003;52:1271–1277. doi: 10.1136/gut.52.9.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinle C, Azpiroz F. Dietary and life-style factors in funcional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:150–157. doi: 10.1038/nrgastro.2012.246. [DOI] [PubMed] [Google Scholar]
- 6.Burri E, Barba E, Huaman JW, et al. Mechanisms of postprandial abdominal bloating and distension in functional dyspepsia. Gut. 2014;63:395–400. doi: 10.1136/gutjnl-2013-304574. [DOI] [PubMed] [Google Scholar]
- 7.Boeckxstaens GE, Hirsch DP, van den Elzen BD, Heisterkamp SH, Tytgat GN. Impaired drinking capacity in patients with functional dyspepsia: relationship with proximal stomach function. Gastroenterology. 2001;121:1054–1063. doi: 10.1053/gast.2001.28656. [DOI] [PubMed] [Google Scholar]
- 8.Feinle C, Azpiroz F. Dietary lipids and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:737–747. doi: 10.1038/ajg.2013.76. [DOI] [PubMed] [Google Scholar]
- 9.Gibson CD, Carnell S, Ochner CN, Geliebter A. Neuroimaging, gut peptides and obesity: novel studies of the neurobiology of appetite. J Neuroendocrinol. 2010;22:833–845. doi: 10.1111/j.1365-2826.2010.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther. 2011;33:880–894. doi: 10.1111/j.1365-2036.2011.04609.x. [DOI] [PubMed] [Google Scholar]
- 11.Weltens N, Zhao D, Van OL. Where is the comfort in comfort foods? Mechanisms linking fat signaling, reward, and emotion. Neurogastroenterol Motil. 2014;26:303–315. doi: 10.1111/nmo.12309. [DOI] [PubMed] [Google Scholar]
- 12.Malagelada C, Accarino A, Molne L, et al. Digestive, cognitive and hedonic responses to a meal. Neurogastroenterol Motil. 2015;27:389–396. doi: 10.1111/nmo.12504. [DOI] [PubMed] [Google Scholar]
- 13.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 14.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 15.Malagelada C, Barba I, Accarino A, et al. Cognitive and hedonic responses to meal ingestion correlate with changes in circulating metabolites. Neurogastroenterol Mot. 2016;28:1806–1814. doi: 10.1111/nmo.12879. [DOI] [PubMed] [Google Scholar]
- 16.Kiviniemi V, Starck T, Remes J, et al. Functional segmentation of the brain cortex using high model order group PICA. Hum Brain Mapp. 2009;30:3865–3886. doi: 10.1002/hbm.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abou-Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V. The effect of model order selection in group PICA. Hum Brain Mapp. 2010;31:1207–1216. doi: 10.1002/hbm.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ystad M, Eichele T, Lundervold AJ, Lundervold A. Subcortical functional connectivity and verbal episodic memory in healthy elderly–a resting state fMRI study. NeuroImage. 2010;52:379–388. doi: 10.1016/j.neuroimage.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 20.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 21.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 23.Ray KL, McKay DR, Fox PM, et al. ICA model order selection of task co-activation networks. Front Neurosci. 2013;7:237. doi: 10.3389/fnins.2013.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 25.Feinle-Bisset C. Modulation of hunger and satiety: hormones and diet. Curr Opin Clin Nutr Metab Care. 2014;17:458–464. doi: 10.1097/MCO.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 26.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J Comp Neurol. 1980;190:259–282. doi: 10.1002/cne.901900205. [DOI] [PubMed] [Google Scholar]
- 28.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennerley SW, Walton ME. Decision making and reward in frontal cortex: complementary evidence from neurophysiological and neuropsychological studies. Behav Neurosci. 2011;125:297–317. doi: 10.1037/a0023575. [DOI] [PMC free article] [PubMed] [Google Scholar]


