Abstract
OBJECTIVES:
Accumulating evidence indicates that the gut microbiota communicates with the central nervous system, possibly through neural, endocrine, and immune pathways, and influences brain function. B. longum 1714™ has previously been shown to attenuate cortisol output and stress responses in healthy subjects exposed to an acute stressor. However, the ability of B. longum 1714™ to modulate brain function in humans is unclear.
METHODS:
In a randomized, double-blinded, placebo-controlled trial, the effects of B. longum 1714™ on neural responses to social stress, induced by the “Cyberball game,” a standardized social stress paradigm, were studied. Forty healthy volunteers received either B. longum 1714™ or placebo for 4 weeks at a dose of 1 × 109 cfu/d. Brain activity was measured using magnetoencephalography and health status using the 36-item short-form health survey.
RESULTS:
B. longum 1714™ altered resting-state neural oscillations, with an increase in theta band power in the frontal and cingulate cortex (P < 0.05) and a decrease in beta-3 band in the hippocampus, fusiform, and temporal cortex (P < 0.05), both of which were associated with subjective vitality changes. All groups showed increased social stress after a 4-week intervention without an effect at behavioral level due to small sample numbers. However, only B. longum 1714™ altered neural oscillation after social stress, with increased theta and alpha band power in the frontal and cingulate cortex (P < 0.05) and supramarginal gyrus (P < 0.05).
DISCUSSION:
B. longum 1714™ modulated resting neural activity that correlated with enhanced vitality and reduced mental fatigue. Furthermore, B. longum 1714™ modulated neural responses during social stress, which may be involved in the activation of brain coping centers to counter-regulate negative emotions.
INTRODUCTION
The existence of bidirectional communication between the gut and brain has long been established. Recently, a body of work has suggested that the commensal gut microbiota plays an important role in modulating the gut-brain axis, possibly through immune, endocrine, and neural system pathways (1). This has led to increased interest in modulating the gut microbiota to target central nervous system (CNS) functions and improve human behavior especially in areas such as stress, mood, anxiety, and cognition. Probiotics may represent a safe and effective way of targeting CNS function. Indeed, an abundance of preclinical studies have shown that probiotics acting through the gut-brain axis can affect brain development, function, and behavior (2). However, the translation of promising preclinical signals into human subjects with certain probiotic strains has proven challenging (3). Therefore, there is a need for more detailed mechanistic insights into the interaction between the gut microbiota and brain function in humans.
One probiotic strain, Bifidobacterium longum 1714™ (Zenflore), has proven effective in modulating CNS functions in animals and humans. B. longum 1714™ has been shown to reduce stress-related behaviors in preclinical studies (4,5) and improve stress responses and cognitive function in healthy volunteers (6). However, the role of B. longum 1714™ in modulating brain function in humans is unclear. Clinical studies with probiotics using neuroimaging methods have started to emerge and provide insight into modulations of CNS functions in healthy volunteers (7–9) and in patients with irritable bowel syndrome (IBS) (10). The first study to demonstrate neural effects of probiotics in humans used functional MRI and showed that brain activations to emotional faces were altered by a 4-week consumption of a fermented dairy drink in comparison to placebo in healthy subjects (7). A more recent study in healthy volunteers showed positive correlations between psychometric measures and brain activity (8) as well as altered functional connectivity (9) in response to emotional processing after a 4-week intervention with a multi-strain probiotic. Of interest, treatment of patients with IBS using B. longum™ NCC3001 for 6 weeks resulted in an improvement in depressions scores and reduction in limbic activity to negative emotional stimuli, but had no effect on gut symptoms (10). Notably, these previous studies used picture-viewing tasks during brain recording that poorly reflect behavioral changes found in animal models, which are commonly used to screen the effects of probiotics on stress and emotions. Furthermore, both studies in healthy volunteers used multi-strain probiotic product making it difficult to identify the bacterial strain responsible for the observed effects and the pathways involved in strain-specific effects. Therefore, further investigations are required using targeting approaches and different stress paradigms.
In this study, we explored the effect of B. longum 1714™ on brain function in response to social stress in a randomized, double-blinded, placebo-controlled trial, using the neuroimaging method magnetoencephalography (MEG). Social stress was induced by social exclusion/rejection (11) through a standardized paradigm, called “Cyberball Game” (CBG) (12). According to a recent review summarizing previous neuroimaging studies on the CBG, regions of the prefrontal cortex (PFC), anterior cingulate cortex (ACC), and temporal cortex are involved in neural processing of this type of social stressor (13). Indeed, social stress during the CBG is consistently associated with altered neural oscillations in certain brain areas, such as alpha band power in frontal cortex and theta band power in the ACC and insula (14–17).
In the current trial, we hypothesized that the probiotic strain B. longum 1714™ alters resting-state brain activity and neurophysiological responses to CBG-induced social stress. We assumed that during the CBG, stress-related neural oscillations of the theta and alpha band power would be changed after probiotic treatment. The results confirmed that B. longum 1714™ modulated neural responses during social stress, suggesting counter-regulation of negative emotions.
METHODS
Participants
Healthy adults were recruited to the study. Criteria for inclusion were (i) man or woman aged 18–50 years; (ii) nonsmoker for at least 3 months; (iii) a body mass index of 18–30; (iv) no chronic allergies; (v) willing to discontinue their normal consumption of probiotics and probiotic-containing foods or potentially immune-enhancing dietary supplements; (vi) receiving no immune-suppressing intervention and not having any immunosuppressive illness within the past year; (vii) receiving no antibiotic therapy within the past 2 months; (viii) having no chronic psychiatric or gastrointestinal disorder; and (ix) having no nonremovable metal parts in the body. Informed consent was obtained from all participants before joining the study. Participants were screened for IBS and psychiatric disorders using the Rome III criteria (18) and the Patient Health Questionnaire (19). During the intervention period, participants were instructed to avoid consumption of food containing probiotics/prebiotics or potentially immune-enhancing dietary supplements. This was supported by providing them with a list of “prohibited” foods (see Information 1, Supplementary Digital Content 1, http://links.lww.com/AJG/A163). Demographic and baseline psychological information was also recorded. The protocol was approved by the Ethics Board of the University of Tübingen Medical School (No. 503/2015BO1, as of August 26, 2015) and registered at ClinicalTrials.gov (identifier No. NCT02793193).
Design
A randomized, double-blinded, parallel-group design was used. After screening, participants were randomly allocated to the probiotic or placebo group for 4 weeks. At baseline (visit 1) and 1 day after completion of the intervention period (visit 2), measurements of resting-state MEG, MEG during CBG, and related questionnaires to measure the level of distress were performed. Structural MR images were acquired on a different day, regardless of the intervention schedule. To record participants health and quality of life status, participants completed the 36-item short-form health survey (SF36) (20) at the beginning of each of the 2 visits. The SF36 includes 8 subscales: physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/vitality, and general health perceptions. The randomization scheme was only unblinded after completion of the experiment and complete data evaluation.
Materials
The probiotic and placebo preparations were provided by Alimentary Health Group, Cork, Ireland. Participants were randomized to receive sachets containing either probiotic (1 × 109 CFU of B. longum 1714™ in 2 g of maltodextrin) or placebo (2 g of maltodextrin). Probiotic and placebo were identically packaged. Participants were instructed to consume one sachet every morning by mixing the content into 50 mL of water. Both subjects and investigators were blinded to the regime administered.
CBG
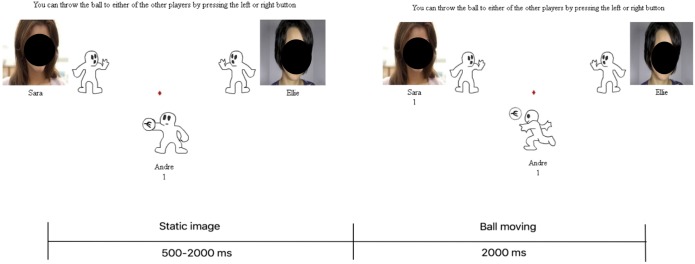
The CBG was used to study the effects of social stress and exclusion as outlined previously by Wang et al. (21). Briefly, during the CBG, participants were asked to play a virtual ball-tossing game with 2 other virtual players programmed. The CBG involves 2 conditions, the so-called inclusion and exclusion. Figure 1 presents a single trial from 1 player throwing the ball to another (Figure 1) (see Information 2, Supplementary Digital Content 1, http://links.lww.com/AJG/A163 for more detail).
Figure 1.
Schematic outline of a trial in the Cyberball Game (CBG).
CBG questionnaires
After the CBG exclusion or inclusion periods, participants completed 3 questionnaires to assess the level of acute distress. We employed the self-report measures of the Need Threat Scale (NTS), the Mood Questionnaire (MQ), and the Subjective “Exclusion Perception” (SEP) (see Information 3, Supplementary Digital Content 1, http://links.lww.com/AJG/A163); all these scales are validated standards for the CBG (12,22). The NTS is designed to measure the feelings and emotional consequences of social rejection, and higher scores related to higher distress level. Its 4 items (rated between 1 and 5 for “weak” to “strong”) comprised self-esteem, belonging, meaningful existence, and control, and combined ratings have been used as a measure of social distress in previous studies. The MQ is used to assess mood, using 8 questions (are you feeling bad, good, happy, sad, pleasant, angry, friendly, and unfriendly), all rated between 1 and 5. The SEP is used to record participants' feeling of being included/ostracized by asking them to rate 2 statements (“I was ignored” and “I was excluded”) between 1 and 5.
MEG recording
Brain magnetic fields were measured with a 275-channel whole-head MEG (CTF Omega, Port Coquitlam, Canada). Participants were studied in sitting position. During each session, 5 minutes resting state was recorded before playing the CBG. During the resting state, participants were instructed to move as little as possible and to stay alert while keeping their eyes closed. During the CBG, task instructions were projected onto a screen in front of the participants using a video projector and a mirror system. MEG signals were sampled at a rate of 585.94 Hz with an anti-aliasing filter set to 146.49 Hz.
To overlay the brain activity derived from MEG on anatomical scans, high-resolution (1 mm, isotropic) T1-weighted structural MR images were acquired using an MPRAGE sequence with a Siemens MAGNETOM Trio 3T scanner (Siemens AG, Erlangen, Germany) (12-channel array head coil) for each participant, but at a separate occasion.
Data analysis
Power calculation.
The primary endpoint was change in brain activity. Based on previously published data (7), we estimated that—with a power of 0.95 for a 2 × 2 repeated measure ANOVA—a minimum sample size of 28 was required to demonstrate an effect size f = 0.23 at α = 0.05 in a parallel-group designed study (see Information 4, Supplementary Digital Content 1, http://links.lww.com/AJG/A163 for more detail).
Data analysis: MEG
Analysis of the MEG data was performed using MATLAB (Mathworks, Natick, MA) and the open-source toolboxes FieldTrip (23). Data from the resting state and during the CBG were analyzed, respectively, after procedures of preprocessing (trial segmentation and artifacts rejection)—Time-frequency analysis (multitaper windowed fast Fourier transform)—Source analysis (dynamic imaging of cortical sources: DICS)—Source statistics (cluster-based permutation statistics).
Data analysis: Questionnaires
Data analysis was conducted using SPSS 21 (IBM, Armonk, NY). To examine whether there was a significant difference in health status between groups at baseline, scores of SF36 during the first visit were entered into a nonparametric independent-sample Mann-Whitney U test of Intervention as between factor (B. longum 1714™ vs placebo) because parametric assumptions of these data were violated. To test the intervention-related changes in participants' health status scored by SF36, changes from before to after the 4-week intervention were computed by subtracting the baseline assessment from the corresponding postintervention values. A nonparametric two-way independent-sample Mann-Whitney U test was used to examine the change of SF36 between Intervention (B. longum 1714™ vs placebo).
To examine whether subjective ratings for the CBG were different between groups at baseline, scores of NTS, MQ, and SEP acquired during the first visit were entered into an independent t test with Intervention as between factor (B. longum 1714™ vs placebo). To control the intervention-related changes in the scores of the NTS, MQ, and SEP during the CBG, changes after each intervention were computed for each condition and entered into a 2 × 2 repeated measure ANOVA with Intervention as a between-factor (B. longum 1714™ vs placebo) × Condition as a within-factor (exclusion vs inclusion). Where significant main effects or interaction were observed, pairwise post hoc comparisons were used with a Bonferroni-adjusted threshold (α = 0.025). Mean data are reported as M ± s.d.
Correlation between stress measures and quality of life assessments with MEG data
To investigate the relationship between changes in neural activity and changes in subjective scores induced by B. longum 1714™, correlation analysis was performed for both, the change in brain activity in the resting state and after the CBG. For the resting state, averaged source power was calculated for the clusters that were significantly different between both visits. The averaged source power was correlated with changes in health status (SF36) for each group separately. For the CBG, for each condition and each intervention, source power within clusters significantly different between both visits was averaged. The averaged source power was correlated with changes in the scores of the NTS, MQ, and SEP separately for each condition and each group, using Pearson correlations.
RESULTS
Subjects and baseline characteristics
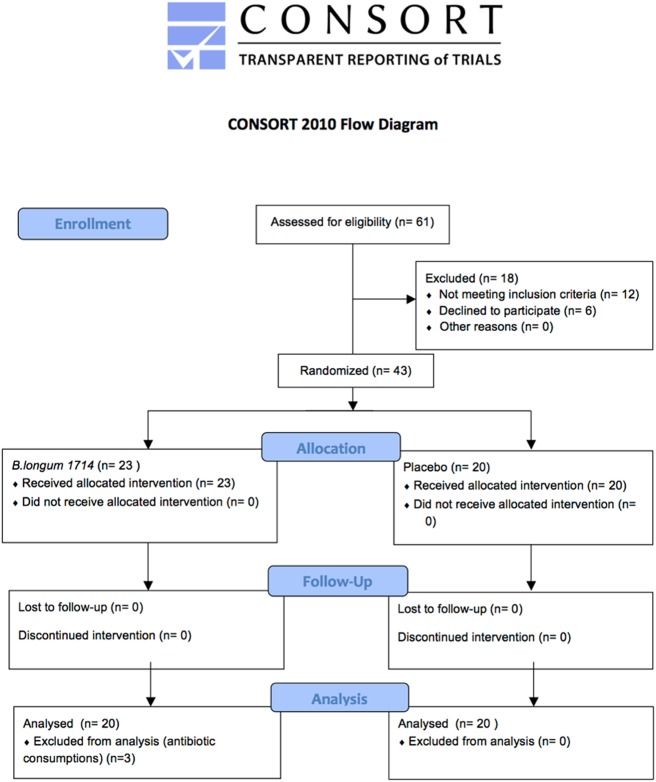
A total of 40 healthy volunteers completed the study. After screening, 43 subjects were randomized to either B. longum 1714™ or placebo. Three subjects could not be included in the final analysis because of use of antibiotics during the intervention period (Figure 2 for detailed trial profile). A total of 40 participants were included in the analysis with n = 20 per intervention group. Sex of participants was matched between groups. Age and body mass index of participants were not significantly different between groups (Table 1 for details).
Figure 2.
The CONSORT flow diagram of the clinical trial. Reprinted from Schulz et al. Copyright BMJ (48). All permission requests for this image should be made to the copyright holder.
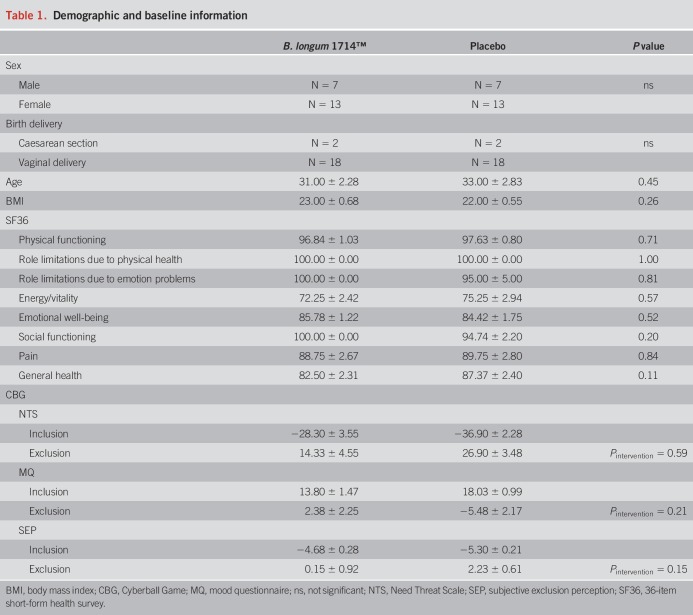
Table 1.
Demographic and baseline information
Administration of social stress (“CBG”) altered brain activity in healthy volunteers at baseline
At baseline, stress effects were analyzed across all participants. No difference between groups at baseline during rest was observed in any frequency band. As expected, source statistics of MEG power in each frequency band showed brain regions that had significantly different activities during the exclusion compared with the inclusion condition (Figure 3). The global NTS (t37 = 13.39, P < 001) and SEP (t38 = 9.99, P < 001) scores were significantly higher, and the MQ scores (t39 = −9.42, P < 001) were lower in the exclusion condition compared with inclusion condition (Table 1). At baseline, there were no significant differences in SF36 scores or CBG subjective scores (NTS, MQ, and SEP) after the social stress between groups (Table 1).
Figure 3.
Neural activities in different frequency bands during exclusion vs inclusion condition. (a) Theta frequency band (6 Hz): increased power in the bilateral cerebellum (CBL), right middle and inferior frontal cortex (MFC and IFC), left fusiform cortex (P = 0.004); decreased power in the left middle frontal and bilateral superior frontal cortex (SFC) (P = 0.02). (b) Alpha frequency band (11 Hz): increased power in the bilateral CBL, left fusiform, right inferior occipital cortex (P = 0.002); decreased power in the right SFC (P < 0.05). (c) Beta-1 frequency band (16 Hz): increased power in the bilateral CBL, left MFC, left inferior temporal, right middle and superior temporal cortex, and right parahippocampal (P = 0.004). (d) Beta-2 frequency band (21 Hz): increased power in the right CBL, left IFC, right inferior and middle temporal cortex (MTC) (P = 0.002). (e) Beta-3 frequency band (26 Hz): increased power in the bilateral CBL, right fusiform, left parahippocampal, left MTC (P = 0.04).
B. longum 1714™ altered resting-state brain activity measured by MEG
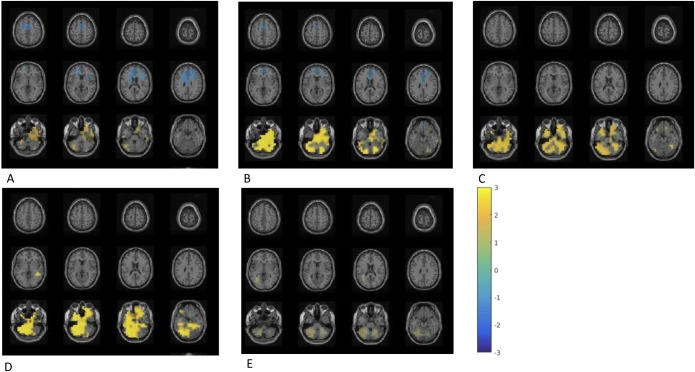
After a 4-week intervention, comparison between probiotic and placebo group showed that B. longum 1714™ feeding increased theta band (6 Hz) power (P < 0.05; Figure 4a) in the bilateral inferior, middle, and superior frontal cortex (IFC, MFC, and SFC) as well as in the bilateral anterior and middle cingulate cortex (ACC and MCC). Furthermore, feeding B. longum 1714™ reduced beta-2 band (26 Hz) power (P < 0.05; Figure 4b) in the bilateral fusiform gyrus, the bilateral hippocampus, the left inferior and superior temporal and bilateral middle temporal cortex, and the left cerebellum.
Figure 4.
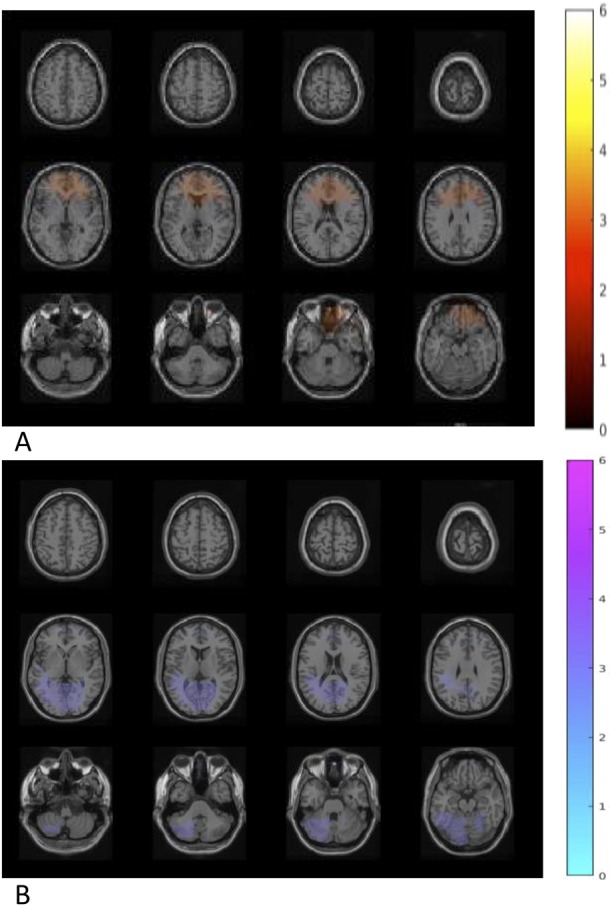
Difference of neural activity change during resting state comparing B. longum 1714™ vs placebo. (a) After the intervention, an increased theta band (6 Hz) power was obtained in a cluster including regions of bilateral inferior frontal cortex, middle frontal cortex, and the bilateral anterior cingulate cortex and middle cingulate cortex, comparing B. longum 1714™ with the placebo group (P < 0.05). (b) After the intervention, reduced beta-2 band (26 Hz) power was obtained in a cluster, consisting of the bilateral fusiform gyrus and hippocampus, left inferior temporal cortex and superior temporal cortex, bilateral middle temporal cortex and left cerebellum, comparing B. longum 1714™ with the placebo group (P < 0.05).
B. longum 1714™–induced change in neural activity correlated with increased vitality
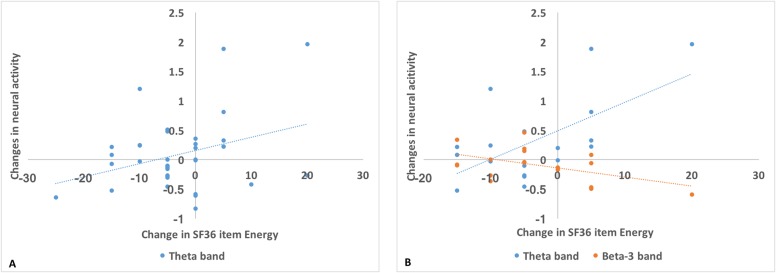
No significant difference was found in changes of SF36 scores after intervention between groups. To investigate the relationship between changes in neural activity during resting state and quality of life, the changes in averaged theta and beta-2 band power with changes in SF36 scores were correlated. In both groups, a significant positive correlation was obtained between changes of the SF36 subscale “Energy/Vitality” with changes of theta band power (r = 0.33, P = 0.04; Figure 5a). Group-specific correlations revealed that only in the B. longum 1714™ group, changes in SF36 score for “Energy/Vitality” positively correlated with changes in averaged theta band power (r = 0.61, P = 0.007), and negatively correlated with changes in beta-3 band power during the resting state (r = −0.50, P = 0.04; Figure 5b).
Figure 5.
Correlation between neural activity change during resting state and SF36 change. (a) In all groups, a positive correlation was obtained between changes of SF36 item “Energy/vitality” with changes of theta band power in the cluster (r = 0.33, P = 0.04). (b) In only B. longum 1714™ group, changes of SF36 item “Energy/vitality” positively correlated with change of averaged theta band power (r = 0.61, P = 0.007), and negatively correlated with change of beta-3 band power in the activated clusters during the resting state, respectively (r = −0.50, P = 0.04). SF36, 36-item short-form health survey.
All groups reported enhanced social stress measured by subjective ratings after a 4-week intervention
Analysis of changes in NTS, MQ, and SEP scores showed significant main effects of condition on NTS (F (1, 33) = 5.91, P = 0.02) and on SEP (F (1, 36) = 5.61, P = 0.02) as determined by ANOVA (Intervention × Condition). Participants in all groups reported increased scores of NTS (exclusion: M = 5.20 ± 2.37; inclusion: M = −4.32 ± 1.85) and SEP (exclusion: M = 0.94 ± 0.42; inclusion: M = −0.30 ± 0.16) in the exclusion compared with inclusion condition, after 4 weeks of intervention. The changes in MQ scores (exclusion: M = −1.87 ± 1.08; inclusion: M = 0.30 ± 0.86) showed no difference after intervention (F (1, 34) = 1.72, P = 0.20) (Figure 6). There were no effects of intervention on the subjective scores of these questionnaires (Table 2).
Figure 6.
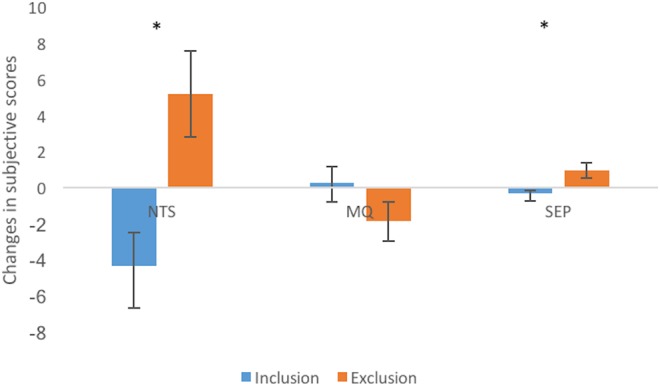
Main effects of condition on NTS and SEP. Participants in all groups reported increased scores of NTS and SEP in the exclusion condition compared with inclusion condition, after a 4-week intervention. MQ, mood questionnaire; NTS, Need Threat Scale; SEP, subjective exclusion perception.
Table 2.
Changes in subjective scores of SF36, NTS, MQ, and SEP
B. longum 1714™–altered brain activity during social stress measured by MEG
After a 4-week intervention, B. longum 1714™ induced changes in source power in theta (6 Hz) band and alpha band (11 Hz) in response to the CBG, compared with placebo. B. longum 1714™ increased theta band power in 1 cluster, consisting of the right IFC and the bilateral MFC and SFC, the left ACC, the bilateral MCC, and the right supramarginal gyrus in both conditions—inclusion and exclusion (P = 0.03; Figure 7a). Furthermore, B. longum 1714™ also increased the alpha band power in the cluster, including regions of the right IFC, the bilateral MFC and SFC, the bilateral ACC and MCC, and the right supramarginal gyrus in both conditions (P = 0.04; Figure 7b). No main effects of condition or interaction of intervention and condition were observed.
Figure 7.
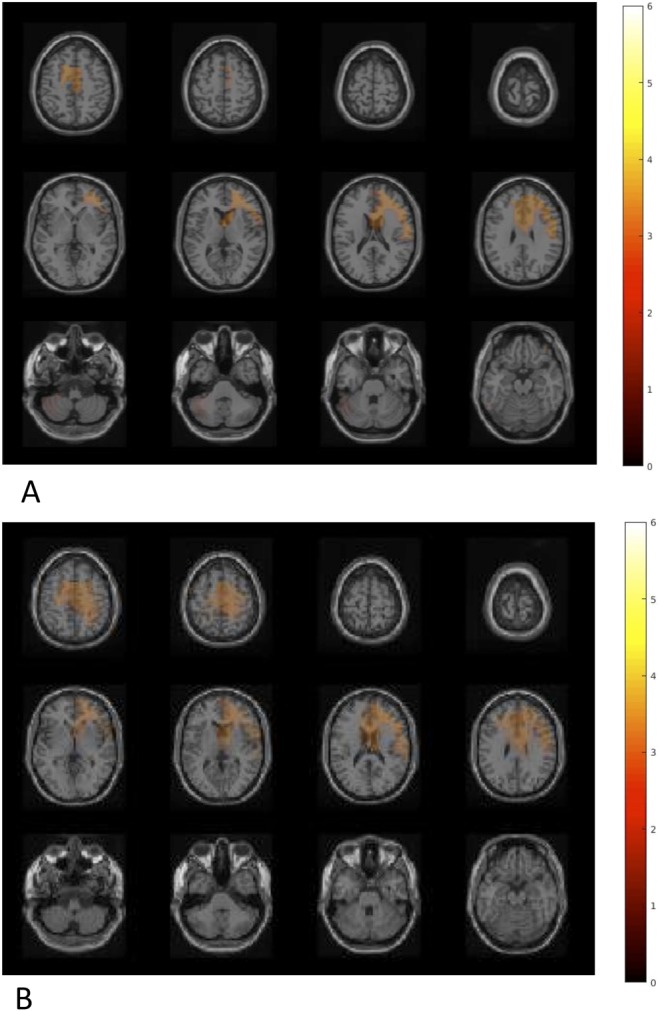
Difference in neural activity change during the Cyberball game comparing B. longum 1714™ vs placebo. (a) Theta band showed an increased power in a cluster, consisting of the right inferior frontal cortex (IFC) and the bilateral middle frontal cortex (MFC) and superior frontal cortex (SFC), the left anterior cingulate cortex (ACC), the bilateral middle cingulate cortex (MCC), and the right supramarginal gyrus (SMG), comparing B. longum 1714 group and the placebo group in both conditions (P = 0.03). (b) Alpha band power also showed an increased power in cluster, including regions of the right IFC, the bilateral MFC and SFC, the bilateral ACC and MCC, and the right SMG, comparing B. longum 1714™ group and the placebo group in both conditions (P = 0.04). No main effects of condition or interaction of intervention and condition were observed.
B. longum 1714™–induced change in neural activity in social stress correlated with changes in distress levels
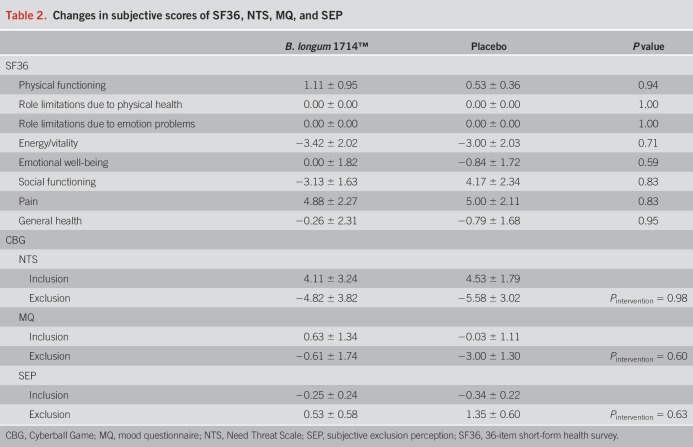
Correlation analysis between changes in neural activities and changes in subjective ratings during the CBG revealed that only with B. longum 1714™ and only during the exclusion condition, NTS changes positively, correlated with changes in the theta band power (r = 0.62, P = 0.008) and alpha band power (r = 0.54, P = 0.03; Figure 8).
Figure 8.
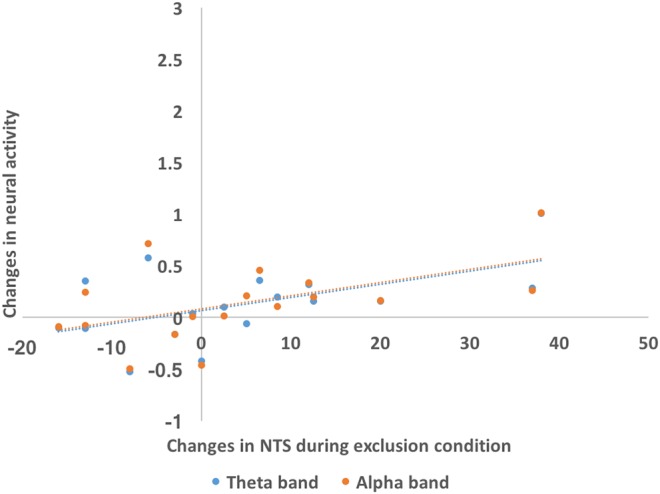
Correlation between neural activity change during the Cyberball game and subjective score changes. Only in B. longum 1714™ group and only during the exclusion condition, NTS changes positively correlated with changes of the theta band power (r = 0.62, P = 0.008) and alpha band power (r = 0.54, P = 0.03). NTS, Need Threat Scale.
Summaries of frequency bands and neuroanatomical areas found to be related to B. longum 1714™ intervention, and associations of changes in neural activity and subjective effects are provided in Tables 3 and 4, respectively.
Table 3.
Summarized neuroanatomical areas and frequency bands of changed neural activities influenced by effect of condition, intervention and/or interaction of condition and intervention
Table 4.
Summarized correlations of changes of averaged power in activated areas with changes of subjective results
DISCUSSION
B. longum 1714™ has been shown to reduce stress-related behaviors in preclinical studies (4,5) and improve stress responses and cognitive function in healthy volunteers (6). The current functional neuroimaging study showed that B. longum 1714™ significantly altered neural activities in the resting state and in response to a social stressor in healthy participants after a 4-week intervention. The alteration in the resting brain activity by B. longum 1714™ was associated with the SF36 scale “Energy/Vitality.” In response to the social stress, the changed neural activities occurred only in the probiotic group. These data suggest that B. longum 1714™ may play a role in managing stress responses by modulating the relevant neural processes.
During the resting state, B. longum 1714™ induced an increase in theta band power in the frontal and cingulate cortex and a decrease in beta-3 band power in the fusiform cortex, hippocampus, temporal cortex, and cerebellum. Although no significant change of SF36 was noted after the 4-week probiotic intake, the increase in theta band and decrease in beta-3 band power found in the resting state was associated with increase in perceived energy/vitality levels, as assessed by the SF36. Energy/vitality in the SF36 measures subjective reception of participants—how full of pep and energetic they felt. Other studies have shown an association between increased power in theta band (whole brain, especially prominent in the frontal regions) on a resting-state EEG with improved attention and arousal after consumption of glucose (24,25) whereas decreased theta band power measured by MEG in cingulate cortex has been correlated with subjective level of fatigue (26). Of interest, an increase in beta band power has been linked to mental fatigue (27), and inversely, reduced beta power could index increased alertness and arousal, accompanied with decreased anxiety and stress (28,29). These data suggest that B. longum 1714™ altered neural activities in a manner associated with enhanced vitality and reduced mental fatigue.
From an evolutionary perspective, humans are programmed to be highly sensitive to social exclusion and have developed monitoring systems to detect cues (30,31). This is necessary for human survival as social isolation is often a fatal threat (32,33). As expected, the CBG induced a social stress effect at baseline across all participants. Social exclusion produced differential neural oscillations in all frequency bands in various areas, such as frontal, temporal, and fusiform cortex. These results were similar to previous EEG and MEG studies, showing modulation of neural oscillations by social exclusion (14,15,17,21). Previously reported ACC activations were related to processing of negative emotions and event appraisal due to the social stress/exclusion, and PFC activation was related to emotional regulation (13,34–36). Theta band oscillations in ACC and insula were described as a marker of social pain in the context of the CBG and was also found during a cold pressor test and physical pain (37,38). The participants reported enlarged subjective distress and decreased mood level during the exclusion condition compared with the inclusion condition, consistent to previous studies (12,14,15,17). After the intervention, as expected, all participants reported higher distress level during the exclusion condition compared with the inclusion condition after 4 weeks, regardless of the type of intervention (probiotic, placebo). However, a clinical effect of B. longum 1714™ on reducing stress was not observed—the study was not powered to detect differences in subjective stress. Furthermore, as with other stress paradigms, the repetition of stress may reinforce the feeling of being excluded and consolidated the memory of the stressful event making it difficult to detect differences with small numbers.
Although both placebo and probiotic groups reported higher subjective distress, changes in neural processing of social stress were observed only after B. longum 1714™ consumption and not placebo. In addition, the correlation between changes in subjective distress and neural activities was seen only with B. longum 1714™. Therefore, these data support the notion that B. longum 1714™ may play a role in managing stress responses by modulating the relevant neural processes; B. longum 1714™ affecting individuals' neurophysiology is a novel finding and was previously only reported for behavioral data in animals and humans (4–6).
However, in comparison to other neurophysiological studies with the CBG (22,23), our study involved repeated exposure to the social stress 4 weeks apart. Indeed, similar changes in theta band activity induced by B. longum 1714™ were observed in the resting-state data (before playing the CBG) of the current study, which correlated with increased vitality. These data suggest that the increase in theta and alpha band power by B. longum 1714™ before the stressor may be to some degree a priming for the stressor and may represent an upregulation of appraisal processes and coping mechanisms in the expectation of being exposed to a similar stressful situation again. Furthermore, lower frontal alpha power has been associated with anxiety (39,40). The increased alpha oscillation activity in our study may indicate the inhibition of limbic activity and thereby counter-regulate negative emotions and stress. Taking this argument further, we suggest that in contrast to placebo, B. longum 1714™ reduced participants' stress response and enabled them to manage the increased distress level by upregulating processes appraising stressful events and downregulating negative emotions.
Other studies, in line with our data, showed neural modulation by different psychobiotic strains, and provided some clues of the potential mechanisms involved. Preclinical studies have reported an increase in serotonin and dopamine level, 2 crucial neurotransmitters regulating mood and emotions, in brain regions of mice such as PFC after interventions with Lactobacillus plantaraum and Lactobacillus helveticus (41–43). Another study showed Lactobacillus rhamnosus JB-1 modulated GABAergic system and reduced stress-related psychiatry-like behaviors in mice, and some of the effects were mediated by the vagus nerve (44). However, effects of the L. rhamnosus JB-1 were not found in a clinical study in healthy volunteers, indicating possibly the challenge of translating preclinical studies into clinical relevance (3). The lack of evidence of translating preclinical to clinical studies was also addressed by a recent meta-analysis on the anxiolytic effect of probiotics (45), suggesting a need to investigate strain specificity and use functional imaging techniques in clinical studies in alternative to subjective questionnaires.
In our recent study, antistress effects of rifaximin on social stress were associated with a reduction of frontal and cingulate beta band power in the insula, frontal and cingulate gyrus, after 1-week intervention (21). Although causing a stress reduction like B. longum 1714™, rifaximin may act through different pathways—different neural oscillations and brain regions. Because the peripheral and central mechanisms by which rifaximin affects stress management are not well understood, one may speculate on an “eubiotic” effect of this antibiotic promoting beneficial bacteria such as Bifidobacteria and Lactobacilli (46,47). Therefore, although effects of probiotics on CNS functions appear to be strain-specific (4), results of existing studies help to understand the possible pathways of the strain we used in the current trial. More studies are required to investigate the effects of different strains on diverse brain activities in response to stress in order to obtain a detailed picture of the effects of probiotics on brain functioning.
CONCLUSION
B. longum 1714™ influenced resting neural activities associated with enhanced vitality and stress-related neural responses, which may be involved in the counter-regulation of negative emotions. Our results further support the role of B. longum 1714™ in reducing stress responses and provides new evidence that this probiotic affects brain function through modulating neural oscillations in certain brain regions. The understanding of neural oscillations induced by stress and their modulation by probiotics is still at its beginning. Studies that examine the mechanisms by which probiotics modulate the gut-brain axis and compare their effects on other CNS functions with other interventions are warranted. To fully understand the effects of B. longum 1714™ on brain function and human behavior, it is necessary to consider the effects of the strain on other CNS functions such as pain sensitivity, mood, and memory in both healthy controls and in patients with psychiatric (depression, anxiety), neurologic (neurodegenerative), and gastrointestinal disorders such as IBS.
CONFLICTS OF INTEREST
Guarantor of the article: Paul Enck, PhD.
Specific author contributions: P.E.: inception of the study and publication of the work. H.W.: contributed to the design of the study, data collection and analysis, drafting of the manuscript, and critical revisions of the manuscript. C.B., E.M., and P.E.: contributed to the design of the study, data analysis, and critical revisions of the manuscript. All authors approved the final version of the manuscript. 1714, 1714-serenitas and Zenflore are trademarks of Alimentary Health.
Financial support: The research leading to these results has received funding from the People Programme of the European Union's Seventh Framework Programme under REA grant agreement no. 607652 (NeuroGut).
Potential competing interests: E.M. is the Technical Director at Alimentary Health Group. Alimentary Health provided the placebo and probiotic containing the B. longum 1714™ strain (also known as 1714-Serenitas), but had no further influence on data collection and data evaluation. P.E. is a consultant for Alimentary Health. The other authors declare no potential conflicts of interest.
Study Highlights.
WHAT IS KNOWN
✓ Accumulating evidence indicates that the gut microbiota communicates with the CNS, possibly through neural, endocrine, and immune pathways, and influences brain function.
✓ Effects of probiotics on the CNS functions are strain specific.
✓ B. longum 1714™ has been shown to reduce stress-related behaviors in preclinical studies and improve stress responses and cognitive function in healthy volunteers.
WHAT IS NEW HERE
✓ B. longum 1714™ altered brain activity of healthy volunteers during social stress as measured by neuroimaging method MEG with high temporal resolution and fine spatial resolution.
✓ B. longum 1714™ influenced resting neural activities associated with enhanced vitality and stress-related neural responses, which may be involved in the counter-regulation of negative emotions.
✓ Our results further support the role of B. longum 1714™ in reducing stress responses in humans and provides new evidence that this probiotic strain affects brain function through modulating neural oscillations in brain regions involved in emotional regulation.
ACKNOWLEDGMENTS
We thank Dr. Gerald Clarke, Prof. John Cryan, and Prof. Ted Dinan at the APC Microbiome Institute, University College Cork, Ireland for support on training and advice on the probiotic selection.
1714™, 1714-Serenitas™, and Zenflore™ are trademarks of Alimentary Health.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/A163
REFERENCES
- 1.Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13(10):701–12. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Lee IS, Braun C, et al. Effect of probiotics on central nervous system functions in animals and humans: A systematic review. J Neurogastroenterol Motil 2016;22(4):589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly JR, Allen AP, Temko A, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun 2017;61:50–9. [DOI] [PubMed] [Google Scholar]
- 4.Savignac HM, Kiely B, Dinan TG, et al. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil 2014;26(11):1615–27. [DOI] [PubMed] [Google Scholar]
- 5.Savignac HM, Tramullas M, Kiely B, et al. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res 2015;287:59–72. [DOI] [PubMed] [Google Scholar]
- 6.Allen AP, Hutch W, Borre YE, et al. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry 2016;6(11):e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–401, 401.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagga D, Reichert JL, Koschutnig K, et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018;9(6):486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagga D, Aigner CS, Reichert JL, et al. Influence of 4-week multi-strain probiotic administration on resting-state functional connectivity in healthy volunteers. Eur J Nutr 2018. [Epub ahead of print May 30, 2018.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto-Sanchez MI, Hall GB, Ghajar K, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology 2017;153(2):448–59.e8. [DOI] [PubMed] [Google Scholar]
- 11.Krieger N. Theories for social epidemiology in the 21st century: An ecosocial perspective. Int J Epidemiol 2001;30(4):668–77. [DOI] [PubMed] [Google Scholar]
- 12.Williams KD, Jarvis B. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behav Res Methods 2006;38(1):174–80. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Braun C, Enck P. How the brain reacts to social stress (exclusion): A scoping review. Neurosci Biobehav Rev 2017;80:80–8. [DOI] [PubMed] [Google Scholar]
- 14.Cristofori I, Harquel S, Isnard J, et al. Monetary reward suppresses anterior insula activity during social pain. Soc Cogn Affect Neurosci 2015;10(12):1668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cristofori I, Moretti L, Harquel S, et al. Theta signal as the neural signature of social exclusion. Cereb Cortex 2013;23(10):2437–47. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto T, Nittono H, Ura M. Cognitive, affective, and motivational changes during ostracism: An ERP, EMG, and EEG study using a computerized cyberball task. Neurosci J 2013;2013:304674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Noordt SJ, White LO, Wu J, et al. Social exclusion modulates event-related frontal theta and tracks ostracism distress in children. Neuroimage 2015;118:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130(5):1480–91. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: A systematic review. Gen Hosp Psychiatry 2010;32(4):345–59. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30(6):473–83. [PubMed] [Google Scholar]
- 21.Wang H, Braun C, Enck P. Effects of Rifaximin on central responses to social stress-a pilot experiment. Neurotherapeutics 2018;15(3):807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastian C, Viding E, Williams KD, et al. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn 2010;72(1):134–45. [DOI] [PubMed] [Google Scholar]
- 23.Oostenveld R, Fries P, Maris E, et al. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An YJ, Jung KY, Kim SM, et al. Effects of blood glucose levels on resting-state EEG and attention in healthy volunteers. J Clin Neurophysiol 2015;32(1):51–6. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Szabo JS, Dykman RA. Effects of a carbohydrate supplement upon resting brain activity. Integr Physiol Behav Sci 2004;39(2):126–38. [DOI] [PubMed] [Google Scholar]
- 26.Ishii A, Tanaka M, Watanabe Y. The neural mechanisms underlying the decision to rest in the presence of fatigue: A magnetoencephalography study. PLoS One 2014;9(10):e109740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka M, Ishii A, Watanabe Y. Neural effects of mental fatigue caused by continuous attention load: A magnetoencephalography study. Brain Res 2014;1561:60–6. [DOI] [PubMed] [Google Scholar]
- 28.Diego MA, Field T, Sanders C, et al. Massage therapy of moderate and light pressure and vibrator effects on EEG and heart rate. Int J Neurosci 2004;114(1):31–44. [DOI] [PubMed] [Google Scholar]
- 29.Field T, Ironson G, Scafidi F, et al. Massage therapy reduces anxiety and enhances EEG pattern of alertness and math computations. Int J Neurosci 1996;86(3-4):197–205. [DOI] [PubMed] [Google Scholar]
- 30.Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull 2005;131(2):202–23. [DOI] [PubMed] [Google Scholar]
- 31.Williams KD. Chapter 6 ostracism: A temporal need–threat model. In: Advances in Experimental Social Psychology, Vol 41 Academic Press: San Diego, CA, 2009, pp. 275–314. [Google Scholar]
- 32.Kling A, Lancaster J, Benitone J. Amygdalectomy in the free-ranging vervet (Cercopithecus aethiops). J Psychiatr Res 1970;7(3):191–9. [DOI] [PubMed] [Google Scholar]
- 33.Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science 2003;302(5648):1231–4. [DOI] [PubMed] [Google Scholar]
- 34.Bolling DZ, Pelphrey KA, Vander Wyk BC. Unlike adults, children and adolescents show predominantly increased neural activation to social exclusion by members of the opposite gender. Soc Neurosci 2016;11(5):475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo S, Yu D, Han S. Genetic and neural correlates of romantic relationship satisfaction. Soc Cogn Affect Neurosci 2016;11(2):337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preller KH, Pokorny T, Hock A, et al. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci USA 2016;113(18):5119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang PF, Arendt-Nielsen L, Chen AC. Dynamic changes and spatial correlation of EEG activities during cold pressor test in man. Brain Res Bull 2002;57(5):667–75. [DOI] [PubMed] [Google Scholar]
- 38.Chen AC, Dworkin SF, Haug J, et al. Topographic brain measures of human pain and pain responsivity. Pain 1989;37(2):129–41. [DOI] [PubMed] [Google Scholar]
- 39.Luijcks R, Vossen CJ, Hermens HJ, et al. The influence of perceived stress on cortical reactivity: A proof-of-principle study. PLoS One 2015;10(6):e0129220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci 2009;10(6):423–33. [DOI] [PubMed] [Google Scholar]
- 41.Liang S, Wang T, Hu X, et al. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015;310:561–77. [DOI] [PubMed] [Google Scholar]
- 42.Liu WH, Chuang HL, Huang YT, et al. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav Brain Res 2016;298(Pt B):202–9. [DOI] [PubMed] [Google Scholar]
- 43.Liu YW, Liu WH, Wu CC, et al. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res 2016;1631:1–12. [DOI] [PubMed] [Google Scholar]
- 44.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 2011;108(38):16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reis DJ, Ilardi SS, Punt SEW. The anxiolytic effect of probiotics: A systematic review and meta-analysis of the clinical and preclinical literature. PLoS One 2018;13(6):e0199041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponziani FR, Scaldaferri F, Petito V, et al. The role of antibiotics in gut microbiota modulation: The eubiotic effects of Rifaximin. Dig Dis 2016;34(3):269–78. [DOI] [PubMed] [Google Scholar]
- 47.Xu D, Gao J, Gillilland M, III, et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014;146(2):484–96.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schulz F, Altman DG, Moher D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]