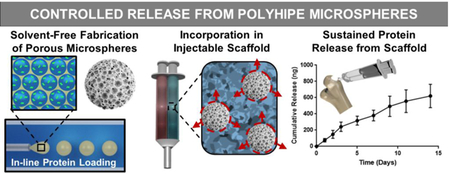
Abstract
Delivery of osteoinductive factors such as bone morphogenetic protein 2 (BMP-2) has emerged as a prominent strategy to improve regeneration in bone grafting procedures. However, it remains challenging to identify a carrier that provides the requisite loading efficiency and release kinetics without compromising the mechanical properties of the bone graft. Previously, we reported on porous, polymerized high internal phase emulsion (polyHIPE) microspheres fabricated using controlled fluidics. Uniquely, this solvent-free method provides advantages over current microsphere fabrication strategies including in-line loading of growth factors to improve loading efficiency. In the current study, we utilized this platform to fabricate protein-loaded microspheres and investigated the effect of particle size (~400 vs ~800 μm) and pore size (~15 vs 30 μm) on release profiles. Although there was no significant effect of these variables on the substantial burst release profile of the microspheres, the incorporation of the protein-loaded microspheres within the injectable polyHIPE resulted in a sustained release of protein from the bulk scaffold over a two-week period with minimal burst release. Bioactivity retention of encapsulated BMP-2 was confirmed first using a genetically-modified osteoblast reporter cell line. A functional assay with human mesenchymal stem cells established that the BMP-2 release from microspheres induced osteogenic differentiation. Finally, microsphere incorporation had minimal effect on the cure and compressive properties of an injectable polyHIPE bone graft. Overall, this work demonstrates that these microsphere-polyHIPE composites have strong potential to enhance bone regeneration through controlled release of BMP-2 and other growth factors.
Keywords: POLYHIPE MICROSPHERES, PROTEIN RELEASE, BMP-2, MESENCHYMAL STEM CELLS
Graphical Abstract
1. Introduction
Limitations of current treatments for large bone defects and non-unions has resulted in significant efforts to develop novel grafting materials with improved regenerative potential.[1, 2] A promising approach has been the incorporation of bone morphogenetic proteins (BMPs) into biomaterial scaffolds. BMPs are a family of potent osteogenic factors active in bone tissue formation during embryonic development and skeletal repair.[3, 4] Of the numerous members of the BMP family that have demonstrated efficacy in bone regeneration, BMP-2 has been the most extensively studied due to its putative role in osteoblastic differentiation, angiogenesis, chemoattraction, and cell signaling during fracture healing.[5–10] As a result, numerous platforms have been investigated as carriers for this potent osteoinductive factor.[11–16]
Recently, delivery of recombinant expressed BMP-2 (rhBMP-2) in a collagen sponge was approved for commercial use by the FDA for treatment of specific spine, tibia, and craniofacial defects.[17, 18] Since its release, this graft has demonstrated strong clinical efficacy and become a leader in the bone grafting market. Despite its regenerative potential, there were several safety concerns including inflammation, ectopic bone formation and neurological deficits.[19] The bolus release of rhBMP-2 from the collagen sponge is rapidly cleared away from the injury and this necessitates the use of supraphysiological dosages to ensure therapeutic levels.[20, 21] Although this bolus release of growth factor has demonstrated improved bone formation, it also leads to undesirable off-target complications.
The dynamic role that BMP-2 plays during various stages of the healing process suggests that a more local and sustained approach may be beneficial. It has been reported that there is an innate upregulation of ectopically osteoinductive BMP expression for several weeks following injury.[22, 23] Sustained BMP levels over this period provides a robust osteogenic effect, allowing appropriate time for osteoprogenitor cell recruitment, retention, and differentiation.[7, 24, 25] As such, numerous systems have been investigated to provide more physiologically relevant growth factor delivery profiles including hydrogels carriers, ceramic materials, and synthetic polymer scaffolds.[26, 27] Although the mild processing conditions of hydrogels make them attractive carriers for growth factors, typical mesh sizes often result in burst release profiles with limited controls of kinetics without the addition of an affinity-based functionality (e.g. heparin).[28–30] Surface-modified ceramics that improve regeneration may experience reduced loading efficiencies during fabrication that raise scale-up concerns.[11, 31, 32] As a result, encapsulation of BMPs into polymeric microspheres has emerged as one of the most promising methods to provide local and controlled delivery of these factors.
The most widely studied of these systems is the fabrication of poly(lactic-co-glycolic acid) (PLGA) microspheres using emulsification-evaporation or porogen-leaching methods.[15, 16, 33–38] These established techniques yield biodegradable particles with a range of porosities and sizes. Delivery of rhBMP-2 in these vehicles has been shown to significantly extend delivery profiles and improve bone regeneration in numerous in vivo models. Kempen et al. reported a marked increase in ectopic and orthotopic bone formation with sustained rhBMP-2 release from a composite PLGA microsphere/poly(propylene fumarate) scaffold.[39] Furthermore, Brown et al. demonstrated that microsphere mediated release could be used to enhance pharmacokinetic profiles in an injectable polyurethane scaffold and improve regeneration over current BMP-2 soaked scaffolds.[40] Despite the strong potential of this traditional microsphere fabrication method, the requisite use of toxic solvents during fabrication poses significant challenges to bioactivity retention and commercialization. In addition to loss of therapeutic activity, protein denaturation resulting from unsatisfactory processing has been shown to introduce immunogenicity and toxicity concerns.[41] Post fabrication loading of growth factors has been explored to minimize processing effects, but often results in reduced loading efficiency and elevated costs.[27, 42] As a result, fabrication strategies that eliminate harsh processing conditions and allow for more efficient loading of costly therapeutics could offer several translational benefits for microsphere delivery of growth factors.
We recently reported a method for solvent-free fabrication of porous microspheres using the principles of emulsion templating and fluid dynamics.[43] This new methodology provides inline loading of therapeutics and independent control over particle size and pore architecture, properties known to strongly influence release kinetics of encapsulated growth factors.[33, 44, 45] Furthermore, the same macromer chemistry can be used for both the microsphere and the porous bone graft, which is expected to improve microsphere-scaffold integration and mechanical integrity. Incorporation of microsphere delivery systems with unmatched chemistries and porosities (e.g. hydrogel microspheres in PLGA graft) has been shown to significantly reduce mechanical properties.[46, 47] As such, this novel fabrication technique provides significant advantages over traditional microsphere methods and has the potential to minimize commercialization and safety concerns.
The focus of the current study was to establish key relationships between microsphere properties and the resulting protein release kinetics. First, loading efficiencies and release kinetics were investigated as a function of microsphere diameter and pore architecture using a model protein. Bioactivity retention of encapsulated rhBMP-2 was then confirmed by monitoring luciferase activity in a BMP-responsive osteoblast reporter cell line. Next, the ability of rhBMP-2 loaded polymerized high internal phase emulsions (polyHIPEs) to induce osteoblastic differentiation of human mesenchymal stem cells (hMSCs) was assessed using alkaline phosphatase (ALP) assays. Finally, the effects of microsphere incorporation on composite scaffold compressive modulus, and yield strength were assessed to ensure no deleterious effects were observed. Collectively, this work aims to highlight the potential of polyHIPE microspheres to improve the regenerative capacity of injectable polyHIPE bone grafts through the controlled release of growth factors.
2. Materials and Methods
2.1. Materials
Polyglycerol polyricinoleate (PGPR 4125) was donated by Palsgaard. Tris buffer was purchased from VWR. Pronase ® protease was purchased from Millipore Sigma. All other chemicals were purchased and used as received from Sigma Aldrich unless otherwise noted. Ethylene glycol dimethacrylate (EGDMA) was filtered through an aluminum oxide column to remove the inhibitor monomethyl ether hydroquinone.
2.2. Fabrication of BSA-FITC Loaded Microspheres
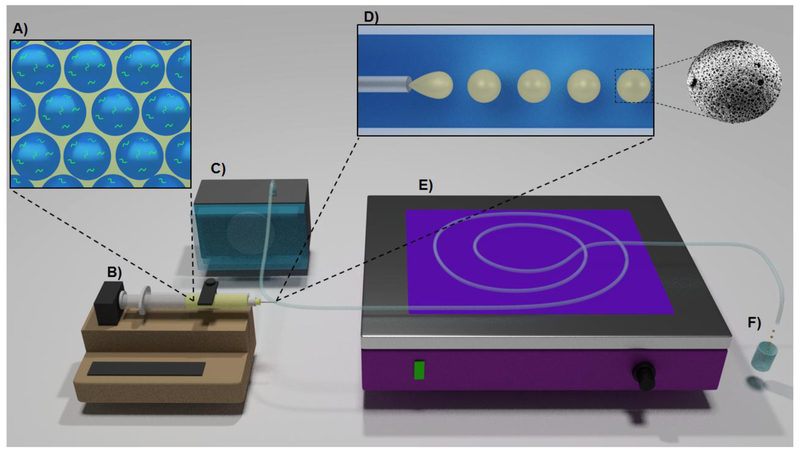
Microspheres were fabricated via a fluidics double emulsion technique (w/o/w) adapted from Moglia et al., Figure 1.[43] Briefly, primary high internal phase emulsions (HIPEs) were fabricated by combining the photocurable macromer EGDMA with PGPR surfactant (10 or 30 wt%) and 2 wt% of the organically soluble photoinitiator, 2,2-Dimethoxy-2-phenylacetophenone (DMPA). Once mixed, an aqueous solution containing the model protein (200 μg/mL), bovine serum albumin – fluorescein isothiocyanate conjugate (BSA-FITC), was added to the organic phase (75% v) and emulsified using a FlackTek Speedmixer DAC 150 FVZ-K. This primary HIPE was then injected dropwise (KD Scientific-100 Infusion Pump) into a continuously flowing external aqueous phase containing 3 wt% poly(vinyl alcohol) (PVA) aqueous solution and passed through UV excitation (UVP High Performance Transilluminator 365 nm) to initiate radical crosslinking. Needle gauge, tubing diameter, aqueous and emulsion flow rate, and surfactant concentration were varied to modulate particle and pore size using previously established relationships outlined in Supplemental Table S1. Collected microspheres were dried in vacuo overnight prior to characterization. Four model compositions were fabricated with target particle diameter of ~800 or ~400 microns and target pore diameter of ~30 or ~15 microns.
Figure 1.
Schematic of microsphere fabrication. Protein is loaded into the aqueous phase of the HIPE (A). Emulsion flow is controlled using a syringe pump (B). PVA solution (3 wt %) flow is controlled using a peristaltic pump (C). The emulsion is injected into a clear tygon tubing (D). Macromer is polymerized after initiator activation by UV irradiation (E). Polymerized particles are collected and filtered using a 100 microns strainer (F).
2.3. Microsphere Microarchitecture and Surface Area Analysis
Average particle and pore diameter of varying compositions was determined using scanning electron microscopy (SEM) (JEOL 6500 and Quanta FEG 650) and image analysis performed using ImageJ. At least 10 particles, from each fabrication batch, were coated with gold, imaged, and particle diameter measured. Pore size measurements were completed on five of these particles using the first ten pores that crossed the median of each representative micrograph. Average particle (n = 30) and pore sizes (n = 150) for 3 batches of each polyHIPE microsphere composition are reported. Brunauer-Emmett-Teller (BET) surface area analysis was performed using Micromeritics 3Flex physisorption equipment with nitrogen adsorption measurements. Microsphere specimens of each composition (~150 mg) were tested in duplicate after immersion in DCM for 48 hours to remove surfactant and vacuum drying for 48 hours.
2.4. Loading Efficiency Model Protein
Loading efficiency of each composition was determined using an accelerated release protocol. Prior to incubation, microspheres were crushed to increase surface area and minimize barriers to diffusion. Specimens were then placed in 2 mL centrifuge tubes, sonicated for 1 hour, and incubated in a mixture of 800 microliters of a 0.1 M Tris buffer, 10 mM CaCl2, at pH 7.6, 100 microliters of DI water and 100 microliters 10mg/mL Pronase® protease, with agitation at 37°C. After 24 hours, specimens were centrifuged to pellet the crushed particles and the aqueous phase removed. The pellet was then re-agitated with 1 mL DI water and incubated an additional 24 hours to remove residual protein. At least four washes were performed until the amount of protein recovered was negligible. Protein concentration was determined using fluorescence spectroscopy (Tecan Infinite 200 Pro) by referencing a standard 12-point calibration curve prepared by measuring fluorescence of known concentrations of BSA-FITC. The concentration of BSA-FITC successfully encapsulated in the microsphere was then calculated and compared to the theoretical concentration loaded into the primary emulsion.
2.5. In Vitro Release Kinetics of Model Protein
Microspheres containing BSA-FITC were placed in 2 mL centrifuge tubes and incubated in 1 mL DI water with agitation at 37°C. At specified time points over 21 days, microsphere releasates were collected and replaced with 1 mL of fresh DI water. Fifty microliters from each sample were diluted in a 400 microliters of a 0.1 M Tris buffer, 10 mM CaCl2, at pH 7.6. Fifty microliters of 10mg/mL Pronase ® protease mixture were added and samples were incubated with agitation at 37°C. Daily protein release was determined using fluorescence spectroscopy (Tecan Infinite 200 Pro) by referencing a standard 12-point calibration curve prepared by measuring fluorescence of known concentrations of BSA-FITC prepared using the same buffer and Pronase concentrations. Cumulative release was determined by normalizing each cumulative time point to the total amount released over the experiment period.
A single microsphere composition was used to investigate the release profile of the microsphere-polyHIPE composite. Five weight percent of small particle diameter - large pore microspheres were incorporated into a secondary polyHIPE scaffold of similar pore size. The scaffold was prepared using deionized water for the aqueous phase. The scaffold weight was 300mg and the amount of protein in the dispersed microspheres was approximately 8 μg of bovine serum albumin – fluorescein isothiocyanate conjugate (BSA-FITC). For comparison, an additional polyHIPE scaffold was prepared by adding the 8 μg of BSA-FITC into the aqueous phase directly into the injectable emulsion without the use of a microsphere delivery vehicle. Release kinetics for direct versus vehicle loaded protein were compared.
2.6. Preparation of rhBMP-2 Loaded Microspheres
Similar to BSA-FITC microsphere fabrication, an aqueous solution containing rhBMP-2 (E. coli expressed, R&D systems) was emulsified with EGDMA and surfactant to yield the primary HIPE. For polyHIPE systems, calcium chloride or other salts are often incorporated into the aqueous phase to minimize Ostwald ripening that broadens the pore size distribution.[48] Similar salt solutions, such as PBS can be utilized to achieve similar effects without introducing concerns for BMP-2 stability. To this end, PBS was utilized to fabricate BMP-2 loaded polyHIPEs for the respective studies. The rhBMP-2 HIPE was then polymerized and immersed in 0.5 mL release medium (αMEM or DMEM containing 0.1% FBS) and allowed to release for 4 days. Releasates were collected and diluted to a concentration of 100 ng/mL for testing of bioactivity.
2.7. Bioactivity Retention of Encapsulated rhBMP-2 with Reporter Cells
A rapid assessment of bioactivity retention of rhBMP-2 loaded into polyHIPE scaffolds was performed by monitoring luciferase activity of a BMP responsive immortalized reporter (BRITER) cell line. Reporter cells were seeded at a density of 100,000 cells/cm2 and cultured in growth media (DMEM + 10% FBS) containing 1 μM 4-hydroxytamoxifen to minimize endogenous BMP expression. After 24 hours, culture media was removed and replaced with polyHIPE releasate (100 ng/mL rhBMP-2) or indicated concentration of stock rhBMP-2 solution (100, 60, 20, 0 ng/mL rhBMP-2). After 3 hours, cells were lysed and luciferase activity measured using a Dual Luciferase Reporter Assay System (Promega). Relative luciferase activity was determined by normalizing BMP-2 dependent firefly luciferase activity to an internal cell density control via renilla luciferase activity.
2.8. Osteogenic Differentiation of Human Mesenchymal Stem Cells
Bone marrow-derived hMSCs were obtained as passage 1 from the Center for the Preparation and Distribution of Adult Stem Cells at Texas A&M Health Science Center College of Medicine, Institute for Regenerative Medicine at Scott & White through NIH Grant # P40RR017447. Cells were cultured to 80% confluency on tissue-culture polystyrene flasks in standard growth media containing Minimum Essential Media α (MEM α, Life Technologies) supplemented with 16.5% fetal bovine serum (FBS, Atlanta Biologicals) and 1% L-glutamine (Life Technologies) prior to passaging. All experiments were performed with cells at passage 3.
Alkaline phosphatase activity of hMSCs cultured with polyHIPE releasate was determined by monitoring the conversion of p-nitrophenyl phosphate (PNPP, Thermo Scientific) to pnitrophenol. hMSCs were seeded at a density of 15,000 cells/cm2 in standard growth media and allowed to adhere. After 24 hours, polyHIPE releasate or fresh rhBMP-2 as positive control (100 ng/mL) was added and changed every 3 days for 14 days following measurement of ALP activity. Osteogenic media (growth media supplemented with 50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, and 10 nM dexamethasone) was also used as positive control and changed as indicated above. Samples were lysed using thermal shock and incubated with PNPP Substrate for 30 min. ALP activity was determined as the rate of PNPP conversion to p-nitrophenyl by measuring the absorbance at 405 nm (Tecan Infinite M200Pro) and normalized to cell number obtained from dsDNA quantification (PicoGreen, Life Technologies).
2.9. Composite Scaffold Fabrication and Characterization
Microspheres-polyHIPE composites were fabricated by loading microspheres into an injectable, redox initiated polyHIPE foam prior to cure. Briefly, redox HIPEs were fabricated according to established protocols with an organic phase comprised of 10 wt% PGPR and 1 wt% benzoyl peroxide (BPO) initiator or trimethylaniline (TMA) as reducing agent.[49] Microspheres were added at 0, 5, 10, or 20 wt% (dried microsphere / HIPE polymer phase) to both initiating and reducing emulsions and mixed to facilitate crosslinking. HIPEs were placed in a 37°C aluminum bead bath to facilitate crosslinking for 24 hours.
The effect of microsphere incorporation on polyHIPE compressive modulus and yield strength was investigated following ASTM D1621–04a standards for mechanical testing of porous foams. PolyHIPEs were sectioned into disks with a 3:1 diameter to height ratio (15 mm diameter, 5 mm thick) using an Isomet® saw. PolyHIPE specimens were compressed using an Instron 3300 at a strain rate of 50 mm/s. The compressive modulus was calculated from the slope of the linear region and the compressive yield strength was identified, after correcting for zero strain, as the stress at the yield point or 10% strain.
2.10. Statistical Analysis
The data are displayed as mean ± standard deviation for each composition. An analysis of variance (ANOVA) comparison was used for multiple composition comparisons with a Tukey’s multiple comparison to analyze the significance of the data. A Student’s t-test was performed to determine any statistically significant differences if only two compositions were present. All tests were carried out at a 95% confidence interval (p<0.05). Microsphere compositions that had a loading efficiency or gel fraction greater than two standard deviations from the average were excluded from further analysis as outliers.
2.11. Ethics Approval
Ethical use of preexisting human mesenchymal stem cells was approved by the Texas A&M University Institutional Review Board (IRB ID: IRB 2017:0276). No research activities involving human subjects as defined by U.S. Department of Health & Human Services and U.S. Food and Drug Administration regulations was performed. No other ethics approvals were required.
3. Results
3.1. Fabrication and Characterization of PolyHIPE Microspheres
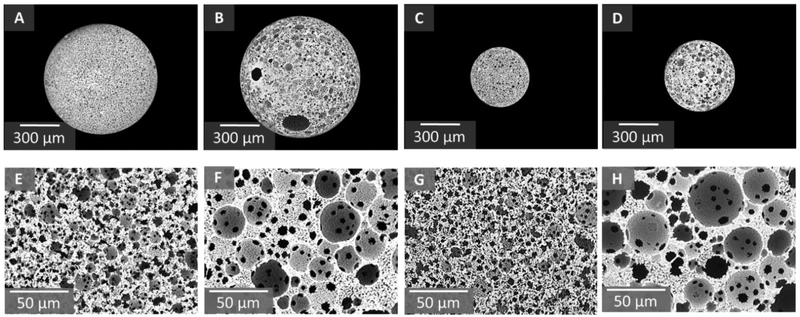
Prior to investigation of encapsulation efficiency and release kinetics, particle diameter and pore architecture was characterized to ensure there were no negative effects on emulsion stability after loading of protein. A factorial design of two particle diameters and two pore sizes was utilized to fabricate four distinct compositions utilizing the parameters outlined in Supplemental Table S1. We previously determined that a strict balance of fabrication parameters including external flow velocity and emulsion injection rates is necessary to ensure laminar flow and uniform particle generation.[43] Here, control over external flow velocity was modulated to generate two distinct particle diameters. Tubing diameter of 2.4 mm was used with a flow rate of 3.7 ml/min to yield a flow velocity of 83 cm/min and large particles approximately 800 microns in size. An elevated flow velocity of 267 cm/min was achieved by decreasing tubing diameter to 1.6 mm and increasing flow rate to 5.1 ml/min. As a result of the increased shear forces at the injection site, small diameter particles approximately 400 microns in diameter were formed. Furthermore, two pore sizes, large ~30 microns and small ~15 microns, were fabricated by increasing surfactant concentrations to 10 wt% and 30 wt%. We have previously demonstrated that increased surfactant concentration serves to decrease interfacial tension and act as a barrier between the organic and aqueous phases, resulting in increased surface area stabilization and a decreased pore size.[50] Representative micrographs of particle diameter and pore size are provided in Figure 2 with quantification of particle and pore size provided in Table 1. Cross-sectional analysis of microspheres confirmed that a uniform pore architecture was maintained throughout the bulk of the particle with no significant differences observed between surface and cross-section pores, Supplemental Figure S1 and Supplemental Table S2. Additionally, protein incorporation had minimal impact on pore size, architecture, or cross-linking efficiency compared to unloaded microspheres, Supplemental Table S3, Supplemental Figure S2 and S3. Finally, BET was performed on varied polyHIPE microsphere compositions and demonstrated a uniform porosity in all compositions with an approximate 1.7 fold increase in surface area for 30% surfactant compositions over 10% surfactant compositions, Table 1.
Figure 2.
Modulated particle diameter of model compositions with representative SEM micrographs (A-D). Modulated pore diameter of model compositions with representative SEM micrographs (E-H). From left to right: large particle-small pore, large particle-large pore, small particle-small pore, small particle-large pore.
Table 1.
Summary table of properties for model compositions including particle diameter (n=30), pore size (n=150), loading efficiency (n=12), gel fraction (n=3) and BET surface area (n=2). All data represents average ± standard deviation.
| Composition | Particle Size (μm) | Pore Size (μm) | Loading Efficiency (%) | Gel Fraction (%) | Surface Area (m2/g) |
|---|---|---|---|---|---|
| Part L-Pore S | 818 ± 61 | 14 ± 6 | 80 ± 4 | 92 ± 1 | 7.9 ± 0.6 |
| Part L-Pore L | 833 ± 59 | 29 ± 12 | 78 ± 5 | 97 ± 2 | 4.7 ± 0.5 |
| Part S-Pore S | 391 ± 60 | 12 ± 6 | 82 ± 6 | 91 ± 3 | 7.5 ± 0.9 |
| Part S-Pore L | 430 ± 58 | 29 ± 17 | 80 ± 8 | 94 ± 4 | 4.5 ± 0.1 |
3.2. Improved Loading Efficiency with In-Line Encapsulation
Encapsulation efficiency of the model protein, BSA-FITC, was determined in each composition by crushing and extracting the protein. In-line loading was achieved through incorporation of a concentrated protein solution as the internal droplet phase during formation of the primary emulsion. After injection into the external phase, the continuous phase was photopolymerized, trapping the protein within the porous structure of the microsphere. All compositions show high encapsulation efficiencies of ~ 80%, Table 1. It is important to note that the fabrication setup was optimized over several iterations to achieve this high encapsulation efficiency. Specifically, microsphere collection was adjusted to minimize flow of the external aqueous phase over microspheres that had already exited the tubing, minimizing undesirable washout of protein after cure. Overall, these studies demonstrated the ability to achieve high encapsulation efficiency of the model protein within a range of particle and pore diameters.
3.3. Effect of Microsphere Composition on Release Kinetics
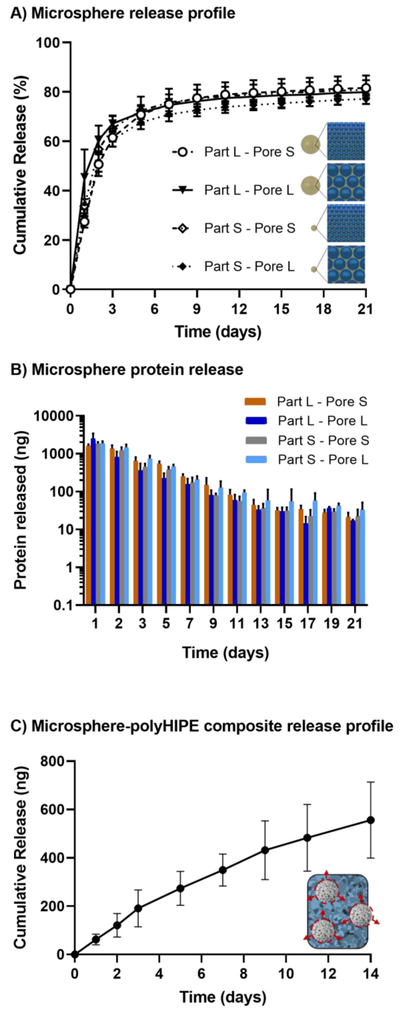
To study the role of several rate-controlling mechanisms on model protein release including surface area-to-volume ratio, tortuosity, and protein adsorption, the delivery profiles of polyHIPE microspheres were monitored over 21 days, Figure 3A, B. The effect of surface area-to-volume ratio on release kinetics was first assessed by fabricating two distinct sets of microspheres, each set containing a large and small pore size (15 vs 30 μm). Then, the effect of the microspheres size on the overall diffusion length was assessed by fabricating two distinct sets of microspheres, each set containing a large and small particle diameter (400 vs 800 μm). Microspheres exhibited a burst release of 60 – 70% of the protein during the first 3 days. After the first seven days the microspheres show an extended protein release between 20 – 100 nanograms up to 21 days. Unexpectedly, minimal differences in release kinetics were observed for the four model compositions tested. Despite significant effects on surface area, decreasing pore size did not significantly alter release kinetics within the 21 days period. Three replicate batches for each composition were tested and exhibited consistent release profiles with minimal batch variability, Supplemental Figure S4.
Figure 3.
Release profiles of BSA-FITC from microspheres (A) and respective protein release for each configuration (B). All data represents average ± standard deviation (3 batches, n = 12). Cumulative release from microsphere-polyHIPE composites (C). Microsphere-polyHIPE composites data represents average ± standard deviation (2 batches, n = 7)
The release kinetics of a microsphere-polyHIPE composite was analyzed to assess the potential of the microspheres to generate a bone graft with controlled release of osteoinductive factors. In contrast to the independent microsphere release profiles, protein release from the composite displayed minimal burst release and sustained release over a period of two weeks, Figure 3C and Supplemental Figure S5. It was hypothesized that the burst release from the microspheres was reduced by subsequent protein adsorption to the polyHIPE scaffold. This was supported by the rapid reduction of protein from a protein solution after a polyHIPE scaffold was placed into the protein solution, Supplemental Figure S6.
3.4. Bioactivity Retention of Encapsulated rhBMP-2
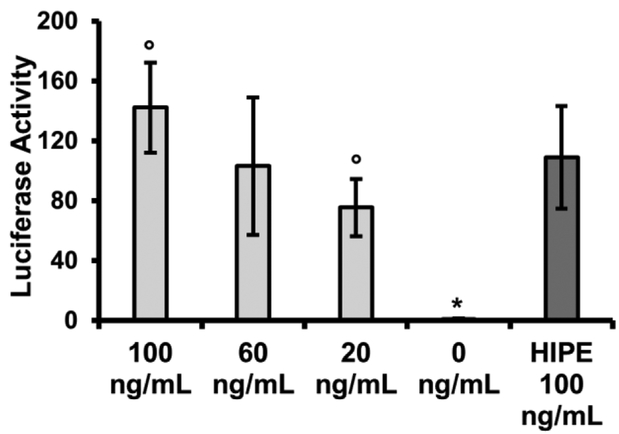
A significant step in the investigation of polyHIPE microspheres as a viable growth factor delivery system is to confirm growth factor loaded into the primary emulsion retains therapeutic activity after fabrication. To this end, a BMP responsive reporter cell (BRITER) was utilized as an initial test of bioactivity, Figure 4. These immortalized calvarial osteoblasts are modified with a BMP responsive dual luciferase reporter construct.[51] Upon exposure to exogenous BMP-2, these cells exhibit a prompt and robust response, allowing for rapid detection of BMP activity and a reduction in time needed to confirm bioactivity. This allows for rapid iteration on carrier property. Luciferase activity was first determined in response to selected concentrations of non-encapsulated rhBMP-2 and utilized to determine a reference activity profile. rhBMP-2 was then encapsulated in high internal phase emulsion and photopolymerized. Encapsulated factor was extracted from the scaffold over 4 days and exposed to reporter cells for 3h followed my measurement of luciferase activity. Protein concentration extracted from primary emulsions was determined using established CBQCA quantification assays and normalized to a concentration of 100 ng/mL. As shown in Figure 4, encapsulated rhBMP-2 retained ~60% activity compared to non-encapsulated BMP-2 of equal concentration. BRITER cells with no exogenous BMP-2 exposure were used as negative control and demonstrated negligible activity.
Figure 4.
Normalized luciferase activity of BRITER cell line treated with stock rhBMP-2 solutions or rhBMP-2 loaded polyHIPE releasate. All data represents average ± standard deviation for n = 4. The * represents significant difference (p<0.05) between the untreated control and all other rhBMP-2 treated samples. The ° represents significant difference (p<0.05) between samples treated with 100 ng/mL and 20 ng/mL rhBMP-2 stock solutions.
3.5. Osteoblastic Differentiation of hMSCs Induced by rhBMP-2 Release
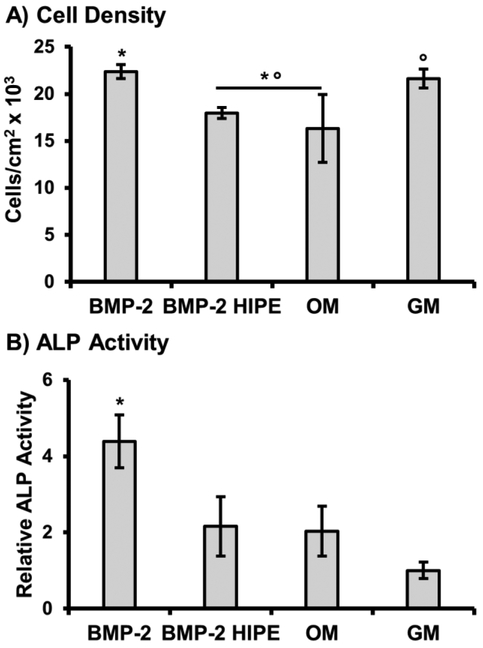
Next, an additional measure of bioactivity was performed to confirm that extracted rhBMP-2 could elicit osteoblastic differentiation of human mesenchymal stem cells. In this study, alkaline phosphatase activity was characterized as an early marker of osteoblastic differentiation, Figure 5. The upregulation of ALP, an enzyme active in mineral formation and dephosphorylation processes of osteoblasts, is a well-established response to exposure of exogenous BMPs.[9] hMSCs were cultured with rhBMP-2 encapsulated and released from polyHIPE scaffolds, with non-encapsulated rhBMP-2 or osteogenic media used as positive controls. Additionally, cells cultured in standard growth media without an osteoinductive agent were used as a negative control. After 2-week exposure, encapsulated rhBMP-2 promoted a similar osteoblastic response as hMSCs cultured in osteogenic (dexamethasone supplemented) conditions. Non-encapsulated rhBMP-2 had the highest activity with a ~4 fold increase over standard growth conditions. In addition, exposure of polyHIPE releasates had no deleterious effects on cell density during the culture period, confirming no toxic leachable are present after microsphere fabrication. Minor differences in density are attributed to changes in proliferation activity of hMSCs observed during osteoblastic differentiation.[52]
Figure 5.
Effect of rhBMP-2 loaded polyHIPE releasate on cell density (A) and alkaline phosphatase activity (B) of hMSCs cultured with releasate for 14 days. Cells were cultured in fresh solution of stock rhBMP-2 (BMP-2) and osteogenic media (OM) as positive control, and growth media (GM) as negative control. All data represents average ± standard deviation for n = 4. The * and ° represent significant difference (p<0.05) between BMP-2 or GM and indicated compositions for density or ALP activity.
3.6. Effect of Microsphere Incorporation on Composite PolyHIPE Properties
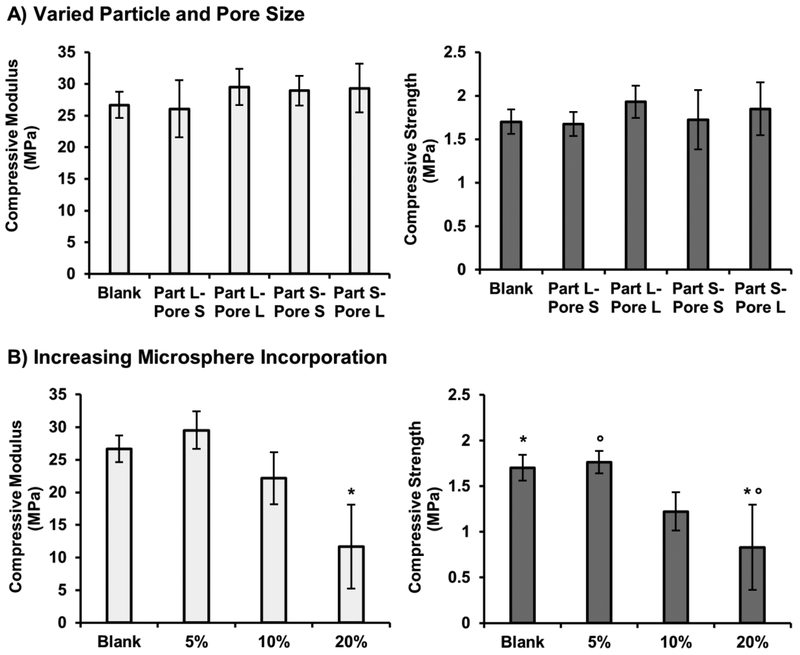
PolyHIPE scaffolds are unique in their ability to combine high porosities with uniform pore structures with mechanical properties that approach those of cancellous bone.[49, 50] Therefore, it is critical that any platform utilized to provide targeted bioactivity not compromise load bearing potential of the graft. To this end, the effect of microsphere incorporation on compressive modulus and strength of injectable scaffold was characterized as a function of microsphere structure and percent incorporation. An initial study of polyHIPE compressive modulus and strength was performed after adding 5 wt% of each of the four model compositions to assess potential effects of particle and pore size. Distribution of microspheres in polyHIPE scaffolds is illustrated in the supplemental information, Supplemental Figure S7. At this level of incorporation, no statistical differences were observed between the blank polyHIPE and the corresponding microsphere-loaded composition, Figure 6A. To further highlight the range of this system, additional studies were performed to identify the effect of increasing amounts of microsphere incorporation on compressive modulus and strength. Microspheres with surfactant concentration matched to that of the polyHIPE scaffold were added at 10 and 20 wt%, Figure 6B. A non-significant decrease in compressive properties was observed for the 10 wt% microsphere-polyHIPE composite and ~50% reduction in compressive properties was observed at 20 wt% incorporation.
Figure 6.
Effect of microsphere particle size and pore size on microsphere-polyHIPE composite scaffold compressive modulus and strength for 5 wt% incorporation using four model compositions (A). Effect of increasing microsphere incorporation (5, 10 and 20 wt%) on microsphere-polyHIPE composite scaffold compressive modulus and strength (B). All data represents average ± standard deviation for n = 3. The * and ° represent significant difference (p<0.05) for 20 wt% compressive modulus or strength and other indicated compositions.
4. Discussion
To establish polyHIPE microspheres as a platform for sustained delivery of bioactive factors, the role of particle size and architecture on encapsulation efficiency and release kinetics of the model protein, BSA-FITC, was investigated. A significant challenge to growth factor incorporation is elevated production costs associated with inefficient loading of therapeutics during the manufacturing process. Microsphere delivery systems fabricated using traditional emulsion-solvent evaporation methods often report encapsulation efficiencies below 70%.[53, 54] Other systems, such as post-loaded scaffolds soaked in concentrated growth factor solutions, require elevated soak times and growth factor concentrations.[55] Therefore, a fabrication method with the ability to efficiently load bioactive factor into delivery vehicles would overcome a significant barrier to clinical translation. These studies demonstrate the advantage of combining emulsion templating with controlled fluidics as growth factor can be in-line loaded directly into the microsphere vehicle during fabrication. It is hypothesized that the rapid polymerization of the prepolymer phase during fabrication trapped the in-line loaded protein, which combined with the lack of additional purification processes allowed for high encapsulation efficiency. The high loading efficiency was achieved for all microspheres configurations.
Achieving physiologically relevant delivery profiles of osteogenic factors is a critical design criterion that has yet to be sufficiently addressed in clinically available systems. Although some level of burst release can be desirable and initiate fracture healing, it has been demonstrated that sustained delivery of osteogenic factor is also needed to allow appropriate time for osteoprogenitor cell recruitment and differentiation.[22] Unlike traditional delivery of small, hydrophobic compounds, release of high molecular weight growth factors from polymeric systems most often proceeds through diffusion of the protein through water filled pores.[56] This work aimed to assess rate-controlling mechanisms over release kinetics of passive diffusion from porous microspheres. The use of a double emulsion, controlled fluids fabrication system, allowed for independent modulation of microsphere property and more systematic study of these mechanisms. Specifically, particle and pore diameter were modulated to investigate the effects of surface area-to-volume ratio, path length/tortuosity, and protein adsorption on release kinetics. SEM and BET analysis were first performed to demonstrate that microsphere property could be modulated to independently control these properties. As highlighted in Table 1 and Figure 2, we successfully reduced particle size and pore size by two-fold by changing the external phase velocity or surfactant concentration. We confirmed that the smaller pore sizes at higher surfactant concentration resulted in an increased surface area without impacting particle size. This level of architectural control provided the means to decouple the effect of these two variables on protein release.
It was originally hypothesized that pore size and particle diameter could serve as tools to modulate tortuosity, a unitless measure of path length through a porous medium relative to the end-to-end path length, and diffusion distance, ultimately allowing for control over protein release kinetics. Contrary to this hypothesis, we did not observe significant differences in release kinetics in response to modulating these properties. One possible reason is that the impact on the overall diffusion path was not large enough to impact diffusion through the highly interconnected porous architecture. Furthermore, the interconnect diameter of ~1 micron is approximately 10 times higher than the protein radius, which most likely failed to hinder protein transport through the pores.[57] Protein-material interactions have also been demonstrated as a potent rate-controlling mechanism in polymeric delivery systems.[58, 59] In addition to increased tortuosity, it was hypothesized that the larger surface area present in the particles with reduced pore sizes would result in greater protein adsorption and slower release kinetics. Although BET confirmed a 1.7X increase in surface area, microspheres displayed similar release kinetics which may be an indicator that protein adsorption did not substantially affect the release profile.
These studies were designed to provide an introductory investigation into the potential of polyHIPE microspheres as controlled delivery vehicles. Additional methods can be explored to further modulate release kinetics, if necessary. Given the slow degradation rates of EGDMA scaffolds, degradation-based release was not hypothesized to play significant role in these studies. However, selection of alternative biodegradable prepolymers fabricated with a potentially closed-pore architecture would allow for introduction of yet another rate-controlling mechanism, further enhancing the tunability of this system.[60] We have previously demonstrated that closed pore morphologies can be achieved in biodegradable systems by changing to an aqueous initiator.[61] In the current application, we developed the microspheres to serve as drug-depots within our injectable polyHIPE bone grafts. Multiple studies have reported on the ability to improve sustained release profiles of growth factor delivered from microsphere vehicles by imbedding into secondary scaffolds.[15, 39, 62] These studies have reported success in promoting osteogenic activity in vitro and in vivo. To this end, we demonstrated that embedding polyHIPE microspheres into an injectable polyHIPE or directly incorporating the protein into the polyHIPE scaffold resulted in reduced burst release and sustained release of protein from the composite scaffold, Figure 3. Although there exists the potential to load growth factor directly into our polyHIPE scaffold, this approach is not considered ideal as it would couple bioactive factor delivery to properties of the bulk scaffold. In contrast, implementing a separate microsphere delivery system into the injectable polyHIPE construct would allow for localized protein-eluting depots to encourage bone regeneration without negatively influencing bulk scaffold properties. This approach enables different microspheres populations with different dosages or growth factors to be loaded into the scaffold to enhance regeneration and tailor the graft to the needs of the patient.
A significant hurdle to translation of BMP-2 loaded scaffolds is developing a fabrication method that retains therapeutic activity of encapsulated factors. Processes that require extensive purification, exposure to heat, or interaction with toxic solvents are prone to reduce bioactivity retention.[63] Uniquely, the photopolymerization method utilized here crosslinks the continuous phase of the primary emulsion within minutes at room temperature and eliminates the need for additional purification. Additionally, the lack of organic solvents in this process provides the advantage of eliminating toxic leachables that may raise concerns over biocompatibility. We have previously demonstrated that polyHIPE scaffolds composed of EGDMA and other biodegradable macromers exhibit high cross-linking efficiencies, resulting in minimal unreacted macromer and high viability of hMSCs seeded on the scaffold surface.[49, 60] Furthermore, ongoing studies are investigating Pickering emulsions that utilize hydroxyapatite nanoparticles in lieu of classic surfactants to further mitigate concerns over components that may reduce bioactivity or cytocompatibility, and provide an additional mechanism to direct cell activity.[64, 65] This work investigated two methods to confirm retention of growth factor activity. First, a rapid throughput method based on genetically modified osteoblast reporter cells was utilized to rapidly assess bioactivity retention, followed by a functional hMSC differentiation protocol to confirm potential therapeutic efficacy. Although this study suggests that there is some reduction in bioactivity, the ability of encapsulated factor to promote measurable osteoblastic differentiation after release from polyHIPE scaffolds demonstrates the utility of the polyHIPE microspheres, Figures 4 and 5. Several mechanisms may explain the observed difference in bioactivity for factor released from polyHIPEs compared to stock rhBMP-2. Most likely, early degradation of the factor occurred during the incubation period utilized for protein extraction. Bone morphogenetic proteins have been shown to possess low stability with therapeutic half-life being a function of incubation conditions such as solvent, temperature, and time in solution.[66–68] Stabilizing saccharides such as trehalose and heparin are often utilized to improve bioactivity retention in microsphere delivery systems studied for bone tissue engineering. Trehalose, a non-reducing disaccharide, serves as thermal protectant and improves stability under lyophilization and storage conditions.[69] Furthermore, Zhao et al. and Bramono et al. demonstrated heparin, a sulfated polysaccharide, could be used to limit degradation and prolong half-life of BMP-2 in media, up to 20-fold in cases.[70, 71] To this end, current focus will be placed on extending therapeutic activity of released rhBMP-2 during duration of the targeted release profiles through investigation of stabilizing molecules.
Ideally, an injectable bone graft contains a porous architecture to facilitate cellular infiltration, and compressive properties sufficient to stabilize the injury and provide mechanical stimuli to encourage regeneration.[72, 73] It was hypothesized that providing similar chemistry and pore architecture to that of the polyHIPE monoliths would limit effects of microsphere incorporation on mechanical properties. The ability to incorporate varied microsphere compositions without significantly impacting scaffold mechanical properties, Figure 6A, is promising as it demonstrates we can independently tune release profiles without impacting scaffold integrity. It is noted that a reduction of mechanical properties was observed at elevated concentrations of microsphere incorporation. It is hypothesized that as the number of particles increased, non-uniform dispersion within the emulsion resulted in the formation of aggregates and limited proper integration with the surrounding, curing HIPE. Uniform dispersion within the scaffold is critical as it has been observed that a lack of interfacial interactions between scaffold and microsphere can result in a decrease of microsphere-polyHIPE composite mechanical properties.[39] Kempen et al. demonstrated that increasing amounts of both PLGA and poly(propylene fumarate) (PPF) microspheres added into a porous PPF scaffold resulted in a significant decrease in compressive properties. Although a decrease was observed with increasing incorporation of PPF microspheres, the availability of covalent, interfacial interactions between scaffold and microsphere of matching chemistry resulted in increased compressive properties over PLGA microspheres. From this, it is anticipated that if uniform dispersion within our scaffold is maintained, allowing for appropriate interfacial bonding between methacrylate functional groups of sphere and emulsion, mechanical effects will be reduced. Representative microsphere-polyHIPE composite scaffold architecture is provided in Supplemental Figure S7. Current studies are exploring modified double barrel syringes that allow for more homogenous mixing and distribution of microspheres in the injectable HIPE. Furthermore, it is not anticipated that 20 wt% microsphere incorporation will be needed to promote an osteogenic response. Microsphere incorporation ranging 1–10 wt% has been demonstrated suitable to promote new bone formation utilizing protein concentrations similar to those presented here.[40, 62, 74, 75] If a more robust response is desired, BMP-2 concentration loaded into the primary emulsion can be readily increased to improve activity as has been observed in similar microsphere delivery systems.[75]
5. Conclusions
The aim of this study was to improve the osteoinductive potential of polyHIPE grafts by utilizing a new polyHIPE microsphere-based growth factor delivery system and establish the key relationships between microsphere properties and resultant protein release kinetics. High loading efficiencies were achieved in all configurations. Although all microsphere configurations in the study exhibited a burst release on the first 3 days, incorporation of the microspheres or protein into the scaffold resulted in a sustained released for at least 14 days with minimal burst release. Bioactivity retention of the encapsulated BMP-2 growth after emulsion fabrication and photopolymerization processes was confirmed with demonstrated ability of the released BMP-2 to induce osteogenic differentiation of hMSCs. Finally, microsphere-polyHIPE composites were characterized to demonstrate that microsphere incorporation up to 5 wt% did not have deleterious effects on compressive properties. Overall, this investigation provides key insights critical to the design of an osteoinductive polyHIPE system capable of providing physiologically relevant delivery profiles.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
This manuscript describes a method for solvent-free fabrication of porous microspheres from high internal phase emulsions using a controlled fluids setup. The principles of emulsion templating and fluid dynamics provide exceptional control of particle size and pore architecture. In addition to the advantage of solvent-free fabrication, this method provides in-line loading of protein directly into the pores of the microspheres with high loading efficiencies. The incorporation of the protein-loaded microspheres within an injectable polyHIPE scaffold resulted in a sustained release of protein over a two-week period with minimal burst release. Retention of BMP-2 bioactivity and incorporation of microspheres with minimal effect on scaffold compressive properties highlights the potential of these new bone grafts.
ACKNOWLEDGEMENTS
Funding was provided by NIH R21 AR057531. Gabriel Rodriguez-Rivera was supported by the Ford Foundation Predoctoral Fellowship. The authors acknowledge Palsgaard USA for providing PGPR 4125. hMSCs were provided by the Texas A&M HSC COM Institute for Regenerative Medicine at Scott & White through a grant from NCRR of the NIH, Grant # P40RR017447. BRITER cells were generously provided by Dr. Daniel Alge of Texas A&M. The authors would like to thank Prof. Hugh Daigle and his lab for the use of the Micromeritics 3Flex gas adsorption equipment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
✯ Part of the Drug Delivery for Musculoskeletal Applications Special Issue, edited by Robert S. Hastings and Professor Johnna S. Temenoff.
Contributor Information
Michael Whitely, Email: whitely.michael@ymail.com.
Gabriel Rodriguez-Rivera, Email: gabriel_rodriguez@utexas.edu.
Christina Waldron, Email: cjwaldron@utexas.edu.
Sahar Mohiuddin, Email: sahar.mohiuddin@gmail.com.
Stacy Cereceres, Email: stacy.cereceres@gmail.com.
Nicholas Sears, Email: nicksears@gmail.com.
Nicholas Ray, Email: nick.ray@utexas.edu.
REFERENCES
- [1].Burchardt H, The biology of bone graft repair, Clinical orthopaedics and related research (174) (1983) 28–42. [PubMed] [Google Scholar]
- [2].Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT, Tissue engineering of bone: material and matrix considerations, JBJS 90 (2008) 36–42. [DOI] [PubMed] [Google Scholar]
- [3].Chen D, Zhao M, Mundy GR, Bone morphogenetic proteins, Growth factors 22(4) (2004) 233–241. [DOI] [PubMed] [Google Scholar]
- [4].Wozney JM, Rosen V, Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair, Clinical orthopaedics and related research (346) (1998) 26–37. [PubMed] [Google Scholar]
- [5].Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs), JBJS 85(8) (2003) 1544–1552. [DOI] [PubMed] [Google Scholar]
- [6].Deckers MM, Van Bezooijen RL, Van Der Horst G, Hoogendam J, van der Bent C, Papapoulos SE, Löwik CW, Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A, Endocrinology 143(4) (2002) 1545–1553. [DOI] [PubMed] [Google Scholar]
- [7].Fiedler J, Röderer G, Günther KP, Brenner RE, BMP‐2, BMP‐4, and PDGF‐bb stimulate chemotactic migration of primary human mesenchymal progenitor cells, Journal of cellular biochemistry 87(3) (2002) 305–312. [DOI] [PubMed] [Google Scholar]
- [8].Kanczler J, Oreffo R, Osteogenesis and angiogenesis: the potential for engineering bone, Eur Cell Mater 15(2) (2008) 100–114. [DOI] [PubMed] [Google Scholar]
- [9].Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S, BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop, Journal of Bone and Mineral Research 18(10) (2003) 1842–1853. [DOI] [PubMed] [Google Scholar]
- [10].Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V, BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing, Nature genetics 38(12) (2006) 1424. [DOI] [PubMed] [Google Scholar]
- [11].Bose S, Tarafder S, Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review, Acta biomaterialia 8(4) (2012) 1401–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee K, Silva EA, Mooney DJ, Growth factor delivery-based tissue engineering: general approaches and a review of recent developments, Journal of the Royal Society Interface 8(55) (2011) 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Young S, Wong M, Tabata Y, Mikos AG, Gelatin as a delivery vehicle for the controlled release of bioactive molecules, Journal of controlled release 109(1–3) (2005) 256–274. [DOI] [PubMed] [Google Scholar]
- [14].Li C, Vepari C, Jin H-J, Kim HJ, Kaplan DL, Electrospun silk-BMP-2 scaffolds for bone tissue engineering, Biomaterials 27(16) (2006) 3115–3124. [DOI] [PubMed] [Google Scholar]
- [15].Kempen DH, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, Dhert WJ, Yaszemski MJ, Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering, Biomaterials 29(22) (2008) 3245–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kretlow JD, Klouda L, Mikos AG, Injectable matrices and scaffolds for drug delivery in tissue engineering, Advanced drug delivery reviews 59(4–5) (2007) 263–273. [DOI] [PubMed] [Google Scholar]
- [17].McKay WF, Peckham SM, Badura JM, A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft), International orthopaedics 31(6) (2007) 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Burkus JK, Gornet MF, Dickman CA, Zdeblick TA, Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages, Clinical Spine Surgery 15(5) (2002) 337–349. [DOI] [PubMed] [Google Scholar]
- [19].Carragee EJ, Hurwitz EL, Weiner BK, A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned, The Spine Journal 11(6) (2011) 471–491. [DOI] [PubMed] [Google Scholar]
- [20].Uludag H, D’Augusta D, Palmer R, Timony G, Wozney J, Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model, Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials 46(2) (1999) 193–202. [DOI] [PubMed] [Google Scholar]
- [21].Seeherman H, Wozney JM, Delivery of bone morphogenetic proteins for orthopedic tissue regeneration, Cytokine & growth factor reviews 16(3) (2005) 329–345. [DOI] [PubMed] [Google Scholar]
- [22].Cho TJ, Gerstenfeld LC, Einhorn TA, Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing, Journal of Bone and Mineral Research 17(3) (2002) 513–520. [DOI] [PubMed] [Google Scholar]
- [23].Dimitriou R, Tsiridis E, Giannoudis PV, Current concepts of molecular aspects of bone healing, Injury 36(12) (2005) 1392–1404. [DOI] [PubMed] [Google Scholar]
- [24].Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, Wozney JM, Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone, Proceedings of the National Academy of Sciences 87(24) (1990) 9843–9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Urist MR, Bone: formation by autoinduction, Science 150(3698) (1965) 893–899. [DOI] [PubMed] [Google Scholar]
- [26].Uludag H, Gao T, Porter TJ, Friess W, Wozney JM, Delivery systems for BMPs: factors contributing to protein retention at an application site, JBJS 83(1_suppl_2) (2001) S128–S135. [PubMed] [Google Scholar]
- [27].Vo TN, Kasper FK, Mikos AG, Strategies for controlled delivery of growth factors and cells for bone regeneration, Advanced drug delivery reviews 64(12) (2012) 1292–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brazel CS, Peppas NA, Mechanisms of solute and drug transport in relaxing, swellable, hydrophilic glassy polymers, Polymer 40(12) (1999) 3383–3398. [Google Scholar]
- [29].Jeon O, Powell C, Solorio LD, Krebs MD, Alsberg E, Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels, Journal of Controlled Release 154(3) (2011) 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bhakta G, Rai B, Lim ZX, Hui JH, Stein GS, van Wijnen AJ, Nurcombe V, Prestwich GD, Cool SM, Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2, Biomaterials 33(26) (2012) 6113–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Crouzier T, Sailhan F, Becquart P, Guillot R, Logeart-Avramoglou D, Picart C, The performance of BMP-2 loaded TCP/HAP porous ceramics with a polyelectrolyte multilayer film coating, Biomaterials 32(30) (2011) 7543–7554. [DOI] [PubMed] [Google Scholar]
- [32].Kuboki Y, Takita H, Kobayashi D, Tsuruga E, Inoue M, Murata M, Nagai N, Dohi Y, Ohgushi H, BMP-induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: topology of osteogenesis, Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and the Australian Society for Biomaterials 39(2) (1998) 190–199. [DOI] [PubMed] [Google Scholar]
- [33].Crotts G, Park TG, Protein delivery from poly (lactic-co-glycolic acid) biodegradable microspheres: release kinetics and stability issues, Journal of microencapsulation 15(6) (1998) 699–713. [DOI] [PubMed] [Google Scholar]
- [34].Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJ, Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration, Biomaterials 30(14) (2009) 2816–2825. [DOI] [PubMed] [Google Scholar]
- [35].Arnold MM, Gorman EM, Schieber LJ, Munson EJ, Berkland C, NanoCipro encapsulation in monodisperse large porous PLGA microparticles, Journal of Controlled Release 121(1–2) (2007) 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yang Y, Bajaj N, Xu P, Ohn K, Tsifansky MD, Yeo Y, Development of highly porous large PLGA microparticles for pulmonary drug delivery, Biomaterials 30(10) (2009) 1947–1953. [DOI] [PubMed] [Google Scholar]
- [37].Cai Y, Chen Y, Hong X, Liu Z, Yuan W, Porous microsphere and its applications, International journal of nanomedicine 8 (2013) 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gentile P, Chiono V, Carmagnola I, Hatton PV, An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering, International journal of molecular sciences 15(3) (2014) 3640–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kempen DH, Lu L, Kim C, Zhu X, Dhert WJ, Currier BL, Yaszemski MJ, Controlled drug release from a novel injectable biodegradable microsphere/scaffold composite based on poly (propylene fumarate), Journal of Biomedical Materials Research Part A 77(1) (2006) 103–111. [DOI] [PubMed] [Google Scholar]
- [40].Brown KV, Li B, Guda T, Perrien DS, Guelcher SA, Wenke JC, Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release, Tissue Engineering Part A 17(13–14) (2011) 1735–1746. [DOI] [PubMed] [Google Scholar]
- [41].Cleland JL, Powell MF, Shire SJ, The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation, Critical reviews in therapeutic drug carrier systems 10(4) (1993) 307–377. [PubMed] [Google Scholar]
- [42].Schliephake H, Application of bone growth factors—the potential of different carrier systems, Oral and maxillofacial surgery 14(1) (2010) 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Moglia R, Whitely M, Brooks M, Robinson J, Pishko M, Cosgriff-Hernandez E, Solvent-Free Fabrication of polyHIPE Microspheres for Controlled Release of Growth Factors, Macromolecular rapid communications 35(14) (2014) 1301–1305. [DOI] [PubMed] [Google Scholar]
- [44].Freiberg S, Zhu X, Polymer microspheres for controlled drug release, International journal of pharmaceutics 282(1–2) (2004) 1–18. [DOI] [PubMed] [Google Scholar]
- [45].Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R, Controlled delivery systems for proteins based on poly (lactic/glycolic acid) microspheres, Pharmaceutical research 8(6) (1991) 713–720. [DOI] [PubMed] [Google Scholar]
- [46].Santoro M, Tatara AM, Mikos AG, Gelatin carriers for drug and cell delivery in tissue engineering, Journal of controlled release 190 (2014) 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tan H, Huang D, Lao L, Gao C, RGD modified PLGA/gelatin microspheres as microcarriers for chondrocyte delivery, Journal of Biomedical Materials Research Part B: Applied Biomaterials 91(1) (2009) 228–238. [DOI] [PubMed] [Google Scholar]
- [48].Carnachan RJ, Bokhari M, Przyborski SA, Cameron NR, Tailoring the morphology of emulsion-templated porous polymers, Soft Matter 2(7) (2006) 608–616. [DOI] [PubMed] [Google Scholar]
- [49].Moglia RS, Whitely M, Dhavalikar P, Robinson J, Pearce H, Brooks M, Stuebben M, Cordner N, Cosgriff-Hernandez E, Injectable Polymerized High Internal Phase Emulsions with Rapid in Situ Curing, Biomacromolecules 15(8) (2014) 2870–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Moglia RS, Holm JL, Sears NA, Wilson CJ, Harrison DM, Cosgriff-Hernandez E, Injectable PolyHIPEs as High-Porosity Bone Grafts, Biomacromolecules 12(10) (2011) 3621–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yadav PS, Prashar P, Bandyopadhyay A, BRITER: a BMP responsive osteoblast reporter cell line, PloS one 7(5) (2012) e37134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lian JB, Stein GS, Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation, Critical Reviews in Oral Biology & Medicine 3(3) (1992) 269–305. [DOI] [PubMed] [Google Scholar]
- [53].Yang Y-Y, Chia H-H, Chung T-S, Effect of preparation temperature on the characteristics and release profiles of PLGA microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method, Journal of Controlled Release 69(1) (2000) 81–96. [DOI] [PubMed] [Google Scholar]
- [54].Kirby GT, White LJ, Rahman CV, Cox HC, Qutachi O, Rose FR, Hutmacher DW, Shakesheff KM, Woodruff MA, PLGA-based microparticles for the sustained release of BMP-2, Polymers 3(1) (2011) 571–586. [Google Scholar]
- [55].Geiger M, Li R, Friess W, Collagen sponges for bone regeneration with rhBMP-2, Advanced drug delivery reviews 55(12) (2003) 1613–1629. [DOI] [PubMed] [Google Scholar]
- [56].Fredenberg S, Wahlgren M, Reslow M, Axelsson A, The mechanisms of drug release in poly (lactic-co-glycolic acid)-based drug delivery systems—a review, International journal of pharmaceutics 415(1–2) (2011) 34–52. [DOI] [PubMed] [Google Scholar]
- [57].Deen W, Hindered transport of large molecules in liquid-filled pores, AIChE Journal 33(9) (1987) 1409–1425. [Google Scholar]
- [58].Crotts G, Sah H, Park TG, Adsorption determines in-vitro protein release rate from biodegradable microspheres: quantitative analysis of surface area during degradation, Journal of controlled release 47(1) (1997) 101–111. [Google Scholar]
- [59].Mumcuoglu D, de Miguel L, Jekhmane S, Siverino C, Nickel J, Mueller TD, van Leeuwen JP, van Osch GJ, Kluijtmans SG, Collagen I derived recombinant protein microspheres as novel delivery vehicles for bone morphogenetic protein-2, Materials Science and Engineering: C 84 (2018) 271–280. [DOI] [PubMed] [Google Scholar]
- [60].Whitely ME, Robinson JL, Stuebben MC, Pearce HA, McEnery MA, Cosgriff-Hernandez E, Prevention of Oxygen Inhibition of PolyHIPE Radical Polymerization Using a Thiol-Based Cross-Linker, ACS biomaterials science & engineering 3(3) (2017) 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Robinson JL, Moglia RS, Stuebben MC, McEnery MA, Cosgriff-Hernandez E, Achieving interconnected pore architecture in injectable polyHIPEs for bone tissue engineering, Tissue Engineering Part A 20(5–6) (2014) 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li B, Yoshii T, Hafeman AE, Nyman JS, Wenke JC, Guelcher SA, The effects of rhBMP-2 released from biodegradable polyurethane/microsphere composite scaffolds on new bone formation in rat femora, Biomaterials 30(35) (2009) 6768–6779. [DOI] [PubMed] [Google Scholar]
- [63].van de Weert M, Hennink WE, Jiskoot W, Protein instability in poly (lactic-co-glycolic acid) microparticles, Pharmaceutical research 17(10) (2000) 1159–1167. [DOI] [PubMed] [Google Scholar]
- [64].Hu Y, Zou S, Chen W, Tong Z, Wang C, Mineralization and drug release of hydroxyapatite/poly (l-lactic acid) nanocomposite scaffolds prepared by Pickering emulsion templating, Colloids and Surfaces B: Biointerfaces 122 (2014) 559–565. [DOI] [PubMed] [Google Scholar]
- [65].Fujii S, Okada M, Furuzono T, Hydroxyapatite nanoparticles as stimulus-responsive particulate emulsifiers and building block for porous materials, Journal of colloid and interface science 315(1) (2007) 287–296. [DOI] [PubMed] [Google Scholar]
- [66].Hsieh C-Y, Hsieh H-J, Liu H-C, Wang D-M, Hou L-T, Fabrication and release behavior of a novel freeze-gelled chitosan/γ-PGA scaffold as a carrier for rhBMP-2, Dental Materials 22(7) (2006) 622–629. [DOI] [PubMed] [Google Scholar]
- [67].Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, Ogamo A, Kamijo R, Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins, Journal of Biological Chemistry 278(44) (2003) 43229–43235. [DOI] [PubMed] [Google Scholar]
- [68].Seto SP, Miller T, Temenoff JS, Effect of selective heparin desulfation on preservation of bone morphogenetic protein-2 bioactivity after thermal stress, Bioconjugate chemistry 26(2) (2015) 286–293. [DOI] [PubMed] [Google Scholar]
- [69].Zhao J, Wang S, Bao J, Sun X, Zhang X, Zhang X, Ye D, Wei J, Liu C, Jiang X, Trehalose maintains bioactivity and promotes sustained release of BMP-2 from lyophilized CDHA scaffolds for enhanced osteogenesis in vitro and in vivo, PLoS One 8(1) (2013) e54645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bramono DS, Murali S, Rai B, Ling L, Poh WT, Lim ZX, Stein GS, Nurcombe V, Van Wijnen AJ, Cool SM, Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2), Bone 50(4) (2012) 954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhao B, Katagiri T, Toyoda H, Takada T, Yanai T, Fukuda T, U.-i. Chung, T. Koike, K. Takaoka, R. Kamijo, Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2, Journal of Biological Chemistry 281(32) (2006) 23246–23253. [DOI] [PubMed] [Google Scholar]
- [72].Karageorgiou V, Kaplan D, Porosity of 3D biomaterial scaffolds and osteogenesis, Biomaterials 26(27) (2005) 5474–5491. [DOI] [PubMed] [Google Scholar]
- [73].Kretlow JD, Young S, Klouda L, Wong M, Mikos AG, Injectable biomaterials for regenerating complex craniofacial tissues, Advanced Materials 21(32–33) (2009) 3368–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lee JW, Kang KS, Lee SH, Kim J-Y, Lee B-K, Cho D-W, Bone regeneration using a microstereolithography-produced customized poly (propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorporating BMP-2 loaded PLGA microspheres, Biomaterials 32(3) (2011) 744–752. [DOI] [PubMed] [Google Scholar]
- [75].Kempen DH, Kruyt MC, Lu L, Wilson CE, Florschutz AV, Creemers LB, Yaszemski MJ, Dhert WJ, Effect of autologous bone marrow stromal cell seeding and bone morphogenetic protein-2 delivery on ectopic bone formation in a microsphere/poly (propylene fumarate) composite, Tissue Engineering Part A 15(3) (2008) 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.