Abstract
The transcription factor Helios is expressed in a large percentage of Foxp3+ regulatory T cells (Tregs) and is required for the maintenance of their suppressive phenotype as mice with a selective deficiency of Helios in Tregs spontaneously develop autoimmunity. However, mice with a deficiency of Helios in all T cells do not exhibit autoimmunity, despite the defect in the suppressor function of their Treg population, suggesting that Helios also functions in non-Tregs. While Helios is expressed in a small subset of CD4+Foxp3− and CD8+ T cells and its expression is upregulated upon T cell activation, its function in non-Treg cells remains unknown. To examine the function of Helios in CD4+Foxp3− T cells, we transferred Helios-sufficient or -deficient naïve CD4+Foxp3− TCR transgenic T cells to normal recipients and examined their capacity to respond to their cognate antigen. Surprisingly, Helios-deficient CD4+ T cells expanded and differentiated into Th1 or Th2 cytokine producing effectors in a manner similar to wild type TCR transgenic CD4+ T cells. However, the primed Helios-deficient cells failed to expand upon secondary challenge with antigen. The tolerant state of the Helios-deficient memory T cells was not cell intrinsic, but was due to a small population of Helios-deficient naïve T cells that had differentiated into antigen-specific peripheral Tregs that suppressed the recall response in an antigen-specific manner. These findings demonstrate that Helios plays a role in the determination of CD4+ T cell fate.
Introduction
Regulatory T (Treg) cells are critical negative regulators of immune activation with important roles in maintaining self-tolerance as well as tolerance to commensal microbiota. Treg cells, defined by the expression of the master regulator Foxp3, can arise in the thymus in response to strong signals to self-peptide MHC complexes presented by thymic epithelial cells, but are also capable of differentiating from naïve T cells in vitro when activated in the presence of TGFβ and IL-2 (1-3) and in the periphery in vivo under various conditions (4-7). The relative composition and functional importance of thymically-derived Treg (tTreg) cells and peripherally derived Treg (pTreg) cells in maintaining immune tolerance is unknown and remains a topic of current investigation.
The transcription factor Helios, a member of the Ikaros family of zinc finger proteins, is noted for its expression in Treg cells (approximately 70% of Treg) and we have previously proposed that Helios expression differentiates tTreg from pTreg cells (8). Moreover, using Foxp3/Helios double reporter mice, we have recently demonstrated that that Helios− Treg are not precursors to Helios+ Treg, but are a separate population with a distinct gene expression profile and TCR repertoire (9). We also demonstrated that Helios− Treg are more unstable, more readily lose Foxp3 expression under lymphopenic conditions, and have reduced suppressive function against autoreactive T cell responses. The functional role of Helios in Helios+ Treg is to impart stability on Tregs in the activated, effector state (10). Mice with a Foxp3-Cre driven deletion of Helios (lkzf2) develop slowly progressive autoimmune disease characterized by splenomegaly, an increased number of B cell follicles, and pan-hypergammaglobulinemia, demonstrating a critical role for the transcription factor in maintaining certain features of Treg cell suppressive function, particularly T follicular regulatory cells. (10, 11). However, Kim et al. concluded that Treg were unstable due to a disruption of the IL-2 STAT5 axis in these mice. We also observed a defect in effector Treg stability in these mice, but concluded that the defect was a result of decreased Bcl2 expression. We have been unable to show any differences in the STAT5 pathway in Helios-deficient Treg (unpublished observations), and the mechanism by which Helios maintains stability in Treg remains poorly defined.
In contrast, mice with a CD4-Cre driven deletion of lkzf2 lack an autoimmune phenotype (8) (and unpublished observations), an unexpected result, given that these mice lack Helios-sufficient Treg cells. However, Helios expression can be detected in approximately 5% of CD4+Foxp3− T cells in wild type (WT) C57BL/6 mice. These observations led us to hypothesize that expression of Helios in CD4+Foxp3− T cells might play a requisite role in initiating or maintaining autoimmune activation. In fact, several studies have proposed that the expression of Helios is related to the activation state or to the strength of the antigenic signal delivered, both for Treg and also for all CD4+ cells (12, 13).
The major goal of this study was to characterize the functional role of Helios in the activation and differentiation CD4+Foxp3− T cells using lkzf2fl/fl × CD4-Cre mice. In agreement with a previous study (14), we found that the expression of Helios is associated with, but not required for, the initial expansion and differentiation following antigen-specific priming in vivo. Surprisingly, we found that antigen-specific Helios deficient CD4+ T cells expanded and differentiated into cytokine producing effectors in a manner similar to WT CD4+ T cells. However, these cells then became tolerant and would not respond to restimulation in vivo. The tolerant state was cell extrinsic and was mediated by a small number of antigen-specific pTreg. The suppressive function of these pTreg was antigen-specific as they only inhibited responses to their cognate antigen, but not to an unrelated antigen. Taken together, our experiments suggest that the upregulation of Helios expression during naïve T cell activation controls the ratio of effector cells and pTreg with critical consequences for the robustness of the memory cell response. This enhanced propensity of Helios deficient CD4+ T cells to develop into pTreg may result in an increase in pTreg specific for self-antigens in lkzf2fl/fl × CD4-Cre mice which protect them from the development of autoimmune disease, even in the presence of defective Helios-deficient tTreg.
Material and Methods
Animals
C57BL/6 mice were purchased from Charles River. We previously generated Ikzf2fl/fl mice (8). These mice were crossed to CD4Cre mice, obtained by The National Institute of Allergy and Infectious Diseases (NIAID) and maintained under contract by Taconic Biosciences (Germantown, NY), to generate Ikzf2fl/fl × CD4Cre mice. Ikzf2fl/fl × CD4Cre mice were then crossed to OT-II × Foxp3GFP mice to generate OT-II-Foxp3GFP × Ikzf2fl/fl -CD4Cre mice. OT-II TCR transgenic mice and Foxp3GFP reporter mice were also obtained by (NIAID) and maintained under contract by Taconic Biosciences (Germantown, NY). Congenic F1 SAP−/− mice were kindly provided by Dr. Pam Schwartzberg (NIAID, NIH). LCMV GP61-80-specific TCR transgenic SMARTA mice (15) were originally obtained from the La Jolla Institute of Allergy and Immunology and then crossed at NIH to RAG1−/− mice that were maintained for NIAID under contract by Taconic Biosciences (Germantown, NY). SMARTA × RAG−/− mice were further crossed in our lab to B6.SJL (CD45.1) mice. B6.SJL, RAG1−/−, B6.SJL OT-II RAG1−/−, and C57BL/6J × B6.SJL F1 mice were obtained by NIAID and were maintained by Taconic Biosciences (Germantown, NY). In all experiments, both male and female mice were used. All animal protocols used in this study were approved by the NIAID Animal Care and Use Committee.
Antibodies and reagents
The following staining reagents were used: APC eFluor780 anti-CD4 (RM4-5) Pacific Blue anti-CD4 (RM4-5), APC anti-Helios (22F6), FITC anti-Foxp3 (FJK-16s), e450 anti-Foxp3 (FJK-16s), Alexa Fluor 700 anti-B220 (RA3.6B2), APC anti-Fas (15A7), e450 anti-B220 (RA3.6B2), APC anti-PD-1 (J43), APC eFluor 780 Fixable Viability Dye, and FITC anti-Helios (22F6) were purchased from eBioscience (San Diego, CA). APC anti-CD45RB (C636-16A), Alexa Fluor 700 anti-CD45.2 (104), PerCP Cy5.5 anti-CD45.1 (A20), BV510 anti-CD44 (IM7), BV421 anti-CXCR5 (L138D7), APC anti-CD45.1 (A20), BV650 anti-CD25 (PC61), BV421 anti-CD4 (RM4-5), BV510 anti-IgD (11-26c.2a), PE anti-GL7 (GL7), e450 anti-B220 (RA3.6B2), APC anti-CD44 (IM7), FITC anti-CD8 (S3-6.7), Fire anti-CD4 (RM4-5), BV421 anti-IFNγ (XMG1.2), PE anti-IL4 (11B11), PE anti-CD4 (RM4-5), PE-Cy7 anti-CD4 (RM4-5), PE anti-CD69 (H1.2F3), and PE-Cy7 anti-CD279 (29F.1A12) were purchased from BioLegend (San Diego, CA). APC anti-CD62L (MEL14) and PE anti-CD44 (IM7) were purchased from BD Biosciences (San Jose, CA). PerCP eFluor 710 Annexin V and PE-Cy7 Ki-67 (SolA15) were purchased from Invitrogen (Waltham, Massachusetts). The eBioscience Foxp3/Transcription Factor Staining Buffer Set was used for intracellular staining. Cells were cultured in complete RPMI (cRPMI: RPMI 1640 supplemented with 10% heat-inactivated FCS, 100 μg/ml penicillin, 100 μg/ml streptomycin. 2mM L-glutamine, 10mM HEPES, 0.1mM nonessential amino acids, 1mM sodium pyruvate, and 50 μM 2-ME). LCMV peptide GP66-77 was made in house (Research Technologies Branch-Peptide Synthesis and Analysis Unit, NIAID). Ovalbumin protein and peptide (323-339) were obtained from Invivogen (San Diego, California). Sheep red blood cells were obtained from Lampire Biological (Everett, Pennsylvania). Anti-OVA Ig ELISA kits were obtained from Chondrex (Redmond, Washington).
Adoptive transfer and immunization
For adoptive transfer experiments 0.25 - 1 × 106 FACS sorted CD4+CD44loCD45RBhi Foxp3-GFP− T cells from Ikzf2fl/fl CD4Cre × OT-II-Foxp3GFP or control CD4Cre × OT-II Foxp3GFP mice were injected r.o. into C57BL/6J × B6.SJL F1 recipients or SAP−/− F1 recipients as indicated. The following day, recipient mice were immunized s.c. with 25 μg OVA in CFA to each flank or mice were immunized i.p. with 100 μg OVA in alum. In some experiments, as indicated, s.c. immunizations to each flank were administered again on day 10 with 25 μg OVA in IFA or 25 μg OVA and 10 μg LCMV peptide (GP66-77) in IFA. In some experiments, recipient mice received naïve CD45.1+ OT-II RAG1−/− or CD45.1+ SMARTA RAG1−/− cells on d9 before the second immunization on day 11. For sheep red blood cell immunizations 1-2 × 108 SRBCs were injected i.p. into either Ikzf2fl/fl × CD4Cre or Ikzf2fl/fl mice and spleens were harvested on day 7 post injection. For intracellular cytokine analysis, cells from the draining nodes were stimulated for 4h with Cell Stimulation Cocktail (eBioscience) prior to fixation and staining. For OVA specific IgG1 and IgE measurements, 100 microliters of blood were taken from a heart puncture on day 6 post immunization with OVA alum
Oral tolerance
Naïve CD4+Foxp3-GFP−CD44loCD45RBhi T cells were FACS sorted from Ikzf2fl/fl × CD4Cre OT-II Foxp3GFP or CD4Cre × OT-II Foxp3GFP mice (1 × 106) and injected r.o. into C57BL/6J × B6.SJL F1 recipients. The mice were given 1.5% ovalbumin supplemented water for 7days, changing the water every two days.
In vitro stimulation and survival analysis
1 × 106 FACS sorted CD4+Foxp3-GFP−CD44loCD45RBhi T cells from Ikzf2fl/fl × CD4Cre OT-II Foxp3GFP or CD4Cre × OT-II Foxp3GFP mice were transferred into C57BL/6J × B6.SJL F1 recipients and subsequently immunized with OVA/CFA in the same manner as described. Four days post immunization, draining lymph nodes were harvested and pooled from each transfer group and FACS sorted for the transferred CD45.2+ OTII cells. The cells were cultured for 24 hours in cRPMI with DC harvested from C57BL/6 mice at a 1:10 ratio in the absence or presence of 1uM OVA peptide. The cells were harvested after 24h and analyzed via flow cytometry for activation and cell survival.
In vitro iTreg conversion
3 × 105 FACS sorted CD4+CD25−CD44loCD45RBhi T cells from Ikz2fl/fl × CD4Cre or CD4Cre control mice were cultured in cRPMI with 3 × 104 DC and with reagents at the following concentrations, unless otherwise indicated: 1 μg/ml anti-CD3 (145-2C11), 2 μg/ml anti-IL-2 (S4B6), 50 U/ml rhIL-2, and 1 ng/ml TGFβ. After 3d, cells were stained with eBioscience Fixable Viability Dye eFluor 780 and the intracellular expression of Foxp3 and Helios was analyzed among live cells via flow cytometry.
Statistical Analysis
Prism 7.0 (GraphPad Software) was used to create graphs and perform statistical analysis. Statistical significance between two groups was determined by using a Student t test. P < 0.05 was considered to be significant.
Results
Helios expression in T cells
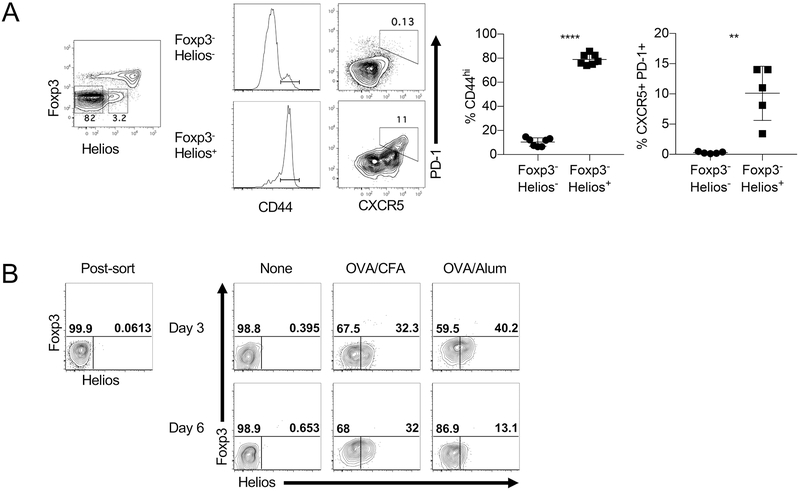
The transcription factor Helios is predominantly expressed in Foxp3+ Treg cells, but we have noted that a small percentage of CD4+Foxp3− cells in normal, unmanipulated mice also express Helios (Fig. 1A). Helios has been reported to be a general marker of activation (12, 13) and another report concluded that Helios was associated with, but not required, for Th2 and TFH differentiation. (14). Analysis of the CD4+Foxp3− cells revealed that the Helios+ cells were predominantly CD44hi while only a small percentage of Helios− cells expressed CD44 (Fig. 1A). Using the TFH markers CXCR5 and PD-1, the analysis indicated that the TFH cells were within the Helios+ population.
FIGURE 1.
Helios is associated with TM and TFH cells. (A) Splenocytes from 10 wk old mice were gated on CD4+ T cells and analyzed for Foxp3 and Helios expression. Foxp3−Helios− and Foxp3− Helios+ cells were analyzed for their expression of CD44 (left) and TFH markers CXCR5 and PD-1 (right). (B) Naive CD4+ T cells (GFP−CD44loCD45RBhi) were isolated from OT-II Foxp3GFP mice and adoptively transferred into congenic F1 recipients which were immunized the following day with the indicated adjuvants. Freshly sorted cells (left) and CD45.2+ cells gated from congenic hosts on days 3 and 6 post immunization (middle and right) were analyzed for expression of Foxp3 and Helios.
To formally test the association of Helios expression and activation, naïve T cells (CD4+GFP−CD44lo) (Supplemental Fig. 1A) were purified from OTII Foxp3-GFP mice and were adoptively transferred into congenic mice and then immunized. Immunization with ovalbumin peptide in either CFA or alum induced the expression of Helios in a subset of the transferred cells which remained Foxp3− (Fig. 1B). Together, these results confirm that Helios is associated with memory and TFH effector subsets and can be transiently induced in naïve T cells upon in vivo priming.
Ikzf2fl/fl × CD4-Cre mice lack an autoimmune phenotype
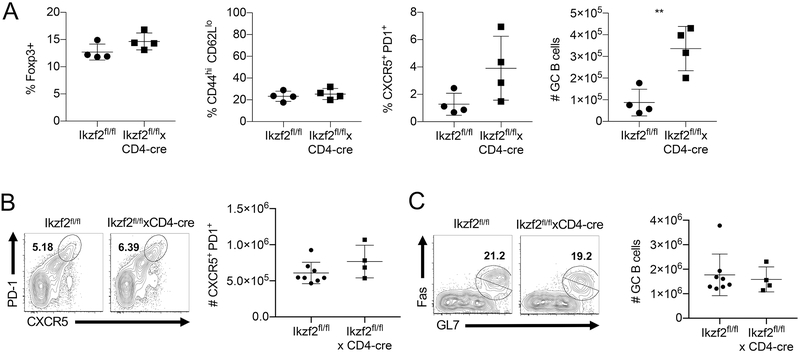
We have previously reported that mice with a Treg-specific deficiency of Helios (Ikzf2fl/fl × Foxp3-Cre) slowly develop a progressive immune activation and signs of systemic autoimmunity (10). However, we previously observed that when Ikzf2fl/fl mice were crossed to CD4-Cre mice, such that Helios is deleted from Treg cells, CD4+Foxp3− T cells and CD8+ cells, the resulting mice lacked the autoimmune phenotype observed in the Ikzf2fl/fl × Foxp3-Cre mice, even at one year of age. Further characterization of these mice revealed no differences in the absolute numbers of splenocytes (data not shown) or the percentage of Treg were observed in Ikzf2fl/fl × CD4-Cre mice (Fig 2A). While activated CD4+Foxp3− and CD8+ T cells could be identified as early as two months of age in Ikzf2fl/fl × Foxp3-Cre mice (10), the level of CD44hiCD62Llo cells remained similar to control mice in Ikzf2fl/fl × CD4-Cre mice (Fig 2A). In unprimed Ikzf2fl/fl × CD4-Cre mice, the percentage of TFH cells was slightly higher, but this increase was not statistically significant. We did note a small, statistically significant increase in the total number of germinal center (GC) B cells in the Ikzf2fl/fl × CD4-Cre mice. However, no differences were noted in the percentages of TFH (Fig. 2B) cells or the absolute number of GC B cells (Fig. 2C) upon immunization with sheep red blood cells (SRBC). Furthermore, histologically no lymphocytic infiltrates were observed in multiple non-lymphoid organs up to one year of age (data not shown). Taken together, it appears the lack of Helios expression in CD4+Foxp3− T cells inhibits their potential to induce autoimmunity in the presence of defective Helios-deficient Treg cells in Ikzf2fl/fl × CD4-Cre mice and that Helios upregulation in CD4+Foxp3− T cells is required for the sustained immune activation necessary for autoimmunity.
FIGURE 2.
Helios deficient mice do not display major defects. (A) Splenocytes from 4-6 mo old Ikzf2fl/fl and Ikzf2fl/fl × CD4Cre mice were gated on CD4+ T cells and analyzed for the frequency of Treg, TM, and TFH cells, as well as the number of GC B cells (B220+IgDloFas+GL7+). (B) Ikzf2fl/fl and Ikzf2fl/fl × CD4Cre mice were immunized i.p. with SRBC. Spleens were harvested 8d post immunization and analyzed for the expression of TFH markers among CD4+ Foxp3− T cells (C) and the number of GC B cells.
Helios is not required for the antigen-specific expansion and differentiation of CD4+Foxp3− T cells
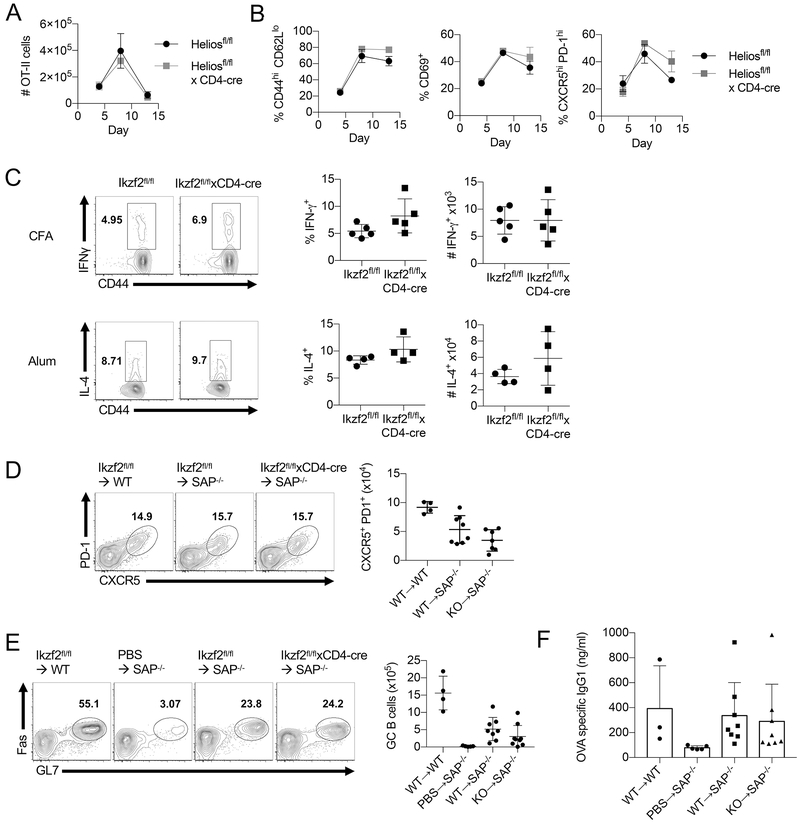
To dissect the role of Helios deficiency on naïve T cell activation, we employed an experimental adoptive transfer system in which TCR transgenic, Helios-deficient naïve CD4+ T cells were isolated and transferred into a congenic host followed by immunization with the cognate antigen. We crossed Ikzf2fl/fl × CD4Cre mice to OT-II Foxp3-GFP mice to create OT-II Foxp3GFP Ikzf2fl/fl × CD4Cre mice that were used as donors in our adoptive transfer system. Adoptively transferred Helios-sufficient and Helios-deficient OT-II cells proliferated comparably following immunization (Fig. 3A), and we saw no defect in the ability of Helios-deficient OT-II cells to become activated as measured by CD44, CD62L and CD69 expression (Fig. 3B). The ability of Helios-deficient OT-II cells to differentiate to functional Th1 and Th2 cells was determined by assessing the production of IFNγ and IL-4 after immunization with OVA and CFA or OVA and alum, respectively. Again, we found no defect in the Helios-deficient OT-II cells, as they produced levels of IFNγ and IL-4 comparable to Helios-sufficient cells (Fig. 3C). Upon immunization, Helios-deficient OT-II cells were also able to differentiate into a TFH effector phenotype (Fig. 3B). It is possible that Helios-deficient OT-II cells are able to express TFH cell markers while exhibiting a functional defect in providing B cell help. To test this possibility, Helios-sufficient or Helios-deficient OT-II cells were adoptively transferred into congenic SAP−/− mice, where the endogenous T cells are unable to form the long-lasting cell-to-cell contacts necessary to provide help to B cells (16). Adoptive transfer into these mice ensures that all B cell differentiation and class switch recombination is the result of the transferred SAP sufficient OT-II cells. Again, we observed no defect in the ability of Helios-deficient T cells to differentiate into TFH cells (Fig. 3D), induce the differentiation of germinal center B cells (Fig. 3E), or to provide help leading to the production of class-switched, OVA-specific antibodies (Fig. 3F). Thus, our results confirm the conclusions of Serre et al and suggest that the absence of Helios does not affect CD4+ T cell priming or effector differentiation.
FIGURE 3.
T cell activation is unaffected by Helios deficiency. (A) Purified naïve CD4+ T cells (GFP−CD44loCD45RBhi) from Ikzf2fl/fl × CD4Cre OT-II Foxp3GFP or control mice were adoptively transferred into congenic F1 recipients. Recipient mice were immunized s.c. the following day with OVA/CFA. Draining LNs were harvested on days 4, 8, and 13 post immunization and the total number of CD45.2+ OT-II cells and (B) the frequency of Teff, CD69+ and TFH cells among the OT-II cells was analyzed by FACS. (C) Naive OT-II cells were adoptively transferred into recipient mice immunized with OVA in CFA (s.c.) or OVA in alum (i.p.). The production of cytokines was assessed by ICS following a 4 h stimulation with PMA/Ionomycin. (D) Naïve OT-II cells were transferred into congenic SAP−/− mice which were immunized with OVA in alum i.p. the following day. Splenocytes were analyzed on d 8 post immunization for TFH cell differentiation of transferred OT-II cells and (E) GC B cell differentiation. (F) Serum was collected from SAP−/− mice on day 8, and OVA-specific IgG1 was measured by ELISA.
Helios is required for the expansion of T cells upon secondary stimulation in vivo
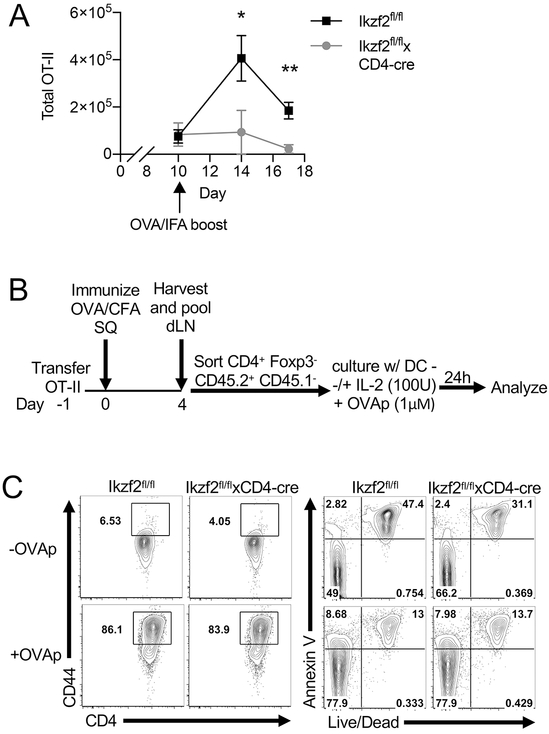
We next investigated the role of Helios in recall responses. Naïve OT-II T cells were adoptively transferred as before and the recipient mice were immunized with OVA in CFA. On day 10 post immunization, the mice were given a second immunization of OVA in IFA and the draining lymph nodes were collected 4 days later to analyze the proliferation of the transferred OT-II cells. The total number of Helios-deficient OT-II T cells was markedly reduced compared to the Helios-sufficient controls, indicating a critical role for Helios for the re-expansion of memory CD4+ T cells (Fig. 4A).
FIGURE 4.
Helios deficient T cells cannot be reactivated. (A) Naïve CD4+ T cells were purified from Ikzf2fl/fl × CD4Cre OT-II Foxp3GFP or control mice and adoptively transferred into congenic F1 recipients. Recipient mice were immunized s.c. the following day with OVA/CFA and boosted s.c. with OVA/IFA on d10 post immunization. Draining LNs were harvested on days 10 (before boost) and days 14 and 17, and the total number of CD45.2+ OT-II cells was analyzed by FACS. (B) Experimental outline of OT-II in vitro restimulation. Naïve OT-II cells were adoptively transferred into F1 congenic mice which were immunized as before. On d4, draining LNs were pooled and CD45.2+ OT-II cells were isolated by FACS and cultured with DCs with or without OVAp (323-339). (C) After 24 h OT-II cell culture was harvested and analyzed for cell activation and cell death by FACS.
One explanation for the failure of the Helios-deficient OT-II cells to expand is that they were more susceptible to activation induced cell death upon secondary challenge. In order to investigate the responses of the deficient cells after primary challenge, naïve OT-II CD4+ T cells were adoptively transferred as before and mice were immunized with OVA in CFA. On day 4 post immunization, the draining LNs were harvested, the transferred OT-II cells isolated and then re-stimulated in vitro as outlined (Fig. 4B). In vitro, Helios deficient T cells were activated comparably to WT cells, as measured by CD44 expression and did not show an increased proportion of apoptotic or nonviable cells as measured by staining with Annexin V and viability dye (Fig. 4C). Similar data were observed in the presence or absence of IL-2. The in vitro re-stimulation assay was also done on d7 post immunization, with no differences noted (data not shown). Thus, primed cells that lack Helios appear to be activated normally and do not exhibit enhanced apoptosis.
We also analyzed Helios-deficient OT-II T cells for various surface markers at day 10 post immunization, as well as at 4 days post boost. We were unable to find differences in the expression of Fas, OX-40, CD30, CD27, or CD120b. Furthermore, we found no difference in the expression of CD73 and FR4 which are reported to define anergic CD4+ T cells (17) (data not shown). Finally, we found no difference in expression of intracellular Bcl-2 or active caspase3, two regulators of apoptosis (data not shown). Thus, the lack of proliferation in Helios-deficient OT-II T cells upon restimulation was not cell intrinsic.
A small percentage of Helios deficient T cells convert into pTreg following priming in vivo
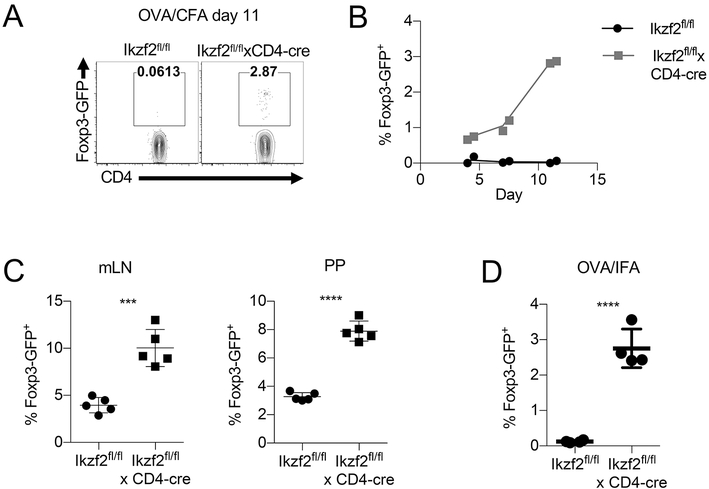
Since the response of primed Helios-deficient OT-II cells was normal on re-stimulation in vitro, the possibility remained that factors in the local environment in the draining lymph node prevented the expansion of the primed Helios-deficient OT-II cells. Since pTreg do not express Helios in normal mice, one possibility is that enhanced pTreg generation from Helios-deficient antigen-specific T cells might explain the defect in proliferation observed after secondary stimulation. To test this idea, we assessed the differentiation of naïve Helios-deficient OT-II cells into pTreg cells after adoptive transfer and immunization with OVA in CFA. Surprisingly, we observed an upregulation of Foxp3-GFP by a small, but consistent fraction of Helios-deficient OT-II cells on day 11 post immunization, but not by WT OT-II cells (Fig. 5A). A time course of adoptive transfer of OT-II cells followed by OVA CFA immunization revealed that Foxp3 upregulation could be observed among Helios deficient OT-II cells as early as day 4 post immunization with OVA/CFA and peaked in frequency on day 10, while Helios-sufficient OT-II cells exhibited virtually no upregulation of Foxp3 at any time point (Fig. 5B). We also examined the ability of Helios deficient CD4+ T cells to differentiate into pTreg in an oral tolerance model and following OVA/IFA immunization, two models that are known to induce pTregs (18-20). In the oral tolerance model, naïve OT-II T cells were adoptively transferred and mice were given ovalbumin in the drinking water. At d7 post-transfer, the transferred cells were analyzed for the conversion to Foxp3+ pTreg. In both the mLN and Peyer’s patches (PP) a small percentage of WT cells converted to pTreg, as expected (Fig. 5C). Moreover, a higher percentage of Helios- deficient cells converted to pTreg. For the IFA model, naïve OT-II CD4+ T cells were adoptively transferred and the mice were immunized with OVA/IFA. As with OVA/CFA, pTreg were generated from Helios-deficient cells, but not from WT cells (Fig. 5D). Therefore, Helios appears to control fate decision of CD4+ T cells and the presence of pTreg generated from Helios deficient OT-II cells could explain the lack of proliferation observed after secondary stimulation.
FIGURE 5.
Helios deficient OT-II cells differentiate into pTregs. (A) Naïve CD4+ T cells from Ikzf2fl/fl × CD4Cre OT-II Foxp3GFP or control mice were adoptively transferred into congenic F1 recipients. Recipient mice were immunized s.c. the following day with OVA/CFA. Draining LNs were harvested on d11 or (B) the indicated days after immunization, gated on CD45.2+ OT-II cells and analyzed for Foxp3 expression. (C) Naïve OT-II T cells were adoptively transferred into congenic F1 recipients. Recipient mice were given OVA in drinking water ad lib. for 7 d. Foxp3 expression in CD45.2+ OT-II cells was measured in mLN and PP. (D) Naïve OT-II cells were adoptively transferred as in (A) and recipient mice were immunized s.c. the following day with OVA/IFA. Foxp3 expression in CD45.2+ OT-II cells was analyzed from the dLN on day 7.
Increased pTreg conversion is not due to differential sensitivity to TGFβ, IL-2 or TCR stimulation in vitro
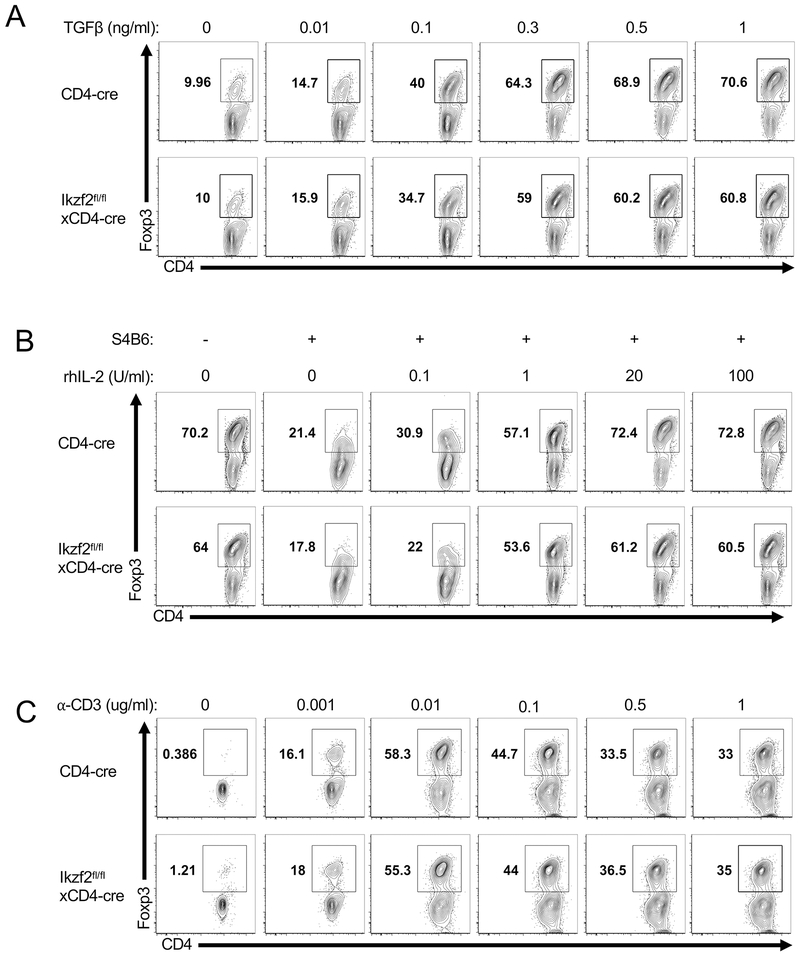
The differentiation of naïve T cells to Treg cells requires activation in the presence of TGFβ and IL-2 (1, 3). To test if the increased in vivo conversion of Helios-deficient OT-II T cells into Treg cells was due to increased sensitivity to TGFβ signaling, we performed a series of in vitro iTreg differentiation assays with titrated concentrations of TGFβ. We compared the capacity of WT CD4+Foxp3− and Helios-deficient CD4+Foxp3− naive T cells to differentiate to Foxp3+ Treg in vitro following stimulation with dendritic cells and soluble anti-CD3 in the presence of IL-2 for 4 days (Fig. 6A). Although Foxp3 expression increased with increasing concentrations of TGFβ, naïve polyclonal CD4 T cells isolated from Ikzf2fl/fl × CD4Cre mice did not show an increased ability to differentiate into iTreg cells at any concentration of TGFβ (Fig. 6A).
FIGURE 6.
Helios deficient CD4 T cells are not differentially sensitive to TGFβ, IL-2 or TCR stimulus. Purified naïve CD4+ T cells (CD25−CD44loCD45RBhi) were cultured with DCs and either (A) IL-2 plus the indicated concentrations of TGFβ or (B) neutralizing anti-mouse IL-2 (S4B6), TGFβ and the indicated concentrations of rhIL-2 or (C) neutralizing anti-mouse IL-2 (S4B6), rhIL-2, TGFβ and the indicated concentrations of soluble α-CD3. Foxp3 expression was analyzed among viable cells on d3.
We additionally tested the ability of Helios deficient naïve T cells to differentiate into iTreg cells under conditions with limited IL-2 availability or limited TCR stimulation by titrating the concentration of IL-2 or anti-CD3, respectively. Again, we were unable to find any concentration of IL-2 (Fig. 6B) or anti-CD3 (Fig. 6C) where Helios-deficient T cells differentiated into iTreg cells with increased frequency compared to their WT counterparts. Together, these results suggest that the observed increase of pTreg conversion by Helios-deficient OT-II cells is not due to an increased sensitivity to TGFβ, IL-2 or TCR signals. However, it remains possible that one of these factors regulates pTreg differentiation in vivo and we are unable to mimic the in vivo situation in vitro or that other unknown variables might affect the conversion of Helios-deficient cells into pTregs in vivo.
Antigen-specific pTreg generated in vivo from Helios-deficient cells manifest antigen-specific suppression
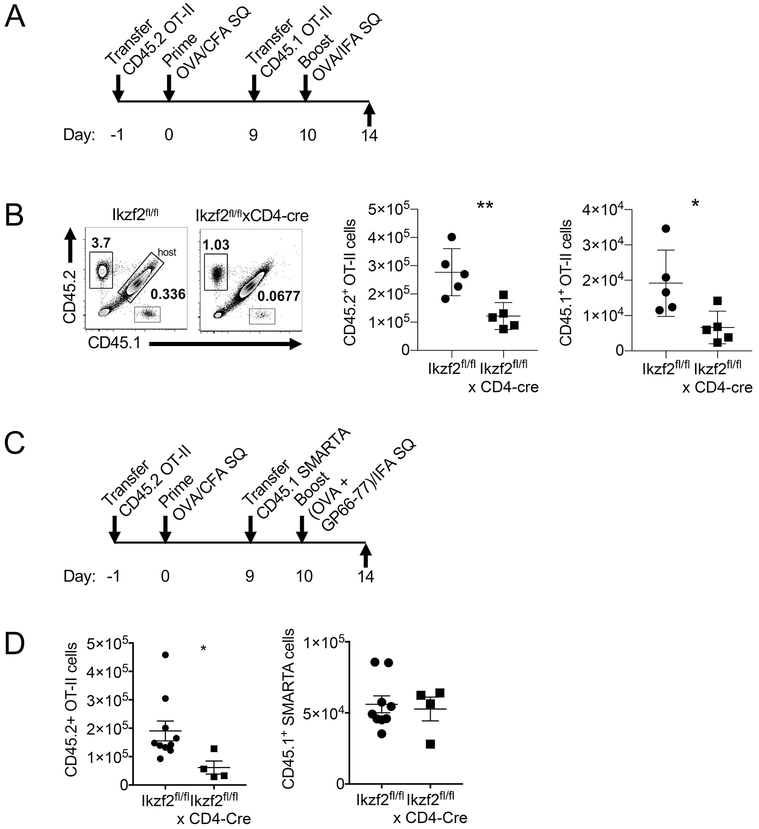
To test if the presence of a small percentage of antigen-specific pTreg was responsible for the lack of proliferation of the primed Helios-deficient OT-II cells upon secondary antigen stimulation, we performed a sequential transfer of congenically labelled OT-II cells as outlined in Fig. 7A. To summarize, CD45.2 Helios-sufficient or Helios-deficient OT-II cells were adoptively transferred into F1 hosts (CD45.1 × CD45.2) and the recipient mice were immunized with OVA/CFA the following day. On day 9 post immunization, freshly sorted WT CD45.1 OT-II cells were transferred, followed by immunization with OVA/IFA on day 10. Draining lymph nodes were examined on d14. The gating strategy is shown in Supplemental Fig 1B. Both the previously primed WT CD45.2 and the freshly transferred WT CD45.1 OT-II cells expanded upon immunization (Fig. 7B). However, both the previously primed CD45.2 Helios-deficient and the freshly transferred WT CD45.1 OT-II cells failed to expand upon secondary immunization. Importantly, suppression was antigen-specific, as there was no defect in the ability of freshly transferred SMARTA T cells (TCR Tg cells specific for NP66-77 of LCMV) on d9 to proliferate in response to immunization with NP66-77 in IFA (Fig. 7C, D). Together, these results demonstrate that OVA-specific pTregs, which arise during the primary antigen response, are able to suppress TCR-mediated stimulation and restimulation.
FIGURE 7.
The generation of pTreg in Helios deficient cells suppresses restimulation in an antigen specific manner. (A) Experimental outline of OT-II sequential transfer. Naïve CD45.2+ OT-II cells were adoptively transferred into congenic F1 mice (CD45.1 × CD45.2), which were immunized s.c. the next day with OVA/CFA. On d9, CD45.1+ OT-II cells were transferred into recipient mice followed by OVA/IFA immunization on day 10. (B) Draining LN were harvested on day 14 and the proliferation of CD45.2+ and CD45.1+ OT-II cells was analyzed by FACS. (C) Experimental outline of OT-II/ SMARTA sequential transfer. As in (C) CD45.1+ SMARTA cells were transferred on d9 followed by s.c. immunization with OVA + GP66-77 in IFA. (D) Draining LNs were harvested on day 14 and the proliferation of CD45.2+ OT-II cells and CD45.1+ SMARTA cells was analyzed.
Discussion
The transcription factor Helios is expressed predominantly in Foxp3+ Treg, but it is also expressed in a small percentage of CD4+Foxp3− T cells in vivo that have a memory phenotype and is transiently induced during antigen priming of naïve cells. Our observation that lkzf2fl/fl × CD4-Cre failed to develop an autoimmune phenotype, while lkzf2fl/fl × Foxp3-Cre mice developed systemic autoimmunity strongly suggested that the expression of Helios in CD4+Foxp3− T cells played a critical role in T effector cell function. In order to understand the role of Helios during the activation of CD4+Foxp3− T cells, we employed a model system in which Helios was deficient in antigen-specific CD4+ T cells. Paradoxically, we have found that Helios is not required for effector cell expansion or differentiation to Th1/ Th2 phenotypes, but that primed, Helios-deficient, antigen-specific cells are tolerant and fail to expand upon restimulation. The tolerance was not T effector cell intrinsic, but was secondary to the predisposition of Helios-deficient T cells to differentiate into pTregs following priming. Thus, the lack of Helios influences the fate of T cells during their initial response to antigen stimulation. In its absence, CD4+Foxp3− T cells can be directed to the pTreg lineage and suppress antigen-specific effector cells of the same specificity.
Only a small percentage (3-5%) of the transferred CD4+Foxp3− T cells converted to pTreg. Nevertheless, these antigen-specific pTreg were potent suppressors of the expansion of both primed OT-II cells and naïve OT-II cells. Thus, the ratio of pTreg to CD4+Foxp3− T cell (~1:20) appears to be sufficient to almost completely inhibit effector cell expansion. In vitro suppression assays with antigen-specific tTreg have shown that complete suppression of proliferation can also be observed with Treg:Teff ratios as high as 1:20 (21). It should also be pointed out that a low percentage of pTregs are generated from polyclonal CD4+Foxp3− T cells in the transfer model of Inflammatory Bowel Disease (IBD), yet they appear to be essential to supplement tTreg suppression in IBD and other models (22, 23). Furthermore, Korn et al have shown that mice immunized with a MOG specific peptide in the absence of IL-6, either through immunization in the presence of IFA or the use of mice deficient in gp130, develop a small percentage of antigen-specific pTreg (2%) that protect mice from EAE (19). Finally, the susceptibility of antigen-specific Helios-deficient CD4+Foxp3− T cells to differentiate to pTreg was also observed in the in vivo oral tolerance model that is widely used to study pTreg generation (7, 24). While pTregs can be generated from Helios-sufficient CD4+Foxp3− T cells under this protocol, higher percentages of pTregs were generated from Helios-deficient CD4+Foxp3− T cells.
It has been widely assumed that Treg activated by one antigen can suppress responses to unrelated antigens in the same environment, so-called “bystander suppression.” This concept has largely been derived from in vitro suppression assays. Recent in vivo experiments from our group have challenged this concept and demonstrated that both antigen-specific iTregs and tTregs, activated by DC doubly pulsed with two different antigens, could only suppress antigen-specific naïve CD4+Foxp3− T cells specific for their cognate antigen, but not an unrelated antigen (25). The pTregs generated from antigen-specific Helios-deficient CD4+Foxp3− T cells appear to function in similar fashion, as pTreg specific for OVA could not suppress the response of SMARTA T cells even though the OVA-specific pTregs were stimulated in the presence of both antigens. We have proposed that the mechanism of suppression used by iTreg and tTregs in vivo involves Treg-mediated depletion of peptide-MHC class II complexes from the DC surface (25), but we have not yet examined the mechanisms used by pTreg generated from Helios-deficient T cells.
The cellular and molecular factors that control pTreg generation in vivo during antigen stimulation remain poorly characterized. In particular, it is unclear why in almost all models only a small percentage of Foxp3− T cells develop into Foxp3+ pTregs. We attempted to determine whether Helios-deficient CD4+ T cells had enhanced responsiveness to TGFβ, IL-2 or TCR signaling in vitro, but we could not discern a difference between WT and Helios-deficient cells in extensive dose-response studies. Although it has been proposed that the absence of inflammatory signals in some models favors pTreg induction (19, 20), in our transfer system, the choice of adjuvant did not affect the generation of pTreg from Helios-deficient cells.
The function of Helios in Tregs remains controversial and the available studies do not offer insights into a potential role for Helios in inhibiting pTreg generation from CD4+Foxp3− T cells. The studies of Kim et al suggested that Helios is required to maintain Treg stability during inflammation by acting on the STAT5 pathway (11). In contrast, we could not identify defects in STAT5 signaling in Helios-deficient Treg and proposed that expression of Helios is necessary for the survival of activated Treg by promoting Bcl2 expression (10). Blaine et al. proposed that Helios regulates IL-2 transcription in Treg and that forced expression of Helios in CD4+Foxp3− results in loss of their ability to produce IL-2 (26). It remains possible that transient Helios expression during activation regulates IL-2 production and deletion of Helios results in the localized production of high concentrations of IL-2 which promotes pTreg induction. However, we have not observed enhanced IL-2 production or enhanced proliferation of Helios-deficient CD4+Foxp3− T cells, at least in vitro, and Helios deficient antigen-specific cells do not demonstrate enhanced expansion in vitro when primed.
One important question raised by our study is whether pTreg induction to self-antigens during development explains the phenotype (or lack of it) in the lkzf2fl/fl × CD4-Cre mouse. The percentages and absolute numbers of Treg are normal in these mice, but, at this time, we have no markers, other than Helios (27), that we believe can definitively distinguish pTreg from tTreg, in order to determine if the lkzf2fl/fl × CD4-Cre mouse possesses a greater proportion of pTreg. We have transferred naïve CD4+ T cells from Ikzf2fl/fl × CD4Cre mice to RAG deficient mice with the hope that they would generate sufficient and competent pTregs to protect against IBD. However, there were no differences in the disease course or severity when compared to Helios sufficient naïve cells (data not shown). Haribhai et al. have demonstrated, though, that pTreg alone are not sufficient to suppress IBD (22), thus the increased pTreg generated from the Helios deficient CD4+ T cells would not be sufficient to suppress IBD. lkzf2fl/fl × CD4-Cre mice also develop EAE with a disease course comparable to WT mice, analogous to the similar activation and expansion seen with the adoptive transfer of OTII cells (data not shown). However, we have not yet examined whether the lkzf2fl/fl × CD4-Cre mice with EAE have developed tolerance that would be manifest upon rechallenge, as we do not have the tools to conclusively determine that the tolerant state is mediated by antigen-specific pTreg.
Lastly, it was recently demonstrated that Helios is expressed during the development of hair cells in the cochlea and that Helios was required for the functional maturation of the terminally differentiated outer hair cells (OHC) (28). Combined with the requirement of Helios for the maintenance of Treg effector function and the requirement of Helios to prevent pTreg differentiation in CD4+Foxp3− cells, these studies suggest that Helios plays a fundamental role in determining CD4+ T cell fate.
Supplementary Material
Key Points.
Mice with a deficiency of Helios in all T cells do not develop autoimmunity.
While antigen-specific Helios−/− CD4+ T cells prime normally, they become tolerant.
pTreg are generated in the absence of Helios and mediate the tolerant state.
Acknowledgements
We thank K. Weng for flow cytometry sorting.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
- 1.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, and Wahl SM. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, and Neurath MF. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 172: 5149–5153. [DOI] [PubMed] [Google Scholar]
- 3.Zheng SG, Wang J, Wang P, Gray JD, and Horwitz DA. 2007. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 178: 2018–2027. [DOI] [PubMed] [Google Scholar]
- 4.Apostolou I, and von Boehmer H. 2004. In vivo instruction of suppressor commitment in naive T cells. J Exp Med 199: 1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, and Waldmann H. 2004. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol 172: 6003–6010. [DOI] [PubMed] [Google Scholar]
- 6.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, and Lafaille JJ. 2004. CD25− T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol 173: 7259–7268. [DOI] [PubMed] [Google Scholar]
- 7.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, and Curotto de Lafaille MA. 2005. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest 115: 1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, and Shevach EM. 2010. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 184: 3433–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornton AM, Lu J, Korty PE, Kim YC, Martens C, Sun PD, and Shevach EM. 2019. Helios(+) and Helios(−) Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur J Immunol 49: 398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sebastian M, Lopez-Ocasio M, Metidji A, Rieder SA, Shevach EM, and Thornton AM. 2016. Helios Controls a Limited Subset of Regulatory T Cell Functions. J Immunol 196: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, Kastner P, Haining WN, and Cantor H. 2015. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 350: 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akimova T, Beier UH, Wang L, Levine MH, and Hancock WW. 2011. Helios expression is a marker of T cell activation and proliferation. PLoS One 6: e24226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross EM, Bourges D, Hogan TV, Gleeson PA, and van Driel IR. 2014. Helios defines T cells being driven to tolerance in the periphery and thymus. Eur J Immunol 44: 2048–2058. [DOI] [PubMed] [Google Scholar]
- 14.Serre K, Benezech C, Desanti G, Bobat S, Toellner KM, Bird R, Chan S, Kastner P, Cunningham AF, Maclennan IC, and Mohr E. 2011. Helios is associated with CD4 T cells differentiating to T helper 2 and follicular helper T cells in vivo independently of Foxp3 expression. PLoS One 6: e20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxenius A, Bachmann MF, Zinkernagel RM, and Hengartner H. 1998. Virus-specific MHC class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. European Journal of Immunology 28: 390–400. [DOI] [PubMed] [Google Scholar]
- 16.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, and Schwartzberg PL. 2006. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. Journal of Experimental Medicine 203: 1551–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez RJ, Zhang N, Thomas SR, Nandiwada SL, Jenkins MK, Binstadt BA, and Mueller DL. 2012. Arthritogenic Self-Reactive CD4(+) T Cells Acquire an FR4(hi) CD73(hi) Anergic State in the Presence of Foxp3(+) Regulatory T Cells. Journal of Immunology 188: 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pabst O, and Mowat AM. 2012. Oral tolerance to food protein. Mucosal Immunol 5: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, and Oukka M. 2008. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 105: 18460–18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aloulou M, Carr EJ, Gador M, Bignon A, Liblau RS, Fazilleau N, and Linterman MA. 2016. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat Commun 7: 10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton AM, and Shevach EM. 2000. Suppressor effector function of CD4(+)CD25(+) immunoregulatory T cells is antigen nonspecific. Journal of Immunology 164: 183–190. [DOI] [PubMed] [Google Scholar]
- 22.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, Wise PM, Chen A, Zheng YQ, Simpson PM, Gorski J, Salzman NH, Hessner MJ, Chatila TA, and Williams CB. 2011. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity 35: 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, and Rudensky AY. 2012. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, and Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. Journal of Experimental Medicine 204: 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akkaya B, Oya Y, Akkaya M, Al Souz J, Holstein AH, Kamenyeva O, Kabat J, Matsumura R, Dorward DW, Glass DD, and Shevach EM. 2019. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat Immunol 20: 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baine I, Basu S, Ames R, Sellers RS, and Macian F. 2013. Helios induces epigenetic silencing of IL2 gene expression in regulatory T cells. J Immunol 190: 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shevach EM 2018. Foxp3(+) T Regulatory Cells: Still Many Unanswered Questions-A Perspective After 20 Years of Study. Front Immunol 9: 1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chessum L, Matern MS, Kelly MC, Johnson SL, Ogawa Y, Milon B, McMurray M, Driver EC, Parker A, Song Y, Codner G, Esapa CT, Prescott J, Trent G, Wells S, Dragich AK, Frolenkov GI, Kelley MW, Marcotti W, Brown SDM, Elkon R, Bowl MR, and Hertzano R. 2018. Helios is a key transcriptional regulator of outer hair cell maturation. Nature 563: 696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.