Abstract
High-dose busulfan (BU) followed by high-dose cyclophosphamide (CY) (BU/CY) before allogeneic hematopoietic cell transplantation (HCT) has long been used as treatment for hematologic malignancies. Administration of phenytoin or newer alternative anti-epileptic medications (AEMs) prevents seizures caused by BU. Phenytoin induces enzymes that increase exposure to active CY metabolites in vivo, whereas alternative AEMs do not have this effect. Lower exposure to active CY metabolites with the use of alternative AEMs could decrease the risk of toxicity but might increase the risk of recurrent malignancy after HCT. Previous studies have not determined whether outcomes with alternative AEMs differ from those with phenytoin in patients treated with BU/CY before allogeneic HCT. We studied a cohort of 2155 patients, 1460 treated with phenytoin and 695 treated with alternative AEMs, who received BU/CY before allogeneic HCT from 2004 through 2014. We found no differences suggesting decreased overall or relapse-free survival or increased risks of relapse, non-relapse mortality, acute or chronic GVHD, or regimen-related toxicity associated with the use of alternative AEMs as compared to phenytoin. The risk of dialysis was lower in the alternative AEM group than in the phenytoin group. Alternative AEMs are safe for prevention of seizures after BU administration and can avoid the undesirable toxicities and drug interactions caused by phenytoin.
Keywords: Busulfan, Cyclophosphamide, Seizure Prophylaxis, Phenytoin, Antiepileptic Medication, Hematopoietic Cell Transplantation, Drug Interactions
INTRODUCTION
High-dose busulfan (BU) is often used to decrease the burden of malignant cells in the recipient before allogeneic hematopoietic cell transplantation (HCT). BU administration can cause seizures.1 Originally, phenytoin was the anti-epileptic medication (AEM; formerly referred to as anti-epileptic drug) most frequently used to prevent BU-induced seizures. Phenytoin is well known as a strong inducer of hepatic drug-metabolizing enzymes, specifically the cytochrome P450 (CYP) enzymes CYP2B6, CYP2C and CYP3A, and the UDP glucuronosyltransferases (UGTs).1 Enzyme induction occurs within 24 hours after administration of phenytoin and lasts for at least a week after administration of phenytoin has ended.2 More recently, newer alternative antiepileptic medications (AEMs) such as levetiracetam have been increasingly used as a replacement for phenytoin to prevent BU-induced seizures. Compared to phenytoin, these alternative AEMs have two advantages: (1) alternative AEMs have fewer potential drug interactions because they do not induce CYPs or UGTs, and (2) they have fewer toxicities.3
BU is often used in combination with high-dose cyclophosphamide (CY) as a conditioning regimen before allogeneic HCT. CY is a prodrug with multiple metabolites (see Supplemental Figure 1 for pharmacokinetic schema of CY and its metabolites).4 Among these, 4-hydroxycyclophosphamide (4HCY) is critical because it is transported intracellularly and spontaneously decomposes to phosphoramide mustard, which covalently cross-links DNA. The 4HCY metabolite carboxyethylphosphoramide mustard (CEPM) is the predominant plasma metabolite after CY administration. Variability in the area under the plasma concentration time curve (AUC) of CY, 4HCY, or other metabolites may account for interpatient differences in the efficacy and toxicity of CY.2, 4–6
Rezvani et al.4 compared the pharmacokinetics of CY, 4HCY, and CEPM in patients treated either with CY followed by targeted BU (CY/TBU) or with targeted BU followed by CY (TBU/CY). To prevent seizures, both groups received phenytoin at the start of BU administration. Phenytoin administered before CY accounts for the greater 4HCY AUC in the TBU/CY group, whereas phenytoin given in conjunction with BU could not have had any effect on CY metabolism in the CY/TBU group.4, 6 Compared to the CY/TBU group, TBU/CY group had a ~0.48-fold lower CY AUC and a 1.7-fold higher 4HCY AUC. In patients treated with CY/TBU, higher 4HCY AUCs were associated with a statistically significant higher risk of mortality.4 Both groups received targeted busulfan (TBU) dosing, where BU doses were personalized by using therapeutic drug monitoring to ensure that patients received the intended BU plasma area under the curve (AUC).7 Therefore, BU itself was unlikely to contribute to the different clinical outcomes between TBU/CY and CY/TBU in the Rezvani trial.4
In addition to phenytoin administration before CY (i.e., BU/CY) increasing 4HCY AUC, BU administration depletes hepatic glutathione, thereby sensitizing the liver to toxic effects of CY and its metabolites.4, 8, 9 In myelofibrosis patients, the CY/TBU regimen was associated with less sinusoidal obstruction syndrome during the first 20 days after HCT, a statistically significant lower risk of non-relapse mortality (NRM) during the first 100 days after HCT, but no statistically significant differences in NRM or overall survival at 2 years.4 On the other hand, in patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS), the CY/TBU regimen was associated with a statistically significant higher risk of relapse after HCT. The cumulative incidence was 44% for patients treated with CY/TBU versus 20% for those treated with TBU/CY. The unadjusted hazard ratio was 2.57 (P = 0.008), and the adjusted hazard ratio was 2.15 (P = 0.02). This observation in the CY/TBU regimen raises concern that relapse rates may be higher when alternative AEMs are administered in patients with AML or MDS, because, unlike phenytoin, they do not increase 4HCY AUC, and intracellular concentrations of the active CY metabolites may be lower in patients treated with alternative AEMs than in those treated with phenytoin.
In addition to its effect on CY metabolism, phenytoin increases the clearance of orally administered BU.10 The effect of phenytoin on intravenous (IV) BU clearance is less clear. The available studies have shown either a slight effect11 or no measurable effect12–14 on IV BU clearance. Therefore, in the absence of targeted BU dosing, replacing phenytoin with an alternative AEM would be expected to increase BU AUC after oral BU administration but not after IV BU administration. Rezvani et al.4 used targeted BU dosing to ensure consistent BU AUCs in comparing the TBU/CY and CY/TBU regimens.
Patients treated with BU/CY differ from those treated with CY/BU in one other potentially important respect. As discussed above, depletion of glutathione during BU administration may sensitize the liver to toxicity after subsequent exposure to CY and its metabolites.8 Therefore, it is difficult to predict whether the use of alternative AEMs and the associated lower intracellular concentrations of active CY metabolites would affect NRM and regimen-related toxicity. Nonetheless, the results of Rezvani et al.4 raise concerns that the use of alternative AEMs may be associated with a higher risk of relapse after HCT in patients treated with BU/CY conditioning regimens.
Many HCT centers have already adopted the use of alternative AEMs to prevent BU-induced seizures. Although alternative AEMs are effective for this indication,1 previous reports with <50 cases have not been powered sufficiently to evaluate whether relapse, NRM or overall survival might be affected by the use of alternative AEMs compared to phenytoin in patients treated with BU/CY conditioning regimens.1, 3, 15–17 Therefore, we conducted a large retrospective study using the Center for International Blood and Marrow Transplant Research (CIBMTR®) registry data to determine whether the use of alternative AEMs was associated with longer-term outcomes when compared to phenytoin in patients treated with BU/CY conditioning regimens before allogeneic HCT.
METHODS
Data Source
The CIBMTR® is a working group of more than 500 transplantation centers worldwide that contribute detailed data on HCT to a statistical center at the Medical College of Wisconsin. CIBMTR® is a research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin. Participating centers are required to report all transplantations consecutively; patients are followed longitudinally, and compliance is monitored by on-site audits. Data quality is ensured, both by computerized checks for discrepancies and by physicians’ review of submitted data. CIBMTR conducts observational studies and complies with all applicable federal regulations that protect human subjects.
Patient Selection
The study cohort included patients who received a first allogeneic hematopoietic cell graft from an HLA-matched sibling or an unrelated donor at a center in the USA during calendar years 2004 through 2014 with the use of BU and CY conditioning. Patients were excluded if they: had a seizure disorder before HCT; had not given consent; received transplants at centers that failed data audits, or if follow-up data after HCT had not been reported. Patients were also excluded if they: underwent HCT for treatment of myelofibrosis in the absence of other hematological malignancy, severe aplastic anemia or other non-malignant diseases; had received total body irradiation or anti-neoplastic medications other than BU and CY in the conditioning regimen before HCT or CY for immunosuppression after HCT; received CY before BU; had missing dates of CY or BU administration. This screen identified 2863 patients from 153 centers who were potentially eligible for the study.
CIBMTR case report forms have not collected information regarding AEMs used to prevent BU-induced seizures. Therefore, HCT centers were invited to participate in the study by completing a survey describing center-specific practices about the use of phenytoin versus alternative AEMs, including the dates of any changes in practice and differences in practices between children and adults. Ninety-two centers returned information (Supplemental Table 1), and additional data review excluded patients who received BU at total doses <8 mg/kg or CY at total doses <100 mg/kg from participating centers. The final cohort included 2155 patients, 1460 who received phenytoin and 695 who received alternative AEMs.
Study Objectives and Definitions
The overall objective was to evaluate outcomes after using alternative AEMs (i.e., any AEM other than phenytoin or fosphenytoin) compared to phenytoin (i.e., phenytoin or fosphenytoin). The efficacy of these AEMs – i.e., how well they prevented BU-induced seizures – could not be assessed, because the CIBMTR repository does not collect data regarding seizures after BU administration. The primary question to be addressed was whether the use of alternative AEMs is associated with a higher risk of recurrent or progressive malignancy (i.e., relapse). Other endpoints included overall survival, survival without recurrent or progressive malignancy (i.e., disease-free survival), NRM (i.e., death without prior recurrent or progressive malignancy), grade II-IV acute graft-versus-host disease (GVHD), grade III-IV acute GVHD, chronic GVHD, renal failure requiring dialysis, idiopathic pneumonia syndrome and sinusoidal obstruction syndrome, all as reported in CIBMTR case-report forms.18, 19
Statistical Analysis
For analysis of the main effect, each patient was categorized into one of two groups based on the AEM used, either phenytoin (i.e., phenytoin or fosphenytoin) or alternative AEMs. Multivariable analysis used Cox proportional hazards model for each endpoint. Candidate variables considered in these analyses are listed in Supplemental Table 2. All variables were tested first for affirmation of the proportional hazards assumption. Factors violating the proportional hazards assumption were adjusted through stratification. Then, a stepwise forward-backward procedure was performed to select the adjusted clinical variables and to build the multivariable models, using a 0.05 threshold of statistical significance for both inclusion and exclusion in the model. Interactions between the main variable ‘AEM group’ (i.e., phenytoin vs. alternative AEM) and the selected adjusted covariates were tested in each model, and no endpoint showed any covariate interactions at a 0.01 threshold of statistical significance. The ‘center’ effect was adjusted in all multivariable models through robust sandwich estimates. All p-values are two-sided. To account for multiple testing, α=0.01 was chosen as the significance level the impact of AEM group on outcomes. Cumulative incidence frequencies and hazard ratios were used to evaluate relapse in the two AEM groups. Because malignancies may differ in their susceptibility to CY and its metabolites, a subset analysis compared the risk of relapse between the two AEM groups separately in patients with AML, chronic myeloid leukemia (CML), MDS, or lymphoid malignancies. A similar analysis compared the risks of relapse between the two AEM groups in adults (age ≥ 18 years) and children (age <18 years). Data analyses were performed using SAS version 9.4 (SAS institute, Cary, NC)
Non-inferiority of the alternative AEM group for the key endpoint of relapse was assessed by comparing the upper limit of the 95% confidence interval of the hazard ratio to a pre-specified noninferiority margin of 1.21, corresponding to an absolute difference in relapse risk of approximately 5% at 2 years. Assuming that the alternative AEM versus the phenytoin groups has a 1.21 hazard ratio for relapse, and given a 30% incidence of relapse at 2 years in the phenytoin group, with 2150 patients distributed in a 2:1 ratio between the phenytoin and alternative AEM groups, we had 83% and 63% power, respectively, to detect the difference based on the log-rank test at 0.05 and 0.01 significance.
RESULTS
Patient and treatment characteristics
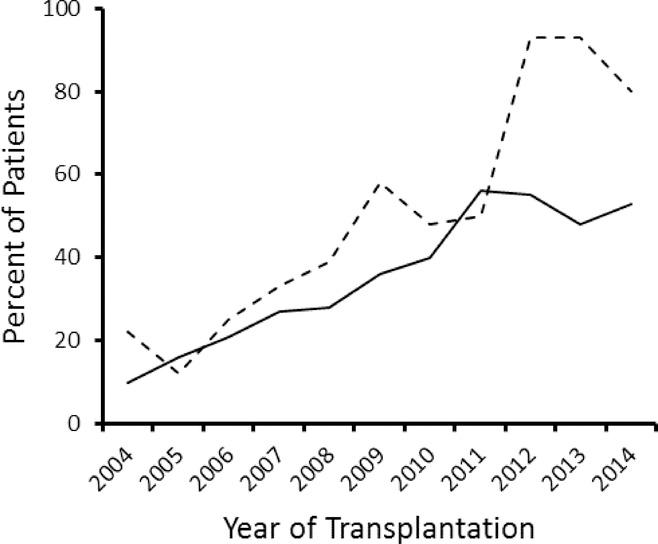
The median age of patients within each AEM group was similar: 46 years for phenytoin group and 47 years for alternative AEM group (Table 1). Most patients were adults (≥18 years of age), 90% in the phenytoin group and 82% in the alternative AEM group. Ursodiol prophylaxis was administered to 6% of the patients in each group. More than half of the patients had AML, and over two-thirds of the patients received unrelated donor grafts. Most patients were treated with IV BU, 77% of patients in the phenytoin group and in 92% of those in the alternative AEM group. Supplemental Table 3 summarizes characteristics of patients subdivided according to BU administration route. BU therapeutic drug monitoring and personalized dose adjustments were used in 21% of patients in the phenytoin group and in 52% of those in the alternative AEM group, but detailed BU pharmacokinetic data are not available. Thus, the impact of AEM group upon BU pharmacokinetics cannot be evaluated. Approximately two-thirds of the patients received growth factor-mobilized blood cell grafts, and less than 10% received cord blood grafts. Most patients received a calcineurin inhibitor with methotrexate for immunosuppression after HCT. In both children and adults, the use of alternative AEMs gradually increased between 2004 and 2011 (Figure 1). The proportion of children treated with alternative AEMs increased sharply in 2012, but the proportion of adults treated with alternative AEMs did not (Figure 1).
Table 1.
Patient characteristics
| Variable | Phenytoin | Alternative AEM |
|---|---|---|
| Number of patients | 1460 | 695 |
| Number of centers | 72 | 59 |
| Patient age, median (range) years | 46 (<1–70) | 47 (<1–71) |
| >18 years, N (%) | 1310 (90) | 572 (82) |
| <18 years, N (%) | 150(10) | 123(18) |
| Male sex, N (%) | 781 (53) | 354 (51) |
| Disease, N (%) | ||
| AML | 799 (55) | 471 (68) |
| MDS | 318 (22) | 126(18) |
| CML | 212 (15) | 63 (9) |
| NHL | 47 (3) | 7 (1) |
| ALL | 39 (3) | 12 (2) |
| Othera | 45 (3) | 16 (3) |
| Donor type, N (%) | ||
| HLA-identical sibling | 498 (34) | 237 (34) |
| Unrelated or umbilical cord blood | 962 (66) | 458 (66) |
| Graft type, N (%) | ||
| Growth factor-mobilized blood | 950 (65) | 481 (69) |
| Bone marrow | 445 (30) | 158 (23) |
| Umbilical cord blood | 65 (4) | 56 (8) |
| BU administration route,b N (%) | ||
| IV | 1130 (77) | 637 (92) |
| Oral | 318 (22) | 57 (8) |
| BU cumulative dose (mg/kg),dmedian (range) | 13 (8–37) | 13 (8–26) |
| BU pharmacokinetics obtained, N (%) | ||
| Missing | 740 (51) | 164 (24) |
| No | 414 (28) | 173 (25) |
| Yes | 306 (21) | 358 (52) |
| CY cumulative dose (mg/kg), median (range) | 120 (100–247) | 120 (101–278) |
| 100–130, N (%) | 1252(86) | 576 (83) |
| 131–170, N (%) | 59 (4) | 22 (3) |
| 171–186, N (%) | 16 (1) | 3 (<1) |
| 187–278, N (%) | 133 (9) | 94(14) |
| Median follow-up of survivors (range), months | 73 (3–139) | 61 (3–123) |
Abbreviations: AEM, anti-epileptic medication; N, number; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; NHL, non-Hodgkin lymphoma; ALL, acute lymphoblastic leukemia; BU, busulfan; IV, intravenous, CY, cyclophosphamide.
Hodgkin disease, myeloproliferative syndrome, myeloma and other leukemia.
Information regarding BU administration route was not available for 12 patients in the phenytoin group and 1 patient in the alternative AEM group.
Doses greater than 8 mg/kg BU - either oral or IV - were used.
Figure 1. Use of alternative AEMs to prevent BU-induced seizures increased between 2004 and 2014.
Plots show the percentages of adult (solid line) and pediatric (dashed line) patients who were treated with alternative AEMs according to year of HCT.
Outcomes in the different AEM groups
The median follow-up of patients after HCT was 73 (range 3–139) months in the phenytoin group and 61 (range 3–1233) months in the alternative AEM group. IV BU use differed between the two AEM groups: 78% in the phenytoin group and 92% in the alternative AEM group (P<0.0001). Thus, for each AEM group, outcomes with IV BU were compared to those with oral BU (Supplemental Table 4). None of the outcomes differed between the two AEM groups at the P=0.01 significance threshold. With the less stringent criteria (P<0.05), in the phenytoin group, grade II-IV acute GVHD and idiopathic pneumonia syndrome were less likely with IV BU compared with oral BU. In the alternative AEM group, NRM and grade III-IV acute GVHD were more likely with IV BU compared with oral BU. Therefore, the analysis of AEM groups was stratified according to BU administration route.
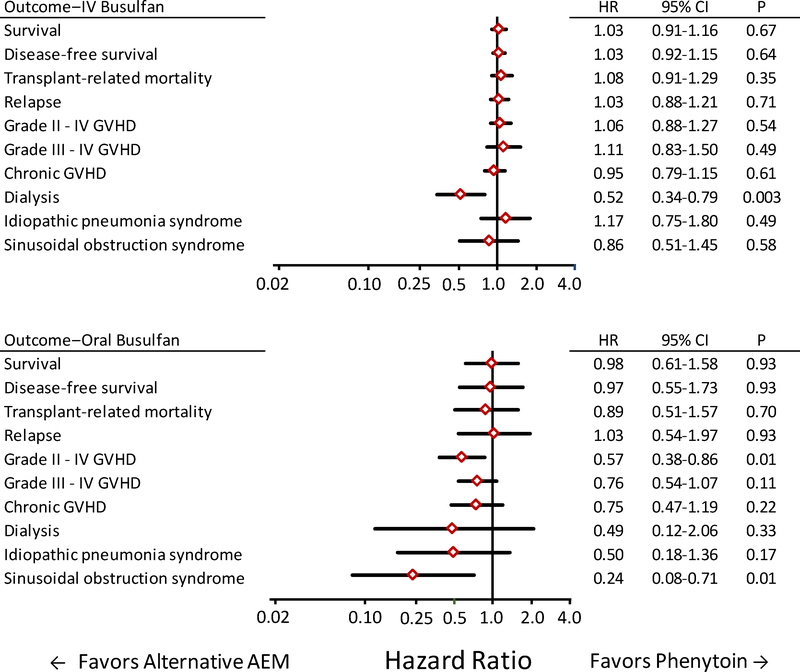
Figure 2 shows results of analyses that incorporated covariate information from all patients in a single model. Among patients treated with IV BU for whom dialysis data were available, the risk of dialysis was lower in the alternative AEM group than in the phenytoin group (HR, 0.52; 95% CI, 0.34–0.79; P=0.003). Among patients included in the model, dialysis was required in 29 of 608 (4%) patients in the alternative AEM group, compared to 70 of 1073 (7%) patients in the phenytoin group. The 0.49 HR point estimate for the risk of dialysis among patients treated with oral BU approximates the 0.52 HR point estimate among patients treated with IV BU. No other outcome between the two groups showed a difference at a 0.01 threshold of statistical significance.
Figure 2. Most outcomes did not differ between patients who received alternative AEMs compared to those who received phenytoin after conditioning with oral or IV BU followed by CY.
Diamonds indicate the hazard ratio point estimates when results for the alternative AEM group were compared to those for the phenytoin group. Bars indicate the 95% confidence intervals. Each statistical model includes risk factor covariate adjustments derived from the entire cohort.
Among patients treated with oral BU, the risks of grades II – IV GVHD and sinusoidal obstruction syndrome were lower in the alternative AEM group than in the phenytoin group (HR, 0.57; 95% CI, 0.38–0.86; P=0.01; and HR, 0.24; 95% CI, 0.08–0.71; P=0.01, respectively). Among patients in the model, grade II – IV GVHD occurred in 22 of 56 (39%) of patients in the alternative AEM group, compared to 182 of 317 (57%) patients in the phenytoin group, and sinusoidal obstruction syndrome was reported in 1 of 57 (2%) patients in the alternative AEM group, compared to 25 of 318 (8%) patients in the phenytoin group. The HR point estimates for these associations were considerably lower among patients treated with oral BU than among patients treated with IV BU. Other outcomes showed no statistically significant differences between the two AEM groups.
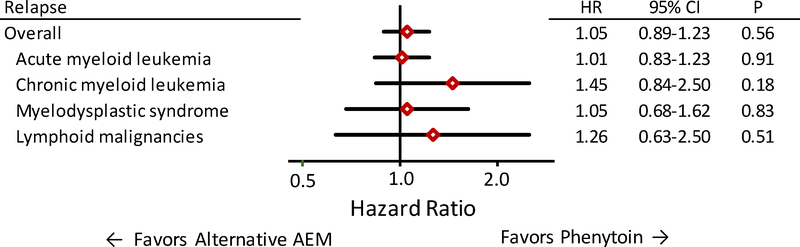
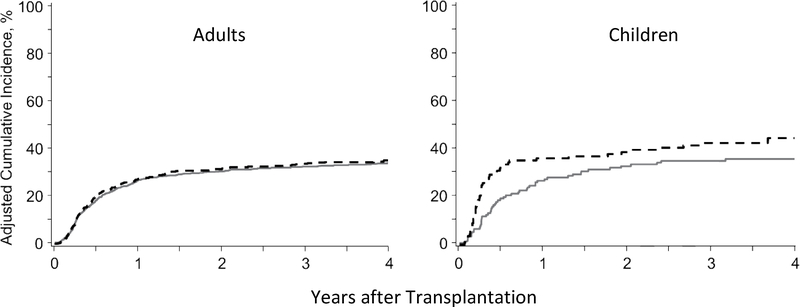
The adjusted HR for relapse in the alternative AEM group compared to the phenytoin group in the entire cohort of 2155 patients was 1.04 (95% CI, 0.89–1.23; P=0.60). The upper limit of the 95% confidence interval is slightly higher than the non-inferiority limit of 1.21 prespecified by the protocol. The risk of relapse did not differ between AEM groups when patients were stratified according to the pre-transplant disease (AML, CML, MDS, lymphoid malignancies) (Figure 3). The adjusted HRs of relapse in the alternative AEM group compared to the phenytoin group were 0.96 (95% CI, 0.80–1.15; P=0.63) among all adult patients and 1.61 (95% CI, 1.00–2.61; P=0.05) among all pediatric patients (Figure 4).
Figure 3. The risk of relapse did not differ between patients who received alternative AEMs compared to those who received phenytoin after conditioning with BU followed by CY for treatment of AML, CML, MDS or lymphoid malignancies.
Diamonds indicate the hazard ratio point estimates of relapse for the alternative AEM group compared to the phenytoin group. Bars indicate the 95% confidence intervals. Each statistical model included risk factor covariate adjustments derived from the entire cohort.
Figure 4. In adult and pediatric patients, the adjusted cumulative incidence of relapse did not differ between those who received phenytoin (solid lines) or alternative AEMs (dashed lines) after conditioning with BU followed by CY.
In pediatric patients, the risk of relapse appeared to differ between the AEM groups, but the p-value of 0.05 did not meet the 0.01 threshold of statistical significance for this study (see Methods).
Outcomes in adults and children
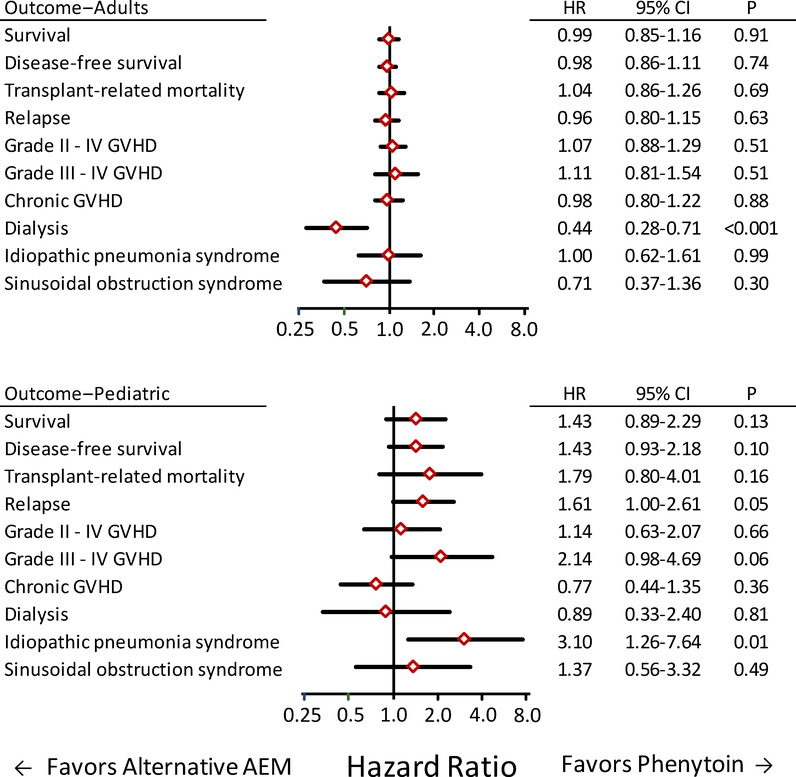
To determine whether results differed between children and adults, we compared outcomes with phenytoin versus alternative AEM group in pediatric and adult patients who received IV BU for treatment of AML, CML, MDS or lymphoid malignancies. The number of children who received oral BU was too small for an informative comparison with adults who received oral BU. As shown in Figure 5, results comparing the phenytoin and alternative AEM groups in adults who received IV BU did not differ from those shown in Figure 2, as expected from the preponderance of adults in the overall cohort. In pediatric patients treated with IV BU, the risk of interstitial pneumonia syndrome was higher in the alternative AEM group than in the phenytoin group (adjusted HR 3.10; 95% CI, 1.26–7.64; P=0.01. In addition, the risk of relapse appeared to be higher in the alternative AEM group compared to the phenytoin group (adjusted HR 1.61; 95% CI, 1.00–2.61; P=0.05) (Figure 5). Doses of BU and CY did not differ between pediatric patients in the alternative AEM group compared to those in the phenytoin group (data not shown). A statistical interaction test did not show that the hazard ratio of relapse differed between adults and children treated with IV BU (P=0.11), but it should be noted that the number of pediatric patients treated with IV BU is small (n=252).
Figure 5. Most outcomes did not differ between patients who received alternative AEMs compared to those who received phenytoin in adult (top) and pediatric (bottom) patients after conditioning with IV BU followed by CY for treatment of AML, CML, MDS or lymphoid malignancy.
Diamonds indicate the hazards ratio point estimates for outcomes when results for the alternative AEM group were compared to those for the phenytoin group. Bars indicate the 95% confidence intervals. Each statistical model included risk factor covariate adjustments derived from the entire cohort. The number of pediatric patients who received oral BU is too small for an informative comparison with adult patients who received oral BU.
DISCUSSION
The results of this large (n=2155), retrospective study support three main conclusions in patients conditioned for allogeneic HCT with BU/CY: 1) AEMs other than phenytoin are safe for use to prevent BU-induced seizures. 2) The use of alternative AEMs does not adversely affect the risk of relapse. 3) The risk of renal failure requiring dialysis is lower in adults receiving IV BU when alternative AEMs are used instead of phenytoin.
Seizures have been reported at frequencies from 2% to 40% in patients receiving BU without the use of an AEM to prevent this complication.20–23 BU freely crosses the blood-brain barrier, and BU concentrations in the central nervous system are similar to plasma concentrations, which most likely accounts for the neurotoxicity associated with BU.22, 24 BU-induced seizures, are typically generalized tonic-clonic in character and usually occur within the period between the second day of BU administration and the first 24 hours after the last BU dose.1 Therefore, prophylaxis for BU-induced seizures should begin before starting treatment with BU and should continue throughout BU administration.
Characteristics of the ideal BU-induced seizure prophylaxis include lack of overlapping toxicity with the conditioning regimen, lack of interference with engraftment of donor cells, and minimal potential for pharmacokinetic drug interactions.1 Given these criteria, phenytoin suffers from possible toxicities and is especially ill-suited because of its drug interactions. The standard of care for prevention of seizures in patients with generalized tonic-clonic seizure disorders shifted from phenytoin to alternative AEMs after their approval by the Food and Drug Administration in the 1990s.25, 26 Acceptance of alternative AEMs to prevent BU-induced seizures in HCT recipients has been slow. As of 2014, phenytoin was still used for approximately 50% of adults at centers who provided data for the current study (Figure 1).
The lower incidence of dialysis associated with the use of alternative AEMs instead of phenytoin in adults could reflect the absence of CYP3A induction. CYP3A is induced by phenytoin may increase dechlorocyclophosphamide and chloroacetaldehyde formation in the kidneys (Supplemental Figure 1). Chloroacetaldehyde has concentration-dependent cytotoxic effects on cultured porcine and rabbit renal tubules and on isolated perfused rat kidneys.27–29 The use of alternative AEMs was not associated with a lower incidence of dialysis in children. On the other hand, the use of alternative AEMs appeared to be associated with a higher risk of idiopathic pneumonia syndrome in children but not in adults (Figure 5). Reasons for these possible age-related differences between adults and children are not apparent.
Interest has emerged in developing novel high-dose conditioning regimens that replace CY with fludarabine (FLU), a purine nucleoside inhibitor that is potentially less toxic yet has similar immunosuppressive and anti-leukemic efficacy as CY.30, 31 Our current results do not apply to patients receiving BU in combination with fludarabine (FLU)30, 31 because the drug-metabolizing enzymes and transporters of CY and FLU differ such that phenytoin would not be expected to affect the pharmacokinetics of FLU or its metabolites. Administration of CY before BU (CY/BU) represents another approach to making the regimen more tolerable, as done by Rezvani et al.4 Our current results do not apply to patients receiving CY/BU because of its different anti-leukemic efficacy and toxicity compared to BU/CY.4 We did not expect to find higher grade II – IV acute GVHD or idiopathic pneumonia syndrome with the use of IV BU as compared to oral BU within the phenytoin group. Several other retrospective analyses have compared outcomes between oral versus IV BU,32–38 but results have been difficult to interpret because of heterogeneity between patient cohorts and insufficient details regarding the AEM, BU dose or BU pharmacokinetics.
This study has limitations. We could not compare the efficacy of seizure prophylaxis in the two AEM groups, because the CIBMTR repository does not collect data regarding seizures after BU administration. Case series have reported the effectiveness of these alternative AEMs,17 as previously reviewed.1 Supplemental Table 5 provides a practical reference of some of the commonly used AEMs to prevent BU-induced seizures. Our results may not reflect actual AEM use across all HCT centers, because not all centers provided data, and patterns of use at centers that provided data might not be representative of those at other centers. Furthermore, detailed information (e.g., drug name, dose, etc.) about the alternative AEM used was not collected. Thus, we cannot recommend a specific alternative AEM. Finally, some variables that could affect clinical outcomes were not available. For example, the risk of sinusoidal obstructive syndrome is inversely correlated with time interval from the last BU dose to the first CY dose,39 and variation in the use of therapeutic drug monitoring, personalized BU dosing and target BU AUC could affect outcomes,7 although we have no reason to suspect that these practices differed between the AEM groups. Future CIBMTR registry studies would benefit if such information is collected.40 A much larger pediatric cohort with information regarding BU dose, administration frequency, and plasma AUC would be needed to determine whether the use of alternative AEMs is associated with an increased risk of relapse (Figure 4). It should be noted that a statistical interaction test did not show that the hazard ratio of relapse differed between adults and children treated with IV BU (P=0.11), although only 273 children (accrued over 10 years) received BU/CY (Table 1). A study designed to address the observed difference between a 0.25 incidence of relapse at 1 year with phenytoin versus a 0.35 incidence with alternative AEMs at 0.8 power and a two-side 0.05 type-1 error with a 1:1 allocation between arms would require approximately 650 patients. At the historical enrollment rate of 27 pediatric patients per year, it would take approximately 24 years to conduct such a study, which is clearly not feasible. To mitigate concerns over relapse with alternative AEM in BU/CY conditioned children, consideration could be given for replacing CY. For example, fludarabine could replace BY since phenytoin would not be expected to affect the pharmacokinetics of FLU or its metabolites.
Although this study had limited power to exclude adverse outcomes associated with the use of alternative AEMs in evaluating low frequency events and in analyzing subgroups of patients, power was sufficient to assure that any differences in the risk of relapse are smaller than might have been expected from the results reported by Rezvani et al.2 We speculate that differences in 4HCY AUC between the alternative and phenytoin AEM groups had very little effect on malignant and normal hematopoietic stem cells because high aldehyde dehydrogenase activity in these cells diverted 4HCY disposition toward CEPM and away from phosphoramide mustard, thereby protecting them from DNA cross-linking and toxicity. In conclusion, we found no statistically significant evidence suggesting worse outcomes with the use of alternative AEMs as compared with phenytoin to prevent BU-induced seizures in patients treated with BU/CY conditioning regimens before allogenic HCT. Our data show no meaningful differences in the available safety outcomes between the two AEM treatment groups. Given the undesirable toxicities and drug interactions caused by phenytoin, the use of alternative AEMs is justified to prevent BU-induced seizures, and the use of phenytoin may be limited to a back-up option.
Supplementary Material
Highlights.
Antiepileptic medications other than phenytoin prevent seizures induced by busulfan.
Alternative antiepileptic medications (AEMs) do not affect relapse risk in adults.
Adults receiving IV busulfan and alternative AEMs have lower risk of renal failure.
ACKNOWLEDGMENTS
We are grateful to: the HCT centers that provided data; to the physicians, nurses, physician assistants, nurse practitioners, pharmacists, and support staff caring for the patients: and to the staff who maintain the CIBMTR repository. Members of the Regimen Related Toxicity Working Committee who reviewed this project include the following: Hisham Abdel-Azim, Muneer Abidi, Allistair Abrahim, Kehinde Adekola, Vaibhav Agrawal, Ibrahim Ahmed, Gorgun Akpek, A Samer Al-Homsi, Arnon Nagler, Jeffery Auletta, Pere Barba, Asad Bashey, Nelli Bejanyan, Christopher Bredeson, Stefan Ciurea, Edward Copelan, Marcos De Lima, Miguel Angel Diaz Perez, Anita D’Souza, John Edwards, Nosha Farhadfar, Shatha Farhan, Haydar Frangoul, Cesar Freytes, Sidhartha Ganguly, Usama Gergis, Eva Guinan, Gregory Hale, Shahrukh Hashmi, Peiman Heimatti, Gerhard Hildebrandt, Leona Holmberg, Sanghee Hong, Ann Jakubowski, Amy Keating, Oscar Lahoud, Jane Liesveld, Mark Litzow, Navneet Majhail, Parinda Mehta, Herman Murthy, Taiga Nishihori, Roomi Nusrat, Richard Olsson, Attaphol Pawarode, Miguel-Angel Perales, Robert Peter Gale, Mohamed Radhi, Voravit Ratanatharathorn, Andrew Rezvani, Dadiv Rizzieri, Seth Rotz, Jacob Rowe, Ayman Saad, Bipin Savani, Harry Schouten, Mathew Seftel, Sachiko Seo, Niketa Shah, Amir Steinberg, Nathan Sunita, Matthew Ulrickson, Celalettin Ustun, John Wagner, Edmund Waller, Kirsten Williams, Mona B. Wirk, Jean Yared.
This work was supported by National Institutes of Health, National Cancer Institute (NCI) grant CA182963. The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the NCI, the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014–17-1–2388 and N0014–17-1–2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Mediware; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
* Corporate Members
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Eberly AL, Anderson GD, Bubalo JS, McCune JS. Optimal prevention of seizures induced by high-dose busulfan. Pharmacotherapy. 2008;28:1502–1510. [DOI] [PubMed] [Google Scholar]
- 2.Mielcarek M, Furlong T, O’Donnell PV, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floeter AE, McCune JS. Levetiracetam for the prevention of busulfan-induced seizures in pediatric hematopoietic cell transplantation recipients. J Oncol Pharm Pract. 2017;23:344–349. [DOI] [PubMed] [Google Scholar]
- 4.Rezvani AR, McCune JS, Storer BE, et al. Cyclophosphamide followed by Intravenous Targeted Busulfan for Allogeneic Hematopoietic Cell Transplantation: Pharmacokinetics and Clinical Outcomes. Biol Blood Marrow Transplant. 2013;19:1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCune JS, Batchelder A, Guthrie KA, et al. Personalized Dosing of Cyclophosphamide in the Total Body Irradiation-Cyclophosphamide Conditioning Regimen: A Phase II Trial in Patients With Hematologic Malignancy. Clin Pharmacol Ther. 2009;85:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCune JS, Batchelder A, Deeg HJ, et al. Cyclophosphamide following Targeted Oral Busulfan as Conditioning for Hematopoietic Cell Transplantation: Pharmacokinetics, Liver Toxicity, and Mortality. Biol Blood Marrow Transplant. 2007;13:853–862. [DOI] [PubMed] [Google Scholar]
- 7.Palmer J, McCune JS, Perales MA, et al. Personalizing Busulfan-Based Conditioning: Considerations from the American Society for Blood and Marrow Transplantation Practice Guidelines Committee. Biol Blood Marrow Transplant. 2016;22:1915–1925. [DOI] [PubMed] [Google Scholar]
- 8.DeLeve LD. Cellular target of cyclophosphamide toxicity in the murine liver: role of glutathione and site of metabolic activation. Hepatology. 1996;24:830–837. [DOI] [PubMed] [Google Scholar]
- 9.DeLeve LD, Wang X. Role of oxidative stress and glutathione in busulfan toxicity in cultured murine hepatocytes. Pharmacology. 2000;60:143–154. [DOI] [PubMed] [Google Scholar]
- 10.Hassan M, Oberg G, Bjorkholm M, Wallin I, Lindgren M. Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. Cancer Chemother Pharmacol. 1993;33:181–186. [DOI] [PubMed] [Google Scholar]
- 11.Kangarloo SB, Naveed F, Ng ES, et al. Development and validation of a test dose strategy for once-daily i.v. busulfan: importance of fixed infusion rate dosing. Biol Blood Marrow Transplant. 2012;18:295–301. [DOI] [PubMed] [Google Scholar]
- 12.Beumer JH, Owzar K, Lewis LD, et al. Effect of age on the pharmacokinetics of busulfan in patients undergoing hematopoietic cell transplantation; an alliance study (CALGB 10503, 19808, and 100103). Cancer Chemother Pharmacol. 2014;74:927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paci A, Vassal G, Moshous D, et al. Pharmacokinetic behavior and appraisal of intravenous busulfan dosing in infants and older children: the results of a population pharmacokinetic study from a large pediatric cohort undergoing hematopoietic stem-cell transplantation. Ther Drug Monit. 2012;34:198–208. [DOI] [PubMed] [Google Scholar]
- 14.Madden T, de Lima M, Thapar N, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant. 2007;13:56–64. [DOI] [PubMed] [Google Scholar]
- 15.Bubalo J, Kovascovics T, Meyers G, al. E. Clonazepam and levetiracetam for prevention of busulfan-induced seizures: A single-center experience. Biol Blood Marrow Transplant. 2008;14:165.18162238 [Google Scholar]
- 16.Soni S, Skeens M, Termuhlen AM, Bajwa RP, Gross TG, Pai V. Levetiracetam for busulfan-induced seizure prophylaxis in children undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2012;59:762–764. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama K, Kume T, Fukaya M, et al. Comparison of levetiracetam with phenytoin for the prevention of intravenous busulfan-induced seizures in hematopoietic cell transplantation recipients. Cancer Chemother Pharmacol. 2018. October;82:717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. [DOI] [PubMed] [Google Scholar]
- 20.Marcus RE, Goldman JM. Convulsions due to high-dose busulphan. Lancet. 1984;2:1463. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann O, Benhamou E, Beaujean F, et al. High-dose busulfan and cyclophosphamide with autologous bone marrow transplantation support in advanced malignancies in children: a phase II study. J Clin Oncol. 1986;4:1804–1810. [DOI] [PubMed] [Google Scholar]
- 22.Vassal G, Deroussent A, Hartmann O, et al. Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. Cancer Res. 1990;50:6203–6207. [PubMed] [Google Scholar]
- 23.Murphy CP, Harden EA, Thompson JM. Generalized seizures secondary to high-dose busulfan therapy. Ann Pharmacother. 1992;26:30–31. [DOI] [PubMed] [Google Scholar]
- 24.Hassan M, Ehrsson H, Smedmyr B, et al. Cerebrospinal fluid and plasma concentrations of busulfan during high- dose therapy. Bone Marrow Transplant. 1989;4:113–114. [PubMed] [Google Scholar]
- 25.Karceski S, Morrell MJ, Carpenter D. Treatment of epilepsy in adults: expert opinion, 2005. Epilepsy & behavior : E&B. 2005;7 Suppl 1:S1–64; quiz S65–67. [DOI] [PubMed] [Google Scholar]
- 26.Wheless JW, Clarke DF, Carpenter D. Treatment of pediatric epilepsy: expert opinion, 2005. Journal of child neurology. 2005;20 Suppl 1:S1–56; quiz S59–60. [DOI] [PubMed] [Google Scholar]
- 27.Springate J, Chan K, Lu H, Davies S, Taub M. Toxicity of ifosfamide and its metabolite chloroacetaldehyde in cultured renal tubule cells. In Vitro Cell Dev Biol Anim. 1999;35:314–317. [DOI] [PubMed] [Google Scholar]
- 28.Mohrmann M, Pauli A, Walkenhorst H, Schonfeld B, Brandis M. Effect of ifosfamide metabolites on sodium-dependent phosphate transport in a model of proximal tubular cells (LLC-PK1) in culture. Ren Physiol Biochem. 1993;16:285–298. [DOI] [PubMed] [Google Scholar]
- 29.Springate JE. Ifosfamide metabolite chloroacetaldehyde causes renal dysfunction in vivo. J Appl Toxicol. 1997;17:75–79. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Barouch S, Cohen O, Vidal L, Avivi I, Ram R. Busulfan fludarabine vs busulfan cyclophosphamide as a preparative regimen before allogeneic hematopoietic cell transplantation: systematic review and meta-analysis. Bone Marrow Transplant. 2016;51:232–240. [DOI] [PubMed] [Google Scholar]
- 31.Rambaldi A, Grassi A, Masciulli A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2015;16:1525–1536. [DOI] [PubMed] [Google Scholar]
- 32.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared to TBI. Blood. 2013;122:3863–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato M, Takahashi Y, Tomizawa D, et al. Comparison of intravenous with oral busulfan in allogeneic hematopoietic stem cell transplantation with myeloablative conditioning regimens for pediatric acute leukemia. Biol Blood Marrow Transplant. 2013;19:1690–1694. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Choi SJ, Lee JH, et al. Decreased incidence of hepatic veno-occlusive disease and fewer hemostatic derangements associated with intravenous busulfan vs oral busulfan in adults conditioned with busulfan + cyclophosphamide for allogeneic bone marrow transplantation. Ann Hematol. 2005;84:321–330. [DOI] [PubMed] [Google Scholar]
- 35.Kim SE, Lee JH, Choi SJ, Lee JH, Ryu SG, Lee KH. Morbidity and non-relapse mortality after allogeneic bone marrow transplantation in adult leukemia patients conditioned with busulfan plus cyclophosphamide: a retrospective comparison of oral versus intravenous busulfan. Haematologica. 2005;90:285–286. [PubMed] [Google Scholar]
- 36.Sobecks RM, Rybicki L, Yurch M, et al. Intravenous compared with oral busulfan as preparation for allogeneic hematopoietic progenitor cell transplantation for AML and MDS. Bone Marrow Transplant. 2012;47:633–638. [DOI] [PubMed] [Google Scholar]
- 37.Lombardi LR1, Kanakry CG1, Zahurak M2, et al. Therapeutic drug monitoring for either oral or intravenous busulfan when combined with pre- and post-transplantation cyclophosphamide. Leuk Lymphoma. 2016;57:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493–500. [DOI] [PubMed] [Google Scholar]
- 39.Hassan M, Ljungman P, Ringden O, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplantation. 2000;25:915–924. [DOI] [PubMed] [Google Scholar]
- 40.McCune JS, Baker KS, Blough DK, et al. Variation in Prescribing Patterns and Therapeutic Drug Monitoring of Intravenous Busulfan in Pediatric Hematopoietic Cell Transplant Recipients. J Clin Pharmacol. 2013;53:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genentech USA Inc. Klonopin (clonazepam) package insert. 2013.
- 42.UCB Inc. Keppra (levetiracetam) package insert. 2017.
- 43.Mylan Institutional Inc. Phenytoin sodium (Dilantin)) package insert. 2012.
- 44.Joerger M, Schellens JH, Beijnen JH. Therapeutic drug monitoring of non-anticancer drugs in cancer patients. Methods Find Exp Clin Pharmacol. 2004;26:531–545. [DOI] [PubMed] [Google Scholar]
- 45.Caudle KE, Rettie AE, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther. 2014;96:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Copyright University of Washington 1999–2016. UW Metabolism and Transport Drug Interaction Database, accessed: January 22, 2016. [DOI] [PMC free article] [PubMed]
- 47.Silverman AK, Fairley J, Wong RC. Cutaneous and immunologic reactions to phenytoin. J Am Acad Dermatol. 1988;18:721–741. [DOI] [PubMed] [Google Scholar]
- 48.Marik P Anticonvulsant hypersensitivity syndrome occurring as sepsis with multiorgan dysfunction. Pharmacotherapy. 1999;19:346–348. [DOI] [PubMed] [Google Scholar]
- 49.Blackburn SC, Oliart AD, Garcia Rodriguez LA, Perez Gutthann S. Antiepileptics and blood dyscrasias: a cohort study. Pharmacotherapy. 1998;18:1277–1283. [PubMed] [Google Scholar]
- 50.Raccor BS, Claessens AJ, Dinh JC, et al. Potential contribution of cytochrome P450 2B6 to hepatic 4-hydroxycyclophosphamide formation in vitro and in vivo. Drug Metab Dispos. 2012;40:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.