Abstract
This article reviews the current status of skin tissue equivalents that have emerged as relevant tools in commercial and therapeutic product development applications. Due to rise of animal welfare concerns, numerous companies have designed skin model alternatives to assess the efficacy of pharmaceutical, skincare and cosmetic products in an in vitro setting, decreasing the dependency on such methods. Several validated test methods are performed on such models to investigate various characteristics including skin irritation, permeation, corrosion and hydration, genotoxicity and absorption. Skin models have also made an impact in determining the root causes of skin diseases and providing a means for developing an effective treatment in certain clinical applications. When designing a skin model there are various chemical and physical considerations that need to be considered to produce a biomimetic design. This includes designing a structure that mimics the structural characteristics and mechanical strength needed for tribological property measurement and toxicological testing. Presently, various commercial products have made significant progress towards achieving a native skin alternative, with focuses on one or more of the aforementioned considerations. Further research perspectives involve development of a functional bi-layered model that mimics the constituent properties such as viscoelasticity and organization of the native epidermis and dermis, as well as incorporation of dynamic elements to mimic skin’s interactions with other organs. In this article, the skin models are divided into three categories: In Vitro Epidermal Skin Equivalents, In Vitro Full Thickness Skin Equivalents, and Clinical Skin Equivalents. A description of skin model characteristics, testing methods, applications, and potential improvements is presented.
Keywords: Skin, Pharmaceutical, Cosmetic, Product Testing, Tribology, Commercial
Graphical Abstract
This article reviews the current status of skin tissue equivalents that have emerged as relevant tools in commercial and therapeutic product development applications with an aim to reduce dependency on animal models. A description of skin model characteristics, testing methods, applications, and potential improvements is presented. With the continuous research and development of such skin models, a fully functional tri-layered model replicating the properties of the epidermis, dermis, and hypodermis can be expected in coming years.
Introduction
Skin models are common tools utilized in in vitro applications for product evaluation and testing. Skin equivalents are applicable in a wide range of industries such as in skincare, cosmetics, and pharmaceutical companies. According to the Global In-Vitro Diagnostics (IVD) Market, an estimate of approximately 76 billion dollars will be spent in product testing by 2023 in the aforementioned markets. [1] In addition to allowing for accurate testing methods for consumer products, skin models also aid in several research efforts to provide treatments for different diseases such as melanoma and psoriasis. Despite previous efforts, there is still an ever-present need for a multi-layered, completely biomimetic, chemically and physically accurate skin equivalent for use in research and product development. Current in vitro models available on the market are largely epidermal only models, including EpiSkin®, EpiDerm®, and SkinEthic®. Although certain companies have sought to develop bi-layered constructs to model both the epidermal and dermal layers, such as Epiderm FT® and StrataTest®, several limitations remain and there are still many future perspectives to further investigate.
Skin Physiology
Skin serves as the critical interface between living organisms and their external environments. Other than functioning as a barrier, it maintains body temperature, recognizes stimuli, and defends against harmful foreign agents among other roles. [2] Within the vertebrate classification, all skin is layered, readily repaired from damage, and includes an extension of the nervous system to add heightened sensory abilities for survival purposes. [2] Human skin in particular is one of the most complex structures when compared to similar organisms, qualifying it as the largest and one of the most complex organs of the human body. Special derived structures including hair follicles, eccrine sweat glands, sebaceous glands, and apocrine glands, along with heterogeneous cells, and extracellular components make the skin a physically diverse entity. [3] Variations of skin type on different locations on our bodies, as well as between different individuals, include skin thickness, composition, density of structures, and biochemical differentiation.[3] Normal and abnormal skin, as well as skin diseases, can be studied through these variations. The human skin can be categorized into three primary layers: the epidermis, dermis and hypodermis. Each of these layers is responsible for the maintenance of homeostasis of the body’s inner environment in response to its outer environment.
Layers of Skin: Epidermis, Dermis, and Hypodermis
The epidermis is the outermost layer of the skin. It is stratified and consists of a renewable epithelium that is the manifestation of progressive differentiation, keratinization and cornification. [3] Keratinocytes, melanocytes, Langerhans, and Merkel cells are examples of differentiated embryonic cells that compose the epidermis. Epidermal homeostasis is determined by proliferation rate, differentiation, apoptosis, cellular interaction, adhesion, and interaction of keratinocytes with the dermal layer below. [3] The stratum corneum is the outermost layer in the skin’s physiology. It is produced through the terminal differentiation of epidermal cells. It’s a complex multi-layered structure that provides effective cellular barrier between external environment and internal milieu of living cells. It comprises, what is traditionally known as a brick and mortar structure. Where bricks are formed of protein rich cells, called corneocytes, which are embedded into a mortar of multi-layered lipid structure. The wall of corneocytes is made of highly crosslinked proteins that is tightly bound to lipids forming cornified lipid envelope (CLE). The CLE provides the template for barrier lipids to connect with lamellar lipid layers forming the composite structure of stratum corneum. Corneocytes are mutually connected with corneodesmosomes. The cellular desquamation or exfoliation is a well-orchestrated process and aberrations result in disease conditions. [3] The melanin-producing cells of the skin are melanocytes and are differentiated from the neural crest. [3] They are generally found along the basal layer of the epidermis and in the hair bulbs. Melanosomes are the characteristic membrane-bound organelles of the melanocytes and are home to enzymatic reactions that lead to the production of melanin, or skin pigment. [3]
The dermis is the connective tissue layer beneath the epidermis and it is well connected with epidermis via dermal-epidermal junction that comprises lamina densa, lamina lucida and several proteins such as collagen anchoring fir. Dermis is the primary source of elasticity, flexibility, and tensile strength of the skin. Its functions include mechanical protection, thermal regulation, and sensory signal propagation. The fibrous connective tissue consists of collagen and elastic connective tissue, while the non-fibrous component consists of fine filamentous, glycoproteins, proteoglycans, and glycosaminoglycans. [4] Collagen composes 75% of skin mass and is integral to its elasticity. The primary collagen type within the dermis is Type I collagen. Type I collagen fiber provides the primary mechanical durability to the dermis. The dermis can be organized into two primary subcategories known as the papillary and reticular dermis. The papillary dermis is made up of small-diameter collagen fibrils and provides the primary defense against mechanical stress. [3] On the other hand, the reticular dermis consists of interwoven bundles of large-diameter collagen fibrils, which allow the skin to be elastic in nature and possess resilient mechanical properties. [4]
The hypodermis is the third skin layer and its primary function is that of protection and cushioning. Unlike the primarily fibrous composition of the dermis above it, the hypodermis is an adipose tissue and lipid rich layer and integrates with the epidermis and dermis through a complex network of nerves and vessels. Sweat glands and the bulbs of hair follicles extend into the hypodermis as well. [3] The blood supply provided to the skin varies depending on region and functionality of that region. The supply comes from cutaneous branches of the subcutaneous arteries and differentiates into three plexii: the subcutaneous plexus, the cutaneous plexus and the subpapillary plexus. Blood flow to various skin regions is studied for the purposes of subcutaneous drug delivery, dermatological studies and toxicology studies. [5]
The Tribology of Skin and Related Studies
Tribology is the study of interacting surfaces in relative motion, which applies to a range of mechanical and chemical engineering applications as well as human skin. Since the 1950s, several reviews have investigated skin’s tribological behavior. [6–10] Numerous factors must be considered when studying skin mechanics such as skin layer, anatomical region, age, skin hydration, mechanical loading type/period, mechanical load direction, and strain rate. [11] In terms of skin’s tribological and mechanical properties, the main characteristics under investigation are surface roughness, friction, adhesion, elastic modulus, and surface charge. [12] Having a thorough understanding of skin’s tribological properties allows for the improvement of friction and lubrication problems during skin-product interactions. [13] The application of a skin cream alters the aforementioned properties, which determines the efficacy of the product depending on its intended purpose. [12]
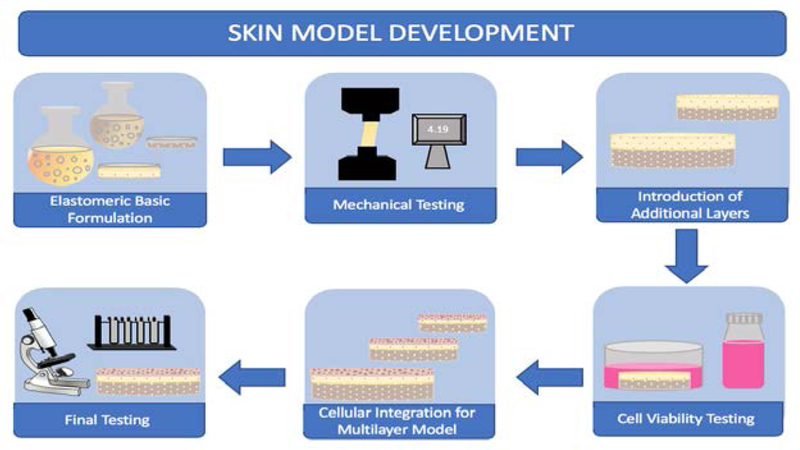
Figure 1 presents the basic example of the sequential stages in skin model development to refine the skin mimic’s characteristics. In order to develop relevant models that can be used for comprehensive applications, the model must have a standardized production process and analytical methods. [94] The process involves first determining an optimal formulation that will mechanically and chemically represent native skin. As these formulations are prepared, numerous characterization methods are used such as tensile testing (shown in Figure 1) to determine proximity of the model to native skin properties. Once the characterization methods have been established, cellular integration and subsequent viability studies will be performed to ensure that the mimic is a viable construct. A number of characterization methods such as atomic force microscopy (AFM) and nanoindentation can be used for tribological assessment. AFM and nanoindentation allow for the analysis of the tribological properties at a nanoscale. AFM utilizes a sharp tip with a radius that is typically less than 10 nm to allow for the simulation of a single point of contact. Nanoindentation acts in a similar way to establish contact at one point on the sample to examine its hardness and young’s modulus. [12] Scanning electron microscopy (SEM) has also been used for examining the surface morphological and microstructural changes upon interaction with a given surface. Additional topics of investigation for tribological properties include the coefficient of friction, contact angle and adhesive forces during topical product application. [12, 14]. Skin tribological properties have been investigated in various disciplines including skin care products, prosthetics, wound healing, and in medical and sports materials. [11, 15, 16]
Figure 1.
Simplified Stages of Skin Model Development
For example, Tang et al. utilized AFM to identify the differences in friction, adhesion, dynamic viscosity and durability between virgin rat skin and various cream-treated rat skins. Depending on the topical agent applied to the skin, the sample exhibited different properties. For instance, the application of an oil-free cream and a common cream resulted in a slippery texture which led to a low viscosity, coefficient of friction and adhesive force. Although several other conclusions were made, the study ultimately showed that the lipid layer of virgin skin does not compare to a cream film as it is too thin. This lack of thickness prevents the protection of skin from dehydration in low humidity causing the virgin skin to accumulate more surface charges than the cream-treated skin and thus have a higher surface potential. [17]
Aside from analyzing the effects of topical creams on skin’s tribological and mechanical properties, a number of comparative studies have also been conducted to determine the efficacy of an artificial skin model in mimicking native skin behavior. [18] Currently, there is a lack of consensus on this issue since there have been works with both positive and negative results. [19, 20] Moreover, it has been reported that many commercially available skin substitutes, listed later in this review, do not incorporate mechanical and textural similarity as their purpose requires imitating the biological and histological skin properties. [21] Nonetheless, a study done by Nachman et al. reported that the artificial skin model did illustrate the friction and deformation behavior of native skin. [20] In contrast, a comparative study conducted by Franklin et al. determined that as a tribological test-bed, the synthetic skin model did not provide an accurate alternative to using human skin. [19]
An additional commercially-available skin model known as SynTissue® was developed by Syndaver® Labs for design validation and efficacy testing of medical devices. [19] Franklin et al. conducted a study which investigated the suitability of the SynTissue® model for assessing skin friction behavior in both dry and moist skin conditions under three different applied surface pressures. The results were not promising as they showed that the synthetic skin model did not have comparable results to human skin in vivo and thus could not serve as an alternative model for testing the sliding contact between skin and medical devices. [19] Thus, since most products developed for dermal use involve a thorough study of their tribological interactions with skin, tribology is a critical consideration in the development of skin models.
Role of Skin Models in Product Testing
When compared to mammalian counterparts, human skin features unique characteristics that render skin behavior comparison between species inaccurate. These unique aspects include pigmentation, the dermal evolution, adipose tissue presence, and skin appendage distribution. [22] The cellular interactions of these layers together form the behavior of skin in response to exposure to certain materials and stimuli. Thus, the penetration and absorption of chemicals and materials into the human skin cannot be completely predicted by studying these phenomena on the skin of other mammals, since the structure and organization varies. This is particularly important within the context of skincare product development, as many companies currently use animal model testing methods in this process.
Early in vitro skin models consisted of two dimensional cell culture to test cell viability and any immediate effects on cells. However, as development continued with efforts towards reconstructed human epidermal (RHE) models, researchers were able to mimic the epidermis through viable and non-viable skin layers including metabolical and biomolecular properties similar to in vivo conditions.[134] Such models were adapted due to their relatively easy reproducibility and high throughput accessibility. Current efforts in skin model development include addition of dermal layer components to create a three dimensional model that can be used to study permeability and absorption. Efforts are also being made towards the improvement of limitations present in current models, such as advancement of their barrier functions.[135]
Numerous companies utilize ex vivo human skin in bioequivalence studies to determine the effects of topical pharmaceutical products. Although using human skin is the gold standard, human skin, like animal models, is subject to high variability , which could make the results of such study less accurate. [23] Current testing methods include in vitro permeation testing (IVPT), in vivo tape stripping or dermatopharmacokinetics, and in vivo microdialysis, also known as microperfusion. [24] IVPT involves the use of human dermatomed skin mounted in diffusion cells. Although there are no approved protocols for conducting IVPT studies, researchers have been able to utilize their own IVPT data as a comparison with previous literature. For example, Yang et al. used IVPT data to analyze in vivo estradiol delivery from drug-loaded patches. [25]
An additional testing method is the in vivo dermatopharmacokinetic (DPK) method which uses adhesive tape strip the stratum corneum layers for analysis of drug penetration. [26] The use of the DPK method has been investigated by the FDA for testing the bioequivalence of topical dermatological products. [27–29] This method is also subject to variability as the results depend on the operator’s technique, which may vary between individuals. [23] Finally, microdialysis is a testing technique that utilizes a very thin, semi permeable, and hollow probe that is inserted into the dermis and exchanges small diffusible molecules from the extracellular fluid into the probe. The fluid exchange allows for the determination of the concentration of unbound drug at the site in a concentration versus time relationship. [23] A more recently developed method that serves a similar purpose to microdialysis is dermal open-flow microperfusion (dOFM). dOFM is different from microdialysis due to the presence of continuous and unfiltered access to the dermal fluid. [30] Both microdialysis and dOFM require user expertise, which makes them more limited to research environments. [23] Therefore, skin models can serve a critical role within the topical drug development and evaluation process in the preclinical testing stages.
Reconstructed skin models have become a useful alternative to both human and animal skin models as they are more reproducible and allow for more consistent results. The parallel artificial membrane permeation assay (PAMPA) is a rapid screening of passive transport through a given membrane. [31] The assay is conducted in a 96-well filter plate that is coated with a liquid artificial membrane to separate the donor and acceptor compartments. The donor compartment contains a buffer solution of the compounds of interest and the other includes a fresh buffer solution. A PAMPA-skin artificial membrane was developed by Ottaviani et al. to compare the permeability of various compounds through human skin and the PAMPA-skin synthetic membrane. The study showed a positive correlation between the permeability of the two models and thus confirms the efficacy of using a synthetic skin membrane. [32]
In addition to testing the efficacy of the models themselves, there are a number of validated test methods that exist for testing specific characteristics such as irritation, corrosion, hydration, genotoxicity, and absorption to determine the efficacy of pharmaceutical and cosmetic products. Initially these testing methods would primarily involve the use of animals, but there have been many developments towards eliminating their use in the future. For example, the Draize test has been used for skin irritation and corrosion testing for several decades. [33] The test involves the single application of a topical product onto the intact skin of an animal such as a rabbit. The test substance is removed after approximately four hours and assessed immediately. The exposure to the test substance continues in specific intervals over the course of several days. Not only is this method subject to animal welfare concerns, but it also has highly variable results. [33] A validated alternative to the Draize test was the EpiSkin® test method which was recognized as the only method that could definitively discriminate between skin irritants and non-irritants. [34] Alépee et al. conducted a study to determine the reliability of the reconstructed human epidermis (RHE) model that was developed by SkinEthic®. The test consisted of topically applying test substances for 42 minutes followed by 42 hours of post-incubation. The SkinEthic® RHE test method showed good reproducibility across three independent laboratories and had reliable results with an accuracy of 85%. The overall accuracy of the SkinEthic® RHE test method resulted in its recognition by the ECVAM Scientific Advisory Committee as the only verified replacement method for the Draize rabbit in vivo test in evaluating the irritancy of test substances. [34] Similar to EpiSkin®, Epiderm® had a validated skin corrosion test protocol. The SkinEthic® RHE model was also accepted as a means to distinguish between corrosive and non-corrosive reference chemicals with an accuracy of 93%. [35]
In addition to irritation and corrosion, the hydrations effects of a test product on human skin is another important topic to investigate. Through the use of a corneometer, one can measure the hydration level or skin moisture. The corneometer is a commonly used instrument that quantifies skin moisture based on changes in the dielectric constant (ie. capacitance) of the material in interacting with the probe. [36, 37] Bazin et al. conducted a study to compare measurements of skin hydration and biomechanical properties on different portions of the face and volar forearm. The study involved two 1-hour studies and two 3- week studies in which female volunteers applied a moisturizing and firming product, respectively. The corneometer was used to measure the capacitance and in turn water content of the stratum corneum following product application. The results showed that the volar forearm did accurately represent the face in terms of hydration and biomechanical properties. [38]
Genotoxicity is another concern when testing the efficacy of cosmetic and pharmaceutical products. In short, genotoxicity is the destructive effect on a cell’s genetic material. Any substance that has a genotoxic effect is known as a genotoxin. Genotoxins include cancer-causing agents, mutation-causing agents, and birth defect-causing agents. [39] In vivo genotoxicity testing was declared impractical at the large scale that it was practiced, and this led to a ban of the testing method for cosmetic ingredients in Europe as well as in other chemical evaluation programs since 2009. [40] In order to replace the in vivo testing method, researchers have sought to develop in vitro genotoxicity assays with the use of human reconstructed skin models such as Epiderm®, which was reviewed by Kirsch-Volders et al.
Genotoxic agents have been reported to induce the formation of micronuclei (MN), which are additional nuclear bodies that contain damaged chromosome fragments that were not integrated into the nucleus following cell division. [41] The human reconstructed skin micronucleus (RSMN) assay utilized the Epiderm® model as genotoxicity test-bed to measure the induction of MN during the division of basal cell keratinocytes. [40, 42] Additional MN assays have been performed on skin models such as Episkin® which has demonstrated its relevance as a tool for studying genotoxicity. [43, 44]
Toxicology studies also involve the use of percutaneous absorption testing for hazard analysis upon skin contact as well as for developing pharmaceutical products for dermal or transdermal application. [45] The Phenion® Full-Thickness Skin Model was utilized in a parallel study with pig skin to quantify the permeation of benzoic acid, nicotine, testosterone and caffeine using Franz-type diffusion cells. [45] The results showed that the Phenion® model closely represented human skin and that it may be useful in percutaneous absorption studies upon further validation. [45] Table 1 summarizes the various testing types used to assess skin models and their relative advantages and disadvantages.
Table 1.
Skin model assessment methods and respective advantages and disadvantages
| Test Type | Examples | Advantages | Disadvantages |
|---|---|---|---|
| In Vitro | In Vitro Permeation Testing (IVPT) | Relatively faster, moderately inexpensive, high throughput methods, good correlation with in vivo clinical studies | Still may not be an accurate representation of human skin performance, currently no approved protocols for IVPT studies |
| In Vivo | Dermatopharmacokinetic (DPK) Method, Microdialysis, Dermal Open-Flow Microperfusion (dOFM) | A moderately accurate means of determining skin performance | Relatively expensive, may be more time consuming, very operator dependent results, within subject variability adverse skin inflammation |
| Pharmaceutical & Toxicology | Parallel Artificial Membrane Permeation Assay (PAMPA), Micronuclei (MN) Assay, Corneometer based hydration studies | Informs decisions on future clinical testing, determines any harmful effects, relative reproducibility of results | Inflammatory responses differ from person to person, may not be an accurate in vivo representation |
| Characterization | Atomic Force Microscopy (AFM), Nanoindentation, Scanning Electron Microscopy (SEM) | Standard characterization methods, provides details on surface properties | Depending on method and model developed, some tests may damage surface of model |
Skin Models: Research and Applications
Early skin models incorporated fibroblasts and keratinocytes onto a nylon mesh in an attempt to recreate the dermal matrix. [46]. Since then, scaffolds for skin cell culture have evolved to include more sustainable and biologically accurate biomaterials, allowing for property mimicking and skin behavior prediction. Skin models are used extensively for research and development purposes and have a number of commercial and clinical applications such as topical product permeation, product irritation, drug delivery and toxicology testing.
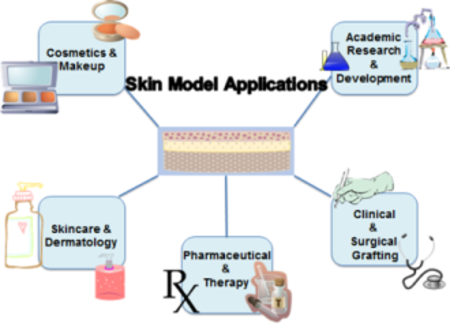
Commercial Applications
The cosmetics and skincare industry is one of the largest growing global markets, due to its continuous development of beauty and hygiene products. [136–137] These two industries can be divided into five categories: skincare products, hair care products, color cosmetics, fragrances, personal care products, and over-the-counter (OTC) products. [138] The cosmetics industry can be further organized into two subcategories: makeup and skin enhancing. Makeup products, also known as color cosmetics, are generally applied for a short term and removed or cleaned after use. Skin enhancing products like hydrating products, testing formulations for bio actives (OTCs), tanning creams or bleaching products are meant to have a more lasting impact on the skin’s appearance and may be present within the skin for a longer period of time. For these reasons, companies that develop such products need skin mimics to determine what concentration of certain compounds or chemicals will meet the product goal, without being harmful to the consumer and meeting regulatory requirements.
Skincare products are another critical application for skin models used in product development and evaluation research. Unlike makeup products and cosmetics, skincare products are meant for cleaning, moisturizing, and refreshing. These products come in the form of lotions, creams, exfoliants, scrubs, and serums among others. Common skin cleansers consist of synthetic detergents (syndets) and have low alkaline pHs, whereas most soaps are anionic surfactants with higher pHs. [47]
Due to the nature of these products, the balance between hygiene and stratum corneum barrier protection must be established. Thus, testing of such products is critical to determining the exact effect of the high charge density of the carboxyl head group as it increases protein binding on the skin surface. Most surfactants cannot distinguish sebum and oil-soluble skin soils from lipophilic substances of the intercellular lipids, thus a need for “smart” cleansers is presented. [47] Synthetic skin models aid in the development of such products through the mimicking of the cleanser-skin interface.
EpiSkin® (L’ Oreal, Lyon, France) is an example of an in vitro epidermal skin equivalent that was developed to mimic the aforementioned cleanser-skin interface among other skin interactions. The EpiSkin® model is available in two subtypes, irritation and penetration, which allows product developers to determine the effect of various topical agents. [96] Additionally, SkinEthic® (SkinEthic), EpiDerm® (MatTek Corp.), VitroSkin (IMS Inc.), Epidermal Skin Test 1000® (CellSystems Biotechnolgies GmbH), CreativeBioArray (Creative Bioarray) are all commercially available products for preliminary toxicity and permeation studies. In vitro full thickness skin equivalents such as EpidermFT® (MatTek Corp.), StrataTest® (StrataTech), and Advanced Skin Test 2000® (CellSystems Biotechnologies GmbH) are also utilized in the aforementioned studies and all of these models will be detailed further in this review.
Clinical Applications
Skin models are frequently used in clinical applications as both grafts and in vitro study models. Electrospun fiber matrices alone or in combination with hydrogels has been developed to deliver bioactive molecules and cells to promote healing. [48–55] Specifically, fibroblast and keratinocyte dermo-epidermal equivalents are used to study wound healing. The models help in the understanding of cell proliferation, re-epithelialization, epidermal differentiation, dermal remodeling and basement membrane reorganization. [56] Regeneration is a critical field of research as it provides a more permanent and self-sufficient alternative to continuous graft replacement and care. Introducing regenerative cells to synthetic skin models is key in modeling the actual regenerative properties of human skin and would reflect wound healing in response to abrasive or corrosive chemicals in products.
Fibroblasts and keratinocytes are isolated from donor skin biopsies and seeded onto bioengineered dermal constructs. To introduce the regenerative component, researchers have been able to derive pluripotent cells from adult keratinocytes and generate keratinocytes from human embryonic stem cells. [57] Therefore, skin models used in clinical applications, where this property would be most critical, incorporate regenerative cells as the derivation process is time consuming and expensive.
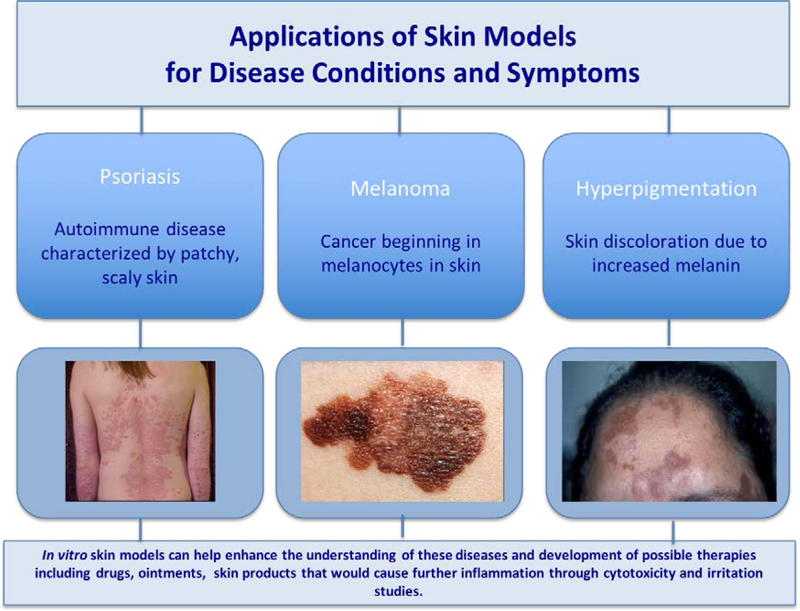
Isolated normal human keratinocytes have been used to generate human skin equivalents to address a number of diseases such as psoriasis (Figure 2). Psoriasis is an inflammatory skin disease that accelerates the life cycle of cells and results from an unfavorable interaction between the immune system and the epithelium. [58, 59] Tjabringa et al. conducted a study that aimed to develop an in vitro reconstructed skin model that would exhibit the characteristics of psoriasis. The experiments conducted on the psoriatic epidermis showed the presence of the characteristic proteins to the disease and thus illustrated the potential of the skin equivalent as an accurate disease model for developing treatments. [59]
Figure 2.
Skin Models in Treating Skin Diseases [64]
A three-dimensional human skin model has also been used to mitigate melanin synthesis in a study conducted by Sugimoto et al. The study evaluated the inhibitory effects of 4-hydroxyphenyl α-glucopyranoside (α-arbutin) on the melanogenesis of a three-dimensional human skin models with cultured human melanoma cells. [60] Hyperpigmentation is the increased production of melanin and tyrosinase. [60, 61] The results showed that α-arbutin did not inhibit cell viability but melanin synthesis in the human skin model did decrease by 40%. This showed that α-arbutin could be effectively used as an ingredient for skin lightening and that the skin model was an effective vehicle to deliver this result. [61] The role of in vitro skin models in disease treatment and prevention has been further investigated in other reviews. [62, 63]
Drug Delivery and Pharmaceutical Applications
Traditional two-dimensional epidermal skin models cannot accurately depict efficacy of a drugs however, due to their usually metabolic nature. In these cases, a three-dimensional skin model is used in testing. Although these models help address the physical distribution of drug, issues remain regarding penetration, metabolism, and action of drug. Research on “organ-on-a-chip” style models includes incorporating vascularization and introduction of gland constructs, as these dermal components play a role in drug distribution. Although a number of companies have now begun developing “full thickness” or dermal incorporated models, further prospects in this area will involve introduction of dynamic cellular factors to properly mimic pharmacokinetic properties of subcutaneously delivered drugs. [65]
Chemical and Physical Considerations of Skin Models
In order to ensure an accurate representation of human skin, there are a number of characteristics that such skin models must possess. From the tissue engineering perspective, in order to support surface cell culture and seeding, the presence of a biodegradable, three-dimensional structured scaffold matrixes is imperative. [66] This also presents a potential for a regenerative approach to skin models; as the cells are seeded and begin to proliferate, the underlying scaffold degrades leaving behind the natural extracellular matrix for cells to structure themselves.
Since most clinical applications require a seeded cell culture on scaffold-based models, it is imperative that cells are reliably sourced and isolated from a dependable line that would provide an accurate representation of in vivo behavior. [66] To ensure proliferation and proper growth, the accompanying growth factors are also an important consideration for model design. [66] Bioreactors are often commonly used in skin model development as they allow cellular growth and proliferation, feature a polymeric matrix for structural support, and contain an in vivo-reflecting media environment. [66]
The matrix scaffold itself is a critical component of a skin model and often include components of natural macromolecules like type I collagen, glycosaminoglycan, and chitosan and synthetic polymers such as aliphatic polyesters, lactide, glycolide, and ε-caprolactone. [66] Recently, hydrogel-based polymer matrices are being researched as a novel component of these scaffolds’ matrices. These hydrogels are commonly made of agarose, alginate, chitosan, collagen, hyaluronan, and fibrin. [67] The advantage of incorporating hydrogels into skin model design are two-fold as they contain structural components similar to the natural extracellular matrix as well as a sufficiently high water content and can thus be used as vehicles for novel drug delivery into the models. [68] Another relatively newer approach to skin model development is the use of decellularization methods. Decellularization is a technique often used in regenerative medicine that results in a natural bioscaffold that can be seeded with host cells and developed. The technique involves the selective removal of cellular tissue from an organ, leaving only the extracellular matrix and vasculature behind. Although not plausible for mass production of skin models due to the limitation of donor organs and excessive time dependency, the decellularization method could be increasingly useful for vasculature-dependent applications, such as product permeation and drug delivery studies. [69]
There are a few critical components of the microenvironment that must be controlled during skin development including the ECM, dermal substrates, fibroblasts and other cellular components. Dermal fibroblasts and epidermal cells both produce the molecules, fibrous proteins, and proteoglycans, which in turn provide strength and flexibility to the overall structure of skin. The ECM also serves as a critical anchor to cell attachment and development and is thus an integral component to many skin models, whether in a natural or synthetic capacity. [70]
Dermal substrates include cellular structures and fibroblast-populated collagen matrix, both of which enhance formation of basement membrane proteins and increase epidermal attachment overall. [71] Contraction of the collagen matrix is a key issue that must be addressed when developing skin models in order to maintain viability of the model over an extended period of time. To combat this, Stark et al. used an esterified hyaluronic material with fibroblast as an alternative to collagenous constructs, which showed improvement over a 12-week cell growth and differentiation period. [72, 73] Due to the three-dimensional nature of cell culture on epidermal-dermal models, alternative cells such as myofibroblasts, endothelial cells, inflammatory cells, and adipocytes all enhance the microenvironment. [70]
When considering the use of immortalized cell lines or primary cells during seeding of skin models, it is important to consider their primary application. Essentially, the primary cells are useful in the evaluation of differences in epithelial maturation, but have a relatively shorter life span, and cannot be used to create multiple duplicates, which are often critical to studies. Immortalized cell lines on the other hand increase reproducibility and decrease variation between samples during testing and are more useful in industrial applications. [70]
Another critical component of in vitro skin equivalent is replication of the immune responses that native skin exhibits, especially in irritation and surface toxicity studies. Since these models are not connected to a complete immune system, this becomes a challenge to overcome. In the skin, Langerhans cells are primarily responsible for initiating an immune response to foreign particles. Incorporating Langerhans cells in in synthetic models is difficult due to their inability to be subcultured and expanded in vitro. [74] Regnier et al. were able to generate dendritic cells/Langerhans cells from CD34+ hematopoietic progenitors that showed similar functionality to native Langerhans cells. [74] In general, skin models for the purpose of in vitro product evaluation have several chemical, biological, and physical considerations that they must meet to be effective as mediums of testing.
Commercially Available In Vitro Skin Models
Reconstructed human epidermal models include the validated EpiDerm® and EpiSkin® models and have a higher degree of standardization. Disadvantages include impairment of the barrier function, low complexity, and a narrow testing period. These models are used primarily for skin irritation, skin corrosion, phototoxicity, epidermal genotoxicity, transdermal drug delivery, skin sensitization, and metabolism. [56] RHE models with melanocytes are used for applications of skin lightening and pigmentation.
Full thickness (FT) models are generally used to test percutaneous absorption, wound healing, and bacterial adhesion. The advantage of these models is that they are standardized and available commercially. However, the drawback to FT models is their cost and inability to sustain long-term cell culture. [93] FT models enhanced with melanocytes are useful for investigation of melanogenetic proteins and assessment of vitiligo pathogenesis. Langerhans enhanced FT cells are useful in assessing allergens and the investigation of the maturation and migration of Langerhans cells. Angiostatic therapies and adipose metabolism can be assessed with FT endothelial and subcutis models. [93] A significant disadvantage of these types of models is the lack of validated and standardized commercial availability as well as their technically demanding nature. [93] FT models with stem cells are used in the assessment of epidermal development; wound healing studies, pigmentation disorders, substance penetration, and enabling of autologous transplantation. Feasibility is yet to be established with such models and require significant preparation steps and nuanced protocol for use. Hair follicles may simultaneously be included in FT models and are important for product penetration studies, but they have lower throughput methods and are generally not considered along with other skin components. [93]
In general, simple polymeric models are used in the study of diffusion and lipid-based models are used for screening. The disadvantage to these artificial membranes is that although they are able to mimic physical properties well, they are still not representative of skin as a whole. [56] Reconstructed skin models are able to target certain properties for research such as the barrier function or disease features, but these models are generally more permeable than natural human skin.
Clinical skin replacements and grafts include Integra®, Apligraf®, and Epicel®, while full thickness skin models include EpidermFT® and StrataTest®; epidermal models include SkinEthic®, EpiSkin®, and EpiDerm®, which will be discussed later. Integra DRT® is developed by Integra Lifesciences and is composed of a thin silicone film with a porous matrix of bovine collagen and glycosaminoglycan. Apligraf® is a product of the Organogenesis company and includes fibroblasts and collagen together in a dermal matrix on to which keratinocytes may be seeded. Epicel®, by Genzyme, incorporates autologous keratinocytes from ex vivo development alongside murine fibroblasts. [66]
Although the gold standard for testing and evaluation is the use of in vivo human skin models, potentially toxic or unknown materials are difficult to evaluate and remain ethically controversial. [23] Ex vivo skin models are however commonly used as a substitute. These models are generally obtained from excess materials from plastic surgery or cadavers, deriving from abdominal, breast, or back skin. [94] Such models are critical for topical drug studies in monitoring absorption and distribution across skin surface, which is important to the pharmacokinetic behavior of drugs.
Artificial and reconstructed skin models are used in preliminary pharmaceutical studies to determine passive transport mechanisms of such topical medications. [94] These models are preferred in preclinical pharmaceutical research and development due to their relative reproducibility and controllable passive transport characteristics. A number of assays are used in conjunction with these artificial models to test the effective absorption and rapid screening of these drugs including a lipid based parallel artificial membrane-permeability assay (PAMPA) and a phospholipid vesicle-based permeation-assay membrane. [23] A prominent challenge with artificial skin models is the difficulty in mimicking permeation of drugs that are delivered transdermally in combination with others. [95] Human skin’s barrier function is complicated and difficult to artificially simulate due to the lack of the equivalents for dermal features such as vasculature, glands, and lipids that all affect permeation.
Reconstructed human skin models are composed of skin cells cultured on an artificial polymeric matrix. Commercially available reconstructed human epidermal models include EpiSkin®, SkinEthic®, and Epiderm®. Living skin equivalents that are commercially available include GraftSkin®, EpidermFT®, and Phenion®. Although living skin equivalents are crucial to specific clinical applications, most in vitro studies employ the synthesized human epidermal models.
In Vitro Epidermal Skin Equivalents
EpiSkin®
EpiSkin® is a model that was acquired by L’Oreal in 1997 and is marketed as a 12 well plate system. [96] It is comprised of a type 1 bovine collagen matrix, type IV human collagen, and passaged human keratinocyte cells. Within a batch of these models the variability is low, but between batches, variation does exist. [96] The viable cells of the EpiSkin® model are organized differently than the native epidermis, there are changes in cell shape in the suprabasal compartment. The basal cells are cubical and the upper cell layers are flat. [96] The lamellar body, as well as the granular cells with keratohyalin is irregular as compared to the native epidermis. The two subtypes of EpiSkin® (irritation and penetration) vary in the thickness packing of the stratum corneum, with the penetration model exhibiting a tighter packed, thicker characteristic.
The phospholipid content of the EpiSkin® irritation model matches that of the human epidermis, however in the penetration model, this correlation of phospholipid presence is reduced. [97] The amount of free fatty acids and cholesterol esters in the models was lower than in native tissue. A large variation of phospholipid content in the models between batches was observed. [97, 98] Although there is no direct correlation model between a particular lipid class and drug transport, an increase in triglycerides and lipid retention within cornified cells is associated with hyperproliferation and impaired barrier function. [96] EpiSkin® also contains certain key biochemical markers such as Keratin 1, 6, and 10, SKALP, SPRR2 and SPRR2. [98]
One irritation study done on the EpiSkin® models shows a correlation in cytotoxic potential (r=0.93; n=23; P<0.00001), impairment of barrier function (r=0.87; n=20; P<0.00001), and cutaneous irritation (r=0.81; n=20; P<0.0001). [99, 100] Another study found that the concentration of surfactants needed to trigger a reaction is the model was lower than was needed in vivo. [96] In permeability studies, caffeine permeation was monitored and found to exhibit the same behavior as human skin. [101]
SkinEthic®
SkinEthic® is an artificial human skin model originating from the Martin Rosdy laboratories in Nice, France. [96, 102] The SkinEthic model consists of a stratum corneum, stratum granulosum, and stratum spinosum. Electron microscopy of the model depicts the presence of lamina densa components and anchoring filaments, which mimic the actual basement membrane. [96] Differing from the native epidermis however the SkinEthic® model has lipid droplets distributed throughout and alternating electron-dense and electron-lucent lipid lamellar sheets. [98] Although the general lipid composition of SkinEthic® matches that of native tissue, there is a higher ceramide 2 presence and ceramide 7 is lacking. [98]
In terms of biochemical markers, the SkinEthic® model contains keratin 1, 10, SPRR, SPRR3, loricrin, involucrin, and transglutaminase, which are native to human epidermis, as well as Keratin 6, and SKALP which are not. [98] Following toxicity studies, the model was able to differentiate between phototoxic and nonphototoxic compounds. [103] Similarly, irritation was tested on the SkinEthic® model using sodium lauryl sulfate, calcipotriol and trans-retinoic acid; cytokine expression and inflammatory levels correlated with results of a similar study done on human skin. [104] Transport studies done on SkinEthic® with lauric acid, caffeine and mannitol showed lower transport rates as compared to those in human skin. [105]
Epiderm®
EpiDerm® is an in vitro skin model marketed by MatTek Corporation from Ashland, MA. Its factsheet describes it as “normal human derived epidermal keratinocytes (NHEK) which have been cultured to form a multilayered, highly differentiated model of the human epidermis”. [96, 106] In terms of its morphology, EpiDerm is similar to the human epidermis with a difference in keratinocytes that are grown on polycarbonate filters and thus do not exhibit Rete ridges present in the native epidermal-dermal junction.
The lipid composition of EpiDerm® is comparable to native human skin, however there was a lower concentration of cholesterolesters and free fatty acids. [98] Biochemical markers include keratin 1, 10, and 6 in both EpiDerm® irritation and penetration models, along with transglutaminase and loricrin. [98] Following toxicity studies, the model accurately determined the presence of certain phototoxic compounds, confirmed with an MTT assay. [107, 108] In irritancy studies however, EpiDerm® cultures did differ from the response of human skin and it was concluded this was due to the suboptimal barrier function of the model compared to native human skin. Thus, it was determined that the concentration of irritable surfactant needed to elicit a reaction was lower for the model. [109] However, another study looking at the reaction of EpiDerm® to 22 cosmetic formulations found a positive correlation between the irritation reaction of the model to that of human skin, deeming it an accurate portrayer of potential cosmetic irritability. [110]
In permeation studies, it was found that the maximum transport rate was reached faster in the EpiDerm® model when compared to native human skin, however, a prolonged application of hydrogel on the skin model leads to a decrease in permeation. Overall the rank order of permeability was about the same between the model and human skin. [101] Another transport study, this time with the lipophilic drug flufenamic acid, demonstrated a higher permeation and transport rate in the model than in native tissue. [65]
Additional In Vitro Epidermal Skin Equivalents
Epidermal Skin Test 1000® (CellSystems Biotechnologie GmbH) is an epidermal reconstructed model composed of primary human keratinocytes, resulting in differentiated cornified skin layers and is primarily used for skin corrosiveness testing. [66, 78] Similarly, CreativeBioArray (Creative Bioarray) is human keratinocytes cultured on inert polycarbonate filters in medium and includes specific markers such fillaggrin, involucrin, loricrin, keratin 10, and keratin 5 which make it ideal for skin hydration, skin irritation, corrosion, and UV testing among others. [79] Biomimiq’s human epidermal equivalent features eight epidermal cell layers consisting of human keratinocytes and features toxicity, corrosiveness, and irritation testing as well. [80] RHE/001 (StratiCell) on the other hand features a polycarbonate cell-seeded filter in culture media and is useful for penetration, absorption, and mechanical testing. [81] For the specific applications of SPF/UVA testing or cosmetics development, VitroSkin (IMS Inc.) is a useful product; it features formulated substrate that mimics skin topography and other properties like pH and surface tension. [82]
Although all of these reconstructed epidermal models present certain similar properties to skin and can be used in preliminary toxicity and permeation studies, they still present a number of limitations. The greatest limitation is their relatively weaker barrier function, which leads to increased permeability and thus inaccurate transport results in comparison. [96] It is evident that a functioning skin model that depicts both the epidermis and dermis is crucial to the complete and accurate depiction of skin characteristics in vivo. [111] However, developing a fully functioning dermal layer in vitro presents unique challenges in itself as it becomes difficult to model internal vasculature and gland compositions that play a significant role in product permeation and transport. [112]
The primary reason for reported differences between synthetic skin mimics and human skin is their maturation process. While reconstructed skin models are generally a result of a temporary incubation, native human skin is a result of a number of internal and external factors over a lifetime, thereby increasing the variation between in vivo samples. [113] Tfayli et al. determined through Raman imaging that lipids of the EpiDerm® model were present as either droplets or in segregated zones, which differs from skin’s natural continuous barrier, citing this as the primary point of different in permeation characteristics. [113]
In Vitro Full Thickness Skin Equivalents
EpidermFT® and StrataTest®
The prevalent current need in the in vitro skin equivalents market is that of a bi-layered epidermis/dermis representative model. MatTek developed a full thickness model called the EpidermFT®, which features neonatal human-derived dermal fibroblasts and neonatal human-derived epidermal keratinocytes in co-culture that form a multi-layered differential epidermal-dermal model. [66] Mallampati et al. evaluated irritation on the EpidermFT-300® model as compared to the in vivo irritation model; both showed an increase in trans epidermal water loss and erythema scores as aliphatic hydrocarbon length increased, proving the EpidermFT-300®’s efficacy in reflecting in vivo irritation behavior. [114]
StrataTest® is a full thickness skin model developed by StrataTech that utilizes a near-diploid human keratinocyte cell line. [66] Rasmussen et al. determined StrataTest® ‘s effectiveness through the detection of reactive oxygen species following exposure to ozone, cigarette smoke, and ultraviolet irradiation. They concluded that the StrataTest® model has a wide variety of applications in toxicological assays. [83] Along with these, multiple companies are attempting to develop a fully functional bi-layered model that can improve in vitro testing significantly and reduce the dependency on animal testing, which is often inaccurate and has much variation due to differences between individual animals used. [115]
Along with their epidermal equivalent, CellSystems Biotechnologies GmbH also has a full thickness model, Advanced Skin Test 2000®.This model features embedded fibroblasts and keratinocytes and is useful in pharmaceutical and chemical product testing. [84] For genotoxicity applications and pigmentation studies, Creative Biorrary’s CreativeBioArray Full Thickness model is ideal due to its incorporation of a dermal component featuring human skin fibroblasts in collagen matrix and keratinocytes. [85] Meanwhile for more topological testing applications, Genoskin has a full thickness model called, NativeSkin, which is a round human skin biopsy prepared for long term testing. [86] Similarly, Cell Applications Inc.’s 3D skin models features human epidermal keratinocytes stratified to squamous epithelium and is useful for study of product absorption and wound healing. [87]
3D Bioprinted Skin Models
Recently, bioprinting has emerged as a high throughput technique for fabrication of engineered full thickness skin models. [116] Even though conventional tissue engineering (TE) methods have been used to mimic the layered skin architecture, these methods are time consuming, expertise dependent and costly. The TE constructs are restricted by size which can be a limiting factor for large surface area wounds. On the other hand, bioprinting can successfully replicate the micro and macro scale features of natural human skin for uneven contours and larger surface area with minimum sample handling and faster fabrication rate. [117]
Both in vitro and in situ bioprinting approaches have been used for skin models (Figure 4). For in vitro bioprinting, first approach is free-form fabrication (FFF) which uses multiple extrusion heads to layer hydrogel pre-polymer, crosslinker and cells suspension to form the stratifies skin structure. The technique was used by Lee et al. to produce in vitro epidermal/dermal skin model on planar and non-planar surface. [118] Ten layers of collagen, and its crosslinker were deposited in a 60 mm petri dish. The two main cell types used in skin models were embedded in the second (fibroblast) and eighth (keratinocyte) collagen layer. After incubation, two distinct cell layers, 75 µm apart, were formed. A layer of crosslinker solution on top and bottom of each collagen layer kept the 3D structure intact after bioprinting. Second in vitro approach is laser based bioprinting which utilizes a donor and collector slide. [119] The donor slide is coated with energy absorbing material above the printing suspension. With each laser pulse, the subjacent biomaterial deposits on the collector slide. This technique is used to fabricate layers of 2D patterns that can sum up to a 3D structure. Michael et al. used this technique to deposit 20 layers of keratinocytes and 20 layers of fibroblasts suspended in collagen on Matriderm©, an acellular skin equivalent. [120] In vitro and in vivo evaluations conducted over 11 days study revealed no gaps between epidermis and dermis layers though keratinocytes showed limited differentiation and thinner epidermis compared to normal human skin. Cited as being slower process, Cubo et al. developed a 3D bioprinting device that made printing process 100 times faster. [121] The skin equivalents used primary keratinocytes and fibroblast in human plasma which improved the epidermal thickness. Other than layering the cells, 3D bioprinting can be used to create graded biomaterial complex with spatial control over cell densities to mimic the complex microenvironment of natural human skin. Efforts are being made to bioprint skin equivalents with accessory features such as pigmentation, glands and hair follicles. Min et al. reported the addition of melanocytes to the 3D printed construct to create epidermal pigmentation. [122] The study showed successful freckle like pigmentation in epidermis without the use of UV or any chemical stimulation. For in situ bioprinting, biomaterial along with cell suspension can be directly printed on the wound site. Recently, in situ bioprinting was used to print stratified skin structure on full thickness wound of a nude mouse using amniotic fluid-derived stem cells suspended in collagen-fibrin gel. The results showed better wound closure rate and angiogenesis compared to acellular collagen gel. [123] These models were tested for full thickness wounds and they have established the basis for 3D bioprinted skin equivalents.
Figure 4.
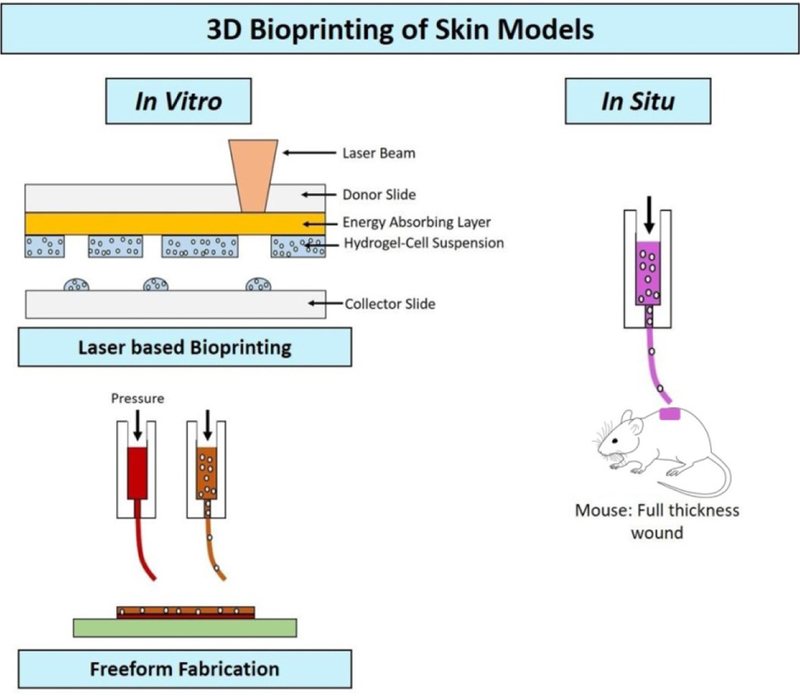
3D bioprinting strategies used for skin model fabrication 117
Clinical Skin Equivalents
Clinical skin models are used to investigate different diseases and ailments as bioequivalent comparisons to the conditions present in a human’s skin. The defects of skin caused by burns, venous or diabetic ulcers, and acute injury can lead additional infections and diseases if not treated properly. A number of artificial skin grafts have been developed and have shown potential in the treatment of injuries that require skin regeneration and repair, but challenges still remain. [124–127]
Apligraf® which was developed by Organogenesis is a dermal lattice that is composed of fibroblasts, collagen, and keratinocyte seeding to mimic the native human epidermis. The skin equivalent allows for continuous wound healing with the use of protective tissue and proteins. [88] Epicel® is an additional model that is used for wound healing and was fabricated by Genzyme. Epicel® enables healing in patients affected by various degrees of burns through the ex vivo growth of autologous keratinocytes with murine or rodent fibroblasts. [89] In addition, FortiCell Bioscience designed Orcel® which also utilizes the culture of human epidermal keratinocytes as well as the dermal fibroblasts on a Type I bovine collagen sponge to aid in limb wound healing that is caused by skin ulcers or diabetes. [90] A porous bovine collagen mechanism is also utilized in the Integra Lifesciences model, Integra® Dermal Regeneration Template, in combination with glycosaminoglycan which is covered with a thin silicone film. The Integra® Dermal Regeneration Template is especially applicable in deep partial thickness or full thickness burn injuries as well as in reconstructive surgery applications. Finally, Ex Vivo Skin is an ex vivo post abdominal surgery derived model designed by Straticell. Ex Vivo Skin was used to investigate percutaneous absorption of different agents that come in contact with skin. [92]
Potential Improvements and Future Investigations
Most improvements being made to the current in vitro skin models involve the incorporation of a dynamic element to the mimics. Atac et al. incorporated a chip-based bioreactor platform to apply varying mechanical shear stress and increasing culture viability through a changeable media construct. [129] The principle used here is that of “organ-on-a-chip.” Organ-on-a-chip systems are essentially microfluidic based biomimetic systems used to study dynamic organ behavior on a small scale, through cell culture for various applications including drug delivery and toxicology. [130] These organs on chip systems can be combined together to study a group of organs’ responses to a single stimulus representative of their in vivo behavior.
Maschmeyer et al. combined co-cultures of human intestine, liver, skin and kidney equivalents to a four-part organ-on-a-chip system to monitor drug delivery, transport, and toxicity through these systems. [131] In fact, organ-on-a-chip systems are common for drug delivery and toxicity studies due to their composition of microfluidic channels and pumps that artificially mimic blood and lymph movement through constant media change and drainage, allowing for an accurate depiction of pharmacokinetic and pharmacodynamics behavior of drugs. [132]
For the purpose of in vitro skin models, a dynamic organ a chip-based culture could mitigate the need for a synthetically developed dermal layer by reproducing its fluidity and transport mechanisms through fluid exchange. Since the dermal layer of human skin is composed of blood vessels, it is crucial to reflect their behavior in any synthetic model. However, incorporating such vessels into a static artificially synthesized model is difficult if the fluid (mimicking blood) is not continually being pumped, thereby mimicking the behavior of the heart. Wufuer et al. developed a skin-on-a-chip model that mimics the epidermal, dermal, and endothelial layers of native skin. The cells for the culture were derived from human skin cells through porous membranes to maintain interlayer communication of cells. Through cytokine expression analysis and permeability studies, their model demonstrated an accurate depiction of drug toxicity levels and shows promise for skin disease modeling and evaluation of cosmetics and pharmaceuticals. [133]
Conclusion
Skin equivalents are critical components of the in vitro testing and evaluation of skincare products and therapeutic applications. They incorporate certain synthetic components along with natural components to mimic native skin tissue’s response to applied pressure, chemicals, and other interactions. The skin models present on the market today are largely representative of the epidermal layer, presenting a further need for development. For example, an improved barrier function in startum corneum, a better representation of the epidermal-dermal junction that represents skin’s viscoelastic properties and incorporation of an accurate immune response are all possible future perspectives in the development of such skin models. Once the models themselves are developed, future perspectives involve the interaction of these models with simulated organs in artificial close looped systems through methods like “organ-on-a-chip.” Along with efficacy, models must be reproducible and demonstrate similar results following numerous irritancies, toxicology, and permeation tests. Once developed, however, skin equivalents will significantly reduce dependency on current animal models and provide an accurate and relatively definitive product testing method. With the continuous research and development of such skin models, a fully functional tri-layered model replicating the properties of the epidermis, dermis, and hypodermis can be expected in coming years.
Figure 3.
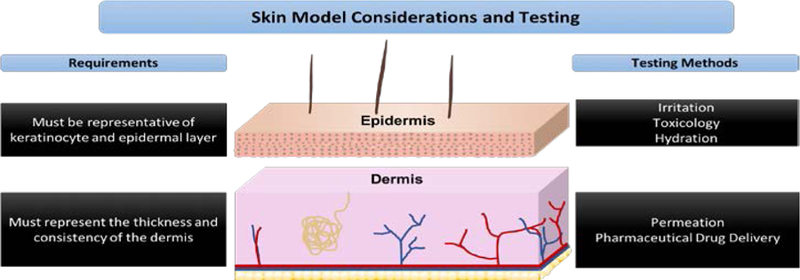
Fundamental skin model considerations and testing of the epidermal and dermal layer
Table 2.
Commercially available skin equivalents
| In Vitro Epidermal Skin Equivalents | |||
|---|---|---|---|
| Model Name | Company | Product Description | Application for Testing |
| SkinEthic Rhe (Reconstructed Human Epidermis)® | SkinEthic | Culture of human keratinocytes on polycarbonate filter in chemical based medium [66] | Skin corrosiveness, skin irritation, UV exposure, bacterial adhesion, DNA damage, omics, permeation [75] |
| Episkin® | SkinEthic | Culture of human keratinocytes on collagen base leading to reconstruction of epidermis with stratum corneum [66] | Skin corrosiveness, skin irritation, UV exposure, bacterial adhesion, DNA damage, omics, permeation [76] |
| Epiderm® | MatTek | Culture of neonatal human-derived epidermal keratinocytes (NHEK) forming differential mimic of human epidermis [66] | Skin corrosiveness skin irritation, hydration, genotoxicity, dermal drug delivery, phototoxicity [77] |
| Epidermal Skin Test 1000® | CellSystems Biotechnologie GmbH | Reconstructed skin equivalent from primary human filter [80] keratinocytes resulting in cornified skin layers and multi-layer differentiation [66] | Skin corrosiveness [78] |
| CreativeBioArray Reconstructed Human Epidermis | Creative Bioarray | Human keratinocytes cultured on inert polycarbonate filter in medium; present with specific markers including filaggrin, involucrin, loricrin, keratin 10, and keratin 5 [79] | Skin hydration, skin irritation, skin corrosion, UV exposure, DNA damage, omics, permeability [79] |
| Biomimiq Epidermal Skin Model | Biomimiq | Eight epidermal cell layers through culture of primary human keratinocytes aired on inert acellular filter [80] | Toxicity, skin infection, skin corrosiveness and skin irritation testing [80] |
| RHE/001 | StratiCell | Polycarbonate cell-seeded filters in culture media reconstituted to match native epidermis [81] | Penetration, absorption, and mechanical testing [81] |
| VitroSkin | IMS Inc. | Formulated substrate that mimics skin topography, pH, critical surface tension, and ionic strength [82] | SPF/UVA testing, cosmetics, tanning development [82] |
| In Vitro Full Thickness Skin Equivalents | |||
| Epiderm FT® | MatTek | Co-culture of neonatal human-derived dermal fibroblasts (NHFB) and NHEK mimicking both epidermic and dermis | Anti-aging, wound healing, skin hydration, UV protection [77] |
| StrataTest® | StrataTech | Culture of near-diploid human keratinocytes cell line [NIKS] | Toxicology, chemical and physical tissue damage [83] |
| Advanced Skin Test 2000® | CellSystems Biotechnologie GmbH | Embedded fibroblasts with superficial layer of keratinocytes resulting in full thickness epidermal/dermal equivalent | Pharmaceutical and chemical compound testing, toxicity, skin irritation [84] |
| CreativeBioArray Full Thickness Model | Creative Bioarray | Dermal compartment of human skin fibroblasts in collagen matrix and human keratinocytes with markers including filaggrin, involucrin, loricrin, and decorin [85] | Skin irritation, skin sensitization, genotoxicity, pigmentation, anti-aging, stress/inflammation [85] |
| NativeSkin | Genoskin | Round human skin biopsies prepared for long term testing and resilience in testing [86] | Topological testing, skin hydration and evaluation |
| 3D Skin Model | Cell Applications Inc. | Three dimensional system of human epidermal Culture of human epidermal keratinocytes and dermal keratinocytes stratified to squaous epithelium [87] | Skin irritation, phototoxicity, absorption, penetration, wound healing [87] |
| Clinical Skin Equivalents | |||
| Apligraf® | Organogenesis | Dermal matrix based combination of fibroblasts, collagen, and keratinocyte seeding to mimic epidermal skin layer | Chronic wound healing includes protective tissue and proteins [88] |
| Epicel® | Genzyme | Ex vivo growth of autologous keratinocytes with murine fibroblasts | Autologous wound healing for burn patients [89] |
| Orcel® | FortiCell Bioscience | Culture of human epidermal keratinocytes and dermal fibroblasts on Type I bovine collagen sponge | Limb wound healing from skin ulcers or diabetes [90] |
| Integra® Regeneration Dermal Template | Integra Lifesciences | Porous bovine collagen and glycosaminoglycan covered with thin silicone film | Deep partial thickness or full thickness burn injuries and reconstructive surgery applications [91] |
| Ex Vivo Skin | StratiCell | Ex vivo post abdominal surgery derived model [92] | Percutaneous absorption study [92] |
Acknowledgements:
The authors acknowledge funding support from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (R01EB020640) and the Connecticut Regenerative Medicine Research Fund (15-RMBUCHC-08). Authors also acknowledge UConn START funding support from the Office of Voice President Research.
Biography

Prof. Sangamesh Kumbar Ph.D., is an Associate Professor in the Departments of Orthopaedic Surgery, Biomedical Engineering and Materials Science & Engineering at UConn. His funded research is focused on synthesis and characterization of novel polymeric biomaterials and micro‐nanostructured matrices for tissue engineering and drug delivery applications. He teaches multiple courses on the subject area of his expertise. He is on the editorial board of Journal of Applied Polymer Science, Journal of Biomedical Materials Research‐Part B, and Journal of Biomedical Nanotechnology. He is an Associate Editor of Journal of Biomedical Nanotechnology and Bioactive Materials. He has co‐edited two text books namely Natural and Synthetic Biomedical Polymers and Bio‐Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine.
References
- [1].R.A.n.d. Ltd, Global In-Vitro Diagnostics (IVD) Market: Analysis and Forecast 2017–2023 (Focus on Product Type, Test Type, Application, and Competitive Landscape)
- [2].Freinkel RK, Woodley DT, The biology of the skin, CRC Press; 2001. [Google Scholar]
- [3].Haake A, Scott GA, Holbrook KA, Structure and function of the skin: overview of the epidermis and dermis, The biology of the skin 2001 (2001) 19–45.
- [4].Nemoto T, Kubota R, Murasawa Y, Isogai Z, Viscoelastic Properties of the Human Dermis and Other Connective Tissues and Its Relevance to Tissue Aging and Aging– Related Disease, Viscoelasticity-from theory to biological applications, InTech2012.
- [5].Riviere JE, Monteiro-Riviere NA, Dermal absorption models in toxicology and pharmacology, CRC Press; 2005. [Google Scholar]
- [6].Comaish S, Bottoms E, The skin and friction: deviations from Amonton’s laws, and the effects of hydration and lubrication, Br J Dermatol 84(1) (1971) 37–43. [DOI] [PubMed] [Google Scholar]
- [7].Dowson D, Bioengineering of the Skin: Skin Imaging and Analysis, Second Edition, illustrated ed., CRC Press; 1996. [Google Scholar]
- [8].Sivamani RK, Wu G, Maibach HI, Gitis NV, Handbook of Cosmetic Science and Technology, Third Edition, CRC Press; 2009. [Google Scholar]
- [9].Dowson D, Neville A, Bio-tribology and bio-mimetics in the operating environment, Proceedings of the Institution of Mechanical Engineers, Part J: Journal of Engineering Tribology 220(3) (2006) 109–123. [Google Scholar]
- [10].N.P.F. D., THE SKIN SURFACE AND FRICTION, British Journal of Dermatology 67(7) (1955) 239–248. [DOI] [PubMed] [Google Scholar]
- [11].Derler S, Gerhardt L-C, Tribology of Skin: Review and Analysis of Experimental Results for the Friction Coefficient of Human Skin, Tribology Letters 45(1) (2012) 1–27. [Google Scholar]
- [12].Bhushan B, Nanotribological and nanomechanical properties of skin with and without cream treatment using atomic force microscopy and nanoindentation, J Colloid Interface Sci 367(1) (2012) 1–33. [DOI] [PubMed] [Google Scholar]
- [13].Van Der Heide E, Zeng X, Masen MA, Skin tribology: Science friction?, Friction 1(2) (2013) 130–142. [Google Scholar]
- [14].Gerhardt L-C, Schiller A, Müller B, Spencer ND, Derler S, Fabrication, Characterisation and Tribological Investigation of Artificial Skin Surface Lipid Films, Tribology Letters 34(2) (2009) 81. [Google Scholar]
- [15].Li W, Kong M, Liu XD, Zhou ZR, Tribological behavior of scar skin and prosthetic skin in vivo, Tribology International 41(7) (2008) 640–647. [Google Scholar]
- [16].Tomlinson SE, Lewis R, Ball S, Yoxall A, Carré MJ, Understanding the effect of finger–ball friction on the handling performance of rugby balls, Sports Engineering 11(3) (2009) 109–118. [Google Scholar]
- [17].Tang W, Bhushan B, Ge S, Friction, adhesion and durability and influence of humidity on adhesion and surface charging of skin and various skin creams using atomic force microscopy, J Microsc 239(2) (2010) 99–116. [DOI] [PubMed] [Google Scholar]
- [18].Bharat B, Wei T, Surface, tribological, and mechanical characterization of synthetic skins for tribological applications in cosmetic science, Journal of Applied Polymer Science 120(5) (2011) 2881–2890. [Google Scholar]
- [19].Franklin SE, Baranowska J, Hendriks CP, Piwowarczyk J, Nachman M, Comparison of the Friction Behavior of Occluded Human Skin and Synthetic Skin in Dry and Moist Conditions, Tribology Transactions 60(5) (2017) 861–872. [Google Scholar]
- [20].Nachman M, Franklin SE, Artificial Skin Model simulating dry and moist in vivo human skin friction and deformation behaviour, Tribology International 97 (2016) 431–439. [Google Scholar]
- [21].Mohd Noor SNA, Mahmud J, A Review on Synthetic Skin: Materials Investigation, Experimentation and Simulation, Advanced Materials Research 915-916 (2014) 858–866. [Google Scholar]
- [22].Rittié L, Cellular mechanisms of skin repair in humans and other mammals, Journal of cell communication and signaling 10(2) (2016) 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Abd E, Yousef SA, Pastore MN, Telaprolu K, Mohammed YH, Namjoshi S, Grice JE, Roberts MS, Skin models for the testing of transdermal drugs, Clinical pharmacology: advances and applications 8 (2016) 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Raney SG, Franz TJ, Lehman PA, Lionberger R, Chen ML, Pharmacokinetics-Based Approaches for Bioequivalence Evaluation of Topical Dermatological Drug Products, Clin Pharmacokinet 54(11) (2015) 1095–106. [DOI] [PubMed] [Google Scholar]
- [25].Yang Y, Manda P, Pavurala N, Khan MA, Krishnaiah YS, Development and validation of in vitro-in vivo correlation (IVIVC) for estradiol transdermal drug delivery systems, J Control Release 210 (2015) 58–66. [DOI] [PubMed] [Google Scholar]
- [26].Ruela ALM, Perissinato AG, Lino M.E.d.S., Mudrik PS, Pereira GR, Evaluation of skin absorption of drugs from topical and transdermal formulations, Brazilian Journal of Pharmaceutical Sciences 52 (2016) 527–544. [Google Scholar]
- [27].Shah VP, Maibach HI, Topical Drug Bioavailability, Bioequivalence, and Penetration, Springer US; 2013. [Google Scholar]
- [28].Alberti I, Kalia YN, Naik A, Bonny JD, Guy RH , In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum, J Control Release 71(3) (2001) 319–27. [DOI] [PubMed] [Google Scholar]
- [29].Shah V, Topical dermatological drug product NDAs and ANDAs—in vivo bioavailability, bioequivalence, in vitro release and associated studies, US Dept. of Health and Human Services, Rockville: (1998) 1–19. [Google Scholar]
- [30].Bodenlenz M, Aigner B, Dragatin C, Liebenberger L, Zahiragic S, Hofferer C, Birngruber T, Priedl J, Feichtner F, Schaupp L, Korsatko S, Ratzer M, Magnes C, Pieber TR, Sinner F, Clinical applicability of dOFM devices for dermal sampling, Skin Res Technol 19(4) (2013) 474–83. [DOI] [PubMed] [Google Scholar]
- [31].Kansy M, Senner F, Gubernator K, Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes, J Med Chem 41(7) (1998) 1007–10. [DOI] [PubMed] [Google Scholar]
- [32].Ottaviani G, Martel S, Carrupt PA, Parallel artificial membrane permeability assay: a new membrane for the fast prediction of passive human skin permeability, J Med Chem 49(13) (2006) 3948–54. [DOI] [PubMed] [Google Scholar]
- [33].Kolle SN, Teubner W, Landsiedel R, Modern Skin Toxicity Testing Strategies, in: Krutmann J, Merk HF (Eds.), Environment and Skin, Springer International Publishing, Cham, 2018, pp. 27–40. [Google Scholar]
- [34].Alepee N, Tornier C, Robert C, Amsellem C, Roux MH, Doucet O, Pachot J, Meloni M, de A Brugerolle de Fraissinette, A catch-up validation study on reconstructed human epidermis (SkinEthic RHE) for full replacement of the Draize skin irritation test, Toxicol In Vitro 24(1) (2010) 257–66. [DOI] [PubMed] [Google Scholar]
- [35].Kandarova H, Liebsch M, Spielmann H, Genschow E, Schmidt E, Traue D, Guest R, Whittingham A, Warren N, Gamer AO, Remmele M, Kaufmann T, Wittmer E, De Wever B, Rosdy M, Assessment of the human epidermis model SkinEthic RHE for in vitro skin corrosion testing of chemicals according to new OECD TG 431, Toxicol In Vitro 20(5) (2006) 547–59. [DOI] [PubMed] [Google Scholar]
- [36].O’Goshi K, Serup J, Inter-instrumental variation of skin capacitance measured with the Corneometer, Skin Res Technol 11(2) (2005) 107–9. [DOI] [PubMed] [Google Scholar]
- [37].Loden M, Hagforsen E, Lindberg M, The presence of body hair influences the measurement of skin hydration with the Corneometer, Acta Derm Venereol 75(6) (1995) 449–50. [DOI] [PubMed] [Google Scholar]
- [38].Bazin R, Fanchon C, Equivalence of face and volar forearm for the testing of moisturizing and firming effect of cosmetics in hydration and biomechanical studies, Int J Cosmet Sci 28(6) (2006) 453–60. [DOI] [PubMed] [Google Scholar]
- [39].Shah S, Importance of Genotoxicity & S2A guidelines for genotoxicity testing for pharmaceuticals, 2012. [Google Scholar]
- [40].Kirsch-Volders M, Decordier I, Elhajouji A, Plas G, Aardema MJ, Fenech M, In vitro genotoxicity testing using the micronucleus assay in cell lines, human lymphocytes and 3D human skin models, Mutagenesis 26(1) (2011) 177–84. [DOI] [PubMed] [Google Scholar]
- [41].Luzhna L, Kathiria P, Kovalchuk O, Micronuclei in genotoxicity assessment: from genetics to epigenetics and beyond, Front Genet 4 (2013) 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mun GC, Aardema MJ, Hu T, Barnett B, Kaluzhny Y, Klausner M, Karetsky V, Dahl EL, Curren RD, Further development of the EpiDerm 3D reconstructed human skin micronucleus (RSMN) assay, Mutat Res 673(2) (2009) 92–9. [DOI] [PubMed] [Google Scholar]
- [43].Flamand N, Marrot L, Belaidi JP, Bourouf L, Dourille E, Feltes M, Meunier JR, Development of genotoxicity test procedures with Episkin, a reconstructed human skin model: towards new tools for in vitro risk assessment of dermally applied compounds?, Mutat Res 606(1–2) (2006) 39–51. [DOI] [PubMed] [Google Scholar]
- [44].Barcham R, Orsini N, Andres E, Hundt A, Luzy AP, Successful proof of concept of a micronucleus genotoxicity assay on reconstructed epidermis exhibiting intrinsic metabolic activity, Mutat Res 829–830 (2018) 75–86. [DOI] [PubMed] [Google Scholar]
- [45].Ackermann K, Borgia SL, Korting HC, Mewes KR, Schafer-Korting M, The Phenion full-thickness skin model for percutaneous absorption testing, Skin Pharmacol Physiol 23(2) (2010) 105–12. [DOI] [PubMed] [Google Scholar]
- [46].Slivka SR, Landeen LK, Zeigler F, Zimber MP, Bartel RL, Characterization, barrier function, and drug metabolism of an in vitro skin model, Journal of Investigative Dermatology 100(1) (1993) 40–46. [DOI] [PubMed] [Google Scholar]
- [47].Draelos ZD, The science behind skin care: Cleansers, Journal of cosmetic dermatology 17(1) (2018) 8–14. [DOI] [PubMed] [Google Scholar]
- [48].Kumbar S, James R, Nukavarapu S, Laurencin C, Electrospun nanofiber scaffolds: engineering soft tissues, Biomedical materials 3(3) (2008) 034002. [DOI] [PubMed] [Google Scholar]
- [49].Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT, Electrospun poly (lactic acid-co-glycolic acid) scaffolds for skin tissue engineering, Biomaterials 29(30) (2008) 4100–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Manoukian OS, Matta R, Letendre J, Collins P, Mazzocca AD, Kumbar SG, Electrospun Nanofiber Scaffolds and Their Hydrogel Composites for the Engineering and Regeneration of Soft Tissues, Biomedical Nanotechnology: Methods and Protocols (2017) 261–278. [DOI] [PubMed]
- [51].Nada AA, James R, Shelke NB, Harmon MD, Awad HM, Nagarale RK, Kumbar SG, A smart methodology to fabricate electrospun chitosan nanofiber matrices for regenerative engineering applications, Polymers for Advanced Technologies 25(5) (2014) 507–515. [Google Scholar]
- [52].Shelke NB, Lee P, Anderson M, Mistry N, Nagarale RK, Ma XM, Yu X, Kumbar SG, Neural tissue engineering: nanofiber‐hydrogel based composite scaffolds, Polymers for Advanced Technologies 27(1) (2016) 42–51. [Google Scholar]
- [53].Kumbar SG, Nair LS, Bhattacharyya S, Laurencin CT, Polymeric nanofibers as novel carriers for the delivery of therapeutic molecules, Journal of nanoscience and nanotechnology 6(9–10) (2006) 2591–2607. [DOI] [PubMed] [Google Scholar]
- [54].Guadalupe E, Ramos D, Shelke NB, James R, Gibney C, Kumbar SG, Bioactive polymeric nanofiber matrices for skin regeneration, Journal of Applied Polymer Science 132(16) (2015). [Google Scholar]
- [55].Jaiswal D, James R, Shelke NB, Harmon MD, Brown JL, Hussain F, Kumbar SG, Gelatin nanofiber matrices derived from Schiff base derivative for tissue engineering applications, Journal of biomedical nanotechnology 11(11) (2015) 2067–2080. [DOI] [PubMed] [Google Scholar]
- [56].Martínez-Santamaría L, Guerrero-Aspizua S, Del Río M, Skin bioengineering: preclinical and clinical applications, Actas Dermo-Sifiliográficas (English Edition) 103(1) (2012) 5–11. [DOI] [PubMed] [Google Scholar]
- [57].Raya Á, Rodríguez-Pizà I, Guenechea G, Vassena R, Navarro S, Barrero MJ, Consiglio A, Castella M, Río P, Sleep E, Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells, Nature 460(7251) (2009) 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, Ruether A, Schreiber S, Weichenthal M, Gladman D, Rahman P, Schrodi SJ, Prahalad S, Guthery SL, Fischer J, Liao W, Kwok PY, Menter A, Lathrop GM, Wise CA, Begovich AB, Voorhees JJ, Elder JT, Krueger GG, Bowcock AM, Abecasis GR, Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways, Nat Genet 41(2) (2009) 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tjabringa G, Bergers M, van Rens D, de Boer R, Lamme E, Schalkwijk J, Development and validation of human psoriatic skin equivalents, Am J Pathol 173(3) (2008) 815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sugimoto K, Nishimura T, Nomura K, Kuriki T, Inhibitory effects of alpha-arbutin on melanin synthesis in cultured human melanoma cells and a three-dimensional human skin model, Biol Pharm Bull 27(4) (2004) 510–4. [DOI] [PubMed] [Google Scholar]
- [61].Briganti S, Camera E, Picardo M, Chemical and instrumental approaches to treat hyperpigmentation, Pigment Cell Res 16(2) (2003) 101–10. [DOI] [PubMed] [Google Scholar]
- [62].MacNeil S, Progress and opportunities for tissue-engineered skin, Nature 445(7130) (2007) 874–80. [DOI] [PubMed] [Google Scholar]
- [63].Semlin L, Schafer-Korting M, Borelli C, Korting HC, In vitro models for human skin disease, Drug Discov Today 16(3–4) (2011) 132–9. [DOI] [PubMed] [Google Scholar]
- [64]. in: P.o. back.jpg (Ed.) Wikimedia Commons, 2018.
- [65].Zghoul N, Fuchs R, Lehr CM, Schaefer UF, Reconstructed skin equivalents for assessing percutaneous drug absorption from pharmaceutical formulations, Altex 18(2) (2001) 103–6. [PubMed] [Google Scholar]
- [66].Zhang Z, Michniak-Kohn BB, Tissue engineered human skin equivalents, Pharmaceutics 4(1) (2012) 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lee KY, Mooney DJ, Hydrogels for tissue engineering, Chem Rev 101(7) (2001) 1869–79. [DOI] [PubMed] [Google Scholar]
- [68].Peppas NA, Carr DA, Impact of Absorption and Transport on Intelligent Therapeutics and Nano-scale Delivery of Protein Therapeutic Agents, Chem Eng Sci 64(22) (2009) 4553–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Baptista PM, Orlando G, Mirmalek-Sani SH, Siddiqui M, Atala A, Soker S, Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering, Conf Proc IEEE Eng Med Biol Soc 2009 (2009) 6526–9. [DOI] [PubMed] [Google Scholar]
- [70].Brohem CA, Cardeal LB, Tiago M, Soengas MS, Barros SB, Maria-Engler SS, Artificial skin in perspective: concepts and applications, Pigment Cell Melanoma Res 24(1) (2011) 35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cooper ML, Hansbrough JF, Spielvogel RL, Cohen R, Bartel RL, Naughton G, In vivo optimization of a living dermal substitute employing cultured human fibroblasts on a biodegradable polyglycolic acid or polyglactin mesh, Biomaterials 12(2) (1991) 243–8. [DOI] [PubMed] [Google Scholar]
- [72].Stark HJ, Willhauck MJ, Mirancea N, Boehnke K, Nord I, Breitkreutz D, Pavesio A, Boukamp P, Fusenig NE, Authentic fibroblast matrix in dermal equivalents normalises epidermal histogenesis and dermoepidermal junction in organotypic co-culture, Eur J Cell Biol 83(11–12) (2004) 631–45. [DOI] [PubMed] [Google Scholar]
- [73].Stark HJ, Boehnke K, Mirancea N, Willhauck MJ, Pavesio A, Fusenig NE, Boukamp P, Epidermal homeostasis in long-term scaffold-enforced skin equivalents, J Investig Dermatol Symp Proc 11(1) (2006) 93–105. [DOI] [PubMed] [Google Scholar]
- [74].Regnier M, Duval C, Schmidt R, Potential cosmetic applications of reconstructed epidermis, Int J Cosmet Sci 21(1) (1999) 51–8. [DOI] [PubMed] [Google Scholar]
- [75].(2019). SkinEthic RHE Reconstructed Human Epidermis Retrieved from http://www.episkin.com/SkinEthicRHE
- [76].(2019). EpiSkin/Human Epidermis Retrieved from http://www.episkin.com/Episkin
- [77].(2019). EpiDermFT in vitro 3D Tissue Retrieved from https://www.mattek.com/products/epidermft/
- [78].Hoffmann J, Heisler E, Karpinski S, Losse J, Thomas D, Siefken W, Ahr HJ, Vohr HW, Fuchs HW, Epidermal-skin-test 1,000 (EST-1,000)--a new reconstructed epidermis for in vitro skin corrosivity testing, Toxicol In Vitro 19(7) (2005) 925–9. [DOI] [PubMed] [Google Scholar]
- [79].(2018). In vitro Reconstructed Human Epidermis (RHE) Retrieved from https://www.creative-bioarray.com/in-vitro-reconstructed-human-epidermis-rhe.htm
- [80].(2018). Aeon Astron/ In vitro Skin Testing Biomimiq Retrieved from http://www.aeonastron.com/product.php?catId=7
- [81].(2019). In Vitro Skin Models Retrieved from https://www.straticell.com/en/models-tools/in-vitro-skin-models
- [82].(2019). Vitro-Skin® Retrieved from https://www.ims-usa.com/vitro-skin-substrates/vitro-skin/
- [83].Rasmussen C, Gratz K, Liebel F, Southall M, Garay M, Bhattacharyya S, Simon N, Vander Zanden M, Van Winkle K, Pirnstill J, Pirnstill S, Comer A, Allen-Hoffmann BL, The StrataTest(R) human skin model, a consistent in vitro alternative for toxicological testing, Toxicol In Vitro 24(7) (2010) 2021–9. [DOI] [PubMed] [Google Scholar]
- [84].(2018). Manual AST2000 Advanced Skin Test Retrieved from http://www.lifelinecelltech.com/pdf/ASTManual.pdf
- [85].(2018). In vitro Full Thickness Skin Model Retrieved from https://www.creative-bioarray.com/in-vitro-full-thickness-skin-model.htm
- [86].(2019). NativeSkin® Human Skin Models Retrieved from https://www.genoskin.com/en/tissue-samples/human-skin-models-nativeskin/
- [87].(2019). 3D Skin Cell Model Retrieved from https://www.cellapplications.com/3d-skin-model
- [88].(2011). Apligraf® Retrieved from http://www.apligraf.com/professional/pdf/APG_Factsheet.pdf
- [89].(2018). Epicel® Retrieved from https://www.epicel.com/patients/
- [90].(2006). OrCel® Wound Sealant Retrieved from https://www.medgadget.com/2006/09/orcel_wound_sea.html
- [91].(2004). INTEGRA® Dermal Regeneration Template Retrieved from https://www.integralife.com/file/general/1453795605.pdf
- [92].(2019). Ex vivo skin explants Retrieved from https://www.straticell.com/en/models-tools/in-vitro-skin-models
- [93].Guenou H, Nissan X, Larcher F, Feteira J, Lemaitre G, Saidani M, Del Rio M, Barrault CC, Bernard F-X, Peschanski M, Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study, The Lancet 374(9703) (2009) 1745–1753. [DOI] [PubMed] [Google Scholar]
- [94].Mathes SH, Ruffner H, Graf-Hausner U, The use of skin models in drug development, Advanced drug delivery reviews 69 (2014) 81–102. [DOI] [PubMed] [Google Scholar]
- [95].Van Gele M, Geusens B, Brochez L, Speeckaert R, Lambert J, Three-dimensional skin models as tools for transdermal drug delivery: challenges and limitations, Expert opinion on drug delivery 8(6) (2011) 705–720. [DOI] [PubMed] [Google Scholar]
- [96].Netzlaff F, Lehr C-M, Wertz P, Schaefer U, The human epidermis models EpiSkin®, SkinEthic® and EpiDerm®: an evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport, European Journal of Pharmaceutics and Biopharmaceutics 60(2) (2005) 167–178. [DOI] [PubMed] [Google Scholar]
- [97].Ponec M, Boelsma E, Weerheim A, Mulder A, Bouwstra J, Mommaas M, Lipid and ultrastructural characterization of reconstructed skin models, International journal of pharmaceutics 203(1–2) (2000) 211–225. [DOI] [PubMed] [Google Scholar]
- [98].Ponec M, Boelsma E, Gibbs S, Mommaas M, Characterization of reconstructed skin models, Skin Pharmacology and Physiology 15(Suppl. 1) (2002) 4–17. [DOI] [PubMed] [Google Scholar]
- [99].Roguet R, Cohen C, Dossou K, Rougier A, Episkin, a reconstituted human epidermis for assessing in vitro the irritancy of topically applied compounds, Toxicology in vitro 8(2) (1994) 283–291. [DOI] [PubMed] [Google Scholar]
- [100].Roguet R, Regnier M, Cohen C, Dossou K, Rougier A, The use of in vitro reconstituted human skin in dermotoxicity testing, Toxicology in vitro 8(4) (1994) 635–639. [DOI] [PubMed] [Google Scholar]
- [101].Dreher F, Fouchard F, Patouillet C, Andrian M, Simonnet J-T, Benech-Kieffer F, Comparison of cutaneous bioavailability of cosmetic preparations containing caffeine or α-tocopherol applied on human skin models or human skin ex vivo at finite doses, Skin Pharmacology and Physiology 15(Suppl. 1) (2002) 40–58. [DOI] [PubMed] [Google Scholar]
- [102].Rosdy M, Pisani A, Ortonne JP, Production of basement membrane components by a reconstructed epidermis cultured in the absence of serum and dermal factors, British Journal of Dermatology 129(3) (1993) 227–234. [DOI] [PubMed] [Google Scholar]
- [103].Bernard F, Barrault C, Deguercy A, De Wever B, Rosdy M, Development of a highly sensitive in vitro phototoxicity assay using the SkinEthicTM reconstructed human epidermis, Cell biology and toxicology 16(6) (2000) 391–400. [DOI] [PubMed] [Google Scholar]
- [104].de Fraissinette A.d.B., Picarles V, Chibout S, Kolopp M, Medina J, Burtin P, Ebelin M, Osborne S, Mayer F, Spake A, Predictivity of an in vitro model for acute and chronic skin irritation (SkinEthic) applied to the testing of topical vehicles, Cell biology and toxicology 15(2) (1999) 121–135. [DOI] [PubMed] [Google Scholar]
- [105].Lotte C, Patouillet C, Zanini M, Messager A, Roguet R, Permeation and skin absorption: reproducibility of various industrial reconstructed human skin models, Skin Pharmacology and Physiology 15(Suppl. 1) (2002) 18–30. [DOI] [PubMed] [Google Scholar]
- [106].M. Corporation, EpiDerm ™ - Overview 2018.
- [107].Liebsch M, Barrabas C, Traue D, Spielmann H, Development of a new in vitro test for dermal phototoxicity using a model of reconstituted human epidermis, Altex 14(4) (1997) 165–174. [PubMed] [Google Scholar]
- [108].Cannon C, Neal P, Southee J, Kubilus J, Klausner M, New epidermal model for dermal irritancy testing, Toxicology in Vitro 8(4) (1994) 889–891. [DOI] [PubMed] [Google Scholar]
- [109].Gibbs S, Vietsch H, Meier U, Ponec M, Effect of skin barrier competence on SLS and water‐induced IL‐1α expression, Experimental dermatology 11(3) (2002) 217–223. [DOI] [PubMed] [Google Scholar]
- [110].Faller C, Bracher M, Dami N, Roguet R, Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics☆, Toxicology in vitro 16(5) (2002) 557–572. [DOI] [PubMed] [Google Scholar]
- [111].Nakamura M, Rikimaru T, Yano T, Moore KG, Pula PJ, Schofield BH, Dannenberg AM, Full-Thickness Human Skin Explants for Testing the Toxicitiy of Topically Applied Chemicals, Journal of Investigative Dermatology 95(3) (1990) 325–332. [DOI] [PubMed] [Google Scholar]
- [112].Walters KA, Watkinson AC, Brain KR, In vitro skin permeation evaluation: the only realistic option, Int J Cosmet Sci 20(5) (1998) 307–16. [DOI] [PubMed] [Google Scholar]
- [113].Tfayli A, Bonnier F, Farhane Z, Libong D, Byrne HJ, Baillet-Guffroy A, Comparison of structure and organization of cutaneous lipids in a reconstructed skin model and human skin: spectroscopic imaging and chromatographic profiling, Exp Dermatol 23(6) (2014) 441–3. [DOI] [PubMed] [Google Scholar]
- [114].Mallampati R, Patlolla RR, Agarwal S, Babu RJ, Hayden P, Klausner M, Singh MS, Evaluation of EpiDerm full thickness-300 (EFT-300) as an in vitro model for skin irritation: studies on aliphatic hydrocarbons, Toxicol In Vitro 24(2) (2010) 669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Welss T, Basketter DA, Schroder KR, In vitro skin irritation: facts and future. State of the art review of mechanisms and models, Toxicol In Vitro 18(3) (2004) 231–43. [DOI] [PubMed] [Google Scholar]
- [116].Vijayavenkataraman S, Lu WF, Fuh JYH, 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes, Biofabrication 8(3) (2016) 032001. [DOI] [PubMed] [Google Scholar]
- [117].Ng WL, Wang S, Yeong WY, Naing MW, Skin bioprinting: impending reality or fantasy?, Trends in biotechnology 34(9) (2016) 689–699. [DOI] [PubMed] [Google Scholar]
- [118].Lee W, Debasitis JC, Lee VK, Lee J-H, Fischer K, Edminster K, Park J-K, Yoo S-S, Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication, Biomaterials 30(8) (2009) 1587–1595. [DOI] [PubMed] [Google Scholar]
- [119].Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, Gaebel R, Polchow B, Reimers K, Stoelting S, Ma N, Laser printing of skin cells and human stem cells, Tissue Engineering Part C: Methods 16(5) (2009) 847–854. [DOI] [PubMed] [Google Scholar]
- [120].Michael S, Sorg H, Peck C-T, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K, Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice, PloS one 8(3) (2013) e57741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Cubo N, Garcia M, del Cañizo JF, Velasco D, Jorcano JL, 3D bioprinting of functional human skin: production and in vivo analysis, Biofabrication 9(1) (2016) 015006. [DOI] [PubMed] [Google Scholar]
- [122].Min D, Lee W, Bae IH, Lee TR, Croce P, Yoo SS, Bioprinting of biomimetic skin containing melanocytes, Experimental dermatology 27(5) (2018) 453–459. [DOI] [PubMed] [Google Scholar]
- [123].Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S, Bioprinted amniotic fluid‐derived stem cells accelerate healing of large skin wounds, Stem cells translational medicine 1(11) (2012) 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gholipourmalekabadi M, Samadikuchaksaraei A, Seifalian AM, Urbanska AM, Ghanbarian H, Hardy JG, Omrani MD, Mozafari M, Reis RL, Kundu SC, Silk fibroin/amniotic membrane 3D bi-layered artificial skin, Biomed Mater 13(3) (2018) 035003. [DOI] [PubMed] [Google Scholar]
- [125].Lee KH, Tissue-engineered human living skin substitutes: development and clinical application, Yonsei Med J 41(6) (2000) 774–9. [DOI] [PubMed] [Google Scholar]
- [126].Zajicek R, Sticova E, Konigova R, A histological analysis of artificial skin in an extensively burned child, 14 years after application: a case report, J Wound Care 27(1) (2018) 14–18. [DOI] [PubMed] [Google Scholar]
- [127].Burke JF, Yannas IV, Quinby WC, Bondoc CC, Jung WK, Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury, Annals of Surgery 194(4) (1981) 413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Dantzer E, Braye FM, Reconstructive surgery using an artificial dermis (Integra): results with 39 grafts, Br J Plast Surg 54(8) (2001) 659–64. [DOI] [PubMed] [Google Scholar]
- [129].Atac B, Wagner I, Horland R, Lauster R, Marx U, Tonevitsky AG, Azar RP, Lindner G, Skin and hair on-a-chip: in vitro skin models versus ex vivo tissue maintenance with dynamic perfusion, Lab Chip 13(18) (2013) 3555–61. [DOI] [PubMed] [Google Scholar]
- [130].Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE, Reconstituting organ-level lung functions on a chip, Science 328(5986) (2010) 1662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ramme AP, Hubner J, Lindner M, Drewell C, Bauer S, Thomas A, Sambo NS, Sonntag F, Lauster R, Marx U, A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents, Lab Chip 15(12) (2015) 2688–99. [DOI] [PubMed] [Google Scholar]
- [132].Bhise NS, Ribas J, Manoharan V, Zhang YS, Polini A, Massa S, Dokmeci MR, Khademhosseini A, Organ-on-a-chip platforms for studying drug delivery systems, J Control Release 190 (2014) 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wufuer M, Lee G, Hur W, Jeon B, Kim BJ, Choi TH, Lee S, Skin-on-a-chip model simulating inflammation, edema and drug-based treatment, Sci Rep 6 (2016) 37471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Danilenko DM, Lewis Phillips GD, & Diaz D (2016). In Vitro Skin Models and Their Predictability in Defining Normal and Disease Biology, Pharmacology, and Toxicity. Toxicologic Pathology, 44(4), 555–563. 10.1177/0192623316632074 [DOI] [PubMed] [Google Scholar]
- [135].Wever BD, Kurdykowski S, & Descargues P (2015). Human Skin Models for Research Applications in Pharmacology and Toxicology: Introducing NativeSkin®, the “Missing Link” Bridging Cell Culture and/or Reconstructed Skin Models and Human Clinical Testing. Applied In Vitro Toxicology,1(1), 26–32. doi: 10.1089/aivt.2014.0010 [DOI] [Google Scholar]
- [136].Łopaciuk A, & Łoboda M (2013, June). Global beauty industry trends in the 21st century. In Management, knowledge and learning international conference (pp. 19–21). [Google Scholar]
- [137].Kumar S, Massie C, & Dumonceaux MD (2006). Comparative innovative business strategies of major players in cosmetic industry. Industrial Management & Data Systems, 106(3), 285–306. [Google Scholar]
- [138].Romanowski P (2017). Product categories in the cosmetic industry Retrieved from https://chemistscorner.com/product-categories-in-the-cosmetic-industry/