Abstract
Introduction:
During vocalization, efference copy/corollary discharge mechanisms suppress the auditory cortical response to self-generated sounds as reflected in the N1 component of the auditory event-related potential (ERP). N1 suppression during talking is reduced in patients with schizophrenia. We hypothesized that these deficits would recover with auditory training that targets the speech processing system.
Methods:
Forty-nine individuals early in the course of a schizophrenia-spectrum illness (ESZ) were randomly assigned to 40 hours of Targeted Auditory Training (TAT; n=23) or Computer Games (CG; n=26). The N1 ERP component was elicited during production (Talk) and playback (Listen) of vocalization. Effects of Treatment on Global Cognition, N1 suppression (Talk-Listen), N1 during Talking and Listening were assessed. Simple effects of the passage of time were also assessed in the HC after 28 weeks.
Results:
There was a Treatment x Time interaction revealing that N1 suppression was improved with TAT, but not with CG. TAT, but not CG, also improved Global Cognition. However, TAT and CG groups differed in their pre-treatment N1 suppression, and greater N1-suppression abnormalities were strongly associated with greater improvement in N1 suppression.
Conclusions:
In this sample of ESZ individuals, targeted auditory training appeared to improve the function of the efference copy/corollary discharge mechanism which tended to deteriorate with computer games. It remains to be determined if baseline N1 suppression abnormalities are necessary for TAT treatment to have a positive effect on efference copy/corollary discharge function or if improvements observed in this study represent a regression to the mean N1 suppression in ESZ.
Keywords: schizophrenia, cognitive training, efference copy/corollary discharge, auditory ERP
1. Introduction
During talking, our brains automatically generate predictions about the sound of our impending vocalizations in order to adjust ongoing speech to better match our intentions (Burnett et al., 1998; Houde and Jordan, 1998; Sitek et al., 2013) and to mirror our social environment (Pardo, 2006). In addition to optimizing performance to match intentions, these rapid comparisons allow us to distinguish between auditory sensations resulting from our own actions, including overt actions (e.g., speech) and possibly covert actions (e.g., thoughts), and externally generated sounds (Crapse and Sommer, 2008; Greenlee et al., 2011). In human (Ford et al., 2007a; Ford and Mathalon, 2005; Ford et al., 2001a; Ford et al., 2001d; Ford et al., 2012; Ford et al., 2007b; Heinks-Maldonado et al., 2005; Heinks-Maldonado et al., 2007) and non-human primates (Eliades and Wang, 2003; Eliades and Wang, 2005; Eliades and Wang, 2008). This is seen as reduced auditory cortical responses to self-generated compared to externally generated sounds. When a vocalization adjustment is needed or when the source is external and possibly important, auditory cortical responsiveness is heightened. These comparisons have been attributed to a putative efference copy/corollary discharge mechanism (Crapse and Sommer, 2008).
In humans, the function of the efference copy/corollary discharge mechanism can be assessed using scalp recorded EEG and the EEG-derived event-related potentials (ERPs). Specifically, the auditory N100 (N1) component of the ERP, which emanates from auditory cortex (Ford et al., 2016), is reduced in amplitude in response to vocalizations as they are being produced relative to when they are played back (Chen et al., 2011; Curio et al., 2000; Ford et al., 2007a; Ford et al., 2001b; Ford et al., 2013; Ford et al., 2007b; Greenlee et al., 2011; Heinks-Maldonado et al., 2005; Heinks-Maldonado et al., 2007; Heinks-Maldonado et al., 2006; Houde et al., 2002; Sitek et al., 2013; Wang et al., 2014).
The amount of suppression is related to precision of the match between the vocalized sound and the expected sound. The precision of the efference copy/corollary discharge mechanism can be studied by pitch-shifting the sound fed back to the ear (Behroozmand et al., 2009; Heinks-Maldonado et al., 2005) and by incidental differences between the modal sound and spoken sound (Sitek et al., 2013). Specifically, Sitek et al found that N1 to speech sounds as they are being spoken was sensitive to the degree of match between the current and the immediately preceding utterance. N1 during passive listening to that sequence of sounds was not sensitive to these small variations in moment to moment sounds. This may reflect a largely unconscious monitoring the spoken sounds and the rapid corrections in articulation to match intentions (Levelt, 1983). This automatic, rapid process is likely instantiated in cerebellar side-loops, capable of rapidly comparing sensory input with intended motor output (Ramnani, 2006).
We have shown that patients with schizophrenia (Ford et al., 2007a; Ford et al., 2001a; Ford et al., 2001c; Ford et al., 2013; Ford et al., 2007b) and people on the psychosis spectrum show less suppression of the auditory N1 in response to self-generated vocalizations than healthy controls, with similar abnormalities in schizophrenia patients early in their disease course (Perez et al., 2012) and people at clinical high risk for psychosis (Mathalon et al., 2018). Together these findings suggest that patients with schizophrenia show attenuated or absent suppression of auditory cortex in response to self-generated sounds, possibly due to deficits in efference copy/corollary discharge mechanisms. These deficits, consequently, may underlie an inability to make predictions about the sensory consequences of self-generated actions and to utilize them to adjust behavior and tag experiences as self-generated.
In this study, we ask if auditory training targeting discrimination of complex speech-related stimuli might restore this system to normal levels in patients with schizophrenia early in their disease course (ESZ). The training exercises target feed-forward auditory perceptual processes by placing implicit, increasing demands on discrimination of basic auditory and verbal stimuli. Feedback attention and cognitive control operations are engaged by signaling correct/incorrect trials and by embedding the psychophysical training within increasingly complex auditory and verbal working memory/verbal learning trials. The mechanism of action is thus posited to be the “re-tuning” of the bi-directional operations between temporally detailed resolution of auditory inputs in auditory cortex, prefrontally-mediated attention, and auditory/verbal memory functions. Indeed, emerging electro- and magneto-encephalographic data indicate that targeted auditory training enhances both early representations in primary auditory cortex and auditory sensory gating (Dale et al., 2016; Dale et al., 2010; Popov et al., 2011), as well as both early and later task-related activity in prefrontal regions (Dale et al., 2016). Improved efficiency in distributed prefrontal-temporal auditory systems is therefore thought to drive improvements in untrained higher-level cognitive operations (Biagianti et al., 2016; Vinogradov et al., 2012), which may have a role in implementing the efference copy/corollary discharge mechanism. Recently, Ramsay et al. (Ramsay et al., 2017) found that schizophrenia patients who underwent 48 hours of working memory focused cognitive remediation training showed increases in thalamo-prefrontal connectivity that correlated with improvements in global cognition.
We hypothesized that individuals in the TAT but not in the CG treatment group would have improved N1 suppression, due to improved functioning of the efference copy/corollary discharge system during vocalization. Improvement with training could be due to these factors that contribute to the successful operation of this system: (i) transmission of this efference copy from frontal lobes to auditory cortex (Chen et al., 2011; Ford et al., 2002; Wang et al., 2014), and (ii) sensory re-afference in auditory cortex. TAT could improve transmission of the efference copy to auditory cortex by improving frontal-temporal connectivity during talking (Ford et al., 2002). As mentioned above, thalamo-prefrontal connectivity in schizophrenia improves with working memory training (Ramsay et al., 2017), supporting the possibility that frontal-temporal connectivity might be improved by training that targets the speech perception system (Whitford et al., 2018). TAT could also improve processing sounds in speech-spectrum and consequently the sensory re-afference of speech sounds. If either is improved, N1 suppression would be improved, as patients could better match the external sound of their own speech to their internal (intended) representation of it (Sitek et al., 2013). More specifically, if the intended sound is more effectively transmitted to auditory cortex via the efference copy, TAT will specifically affect N1 during talking. If sensory processing is improved, TAT will specifically affect N1 during listening.
In this study, we randomly assigned individuals with recent onset schizophrenia-spectrum illness to targeted auditory training (TAT) or computer games (CG), which was a strong, active control condition. Cognition and N1 suppression during talking were assessed before and after 40 hours of self-paced TAT or CG with 5 hours per week as the recommended pace. Healthy controls were also tested twice to assess the simple effects of the passage of time.
2. Methods and Materials
2.1. Participants
Forty-nine participants early in the illness course of schizophrenia, schizophreniform, or schizoaffective disorder (ESZ), determined by Structured Clinical Interview for the DSM (SCID)-IV diagnosis (First and Frances, 1995), completed EEG assessment before and after a computer-based treatment study protocol at the University of California, San Francisco (ClinicalTrials.gov NCT00694889). In addition, 29 (22.5 ± 5.9 years of age, 14 Female, 28 right-handed) healthy controls (HC) were tested twice to assess the simple effects of time. These samples comprise the subset of participants who agreed to participate in the EEG experiment from the total sample (which has been reported upon (Fisher et al., 2015)). Demographic data are shown in Table 1.
Table 1.
Treatment Group Demographic Dataa
| Targeted Auditory Training (N=23) | Computer Games Control (N=26) | Between-group comparison (p-value) | |
|---|---|---|---|
| Age (years) | 23.4 (4.3) [16 – 35] | 21.0 (3.9) [14 – 30] | .051 |
| Gender | 7F, 16M | 6F, 20M | .796 |
| Average Parental SESb | 34.2 (12.3) | 30.06 (16.9) | .333 |
| Handednessc | 21R, 1L, 1A | 24R, 2L | 1.0 |
| Estimated IQd | 106.5 (10.42) | 104.6 (9.48) | .500 |
| Time between EEG sessions (weeks) | 19.9 (9.1) | 21.33 (8.0) | .556 |
| Antipsychotic medication class | 2U, 20A, 1A+T | 3U, 22A, 1T | |
| Chlorpromazine Equivalent Dosagee (mg) | 277.4 (205.7) | 422.8 (411.2) | .152 |
| Time 1, Time 2 | Time 1, Time 2 | Time 1, Change | |
| Number of Vocalizations | 97.7 (29.2), 106.7 (32.4) | 97.0 (24.2), 104.8 (32.4) | .923, .866 |
| SANS Global Attention | 2.09 (1.20), 1.96 (1.11) | 2.56 (1.33), 1.76 (1.27) | .203, .117 |
| SANS Anhedonia | 2.52 (1.20), 2.65 (0.98) | 2.76 (1.20), 2.36 (1.38) | .482, .117 |
| SANS Alogia | 1.35 (1.37), 1.22 (1.28) | 1.29 (1.65), 1.21 (1.32) | .899, .902 |
| SANS Avolition | 2.52 (1.24), 2.61 (1.20) | 1.88 (1.48), 1.84 (1.46) | .112, .756 |
| SANS Affective Flattening | 1.83 (1.44), 2.35 (1.27) | 1.96 (1.43), 1.54 (1.38) | .753, .031 |
| SAPS Hallucinations | 1.13 (1.58), 1.30 (1.52) | 1.04 (1.62), 1.36 (1.38) | .846, .771 |
| SAPS Delusions | 1.0 (1.24), 1.52 (1.47) | 2.44 (1.56), 1.79 (1.53) | <.001, .004 |
| SAPS Thought Disorder | 0.74 (0.86), 0.52 (0.85) | 0.67 (1.20), 0.72 (1.21) | .814, .405 |
| SAPS Bizarre Behavior | 0.48 (0.90), 0.35 (0.57) | 0.64 (0.91), 0.48 (0.82) | .538, .915 |
Note. Values are given as number gender, handedness, clinical high-risk criteria, and antipsychotic type. Group means with the standard deviation for age, parental socioeconomic status, intelligence quotient, SANS, and SAPS are reported. Gender and handedness were analyzed with Pearson chi-square and Fisher’s Exact tests, respectively. Age, parental socioeconomic status, and intelligence quotient were analyzed with independent samples t-tests. SANS and SAPS baseline ratings and ratings change scores were analyzed with independent samples t-tests.
The Hollingshead (1975) four-factor index of parental socioeconomic status (SES) is based on a composite of maternal education, paternal education, maternal occupational status, and paternal occupational status. Lower scores represent higher SES. SES values are missing from 1 Targeted Auditory Training subject.
The Crovitz-Zener (1962) questionnaire was used to measure handedness and categorize as right (R), left (L), or ambidextrous (A).
The Wechsler Adult Intelligence Scale (WAIS-III) full-scale intelligence quotient (FSIQ) was estimated based on the Wechsler Test of Adult Reading (WTAR) for native English-speaking subjects who were 16 years of age or older at testing (N=47) or Wechsler Abbreviated Scale of Intelligence (WASI-II) two-subtest (Vocabulary and Matrix Reasoning) T scores for all other subjects (N=2).
Chlorpromazine equivalent dosage, in milligrams (mg), are calculated based on Andreasen et al., 2010. Unmedicated subjects were excluded from these statistics.
Abbreviations: U, unmedicated; A, atypical antipsychotic; T, typical antipsychotic; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms;
ESZ met the following inclusion/exclusion criteria: (1) onset of first psychotic episode or initiation of antipsychotic medication within the past 5 years; (2) age 14–36 years; (3) fluent and proficient in English; (4) intelligence quotient (IQ) ≥ 70; (5) no neurological disorder; and (6) no DSM-IV substance dependence in the past year. All ESZ participants had achieved outpatient status for at least 3 months, and participants taking antipsychotic medications (n=44) were on a stable dose for at least one month prior to study participation. Five participants were not taking antipsychotic medications during the study. The study was approved by the Institutional Review Board of University of California, San Francisco. Adult participants and parents of minors provided written informed consent, and minors provided written assent.
ESZ were assessed using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) (Nuechterlein et al., 2008). We used the Global Cognition score (average z-score across speed of processing, working memory, verbal learning, verbal memory, visual learning, visual memory, and problem solving MCCB measures) as our primary cognitive outcome. Symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987), the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984), and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983). Clinical interviews were conducted by a trained research assistant or clinical psychologist. All assessment staff were blind to group assignment.
HC participants were recruited from the community and did not meet criteria for any DSM-IV Axis I diagnosis based on the SCID, or for participants 16 years of age, the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version (Kaufman et al., 1997). HC had no history of substance abuse within the past year based on a SCID interview and no first-degree relative with a psychotic disorder. HC participants completed a second EEG session approximately 6 months (6.7±1.8) after their baseline EEG session.
Exclusion criteria for both groups included estimated intelligence quotient less than 70, a history of significant medical or neurological illness, or a history of head injury resulting in loss of consciousness. The study was approved by the institutional review board of UCSF, and adult participants provided written informed consent. In the case of minors, parents provided written informed consent and minors provided written informed assent. All interviews were conducted by trained interviewers, including a clinical psychologist, clinical psychology pre-doctoral intern, clinical social worker, or research assistant.
2.2. Treatment protocol
Eligible ESZ participants completed a battery of clinical, neuropsychological and EEG assessments, some of which have been previously described (Biagianti et al., 2017). Baseline assessments were conducted prior to randomization. Using computer-based randomization, ESZ participants were randomly assigned to TAT(n=23) or CG (n=26), both of which involved 40 hours of training.
TAT was provided by Posit Science, Inc, and consisted of adaptive computerized exercises designed to improve the speed and accuracy of early auditory processing while engaging in auditory and verbal working memory tasks (Fisher et al., 2009). Such tasks seek to target early sensory processes that rely on thalamic integration and connectivity with prefrontal and auditory cortices to carry out higher-order auditory and cognitive processes (Lee et al., 2013). TAT exercises were individually adapted in difficulty level to maintain 80–85% accuracy. In each session, participants completed 4 to 6 exercises over the course of one hour. Exercises initially focused on basic sensory processes, such as phoneme distinction and frequency modulation sweeps, before progressing onto more complicated tasks of auditory working memory, requiring adequate integration of more basic sensory processing skills. Correct trials on these tasks were rewarded with points and small animations. Participant compliance was monitored remotely via electronic data upload.
The CG control condition was matched to the TAT condition for computer exposure, contact with research personnel, monetary incentives, and non-specific engagement of attention. CG participants played 16 different commercially available computer games, playing 4–5 different games per training day. Games were predominantly visual. Participants completed an average of 36.87 (SD=6.66) hours of training in the TAT condition, and 39.58 (SD=1.43) hours in the CG condition. Number of days to complete training (and subsequent time between EEGs) did not differ between the TAT (M=139.29; SD=63.5) and CG (M=149.31; SD=56.19) conditions p=.556).
All ESZ participants received compensation for the treatment study, and payment was contingent on study participation and not performance (for details about the payment schedule, see Fisher et al. (Fisher et al., 2015)). During the intervention, ESZ participants were free to receive treatments by clinicians who were not involved in the study (e.g. medication management, psychoeducation, psychotherapy).
2.3. Vocalizing Paradigm
2.3.1. Procedure.
Participants completed the Talk-Listen paradigm, as described previously (Ford et al., 2010), using Presentation software (www.neurobs.com/presentation). In the Talk condition, participants were trained to pronounce short (<300ms), sharp vocalizations of the phoneme “ah” repeatedly in a self-paced manner, about every 1–2s, for 187s. The speech was recorded using a microphone connected to the stimulus presentation computer and transmitted back to subjects through Etymotic ER3-A insert earphones in real time (zero delay). In the Listen condition, the recording from the Talk condition was played back, and participants were instructed simply to listen. The number of “ahs” generated did not differ between treatment groups at baseline (t(47) = 0.097, p = .923) or follow up (t(47) = 0.212, p = 0.833).
2.3.2. Data Acquisition and Pre-Processing.
EEG data were recorded from 64 channels using a BioSemi ActiveTwo system (www.biosemi.com). Electrodes placed at the outer canthi of both eyes, and above and below the right eye, were used to record vertical and horizontal electro-oculogram data. EEG data were continuously digitized at 1024Hz and referenced offline to averaged earlobe electrodes before applying a 1Hz high-pass filter using EEGlab (Delorme and Makeig, 2004). Data were next subjected to Fully Automated Statistical Thresholding for EEG artifact Rejection (FASTER) using a freely distributed toolbox (Nolan et al., 2010), as in our previous report (Perez et al., 2012). The FASTER processing approach was modified to include canonical correlation analysis (CCA). CCA was used as a blind source separation technique to remove broadband or electromyographic noise from single trial EEG data, generating de-noised EEG epochs. This approach is similar to the CCA method described by others (De Clercq et al., 2006; Ries et al., 2013), with some important differences as we have done in other ERP studies using this paradigm (Kort et al., 2017; Mathalon et al., 2018).
Epochs were time-locked to the onset of each “ah” and baseline corrected using the −100 to 0 ms baseline preceding vocalization. ERP averages were generated using a trimmed means approach, excluding the top and bottom 5% of single trial values at every data sample in the epoch before averaging to produce a more robust mean estimation (Leonowicz et al., 2005).
To remove any remaining baseline contamination by speech-related artifacts, a temporal, promax-rotated principal components analysis (PCA) was performed on the ERP data (Kayser and Tenke, 2003; Sinai and Pratt, 2002). ERPs were reconstructed after excluding factors that had a maximum loading during the temporal baseline window preceding “ah” onset or that accounted for less than 0.5% of the variance. N1 was identified in the ERP as the most negative peak between 60 and 140 ms after “ah” onset. The N1 Talk-Listen suppression effect was estimated using the N1 peak amplitude Talk-Listen difference score at Cz, following the method we used in our prior report (Mathalon et al., 2018).
2.4. Statistical Correction for Normal Aging Effects
To control for the effects of normal brain maturation and aging, N1 measures (N1 Talk, N1 Listen, N1 Talk-N1 Listen) at Cz were regressed on age in a larger group of HC (Mathalon et al., 2018), and the resulting regression equation was used to calculate age-corrected N1 measures for all subjects and both time points in this study. This was done by subtracting the predicted N1 measure based on a subject’s age from his/her observed score, and then dividing by the standard error of regression associated with the age-regression model run previously in HC. The resulting age-corrected z-scores are deviations from the value expected for a healthy individual at a specific age. This method has been used previously (Mathalon et al., 2018; Perez et al., 2012), and it is preferable to using age as a covariate in an analysis of covariance (ANCOVA) model because it only removes normal aging effects whereas ANCOVA tends to also remove pathological aging effects from the patient data.
2.5. Statistical Analysis
Treatment effects on Global Cognition and N1 suppression z-scores were assessed using mixed models (SAS v9.4) with Treatment Group (TAT, CG) as a between-subjects factor, Time (Baseline, Post-treatment) as a within-subjects factor, and Subject nested within Treatment Group as a random factor. Interactions were followed up with contrasts of relevant comparisons. Suppression effects were followed up with separate tests of the Talk and Listen conditions to isolate the locus of effects.
To test whether changes in N1 suppression z-scores predicted changes in Global Cognition scores, we performed a regression analysis, including effects of Treatment Group (AT vs CG), change in N1 suppression z-score, and their interaction (change in N1 z-score * Treatment Group). The interaction effect tests whether the slope of the relationship between Global Cognition change and suppression z-score change values significantly differs between treatment groups. A similar regression approach was used to test whether changes in SAPS global symptom ratings could be predicted by changes in N1 suppression z-scores. Alpha was set to p = 0.05, two-tailed, for all statistical tests, with Bonferroni correction applied to the symptom rating models (n=4, corrected-p = 0.0125).
The simple effects of time on N1 measures were assessed in the HC with paired t-tests. Intra-class correlation coefficients (ICCs) were also calculated to assess the test-retest stability of N1 measures in HC over a 6-month period.
3. Results
3.1. Treatment effects
Grand average waveforms from electrode Cz are presented in Figure 1. N1 suppression z-scores were not affected by Treatment (F(1,47) = 1.46, p=.23) or Time (F(1,47) = 0.07, p=.8), but were affected by a Treatment x Time interaction (F(1,47) = 7.25, p=.0098). As can be seen in Figure 2, inspection of this interaction revealed that N1-suppression was improved by TAT (t(47) = 2.02, p=.0487), but was marginally worsened by CG (t(47) = −1.78, p=.0817). The treatment groups differed from each other in baseline N1 suppression z-scores (t(47) = 2.64, p = .011) but not in post-treatment N1 suppression z-scores (t(47) = −0.77, p = 0.45). It is also worth noting that simple contrasts of post-treatment N1 suppression z-scores against 0 (i.e., the expected N1-suppression z-score value for HC across the study age range) demonstrated that both treatment groups still had significantly reduced N1-suppression at the second time point (CG: t(47) = −4.31, p < .0001; TAT: t(47) = −3.00, p = 0.0043). Thus, despite the improvement in N1-suppression z-scores observed in the TAT group, a significant abnormality remained. These interaction effects were mirrored by Talk N1 z-scores (F(1,47) = 7.89, p=.0072) but not Listen z-scores (F(1,47) = 0.05, p=.82), as can be seen in Table 2.
Figure 1.
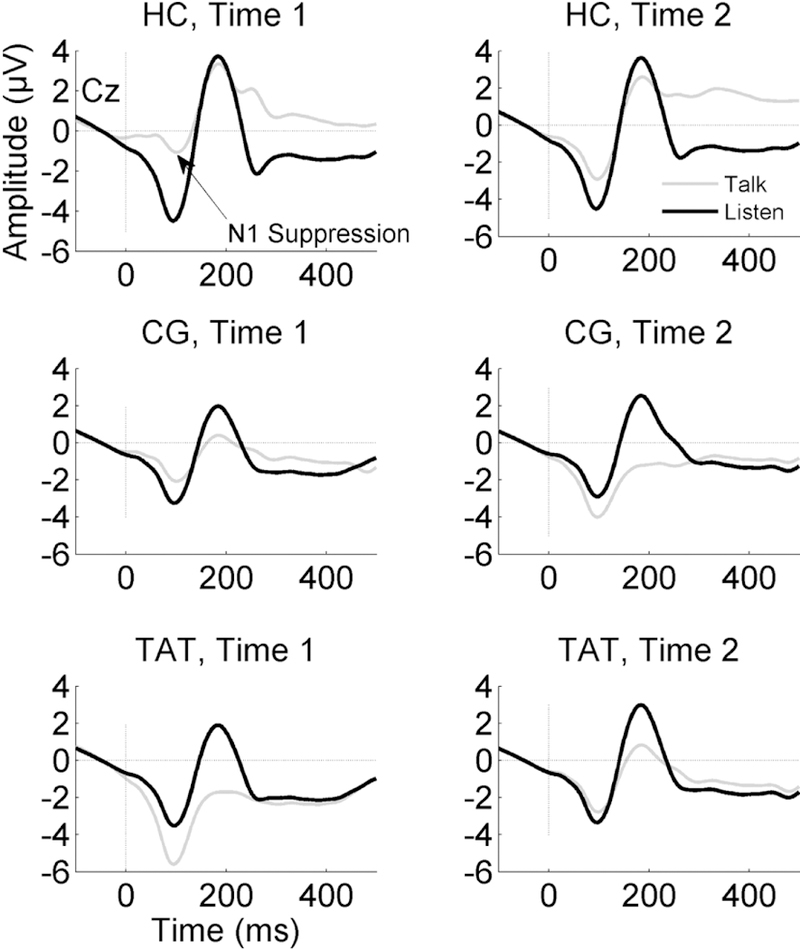
ERP waveforms for Talk and Listen conditions show the N1 component during the Talk (grey) and Listen (black) conditions recorded at Cz. The N1 amplitude during Talk is reduced relative to Listen in Healthy Controls (HC, top). This effect is attenuated in the Early Schizophrenia Patients who were enrolled in either Targeted Auditory Training (TAT, bottom) or an active Computer Games control condition (CG, middle). Waveforms on the left-hand side show Time 1 (pre-treatment) data, while Time 2 (post-treatment for patients and approximately 6 months following Time 1 for HC) are plotted on the right-hand side.
Figure 2.
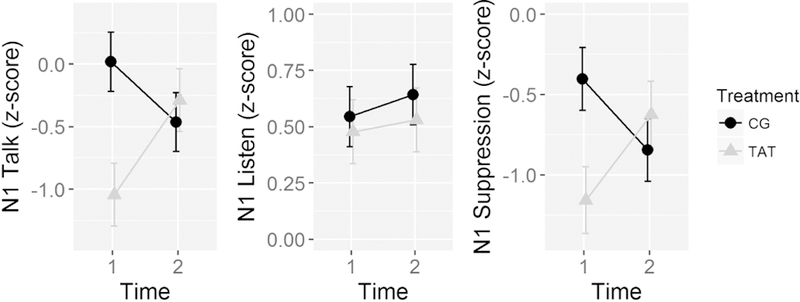
Line graphs show the electrode Cz mean N1 Talk (left), N1 Listen (middle), and N1 Suppresison (Talk-Listen difference, right) z-scores pre- (Time 1) and post-treatment (Time 2) for the Targeted Auditory Training (TAT) and active Computer Games control (CG) treatment groups. The z-scores are based on age-correction done using a healthy control (HC) group, such that the 0 is the expected mean value for the HC group, controlling for age. Negative Talk z-scores reflect larger (i.e., more negative) N1 amplitude, positive Listen z-scores reflect smaller (i.e., less negative) N1 amplitude, and negative Talk-Listen suppression z-scores reflect reduced suppression.
Table 2.
N1 ERP Treatment Effects.
| N1 peak suppression (Talk-Listen) z-scored values at Cz | |||||||
|---|---|---|---|---|---|---|---|
| ANOVA | Within Treatment Group Contrasts | ||||||
| df | F | Sig. | Time2 - Time1 | Mean Difference | t | p-value | |
| Treatment Group | 1, 47 | 1.46 | 0.2323 | TAT | 0.5325 | 2.02 | 0.0487 * |
| Time | 1, 47 | 0.07 | 0.7997 | CG | −0.4403 | −1.78 | 0.0817 |
| Treatment X Time | 1, 47 | 7.25 | 0.0098 * | ||||
| N1 peak amplitude during Talk z-scored values at Cz | |||||||
| ANOVA | Within Treatment Group Contrasts | ||||||
| df | F | Sig. | Time2 - Time1 | Mean Difference | t value | Sig. | |
| Treatment Group | 1, 47 | 2.82 | 0.0996 | TAT | 0.7565 | 2.36 | 0.0227 |
| Time | 1, 47 | 0.39 | 0.5362 | CG | −0.302 | −1.6 | 0.1173 * |
| Treatment X Time | 1, 47 | 7.89 | 0.0072 * | ||||
| N1 peak amplitude during Listen z-scored values at Cz | |||||||
| ANOVA | Within Treatment Group Contrasts | ||||||
| df | F | Sig. | Time2 - Time1 | Mean Difference | t value | Sig. | |
| Treatment Group | 1, 47 | 0.28 | 0.5968 | TAT | 0.5325 | 2.02 | 0.0487 |
| Time | 1, 47 | 0.56 | 0.4567 | CG | −0.4403 | −1.78 | 0.0817 * |
| Treatment X Time | 1, 47 | 0.05 | 0.8913 | ||||
Given the baseline N1 suppression differences between treatment groups, additional ANCOVA models were run to test if the treatment effect on N1 suppression change scores (i.e., the Treatment x Time interaction effect) remained after controlling for N1 suppression at baseline. There was no evidence of different relationships between the N1 suppression change scores and treatment group (t(45) = −1.1, p = 0.277), but the reduced ANCOVA model showed a strong, negative association between N1 suppression change and baseline N1 suppression (t(46) = - 6.28, p < 0.0001). That is, the greater an ESZ subject’s N1 suppression at baseline, the greater N1 suppression reduction for that subject, and the more reduced an ESZ subject’s N1 suppression at baseline, the greater the N1 improvement for that subject. The treatment effect on N1 suppression change scores was non-significant in this model (t(46) = 1.25, p = 0.22).
In this sub-sample of participants from a larger study (Fisher et al., 2015), Global Cognition was affected by a significant Treatment × Time interaction (F(1,47) = 4.14, p = 0.0474; Cohen’s d=0.38), with no main effects of Treatment (F(1,47) = 0.35, p=.56) or Time (F(1,47) = 3.2, p=.08). Follow-up contrasts assessed the effect of Time separately in the TAT and CG groups. The effect of Time was significant in TAT (t(47) = 2.62, p=.012) but not CG (t(47) = 0.18, p=.86). We also assessed Group differences before and after treatment and found they were not different either before (t(47) = −0.18, p=.95) or after (t(47) = 1.19, p=.24) treatment.
3.2. Associations between N1 suppression z-score change and Global Cognition change
The initial model including Treatment Group, change in N1 suppression z-scores, and their interaction showed no significant interaction effects (F(1,45) = 1.89, p = 0.176) on Global Cognition, indicating that the common slope, reduced model was more appropriate. In the reduced model, there was no relationship between the changes in N1 suppression z-scores and Global Cognition change scores (t(46) = −0.705, p = 0.485).
3.3. Associations between changes in N1 z-scores and SAPS Global symptom ratings
Three out of four initial models comparing slopes between treatment groups revealed no significant R-squared change F-tests (all p-values > .194), meaning these more complicated models failed to improve the overall fit, and reduced models allowing for common slopes across Treatment groups were more appropriate. However, the SAPS bizarre behavior global symptom rating change model did exhibit a statistically significant difference in its relationship with N1 suppression z-score change between treatment groups (F(1, 45) = 8.873, p = 0.004). Inspection of individual Betas revealed that TAT was associated with a more positive slope (β = 1.12), meaning that greater N1 suppression improvement was associated with a worsening of bizarre behavior symptom ratings scores. However, the correlation within the TAT group (r = .563, p = 0.0051) was driven by two subjects. One who had the greatest improvement in N1 suppression and the worst (i.e., most increased) change in symptoms, while another had nearly the worst N1 suppression change and the greatest improvement (3-point decrease) in the global symptom rating, and the majority of the remaining subjects (n=17) had zero change in symptoms. None of the three reduced SAPS rating change models showed any trend towards an association between SAPS global symptom rating change scores and change in N1 suppression z-scores (all p-values >= 0.33).
3.4. Simple effects of Time and N1 stability in HC
N1 suppression z-scores did not change over time in the HC group (t(28) = −1.3315, p = 0.19). There were no differences in the raw N1 suppression (p = 0.23), N1 Talk (p = 0.23), or N1 Listen (p = 0.97) amplitudes between test occasions. ICCs were low but equivalent for both raw N1 suppression and suppression z-scores (ICC = 0.4), poor for N1 Talk amplitude (ICC = 0.17), and good for N1 Listen amplitude (ICC = 0.73).
4. Discussion
In this study, we directly compared the effects of targeted auditory training (TAT) and computer games (CG) on N1 suppression during vocalization in schizophrenia patients early in their illness. We found N1 suppression was improved following TAT. The improvement in N1 suppression with TAT was driven by improvement in N1 to spoken sounds during talking but not listening. We suggest that TAT may have improved transmission of the efference copy during talking, allowing the brain to suppress sensations resulting from its own actions, a hallmark of successful functioning of the mechanism. We have previously shown that N1 suppression is related to white matter integrity in the fasciculus connecting frontal lobes and auditory cortex (Whitford et al., 2018). Further, we know cognitive training focusing on working memory improves connectivity between thalamus and prefrontal cortex (Ramsay et al., 2017). Together, these findings suggest that training focused on speech discrimination may have improved frontal-temporal connectivity known to be affected by schizophrenia (Ford et al., 2002) and critical in N1 suppression (Chen et al., 2011; Wang et al., 2014).
The auditory training used in the current study included exercises to enhance discrimination of frequency-modulated tones in the 761–2000 Hz range, as well as discrimination of more complex speech-related stimuli. While this training did affect N1 during talking, it was surprising that it did not affect N1 during passive listening. If each subject’s own speech sound had been used for the auditory training, it is possible that we might have found training increased N1 during listening. However, at least one study that did use identical auditory stimuli for training and testing still failed to show a change in ERP amplitude (Kärgel C et al., 2016).
It is important to emphasize that the patients who underwent ERP assessments are a sub-sample of participants recruited for a larger trial that demonstrated stronger effects of auditory training on Global Cognition compared to those observed in this analysis (Fisher et al., 2015). Nevertheless, both Global Cognition and N1 suppression improved with auditory training yet they were not related to each other, suggesting separate mechanisms may be contributing to the improvement in each. A likely explanation is that the verbal memory training modules effected improvement in Global Cognition performance and the speech discrimination training modules effected improvement in N1 suppression. Unfortunately, it is impossible to isolate the separate effects of the different modules of the auditory training in the current data set.
Although patients were randomly assigned to the two treatment groups, they differed in the amount of N1 suppression at baseline, before the treatments began. Nevertheless, as indicated by a Time x Treatment interaction for N1 suppression, there was marginal worsening with computer games and a significant improvement with targeted auditory training. However, despite these improvements, a significant N1 suppression abnormality was still evident post-training, falling below the expected z-score value (i.e., 0) for HC, controlling for age.
N1 amplitude was stable during passive listening but not during vocalization. We suggest that the poor stability of N1 during vocalization may be reflect its sensitivity to subtleties in speech sounds from one trial to the next during vocalization but not during listening (Sitek et al., 2013). The variability of pitch from one trial to the next would contribute to error variance and poor reliability. Better stability might be seen for those vocalizations that do not vary much from one trial to the next.
4.1. Limitations
One limitation of this study is our failure to find a relationship between N1 suppression and psychotic symptoms, or between a change in psychotic symptoms and change in N1 suppression. Throughout our work with this paradigm, we have found N1 suppression to be reduced in chronic (Ford et al., 2007a; Ford et al., 2001d) and early illness schizophrenia (Mathalon et al., 2018; Perez et al., 2012), psychotic bipolar and schizoaffective disease (Ford et al., 2013), schizotypy (Oestreich et al., 2015), and youth at clinical high risk for psychosis (Mathalon et al., 2018). We found reduction of N1 suppression in first degree family members was intermediate between probands and healthy controls (Ford et al., 2013). While a deficit in N1 suppression during talking appears to be a vulnerability marker of psychosis, it was not related to the severity of psychotic symptoms in any of these populations. To the extent that N1 suppression during talking is an assay of corollary discharge function, our failure to find a relationship with hallucinations is counter to the hypothesis that corollary discharge dysfunction underlies the positive psychotic symptoms of schizophrenia (Feinberg, 1978). We are currently decomposing the N1 amplitude into its constituent parts (EEG power and inter-trial coherence) to determine if their suppression is more sensitive than N1 amplitude suppression to psychotic symptoms. In the past, we found relationships between auditory hallucinations and frontal-temporal coherence in the theta band during speaking (Ford et al, 2002) and inter-trial coherence in the beta band preceding speech onset (Ford et al, 2007).
Another limitation is the marginal, non-significant, worsening of N1 suppression with computer games. Though few studies have examined the effects of exposure to computer or video games on verbal memory in either healthy or cognitively impaired individuals, one study found a significant reduction in verbal memory after a single day of exposure to excessive computer game playing and TV in healthy school-age children (Dworak et al., 2007). Participants in our study were told to play computer games 5 hours a week, but if they played more it could have contributed to the worsening of N1 suppression. Also, there is evidence that the impact of exercising certain networks or functions for people with impaired or vulnerable neural systems (e.g., those involved in visual perception and attention, visual working memory, and visuomotor processing) may have compensatory consequences on other neural systems (Bernstein et al., 2014). It could be the case that visuospatial processing from an intensive course of CG resulted in competitive interference for limited neural resources (Fisher et al., 2015), causing worse N1 suppression, which parallels previous reports of verbal processing/verbal memory performance declines after CG in chronic schizophrenia patients (Fisher et al., 2009) as well as individuals at clinical high risk for psychosis (Loewy et al., 2016). Finally, the slight worsening we see could be attributed to the passage of time, as healthy controls also experienced a slight, non-significant worsening. While speculations abound about why computer games might impair normal brain function, we must remember that the ‘worsening’ in N1 suppression was not significant.
When baseline N1 suppression was controlled for in an ANCOVA model of N1 suppression change, the treatment effect was no longer significant, and there was a strong, negative association between N1 suppression change and baseline N1 suppression. There are two plausible explanations for these ANCOVA results. Given the difference in baseline N1 suppression between the two treatment groups and the lack of such difference in post-treatment N1 suppression, one hypothesis is that the observed improvement in N1 suppression with TAT and marginal worsening of N1 suppression with CG can simply be explained as regression to the mean. The competing explanation is that N1 suppression improvement in TAT and marginal reduction in CG treatment groups may be true treatment effects. That is, N1 suppression abnormalities are necessary at baseline for there to be improvement with treatment. Matching treatment groups on baseline N1 suppression in a future study would allow for a better-controlled assessment of these competing hypotheses.
Still another limitation is that most patients were on medications. Although even the unmedicated patients were stable, 11 patients in the CG group and 8 in the TAT group did have medication changes during the study. However, by the end of the study, CPZ equivalents were not different between the two groups, and there was no significant increase in CPZ in either group.
5. Conclusions
In conclusion, 40 hours of targeted auditory training in schizophrenia patients early in their illness improved Global Cognition and may improve efference copy/corollary discharge function. Given that N1 suppression is unstable over a period of 6 months in healthy controls, it is important that the observed improvement of N1 suppression with auditory training be replicated. We did not assess the long-term effects of these interventions. However, Fisher et al. (Fisher et al., 2010) reported that 50 hours of computerized cognitive training is sufficient to drive improvements in verbal learning/memory and cognitive control that endure 6 months beyond the intervention. Whether the enduring effects would extend to N1 suppression is not known. Finally, we do not know whether other assays of the corollary discharge system would also be affected by TAT, such as pushing a button to hear a tone (Baess et al., 2011; Baess et al., 2009; Bass et al., 2008; Ford et al., 2014) or a sound (Ford et al., 2007a). Because N1 suppression in these paradigms is also affected by schizophrenia (Ford et al., 2007a; Ford et al., 2014), it may also be affected by TAT.
Highlights.
Corollary discharge function may improve with auditory training in schizophrenia
This improvement does not fully rescue abnormal corollary discharge function
Corollary discharge function does not improve with computer games
Acknowledgements
Funding
This study was supported by the National Institute of Mental Health under Award Numbers K02MH067967 (PI: JMF), R01MH058262 (PI: JMF), 5R01MH081051 (PI: SV), R01MH076898 (PI: DHM); by the Stanley Medical Research Institute under Award Number 06TAF-972 (PI: SV), by the Department of Veterans Affairs (PIs: SV, JMF, and a Senior Research Career Scientist award to JMF), and by the Brain and Behavior Foundation (PI: DHM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Abbreviations
- ESZ
early in the course of a schizophrenia-spectrum illness
- HC
Healthy Controls
- EEG
Electroencephalogram
- TAT
Targeted Auditory Training
- CG
Computer Games
- ERP
Event-related potential
- RCT
randomized clinical trial
- MCCB
Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: ClinicalTrials.gov NCT00694889. Registered 1 August 2007.
Competing interests
At the time of study completion, BB was a post-doctoral research fellow partially funded by Posit Science. The auditory training software used in this study was supplied free of charge by Posit Science. SV is a site PI on an SBIR grant to Posit Science, a company with a commercial interest in the training software used in these studies. None of the other authors have any financial interest in Posit Science. All authors declare no other conflicts of interest.
Contributor Information
Brian J. Roach, Email: brian.roach@ncire.org.
Bruno Biagianti, Email: bruno.biagianti@positscience.com.
Holly K. Hamilton, Email: holly.hamilton@ucsf.edu.
Ian S. Ramsay, Email: ramsa045@umn.edu.
Melissa Fisher, Email: mafisher@umn.edu.
Rachel Loewy, Email: rachel.loewy@ucsf.edu.
Sophia Vinogradov, Email: svinogra@umn.edu.
Daniel H. Mathalon, Email: daniel.mathalon@ucsf.edu.
References
- Andreasen NC, 1983. The Scale for the Assessment of Negative Symptoms (SANS) University of Iowa, Iowa City, IA. [Google Scholar]
- Andreasen NC, 1984. Scale for the Assessment of Positive Symptoms University of Iowa, Iowa City, IA. [Google Scholar]
- Baess P, Horvath J, Jacobsen T, Schroger E, 2011. Selective suppression of self-initiated sounds in an auditory stream: An ERP study. Psychophysiology 48, 1276–1283. [DOI] [PubMed] [Google Scholar]
- Baess P, Widmann A, Roye A, Schroger E, Jacobsen T, 2009. Attenuated human auditory middle latency response and evoked 40-Hz response to self-initiated sounds. Eur. J. Neurosci 29, 1514–1521. [DOI] [PubMed] [Google Scholar]
- Bass P, Jacobsen T, Schroger E, 2008. Suppression of the auditory N1 event-related potential component with unpredictable self-initiated tones: evidence for internal forward models with dynamic stimulation. Int. J. Psychophysiol 70, 137–143. [DOI] [PubMed] [Google Scholar]
- Behroozmand R, Karvelis L, Liu H, Larson CR, 2009. Vocalization-induced enhancement of the auditory cortex responsiveness during voice F0 feedback perturbation. Clin Neurophysiol 120, 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein LE, Eberhardt SP, Auer ET Jr., 2014. Audiovisual spoken word training can promote or impede auditory-only perceptual learning: prelingually deafened adults with late-acquired cochlear implants versus normal hearing adults. Front Psychol 5, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagianti B, Fisher M, Neilands TB, Loewy R, Vinogradov S, 2016. Engagement with the auditory processing system during targeted auditory cognitive training mediates changes in cognitive outcomes in individuals with schizophrenia. Neuropsychology 30, 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagianti B, Roach BJ, Fisher M, Loewy R, Ford JM, Vinogradov S, Mathalon DH, 2017. Trait aspects of auditory mismatch negativity predict response to auditory training in individuals with early illness schizophrenia. Neuropsychiatr Electrophysiol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett TA, Freedland MB, Larson CR, Hain TC, 1998. Voice F0 responses to manipulations in pitch feedback. The Journal of the Acoustical Society of America 103, 3153–3161. [DOI] [PubMed] [Google Scholar]
- Chen CM, Mathalon DH, Roach BJ, Cavus I, Spencer DD, Ford JM, 2011. The Corollary Discharge in Humans Is Related to Synchronous Neural Oscillations. J Cogn Neurosci 23, 2892–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse TB, Sommer MA, 2008. Corollary discharge across the animal kingdom. Nature Reviews. Neuroscience 9, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curio G, Neuloh G, Numminen J, Jousmaki V, Hari R, 2000. Speaking modifies voice-evoked activity in the human auditory cortex. Human Brain Mapping 9, 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CL, Brown EG, Fisher M, Herman AB, Dowling AF, Hinkley LB, Subramaniam K, Nagarajan SS, Vinogradov S, 2016. Auditory Cortical Plasticity Drives Training-Induced Cognitive Changes in Schizophrenia. Schizophrenia bulletin 42, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CL, Findlay AM, Adcock RA, Vertinski M, Fisher M, Genevsky A, Aldebot S, Subramaniam K, Luks TL, Simpson GV, Nagarajan SS, Vinogradov S, 2010. Timing is everything: neural response dynamics during syllable processing and its relation to higher-order cognition in schizophrenia and healthy comparison subjects. Int J Psychophysiol 75, 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq W, Vergult A, Vanrumste B, Van Paesschen W, Van Huffel S, 2006. Canonical correlation analysis applied to remove muscle artifacts from the electroencephalogram. IEEE Trans Biomed Eng 53, 2583–2587. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S, 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Dworak M, Schierl T, Bruns T, Struder HK, 2007. Impact of singular excessive computer game and television exposure on sleep patterns and memory performance of school-aged children. Pediatrics 120, 978–985. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X, 2003. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiol 89, 2194–2207. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X, 2005. Dynamics of auditory-vocal interaction in monkey auditory cortex. Cereb Cortex 15, 1510–1523. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X, 2008. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature 453, 1102–1106. [DOI] [PubMed] [Google Scholar]
- Feinberg I, 1978. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophrenia Bulletin 4, 636–640. [DOI] [PubMed] [Google Scholar]
- First MB, Frances A, 1995. DSM-IV handbook of differential diagnosis American Psychiatric Press, Washington, DC. [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S, 2009. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. The American journal of psychiatry 166, 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S, 2010. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophrenia bulletin 36, 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Loewy R, Carter C, Lee A, Ragland JD, Niendam T, Schlosser D, Pham L, Miskovich T, Vinogradov S, 2015. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophrenia bulletin 41, 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Gray M, Faustman WO, Roach BJ, Mathalon DH, 2007a. Dissecting corollary discharge dysfunction in schizophrenia. Psychophysiology 44, 522–529. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, 2005. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? International Journal of Psychophysiology 58, 179–189. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Heinks T, Kalba S, Faustman WO, Roth WT, 2001a. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. American Journal of Psychiatry 158, 2069–2071. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Heinks T, Kalba S, Roth WT, 2001b. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. American Journal of Psychiatry 158, 2069–2071. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT, 2001c. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biological Psychiatry 50, 540–549. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT, 2001d. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biological psychiatry 50, 540–549. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Roach BJ, Keedy SK, Reilly JL, Gershon ES, Sweeney JA, 2013. Neurophysiological evidence of corollary discharge function during vocalization in psychotic patients and their nonpsychotic first-degree relatives. Schizophrenia bulletin 39, 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT, 2002. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biological psychiatry 51, 485–492. [DOI] [PubMed] [Google Scholar]
- Ford JM, Palzes VA, Roach BJ, Mathalon DH, 2014. Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophrenia bulletin 40, 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Perez VB, Mathalon DH, 2012. Neurophysiology of a possible fundamental deficit in schizophrenia. World psychiatry : official journal of the World Psychiatric Association 11, 58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH, 2007b. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry 164, 458–466. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Mathalon DH, 2010. Assessing corollary discharge in humans using noninvasive neurophysiological methods. Nat Protoc 5, 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Palzes VA, Mathalon DH, 2016. Using concurrent EEG and fMRI to probe the state of the brain in schizophrenia. Neuroimage Clin 12, 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee JD, Jackson AW, Chen F, Larson CR, Oya H, Kawasaki H, Chen H, Howard MA 3rd, 2011. Human auditory cortical activation during self-vocalization. PloS one 6, e14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Mathalon DH, Gray M, Ford JM, 2005. Fine-tuning of auditory cortex during speech production. Psychophysiology 42, 180–190. [DOI] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM, 2007. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry 64, 286–296. [DOI] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Nagarajan SS, Houde JF, 2006. Magnetoencephalographic evidence for a precise forward model in speech production. Neuroreport 17, 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde JF, Jordan MI, 1998. Sensorimotor adaptation in speech production. Science 279, 1213–1216. [DOI] [PubMed] [Google Scholar]
- Houde JF, Nagarajan SS, Sekihara K, Merzenich MM, 2002. Modulation of the auditory cortex during speech: an MEG study. J Cogn Neurosci 14, 1125–1138. [DOI] [PubMed] [Google Scholar]
- Kärgel C, Sartory G, Kariofillis D, Wiltfang J, BW., M., 2016. The effect of auditory and visual training on the Mismatch negativity in schizophrenia. International Journal of Psychophysiology 102. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13, 261–276. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, 2003. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clin Neurophysiol 114, 2307–2325. [DOI] [PubMed] [Google Scholar]
- Kort NS, Ford JM, Roach BJ, Gunduz-Bruce H, Krystal JH, Jaeger J, Reinhart RM, Mathalon DH, 2017. Role of N-Methyl-D-Aspartate Receptors in Action-Based Predictive Coding Deficits in Schizophrenia. Biological psychiatry 81, 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Redoblado-Hodge MA, Naismith SL, Hermens DF, Porter MA, Hickie IB, 2013. Cognitive remediation improves memory and psychosocial functioning in first-episode psychiatric out-patients. Psychol Med 43, 1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonowicz Z, Karvanen J, Shishkin SL, 2005. Trimmed estimators for robust averaging of event-related potentials. J Neurosci Methods 142, 17–26. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, 1983. Monitoring and self-repair in speech. Cognition 14, 41–104. [DOI] [PubMed] [Google Scholar]
- Loewy R, Fisher M, Schlosser DA, Biagianti B, Stuart B, Mathalon DH, Vinogradov S, 2016. Intensive Auditory Cognitive Training Improves Verbal Memory in Adolescents and Young Adults at Clinical High Risk for Psychosis. Schizophrenia bulletin 42 Suppl 1, S118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Roach BJ, Ferri JM, Loewy RL, Stuart BK, Perez VB, Trujillo TH, Ford JM, 2018. Deficient auditory predictive coding during vocalization in the psychosis risk syndrome and in early illness schizophrenia: the final expanded sample. Psychol Med, 1–8. [DOI] [PubMed]
- Nolan H, Whelan R, Reilly RB, 2010. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. Journal of neuroscience methods 192, 152–162. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 165, 203–213. [DOI] [PubMed] [Google Scholar]
- Oestreich LK, Mifsud NG, Ford JM, Roach BJ, Mathalon DH, Whitford TJ, 2015. Subnormal sensory attenuation to self-generated speech in schizotypy: Electrophysiological evidence for a ‘continuum of psychosis’. Int J Psychophysiol 97, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JS, 2006. On phonetic convergence during conversational interaction. The Journal of the Acoustical Society of America 119, 2382. [DOI] [PubMed] [Google Scholar]
- Perez VB, Ford JM, Roach BJ, Loewy RL, Stuart BK, Vinogradov S, Mathalon DH, 2012. Auditory cortex responsiveness during talking and listening: early illness schizophrenia and patients at clinical high-risk for psychosis. Schizophrenia bulletin 38, 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov T, Jordanov T, Rockstroh B, Elbert T, Merzenich MM, Miller GA, 2011. Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biological psychiatry 69, 465–471. [DOI] [PubMed] [Google Scholar]
- Ramnani N, 2006. The primate cortico-cerebellar system: anatomy and function. Nat. Rev. Neurosci 7, 511–522. [DOI] [PubMed] [Google Scholar]
- Ramsay IS, Nienow TM, MacDonald AW 3rd, 2017. Increases in Intrinsic Thalamocortical Connectivity and Overall Cognition Following Cognitive Remediation in Chronic Schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging 2, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries S, Janssen N, Burle B, Alario FX, 2013. Response-locked brain dynamics of word production. PloS one 8, e58197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai A, Pratt H, 2002. Electrophysiological evidence for priming in response to words and pseudowords in first and second language. Brain Lang 80, 240–252. [DOI] [PubMed] [Google Scholar]
- Sitek KR, Mathalon DH, Roach BJ, Houde JF, Niziolek CA, Ford JM, 2013. Auditory cortex processes variation in our own speech. PloS one 8, e82925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, de Villers-Sidani E, 2012. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology 37, 43–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mathalon DH, Roach BJ, Reilly J, Keedy SK, Sweeney JA, Ford JM, 2014. Action planning and predictive coding when speaking. Neuroimage 91, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Oestreich LKL, Ford JM, Roach BJ, Loewy RL, Stuart BK, Mathalon DH, 2018. Deficits in Cortical Suppression During Vocalization are Associated With Structural Abnormalities in the Arcuate Fasciculus in Early Illness Schizophrenia and Clinical High Risk for Psychosis. Schizophrenia bulletin 44, 1312–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]


