Abstract
Background:
There are distinct trajectories to cognitive impairment among participants in the Multicenter AIDS Cohort Study (MACS). Here we analyzed the relationship between regional brain volumes and the individual trajectories to impairment in a subsample (n = 302) of the cohort.
Methods:
302 (167 HIV-infected; mean age = 55.7 yrs.; mean education: 16.2 yrs.) of the men enrolled in the MACS MRI study contributed data to this analysis. We used voxel-based morphometry (VBM) to segment the brain images to analyze gray and white matter volume at the voxel-level. A Mixed Membership Trajectory Model had previously identified three distinct profiles, and each study participant had a membership weight for each of these three trajectories. We estimated VBM model parameters for 100 imputations, manually performed the post-hoc contrasts, and pooled the results.
Results:
We examined the associations between brain volume at the voxel level and the MMTM membership weights for two profiles: one considered “unhealthy” and the other considered “Premature aging.” The unhealthy profile was linked to the volume of the posterior cingulate gyrus/precuneus, the inferior frontal cortex, and the insula, whereas the premature aging profile was independently associated with the integrity of a portion of the precuneus.
Conclusions:
Trajectories to cognitive impairment are the result, in part, of atrophy in cortical regions linked to normal and pathological aging. These data suggest the possibility of predicting cognitive morbidity based on patterns of CNS atrophy.
Keywords: HIV, Dementia, Multiple Imputation, Brain Structure, Mixed Membership Trajectory
Introduction
HIV-mediated neural damage results in neurobehavioral disturbances and HIV-associated neurocognitive disorders (HAND) (Antinori et al., 2007), which include asymptomatic neurocognitive impairment, mild neurocognitive impairment, and HIV-associated dementia (Saylor et al., 2016). However, in spite of the success of combination antiretroviral therapy (cART) at reducing the risk of AIDS-defining illnesses and increasing the life span of individuals with HIV disease, the impact of these treatment regimens on neurological and neuropsychological impairments among infected individuals remains unclear. Although HIV associated dementia has all but disappeared among individuals with access to appropriate medical care and management, a milder form of impairment (the “mild cognitive impairment” syndrome) remains prevalent (Sacktor et al., 2002). Understanding the central nervous system (CNS) basis of these impairments remains a high priority research agenda.
One way to study cognition-related syndromes in the context of HIV disease is to analyze trajectories to impairment. That is, what is an individual’s risk of impairment over their lifetime involvement in a longitudinal study, and how do individual factors predict or modify risk of developing impairment? The present study builds on our recent findings (Molsberry et al., 2015) from an analysis using the novel, data-driven Mixed Membership Trajectory Model (MMTM) technique (Manrique-Vallier, 2014) to describe the development of mild and severe cognitive impairment. MMTMs combine features of longitudinal Multivariate Latent Trajectory Models to identify distinct, canonical profiles, with features of cross-sectional Grade of Membership Models (Connor, 2006) to allow individuals to have weighted memberships in each profile(E.A. Erosheva, 2005; E. A. Erosheva, Fienberg, & Joutard, 2007). The utility of this method was shown in an analysis of disability data from the National Long Term Care Survey (NLTCS) (Manrique-Vallier, 2014), finding that most individuals followed a trajectory that implied a late onset of disability; younger cohorts tended to develop disabilities at a later stage in life. An advantage of the MMTMs relative to other trajectory modeling techniques is that the MMTM also expresses each individual participant’s pathway as a weighted combination of the canonical trajectories. In addition to expressing an individual’s closeness to the canonical trajectories (or profiles), the membership weights can also be interpreted as reflecting each individual’s health propensities (in this case, cognitive impairment).
Using the neuropsychological data from the MACS participants (both infected and uninfected) Molsberry and colleagues identified three canonical profiles that we descriptively labelled “normal aging,” “premature aging,” and “unhealthy.” The MMTM expressed each individual’s trajectory as the weighted combination of the three canonical trajectories. The model used predictor variables previously identified as risk factors for HAND to determine individuals’ “closeness” to the canonical profiles. The analysis found that hepatitis-C infection, depression, race, MACS recruitment cohort and confounding conditions all affected individual’s closeness to these trajectories. In addition, clinically defined AIDS, and not simply HIV disease, was associated with closeness to the premature aging trajectory. Thus, an individual participant’s closeness to one of the canonical trajectories is affected by multiple subject-specific characteristics (See (Molsberry et al., 2015), for details).
In order for these trajectories to have the most meaning in terms of understanding the pathobiology of the development of HAND, there should be some association between each individual’s overall (or summary) closeness to each trajectory, and a measure of central nervous system integrity. We took advantage of the fact that there is a subset of individuals (n = 302) within the MACS who are also enrolled in a study involving structural brain imaging and cognition. We utilized these MRI data to determine the extent to which there was an association between brain structural integrity and each individual participant’s closeness to the three canonical trajectories identified in our prior report (Molsberry et al., 2015).
Our primary objective in this study was to investigate the relationship between trajectory membership weights (“closeness”) and brain structural integrity, in order to identify those brain regions linked to the more abnormal trajectories. In order to accomplish this goal, we would ordinarily input the observed/measured data and fit the model in the software of choice, such as SPM or FSL. However, the membership weights from the MMTM are not observable quantities but are random variables; each membership weight is a mean value with a standard deviation. Thus, we could not simply input their point estimates without biasing the results. Therefore, we approached this analysis as a variation of a missing data problem and used multiple imputation methodologies to overcome the problem.
Methods
This research was reviewed and approved by the Institutional Review Boards at all four MACS clinic sites – Johns Hopkins University, Northwestern University, University of California Los Angeles, and the University of Pittsburgh. Each participant signed a written statement of informed consent prior to starting any research-related activities.
Subjects and Brain Imaging:
The MACS is a four-center study of the natural and treated history of HIV infection among men who have sex with men (Kaslow et al., 1987) that tracks the cognitive test performance of the study volunteers. There were three distinct recruitment stages that focused on groups of infected men with different demographic characteristics, or men at risk for infection. Study participants were enrolled at four sites (Los Angeles, Pittsburgh, Chicago, Baltimore/Washington) in three waves: 1984/85, 1987/90 and 2001/03. The men who enrolled in 1984–85 are Cohort 1, those who enrolled in 1987–91 are Cohort 2 and those who enrolled between 2001 and 2003 are Cohort 3. Cohort 1 was the original sample of 4954 men and Cohort 2 was a ‘new recruit cohort’ that focused on enrolling minority and special target groups such as the partners of the men in C1. Cohort 3 focused on recruiting racial/ethnic minorities as well as a special target group of uninfected men who had been censored from C1 in 1995. Because the characteristics of the men in Cohorts 1 and 2 were similar, we refer to a combined Cohort 1 (C1) and a separate Cohort 2 (C2, 2001–2003 enrollees only). This dichotomous variable was used in data analyses (see below).
A subset of 302 participants from across the four clinical centers had 3D magnetic resonance (MR) brain images and contributed these MRI data for this analysis (See Tables 1 and 2, and (Becker et al., 2011)) for details of initial MRI study enrollment. These men had undergone high-resolution anatomical brain imaging (MP-RAGE at 3Tesla field strength). There was no association between HIV status and trajectory closeness (X2 = 1.22, df=2, p= .54).
Table 1:
Characteristics of Participants as a Function of MMTM Classification
| Closest to | |||
|---|---|---|---|
| Healthy Profile | Unhealthy Profile | Premature Aging Profile | |
| N= | 231 (76.5) | 57 (18.9) | 14 (4.6) |
| Age | 57.1 (6.1) | 56.0 (5.7) | 57.4 (7.8) |
| Race (Caucasian) | 173 (74.9) | 35 (61.4) | 9 (64.3) |
| HIV Infected | 124 (54.6) | 35 (64.3) | 9 (64.3) |
| AIDS1 | 16 (12.9) | 7 (20.0) | 1 (11.1) |
| Depressed ever | 173 (74.9) | 43 (74.4) | 10 (71.4) |
| Hepatitis C Infection | 20 (8.7) | 18 (32.6) | 2 (14.3) |
Mean (+ s.d.) for continuous data. Number and percent for categorical data;
expressed as a percent of infected men
Table 2:
Characteristics of Study Sample at the Time of MRI Scan1
| Seronegative | Seropositive | Effect Size2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Trajectory | Normal | Premature Aging | Abnormal | Normal | Premature Aging | Abnormal | Serostatus | Trajectory |
| Number | 103 | 22 | 5 | 124 | 35 | 9 | ||
| Age3 (mean, S.D., Range) | 59.3 (7.1) (50.3–78.5) |
58.9 (5.8) (51.1–76.1) |
53.6 (3.0) (51.6–64.5) |
56.2 (4.5) (48.3–76.5) |
56.0 (5.2) (50.2–76.4) |
56.9 (5.8) (52.4–67.0) |
.002 | .007 |
| Education | 16.8 (2.5) | 16.9 (2.4) | 16.7 (2.3) | 15.7 (2.3) | 16.5 (4.0) | 15.6 (1.7) | .007 | .006 |
| Cohort (%(n) Cohort 3) | 13.6 | 27.3 | 0.0 | 29.0 | 48.6 | 7.0 | .16* | .21* |
| Race (%(n) Caucasian) | 87.4 | 81.8 | 100 | 75.0 | 54.3 | 66.7 | .15* | .20* |
| Diabetes | 9.7 | 31.8 | 0.0 | 14.5 | 20.0 | 0.0 | .16* | .03 |
| Hypertension | 45.6 | 50.0 | 60.0 | 41.9 | 42.9 | 55.6 | .06 | .04 |
| Depressed | 14.7 | 15.0 | 0.0 | 24.1 | 24.2 | 0.0 | .10 | .11 |
| Cocaine | 9.7 | 13.6 | 20.0 | 25.8 | 37.1 | 44.4 | .12 | .22* |
| Amphetamines | 4.9 | 0.0 | 0.0 | 14.5 | 14.3 | 0.0 | .07 | .17* |
| AIDS | n/a | n/a | n/a | 12.9 | 20.0 | 11.1 | .10 | .11 |
| Detectable Virus | n/a | n/a | n/a | 16.4 | 30.4 | 37.5 | n/a | .09 |
| Current CD4+ | n/a | n/a | n/a | 691.1 (297) | 764.1 (477) | 973.1 (420) | n/a | .03* |
| Nadir CD4+ | n/a | n/a | n/a | 277.5 (156) | 264.1 (188) | 294.2 (255) | n/a | .002 |
| Current Viral Load | n/a | n/a | n/a | 1.60 (.84) | 1.83 (1.1) | 2.11 (1.3) | n/a | .03 |
| Peak Viral Load | n/a | n/a | n/a | 4.78 (.601) | 4.80 (.73) | 5.13 (.56) | n/a | .02 |
| CO WAT3 | 55.1 (1.4) | 45.7 (3.5) | 50.3 (6.7) | 53.4 (1.2) | 45.0 (2.3) | 46.9 (4.4) | .002 | .073* |
| Rey Osterreith Figure-Copy | 33.5 (.88) | 30.8 (2.3) | 29.0 (4.3) | 29.8 (.78) | 26.9 (1.5) | 30.4 (2.8) | .005 | .019 |
| Immediate Recall | 25.5 (.94) | 15.1 (2.4) | 17.0 (4.6) | 21.6 (.83) | 14.4 (1.6) | 17.1 (3.0) | .002 | .144* |
| Delayed Recall | 25.2 (.94) | 14.3 (2.4) | 18.2 (4.6) | 21.3 (.83) | 14.6 (1.6) | 16.1 (3.0) | .004 | .144* |
| Stroop Test-Interference | 95.8 (4.7) | 123.5 (12) | 147.3 (23) | 94.0 (4.1) | 119.2 (7.8) | 110.7 (15) | .009 | .072* |
| Grooved Pegboard-Dominant3 | 55.0 (1.8) | 42.0 (4.7) | 52.7 (9.0) | 51.4 (1.6) | 39.3 (3.1) | 45.1 (5.9) | .006 | .076* |
| Non-Dominant3 | 54.3 (1.8) | 43.7 (4.6) | 49.3 (8.8) | 49.8 (1.6) | 40.3 (3.0) | 49. (5.8) | .002 | .052* |
| Trailmaking A3 | 64.8 (2.2) | 53.9 (5.6) | 59.3 (11) | 58.3 (1.9) | 55.8 (3.6) | 58.0 (7.0) | .001 | .017* |
| Trailmaking B3 | 70.8 (2.2) | 55.3 (5.6) | 58.3 (11) | 59.6 (1.9) | 54.8 (3.6) | 54.0 (7.0) | .006 | .042* |
Continuous (mean + s.d.) and discrete (percent) variables.
(for continuous data) or Phi (for categorical data)
Mean (± s.d.) and range
p<.05
The acquired brain images were preprocessed using a non-parametric correction of intensity nonuniformity (Sled, Zijdenbos, & Evans, 1998). We used a custom-made template of tissue priors, which we had created with the Template-O-Matic (TOM8) toolbox and data from the Information eXtraction from Images (IXI) project. We processed the images with voxel-based morphometry (VBM8) in Statistical Parametric Mapping (SPM12) software running in MATLAB. Brains were affine-registered, segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid volumes (CSF), and then normalized with DARTEL. Our analysis used the smoothed (8 × 8 × 8mm FWHM) modulated, normalized, log-transformed GM and WM images that passed our quality control check.
Mixed Membership Trajectory Model:
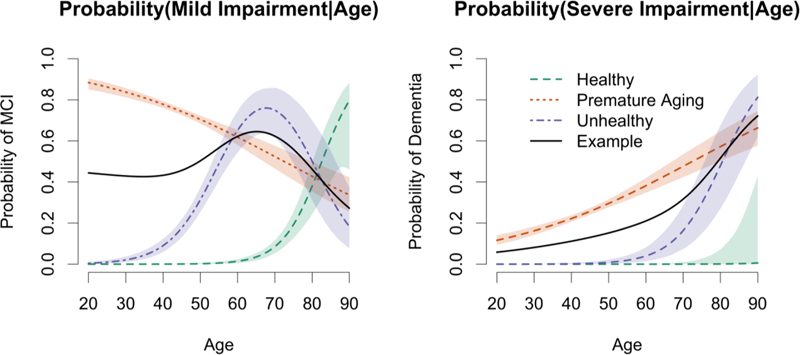
MMTMs assume there is a finite, usually small number of distinct patterns that are called “canonical profiles,” and individual trajectories are modeled as a weighted mixture of those canonical profiles. Molsberry and colleagues (Molsberry et al., 2015) modeled the canonical profiles of 3,892 men (2,099 infected) in the MACS and estimated the membership weights, all within a hierarchical Bayesian framework (Molsberry et al., 2015). Individuals’ cognitive classification (Normal, Mild Impairment, Severe Impairment), recruitment cohort (2001–2003 vs. 1985–1993), AIDS, depression, hepatitis C infection, confounding medical conditions (e.g., hypertension, diabetes, cancer, etc.), race, and death) were used to estimate the parameters of the MMTM. There were three canonical profiles (See Figure 1): we arbitrarily refer to canonical profile 1, for which the probability of normal cognition is initially very high, as the ‘normal aging’ profile; profile 2, for which the probability of mild impairment begins to climb at age 45–50 years, as the ‘premature aging’ profile; and profile 3, for which the probability of normal cognition is near zero even at the youngest age, as the ‘unhealthy’ profile.
Figure 1:
The example trajectory (black line) from a single individual is a weighted mixture of the three extreme profiles (2% Healthy, 50% Premature Aging, 48% Unhealthy) developed by Molsberry and colleagues (Molsberry, et al., 2015) from 25,471 observations from 3892 MACS participants (an average of 6.54 observations per individual). The dashed lines represent the three canonical profiles with pointwise posterior 95% credible bands for each cognitive classification. The x-axis represents assembled cross-sectional probabilities of the three states (normal, mildly, and severely impaired) across time/age. At any given age, the sum of the three probabilities is equal to 1.00. The y-axis represents the age of the men in the cohort at the time of the examination.
Voxel-Level Data Analysis:
Multiple imputation (MI) is a method to obtain valid inferences from imputed data (Rubin, 1986), since naïve imputation methods bias estimates and distort standard errors. MI works by simulating multiple versions of a complete dataset, and in each version the missing values are imputed using some model of data generation. Each simulated complete dataset is analyzed using standard methods and the results are pooled together using special formulas that account for between– and within–imputation variability, producing estimates that incorporate missing data uncertainty. Figure 2 illustrates this process.
Figure 2:
Overview of Multiple Imputation procedure.
We modeled y, the log-transformed tissue volume, at voxel level by multiple regression:
| (1) |
where g is the individual’s closeness to a profile obtained by the MMTM. We generated m=100 MI datasets containing Monte Carlo Markov Chain draws of the membership weights from the posterior multivariate distribution. Each multiply imputed dataset was then fed into SPM8 for analysis (estimation of βand σ2). We were interested in performing post-hoc contrasts, which are defined as cT β where c is a column vector of L weights. For example, c = [0, 0, 1]T (as shown here c may not formally be a contrast vector -- the elements may not sum to zero -- but may be a dummy coding or other coding to isolate parts of β that we are interested in). The estimate has the following distribution (Friston, Ashburner, Kiebel, Nichols, & Penny, 2007):
| (2) |
To apply MI, we let Q = cT βbe the quantity of interest estimated by and U σ2 cT (XT X)−1c, where X is a (simulated) complete set of covariates, and then proceeded as described in the Supplemental Materials. SPM was then used to pool the results of 100 separate analyses. Each voxel had a mean and standard deviation from which we calculated F-maps and degrees of freedom at the voxel level. From these we created 3-D maps of p-values –|P(F1,u ≥ F*|H0)|– at the voxel level. This has the consequence that the multiple comparison problem (i.e., tens of thousands of comparisons) doesn’t exist in the way that it would in a more standard use of SPM.
Results
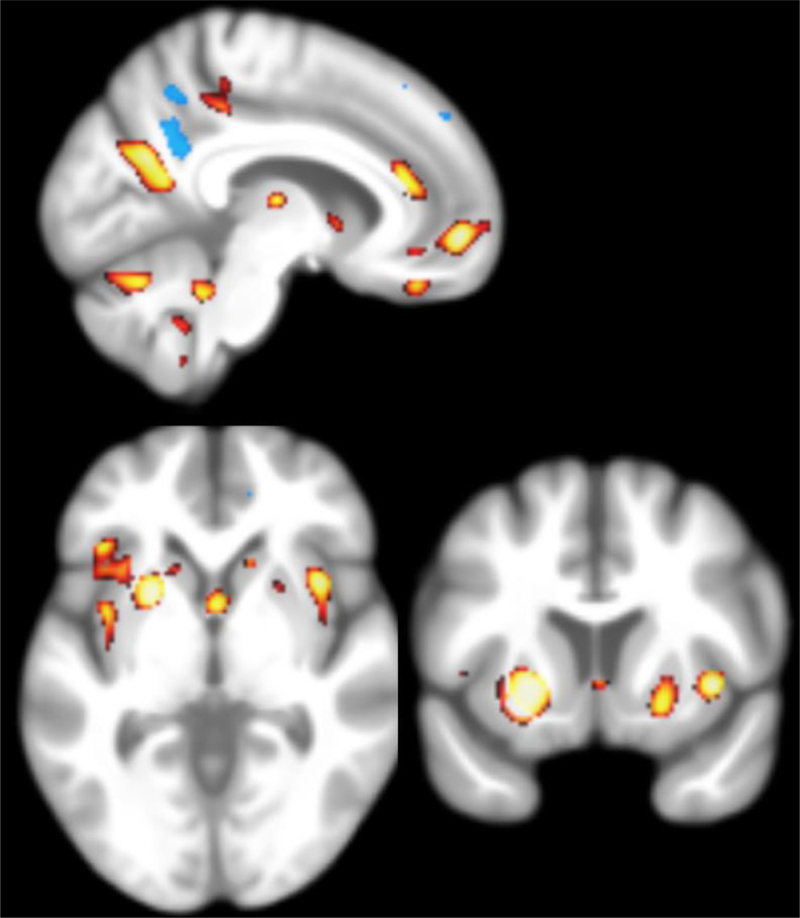
The results of the analyses are shown in Figure 3; the regions significantly associated with the Unhealthy profile (yellow/red) and the Premature Aging profile (blue) are projected onto a mean brain image created from the MACS MRI database. The closer that an individual is to either of these profiles was associated with lower volume of the posterior cingulate/precuneus grey matter. In addition, closeness to the Unhealthy profile is also associated with decreased volume in the putamen, insula, inferior frontal cortex, and caudate nucleus.
Figure 3:
For the “premature aging” profile closeness, the blue-highlighted regions are p-values smaller than 0.01. For the “unhealthy” profile closeness, the yellow/red-highlighted regions are p-values smaller than 0.005.
Discussion
We show here that we were able to generate a method for associating random variables with a mean and standard deviation (in this case, closeness to a trajectory) with grey matter volume treating the analysis as a variation of a missing data problem. From this novel analysis we found that closeness to the Unhealthy trajectory was associated with the volume of posterior cingulate/precuneus grey matter as well as the putamen, insula, inferior frontal cortex, and caudate nucleus. The former is important because it provides another method for integrating leading edge statistical tools with brain imaging data. The latter is important because it provides concurrent validity to the results of the original MMTM analysis (Molsberry et al., 2015), and because the brain regions involved are associated with cognitive functions.
The trajectories to cognitive impairment in our study sample are associated, in part, with atrophy in brain regions linked to HIV disease (i.e., basal ganglia), as well as cortical regions linked with normal and pathological aging (i.e., precuneus). These data suggest the possibility of predicting cognitive morbidity based on patterns of CNS atrophy. According to this view, given that closeness to the trajectories was affected by HIV and (separately) AIDS (Molsberry et al., 2015), it is not unreasonable to suppose that a brain region linked to HIV Disease is associated with the membership weights (however, see below). Further, as the risk of impairment in the three trajectories was expressed as a function age, it is also not unreasonable to suppose that the closeness to the Unhealthy trajectory is linked to those brain regions that are associated with aging and dementia (e.g., (Bailly et al., 2015; Jones et al., 2006; Karas et al., 2007).
There is consistent evidence among studies of brain structural integrity among individuals with HIV Disease that brain regional atrophy is linked to performance on neuropsychological tests (e.g., (Ances & Hammoud, 2014; Ances, Ortega, Vaida, Heaps, & Paul, 2012; Fennema-Notestine et al., 2013; Kallianpur et al., 2013; Lepore et al., 2008; Paul, Cohen, Navia, & Tashima, 2002; Ragin et al., 2012; Thompson et al., 2006; Thompson et al., 2005; Thurnher & Post, 2008; Towgood et al., 2012; Wang et al., 2009)) as well as HIV Disease (when a seronegative control group was present) and immunological and virological markers of disease status (when a control group was not available). Thus, even with access to cART, HIV is associated with measurable brain atrophy (Cardenas et al., 2009; Cohen & Gongvatana, 2011; Jernigan et al., 2011; Kuper et al., 2011; O’Connor, Jaillard, Renard, & Zeffiro, 2017; O’Connor, Zeffiro, & Zeffiro, 2017; Sanford et al., 2017).
The data presented here complement those findings by showing that regional brain volumes are related to temporal trajectories to cognitive dysfunction which themselves were related to a combination of factors including HIV Disease, AIDS, hepatitis-C infection, depression, race, and MACS recruitment cohort (Molsberry et al., 2015). The multi-factorial nature of the predictors, and the link between trajectory membership and brain structure reinforces the view that a range of comorbidities that occur in the context of risk for HIV infection must be considered when interpreting analyses of brain structure and cognitive function (see reviews by (Ances & Hammoud, 2014; Masters & Ances, 2014; O’Connor, Jaillard, et al., 2017; O’Connor, Zeffiro, et al., 2017; Saylor et al., 2016).
The prevalence of mild cognitive disorders in the context of HIV Disease remains high, although the prevalence estimates vary widely. One explanation for the “residual” impairment may be a legacy effect or “burnt-out” brain (Manji, Jager, & Winston, 2013). Related to these alternatives is the possibility that enrollment cohort plays a critical role. Individuals who became infected more recently (i.e., many of the men in C2) had the opportunity to receive cART as the first line of therapy, likely had therapy initiated at an earlier point in the natural history of the infection and are less likely to have had clinical AIDS (cf., (Miller, Selnes, & McArthur, 1990)). Alternatively, there may be a low grade, chronic process that alters brain structure even when peripheral measures of viral load and immunocompetence are within acceptable limits; brain metabolic abnormalities in HIV+ patients support this conclusion (e.g., (Chang et al., 2003; Cohen et al., 2010; Cysique et al., 2013; Ernst, Jiang, Nakama, Buchthal, & Chang, 2010; Harezlak et al., 2011; Kallianpur et al., 2013; Valcour et al., 2013; Yiannoutsos et al., 2004)). There is also growing evidence that abnormal cellular inflammation may play a key role in determining CNS structural and functional competence, and ultimately cognitive functions (e.g., (Underwood et al., 2017).
A variety of factors can affect brain structure and cognition in the context of HIV disease. Early in the epidemic, we could reasonably assume that there was but a single trajectory, or pathway to cognitive impairment among individuals with HIV disease. In the current era, this assumption seems much less reasonable. With the variety of factors that can potentially alter brain health and cognition, it seems very likely that there are multiple trajectories to impairment and that these trajectories may be represented by different patterns of CNS damage. Here we took advantage of the data from the MACS to demonstrate that there is a biological basis to these previously described trajectories. We have demonstrated that these empirically derived pathways to cognitive impairment – that are related to both HIV- and non-HIV-related factors - are associated with measures of brain regional volume. Thus, these data add to the growing body of evidence that critical risk factors such as hypertension and diabetes not only affect brain structure in HIV-infected individuals (as they do in uninfected individuals), but may (along with other non-medical factors) interact with infection status to produce CNS abnormalities (e.g., (Lake et al., 2017; Spies, Ahmed-Leitao, Fennema-Notestine, Cherner, & Seedat, 2016; Thames et al., 2018; Thames et al., 2017; Underwood et al., 2017)).
A recent meta-analysis (O’Connor, Zeffiro, et al., 2017) identified consistencies in structural brain imaging data, as well as some important qualifiers. The standardized mean for total brain volume, gray matter volume, white matter volume, and for CSF volume were significantly related to HIV Disease. However, in spite of evidence from several structural and functional imaging studies of their sensitivity to the presence of virus, the volume of basal ganglia was not reliable in the meta-analysis. And, perhaps as a consequence of the earlier use of cART, publication year was associated with reductions in the impact of HIV Disease on brain structural (See their Figure 7). However, this meta-analysis was made more difficult by the fact that the estimates of between-study heterogeneity suggested that much of the observed variance was between studies making many comparisons difficult or impossible.
While the analysis of brain structure such as this one have provided a great deal of information regarding the impact of HIV disease on the brain, “Changes in brain structure are lagging indicators of advancing [neurodegenerative] disease state.”((Rosen, Huang, & Stufflebeam, 2015), pg. 1628). Functional brain imaging, and particularly functional connectivity within brain regional networks likely provides information regarding pathological changes in the brain prior to measurable structural change or clinical expression. Thus, as the pathophysiological basis of the milder forms of cognitive dysfunction in HIV disease becomes the focus of new research, we likely need to expand our analysis methods to include sensitive measures of neural function (e.g., (Becker, Bajo, et al., 2012; Becker, Cuesta, et al., 2012; Wilson et al., 2013; Wilson et al., 2015)).
Supplementary Material
Acknowledgements
The preparation of this manuscript and the collection of the MRI data were supported in part by funds from the NIH to J.T.B. (AG034852 and MH098745).
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Adrian Dobs, Michelle Estrella, W. David Hardy, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, John P. Phair, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (PI), Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Lawrence A. Kingsley, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (PI), Alison Abraham, Keri Althoff, Michael Collaco, Priya Duggal, Sabina Haberlen, Eithne Keelaghan, Heather McKay, Alvaro Muñoz, Derek Ng, Anne Rostich, Eric C. Seaberg, Sol Su, Pamela Surkan, Nicholas Wada. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Disclosure: Eric N. Miller is the author of the reaction time software used in this study (CalCAP) and has a financial interest in the software.
The members of the Neuropsychology Working Group include Francine Barrington, James T. Becker, Pim Brouwers, Velpandi Ayyavoo, Karl Goodkin, Robin Huebner, Eithne Keelaghan, Andrew J. Levine, Eileen M. Martin, Cynthia Munro, Ann Ragin, Leah Rubin, Ned Sacktor, Eric Seaberg, and Carlie Williams.
Mikhail Popov is currently at the Wikimedia Foundation, and Fabrizio Lecci is at Uber (New York). Samantha Molsberry is a student in Population Health Sciences at Harvard University.
Funding: AG034852, MH098745, U01-AI35042, U01-AI35039, U01-AI35040, UM1-AI35043, U01-AI35041
Footnotes
Conflict of Interest: None.
Ethical approval: This research was reviewed and approved by the Institutional Review Boards at all four MACS clinic sites – Johns Hopkins University, Northwestern University, University of California Los Angeles, and the University of Pittsburgh.
Informed consent: Each participant signed a written statement of informed consent prior to starting any research-related activities.
References
- Ances BM, & Hammoud DA (2014). Neuroimaging of HIV-associated neurocognitive disorders (HAND). Curr Opin HIV AIDS, 9(6), 545–551. doi: 10.1097/COH.0000000000000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, & Paul R (2012). Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr, 59(5), 469–477. doi: 10.1097/QAI.0b013e318249db17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, … Wojna VE (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69(18), 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly M, Destrieux C, Hommet C, Mondon K, Cottier JP, Beaufils E, … Ribeiro MJ (2015). Precuneus and Cingulate Cortex Atrophy and Hypometabolism in Patients with Alzheimer’s Disease and Mild Cognitive Impairment: MRI and (18)F-FDG PET Quantitative Analysis Using FreeSurfer. Biomed Res Int, 2015, 583931. doi: 10.1155/2015/583931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Bajo R, Fabrizio M, Sudre G, Cuesta P, Aizenstein HJ, … Bagic A (2012). Functional connectivity measured with magnetoencephalography identifies persons with HIV disease. Brain Imaging and Behavior, 6(3), 366–373. doi: 10.1007/s11682-012-9149-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Cuesta P, Fabrizio M, Sudre G, Vergis EN, Douaihy A, … Bagic A (2012). Brain structural and functional recovery following initiation of combination antiretroviral therapy. Journal of neurovirology, 18(5), 423–427. doi: 10.1007/s13365-012-0115-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Maruca V, Kingsley LA, Sanders JM, Alger JR, Barker PB, … Selnes O (2011). Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology. doi: 10.1007/s00234-011-0854-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Meyerhoff DJ, Studholme C, Kornak J, Rothlind J, Lampiris H, … Weiner MW (2009). Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol, 15(4), 324–333. doi: 10.1080/13550280902973960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Ames N, Walot I, Jovicich J, DeSilva M, … Miller EN (2003). Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antivir Ther, 8(1), 17–26. [PubMed] [Google Scholar]
- Cohen RA, & Gongvatana A (2011). The persistence of HIV-associated neurocognitive dysfunction and the effects of comorbidities. Neurology, 75(23), 2052–2053. doi:75/23/2052 [pii] 10.1212/WNL.0b013e318200d833 [DOI] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Gongvatana A, Buchthal S, Schifitto G, Clark U, … Navia B (2010). Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. J Neurovirol, 16(6), 435–444. doi: 10.3109/13550284.2010.520817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JT (2006). Multivariate Mixture Models to Describe Longitudinal Patterns of Frailty in American Seniors. Pittsburgh, PA: Carnegie Mellon University, ProQuest, UMI Dissertations Publishing; 3275170. [Google Scholar]
- Cysique LA, Moffat K, Moore DM, Lane TA, Davies NW, Carr A, … Rae C (2013). HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: a (1)H MRS study. PLoS One, 8(4), e61738. doi: 10.1371/journal.pone.0061738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Jiang CS, Nakama H, Buchthal S, & Chang L (2010). Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging, 32(5), 1045–1053. doi: 10.1002/jmri.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erosheva EA (2005). Comparing Latent Structures of the Grade of Membership, Rasch, and Latent Class Models. Psychometrika, 70(4), 619–628. doi: 10.1007/s11336-001-0899-y [DOI] [Google Scholar]
- Erosheva EA, Fienberg SE, & Joutard C (2007). Describing Disability through Individual-Level Mixture Models for Multivariate Binary Data. The annals of applied statistics, 1(2), 346–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Ellis RJ, Archibald SL, Jernigan TL, Letendre SL, Notestine RJ, … Group C (2013). Increases in brain white matter abnormalities and subcortical gray matter are linked to CD4 recovery in HIV infection. J Neurovirol, 19(4), 393–401. doi: 10.1007/s13365-013-0185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Kiebel SJ, Nichols TE, & Penny WD (Eds.). (2007). Statistical Parametric Mapping: The Analysis of Functional Brain Images: Academic Press. [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, … Navia B (2011). Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS, 25(5), 625–633. doi: 10.1097/QAD.0b013e3283427da7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, … Grant I (2011). Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol, 17(3), 248–257. doi: 10.1007/s13365-011-0032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BF, Barnes J, Uylings HB, Fox NC, Frost C, Witter MP, & Scheltens P (2006). Differential regional atrophy of the cingulate gyrus in Alzheimer disease: a volumetric MRI study. Cereb Cortex, 16(12), 1701–1708. doi: 10.1093/cercor/bhj105 [DOI] [PubMed] [Google Scholar]
- Kallianpur KJ, Shikuma C, Kirk GR, Shiramizu B, Valcour V, Chow D, … Sailasuta N (2013). Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology, 80(19), 1792–1799. doi:WNL.0b013e318291903f [pii] 10.1212/WNL.0b013e318291903f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas G, Scheltens P, Rombouts S, van Schijndel R, Klein M, Jones B, … Barkhof F (2007). Precuneus atrophy in early-onset Alzheimer’s disease: a morphometric structural MRI study. Neuroradiology, 49(12), 967–976. [DOI] [PubMed] [Google Scholar]
- Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, & Rinaldo CR (1987). The Multicenter AIDS Cohort Study (MACS): Rationale, organization, and selected characteristics of the participants. American Journal of Epidemiology, 126, 310–318. [DOI] [PubMed] [Google Scholar]
- Kuper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, & Obermann M (2011). Structural gray and white matter changes in patients with HIV. J Neurol. doi: 10.1007/s00415-010-5883-y [DOI] [PubMed] [Google Scholar]
- Lake JE, Popov M, Post WS, Palella FJ, Sacktor N, Miller EN, … Becker JT (2017). Visceral fat is associated with brain structure independent of human immunodeficiency virus infection status. J Neurovirol, 23(3), 385–393. doi: 10.1007/s13365-016-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore N, Brun CA, Chou YY, Chiang MC, Dutton RA, Hayashi KM, … Thompson PM (2008). Generalized tensor-based morphometry of HIV/AIDS usingmultivariate statistics on strain matrices and their application to HIV/AIDS. IEEE Transactions on Medical Imaging, Special Issue on Computational Neuroanatomy, 27(1), 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji H, Jager HR, & Winston A (2013). HIV, dementia and antiretroviral drugs: 30 years of an epidemic. J Neurol Neurosurg Psychiatry, 84(10), 1126–1137. doi: 10.1136/jnnp-2012-304022 [DOI] [PubMed] [Google Scholar]
- Manrique-Vallier D (2014). Mixed Membership Trajectory Models In Airoldi E, Blei D, E. E, & S. F (Eds.), Handbook of Mixed Membership Models and Their Applications (pp. 173–188). New York: Chapman-Hall. [Google Scholar]
- Masters MC, & Ances BM (2014). Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol, 34(1), 89–102. doi: 10.1055/s-0034-1372346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EN, Selnes OA, & McArthur MB (1990). Neuropsychological test performance in HIV1-infected homosexual men: The Multicenter AIDS Cohort Study (MACS). Neurology, 40, 197–203. [DOI] [PubMed] [Google Scholar]
- Molsberry SA, Lecci F, Kingsley L, Junker B, Reynolds S, Goodkin K, … Becker JT (2015). Mixed membership trajectory models of cognitive impairment in the multicenter AIDS cohort study. AIDS, 29(6), 713–721. doi: 10.1097/QAD.0000000000000561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EE, Jaillard A, Renard F, & Zeffiro TA (2017). Reliability of White Matter Microstructural Changes in HIV Infection: Meta-Analysis and Confirmation. AJNR Am J Neuroradiol, 38(8), 1510–1519. doi: 10.3174/ajnr.A5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EE, Zeffiro TA, & Zeffiro TA (2017). Brain Structural Changes following HIV Infection: Meta-Analysis. AJNR Am J Neuroradiol. doi: 10.3174/ajnr.A5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Cohen R, Navia B, & Tashima K (2002). Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neuroscience and Behavioral Reviews, 26, 353–359. [DOI] [PubMed] [Google Scholar]
- Ragin AB, Du H, Ochs R, Wu Y, Sammet CL, Shoukry A, & Epstein LG (2012). Structural brain alterations can be detected early in HIV infection. Neurology, 79(24), 2328–2334. doi: 10.1212/WNL.0b013e318278b5b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BR, Huang SY, & Stufflebeam SM (2015). Pushing the Limits of Human Neuroimaging. JAMA, 314(10), 993–994. doi: 10.1001/jama.2015.10229 [DOI] [PubMed] [Google Scholar]
- Rubin DB (1986). Multiple imputation for nonresponse in surveys: John Wiley & Sons. [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, … Epstein L (2002). HIV-associated cognitive impairment before and after the advent of combination therapy. Journal of NeuroVirology, 8, 136–142. [DOI] [PubMed] [Google Scholar]
- Sanford R, Fernandez Cruz AL, Scott SC, Mayo NE, Fellows LK, Ances BM, & Collins DL (2017). Regionally Specific Brain Volumetric and Cortical Thickness Changes in HIV-Infected Patients in the HAART Era. J Acquir Immune Defic Syndr, 74(5), 563–570. doi: 10.1097/QAI.0000000000001294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, … McArthur JC (2016). HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol, 12(4), 234–248. doi: 10.1038/nrneurol.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, & Evans AC (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE transactions on medical imaging, 17(1), 87–97. doi: 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- Spies G, Ahmed-Leitao F, Fennema-Notestine C, Cherner M, & Seedat S (2016). Effects of HIV and childhood trauma on brain morphometry and neurocognitive function. J Neurovirol, 22(2), 149–158. doi: 10.1007/s13365-015-0379-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Kuhn TP, Mahmood Z, Bilder RM, Williamson TJ, Singer EJ, & Arentoft A (2018). Effects of social adversity and HIV on subcortical shape and neurocognitive function. Brain Imaging Behav, 12(1), 96–108. doi: 10.1007/s11682-017-9676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Kuhn TP, Williamson TJ, Jones JD, Mahmood Z, & Hammond A (2017). Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV- adults. Drug Alcohol Depend, 170, 120–127. doi: 10.1016/j.drugalcdep.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, … Becker JT (2006). 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage, 31(1), 12–23. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayaski KM, Toga AW, Lopez OL, Aizenstein HJ, & Becker JT (2005). Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T-lymphocyte decline. PNAS, 102(43), 15647–15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnher MM, & Post MJD (2008). The uses of structural neuroimaging in the brain in HIV1-infected patients In Goodkin K, Shapshak P, & Verma A (Eds.), The Spectrum of Neuro-AIDS Disorder: Pathophysiology, Diagnosis and Treatment (pp. 247– 272). Washington DC: ASMPress. [Google Scholar]
- Towgood KJ, Pitkanen M, Kulasegaram R, Fradera A, Kumar A, Soni S, … Kopelman MD (2012). Mapping the brain in younger and older asymptomatic HIV-1 men: frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex, 48(2), 230–241. doi:S0010–9452(11)00070–0 [pii] 10.1016/j.cortex.2011.03.006 [DOI] [PubMed] [Google Scholar]
- Underwood J, Cole JH, Caan M, De Francesco D, Leech R, van Zoest RA, … Comorbidity in Relation to, A. C. (2017). Gray and White Matter Abnormalities in Treated Human Immunodeficiency Virus Disease and Their Relationship to Cognitive Function. Clin Infect Dis, 65(3), 422–432. doi: 10.1093/cid/cix301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Busovaca E, Wendeken L, Esmareili P, Rankin KP, Lobach I, & Rosen H (2013). Brain white matter lesions link to CVD risk but not HIV factors in HIV over age 60. J Neurovirol, 19 S1, S85.24101299 [Google Scholar]
- Wang Y, Zhang J, Gutman B, Chan TF, Becker JT, Aizenstein HJ, … Thompson PM (2009). Multivariate Tensor-based Morphometry on Surfaces: Application to Mapping Ventricular Abnormalities in HIV/AIDS. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Fox HS, Robertson KR, Sandkovsky U, O’Neill J, Heinrichs-Graham E, … Swindells S (2013). Abnormal MEG oscillatory activity during visual processing in the prefrontal cortices and frontal eye-fields of the aging HIV brain. PLoS One, 8(6), e66241. doi: 10.137/journal.pone.0066241 PONE-D-13–07244 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Becker KM, Aloi J, Robertson KR, Sandkovsky U, … Swindells S (2015). Multimodal neuroimaging evidence of alterations in cortical structure and function in HIV-infected older adults. Hum Brain Mapp, 36(3), 897–910. doi: 10.1002/hbm.22674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiannoutsos CT, Ernst T, Chang L, Lee PL, Richards T, Marra CM, … Ellis RJ (2004). Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage, 23(3), 928–935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.