Abstract
Background: Postoperative cognitive dysfunction (POCD) is a great problem for anesthetized subjects and is associated with poor short- and long-term outcomes. We explored promising predictors for POCD in elderly patients after hip fracture surgery.
Methods: Elderly subjects (aged ≥65 years) undergoing surgery for hip fracture were consecutively recruited. Neuropsychological assessments were performed 1 day preoperatively (baseline) and 7 days postoperatively, and POCD was defined using the ‘Z scores’ method. Clinical data and laboratory tests were compared between patients with and without POCD development. Binary univariate and multivariate logistic regression analyses were conducted for risk factor assessment. Receiver operating characteristic (ROC) curve analysis was performed to investigate the predictive value of malondialdehyde (MDA) on postoperative day 1 (POD1) for POCD.
Results: A total of 198 patients were finally enrolled in the analysis and 51 patients exhibited POCD within 7 postoperative days, with an incidence rate of 25.8%. MDA expression on POD1 (OR: 1.12, 95%CI: 1.03–1.23, P=0.017) was the only independent risk factor for POCD according to the final multivariate logistic regression analysis. ROC curve analysis indicated that MDA on POD1 was a predictor for POCD, with an area under the curve (AUC) of 0.683 and 95%CI of 0.590–0.775 (P<0.001).
Conclusions: In conclusion, we demonstrated that MDA on POD1 was an independent risk factor for POCD in elderly subjects undergoing hip fracture surgery.
Keywords: Postoperative cognitive dysfunction, hip fracture, malondialdehyde, oxidative stress, biomarker
Introduction
Postoperative cognitive dysfunction (POCD), a condition that has been poorly recognized and defined for decades, is one of the most common postoperative complications, especially for the elderly [1]. As defined by cognitive impairment following surgery with unclear etiology, POCD is a great problem for anesthetized subjects and an important determinant for poor outcomes both short- and long term [2]. Due to significant improvements in medical technology and therapies, life span has been extended, and quality of life has been improved, and requiring more elective surgery than ever. As reported by previous studies, the incidence of POCD varies from 8.9% to 46.1% [3]. Another study reported that the prevalence of POCD in adult patients undergoing noncardiac surgery ranges from 19% to 41% [4]. The occurrence of POCD is significantly associated with a delay in functional recovery and increases in economic burden and mortality rate [5]. Without an adequate plan to address potential cognitive dysfunction, there is a global health risk of increased cognitive decline. The prognosis can be significantly improved if POCD is recognized early and managed effectively [6]. For elderly subjects after hip fracture surgery, POCD is a frequent complication that is closely associated with prolonged hospital stay and increased morbidity and mortality [7]. Due to a lack of understanding of POCD and its multifactorial causes, specific prediction of POCD and its therapy are quite difficult [8]. As a result, preventing the development of POCD is of great importance.
Recently, oxidative stress-induced neuroinflammation has been widely postulated to be closely associated with synapse dysfunction, and oxidative stress has been speculated to underlie the development of POCD in elderly subjects [9]. Operation is hypothesized to be a major cause of the oxidative response in the CNS [10]. Oxidative stress has been widely considered an important part of the postoperative stress response [11]. A previous study which was conducted in experimental POCD model rats reported that oxidative stress contributes to the etiology of POCD [12]. However, no consensus has been reached about the relationship between oxidative stress and POCD until now. MDA, a bioactive aldehyde generated by free radical-mediated lipid peroxidation, is an oxidative stress marker [13]. Superoxide dismutase (SOD) is an important enzyme that catalyses the dismutation of superoxide radicals into hydrogen peroxide (H2O2) or oxygen (O2) [14]. MDA and SOD are two classical indexes reflecting systemic redox homeostasis.
The present study aimed to investigate whether these biomarkers of redox status could independently predict POCD in elderly subjects undergoing hip fracture surgery.
Materials and methods
Patients
This current investigation was approved by the Medical Institutional Ethics Committee of the affiliated hospital of medical school, Ningbo University and Zhejiang province. This prospective single-center observational investigation was performed in the Department of anesthesiology from October 2014 to May 2018. Elderly subjects (aged ≥65 years) undergoing surgery for hip fracture were consecutively recruited. All the participants were required to offer written informed consent. The exclusion criteria were described as follows: (1) with preexisting psychiatric disorder (e.g. dementia, depressive illness, delirium, etc.); (2) with present or prior neurological diseases; (3) with a Mini-Mental State Examination (MMSE) score less than 24 before surgery; (4) with a previous history of neurosurgical surgery; (5) with alcoholism or drug dependence; (6) with auditory or visual disorders, inability to communicate with Chinese language; (7) not willing to participate in the present study; (8) with data missed.
Neuropsychological assessment and POCD definition
A same experienced anesthetist who was blinded to the present study was invited to perform the neuropsychological assessment on 1 day preoperatively (baseline) and 7 day postoperatively. The neuropsychological assessments comprised the widely described tests including MMSE, Word recognition memory test, Digit span test, Trail making test (part A), Symbol digit test, and Verbal fluency test [15]. A Z score for each test was then calculated using the baseline levels and test results according to the ISPOCD1 study by Moller et al. [16]. In this current study, POCD was defined using ‘Z scores’ according to the established method in the ISPOCD1 study [16]. If two (or more) Z scores in individual tests or the combined Z score were ≥1.96, POCD was diagnosed.
Clinical data collection
The demographic data including age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) grade, and smoking status were recorded at initial recruitment. We also collected the preoperative comorbidities (diabetes, hyperlipidemia and hypertension) and calculated the MMSE scores before the surgery. In addition, the clinical data associated with the surgery including type of fracture, anesthesia and surgery, delay of surgery, duration of anesthesia and surgery, recovery time, and perioperative blood transfusion rate were also recorded in details.
Laboratory tests
Fasting blood samples on 1 day before surgery (baseline) and postoperative day 1 (POD1) were collected. The whole blood samples were then centrifuged at 3000 rpm for 10 min and the obtained serum samples were stored at −80°C. MDA level was measured by spectrophotometric method using thiobarbituric acid (TBA) and MDA kits (Jiancheng Bioengineering, Nanjing, Jiangsu, China). The determination of MDA levels relied on the reaction with TBA to generate the products ‘thiobarbituric acid reactive substances’ (TBARS) that could be measured by the method of fluorimetry (excitation at 532 nm and emission at 553 nm) or colorimetry (532 nm). The level of MDA was measured colorimetrically in this current study. SOD activity was measured by the hydroxylamine method using SOD kits (Jiancheng Bioengineering, Nanjing, Jiangsu, China). The determination of SOD activity was based on the suppression role of SOD on the superoxide anion through a dismutation reaction. C-reactive protein (CRP) was measured by the method of enzyme-linked immunosorbent assay (ELISA) using kits (R&D Systems, Minneapolis, MN, U.S.A.). The biochemical determinations were carried out in duplicates in triplicate and the mean value was recorded. Hemoglobin, white blood cell, albumin, urea and creatinine were also measured using the preoperative blood samples in the laboratory of our hospital.
Statistical analysis
Data analyses were performed using GraphPad Prism 5.0 (GraphPad Inc., CA, U.S.A.) and SPSS 19.0 (SPSS Inc., IA, U.S.A.). Before the study, we performed a sample size estimation and 165 patients would be required with a 5% significance level and 80% power. Our final analysis included a sample size of 198 patients and the power value was calculated to be 0.86. All continuous data were reported as mean with standard error (S.E.M) and categorical data as number (n) with percentage (%), respectively. Statistical analyses were investigated using Mann–Whitney U-test or t-test for continuous data, Chi-square test or Fisher exact test for categorical data as appropriate. Binary univariate and multivariate logistic regression analyses were conducted for risk factors assessment. Receiver operating characteristic (ROC) curve analysis was performed to investigate the predictive value of MDA on POD1 for POCD and the cut-off value. A two-sided P-value < 0.05 was considered significantly different.
Results
Patient characteristics
During the inclusion period, we enrolled 224 elderly patients undergoing hip fracture surgery. Twenty-six were excluded due to the exclusion criteria (five preoperative MMSE score <24, three with preexisting psychiatric disorder, three with a history of neurosurgical surgery, four with auditory or visual disorders, five not willing to cooperate, and six with data missed) and a total of 198 patients were finally enrolled into the analysis. Ultimately, 51 patients have exhibited POCD within 7 postoperative days, with an incidence rate of 25.8%. The detailed clinical characteristics are presented in Table 1. The age and ASA grade were significantly higher in the POCD group (P=0.045 and P=0.034, respectively). The development of POCD also seemed to be closely associated with a lower preoperative MMSE score (P=0.034). The presence of preoperative comorbidities including diabetes (P=0.036) and hypertension (P=0.027) was significantly associated with an increased risk of POCD. Those patients who underwent surgery under general anesthesia (P=0.010) or those who had a longer duration of surgery (P=0.042) or anesthesia (P=0.026) were more likely to suffer POCD. No significant differences were observed in gender distribution, smoking status, type of fracture and surgery, delay of surgery, recovery time, perioperative blood transfusion, and baseline neuropsychological assessments (including Digit span test, Verbal fluency test, etc.) between the patients with or without POCD (P>0.05).
Table 1. Demographic and clinical data and POCD.
| Variables | POCD (n=51) | Non-POCD (n=147) | P-value |
|---|---|---|---|
| Age (year) | 72.3 ± 3.2 | 71.3 ± 3.0 | 0.045* |
| Gender, n (%) | 0.71 | ||
| Male | 22 (43.1) | 59 (38.1) | – |
| Female | 29 (56.9) | 88 (61.9) | – |
| BMI (kg/m2) | 23.2 ± 2.1 | 22.9 ± 1.9 | 0.35 |
| ASA physical status, n (%) | 0.034* | ||
| I | 3 (5.9) | 18 (12.2) | – |
| II | 20 (39.2) | 78 (53.1) | – |
| III | 28 (54.9) | 51 (34.7) | – |
| Active smoker, n (%) | 10 (19.6) | 24 (16.3) | 0.59 |
| Preoperative comorbidities, n (%) | – | ||
| Diabetes | 13 (25.5) | 19 (12.9) | 0.036* |
| Hyperlipidemia | 9 (17.6) | 25 (17.0) | 0.92 |
| Hypertension | 17 (33.3) | 27 (18.4) | 0.027* |
| Preoperative medications, n (%) | |||
| Hypoglycemic drugs | 10 (19.6) | 16 (10.9) | 0.11 |
| Beta blocker | 6 (11.8) | 11 (7.5) | 0.35 |
| ACEI/ARB | 9 (15.7) | 16 (10.9) | 0.21 |
| Calcium channel blocker | 6 (11.8) | 9 (6.1) | 0.35 |
| Lipid-lowering medication | 6 (11.8) | 15 (10.2) | 0.76 |
| Type of fracture | 0.85 | ||
| Femoral neck fracture | 28 (54.9) | 83 (56.5) | – |
| Intertrochanteric fracture | 23 (45.1) | 64 (43.5) | – |
| Type of anesthesia | 0.010* | ||
| Spinal | 21 (41.2) | 91 (61.9) | – |
| General | 30 (58.8) | 56 (38.1) | – |
| Type of surgery | 0.28 | ||
| Arthroplasty | 41 (80.4) | 107 (72.8) | – |
| Internal fixation | 10 (19.6) | 40 (27.2) | – |
| Delay of surgery (days) | 3.7 ± 1.5 | 3.5 ± 1.8 | 0.48 |
| Duration of surgery (min) | 98.3 ± 16.2 | 93.5 ± 13.8 | 0.042* |
| Duration of anesthesia (min) | 110.5 ± 18.1 | 104.8 ± 14.8 | 0.026* |
| Recovery time (min) | 35.5 ± 8.8 | 36.1 ± 10.1 | 0.71 |
| Perioperative blood transfusion, n (%) | 14 (27.5) | 33 (22.4) | 0.47 |
| Preoperative neuropsychological assessments | |||
| MMSE score | 27.5 ± 1.5 | 28.1 ± 1.8 | 0.034* |
| Digit span test | |||
| Correct order | 8.4 ± 0.7 | 8.3 ± 0.8 | 0.43 |
| Reverse order | 4.5 ± 1.3 | 4.4 ± 1.1 | 0.59 |
| Trail making test A (s) | 19.1 ± 8.7 | 18.2 ± 6.9 | 0.46 |
| Verbal fluency test | 15.2 ± 3.5 | 16.2 ± 3.9 | 0.11 |
| Word recognition memory tests | 1.4 ± 1.0 | 1.3 ± 0.9 | 0.51 |
| Symbol digit test | 31.5 ± 10.8 | 32.4 ± 9.7 | 0.50 |
*P<0.05.
Laboratory characteristics and POCD
The laboratory tests in patients with or without POCD are listed in Table 2. The content of hemoglobin, white blood cell, albumin, creatinine, and urea were not statistically significant (P>0.05). The preoperative levels of CRP, MDA, and SOD in patients with POCD also did not differ from those without POCD (P>0.05). Those patients with POCD exhibition showed significantly increased serum levels of CRP and MDA and decreased activity of SOD on POD1 (P<0.05).
Table 2. The laboratory tests and POCD.
| Variables | POCD (n=51) | Non-POCD (n=147) | P-value |
|---|---|---|---|
| Hemoglobin (g/dl) | 11.1 ± 1.9 | 11.5 ± 1.7 | 0.16 |
| White blood cell (×109/L) | 7.6 ± 1.9 | 7.4 ± 1.7 | 0.48 |
| Albumin (g/ml) | 39.7 ± 3.8 | 39.5 ± 3.3 | 0.72 |
| Creatinine (mmol/L) | 82.1 ± 13.3 | 81.6 ± 14.1 | 0.82 |
| Urea (mmol/L) | 6.4 ± 1.8 | 6.2 ± 1.5 | 0.43 |
| Preoperative CRP (mg/L) | 11.2 ± 3.6 | 10.9 ± 4.1 | 0.64 |
| Preoperative MDA (nmol/ml) | 4.5 ± 0.5 | 4.4 ± 0.6 | 0.28 |
| Preoperative SOD (U/ml) | 94.7 ± 2.9 | 95.0 ± 2.8 | 0.51 |
| CRP on POD1 (mg/L) | 55.7 ± 22.5 | 47.4 ± 19.3 | 0.012* |
| MDA on POD1 (nmol/ml) | 5.9 ± 0.9 | 5.4 ± 0.7 | 0.001* |
| SOD on POD1 (U/ml) | 78.7 ± 3.9 | 80.5 ± 4.3 | 0.009* |
*P<0.05.
Risk factors for POCD
As mentioned above, all the related risk factors (P<0.05 in Tables 1 and 2) were included in the univariate logistic regression analysis. As illustrated in Table 3, age, type of anesthesia, CRP, MDA, and SOD on POD1 were five potential risk factors for POCD (P<0.05). Of these five factors, the MDA level on POD1 (OR: 1.12, 95%CI: 1.03–1.23, P=0.017) was the only independent predictor for POCD according to the final multivariate logistic regression analysis.
Table 3. Univariate and multiple logistic regression analyses for POCD.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR(95%CI) | P-value | OR(95%CI) | P-value | |
| Age | 2.23(1.07–4.63) | 0.022* | 1.44(0.29–2.51) | 0.57 |
| ASA grade (I/II vs III) | 1.23(0.64–2.36) | 0.58 | ||
| Diabetes | 1.05(0.95–1.17) | 0.24 | ||
| Hypertension | 2.24(0.46–5.37) | 0.28 | ||
| Preoperative MMSE | 0.96(0.75–1.24) | 0.57 | ||
| Type of anesthesia (spinal vs general) | 2.58(1.29–5.11) | 0.009* | 1.48(0.93–2.38) | 0.17 |
| Duration of surgery | 1.09(0.54–2.13) | 0.76 | ||
| Duration of anesthesia | 1.69(0.81–3.53) | 0.17 | ||
| CRP on POD1 | 2.08(1.07–4.01) | 0.033* | 1.38(0.72–2.91) | 0.13 |
| MDA on POD1 | 1.14 (1.06–1.24) | 0.002* | 1.12(1.03–1.23) | 0.017* |
| SOD on POD1 | 1.59 (1.17–2.18) | 0.015* | 1.56(0.84–2.87) | 0.15 |
CI, confidence interval; OR, odds ratio. *P<0.05.
MDA on POD1 and POCD
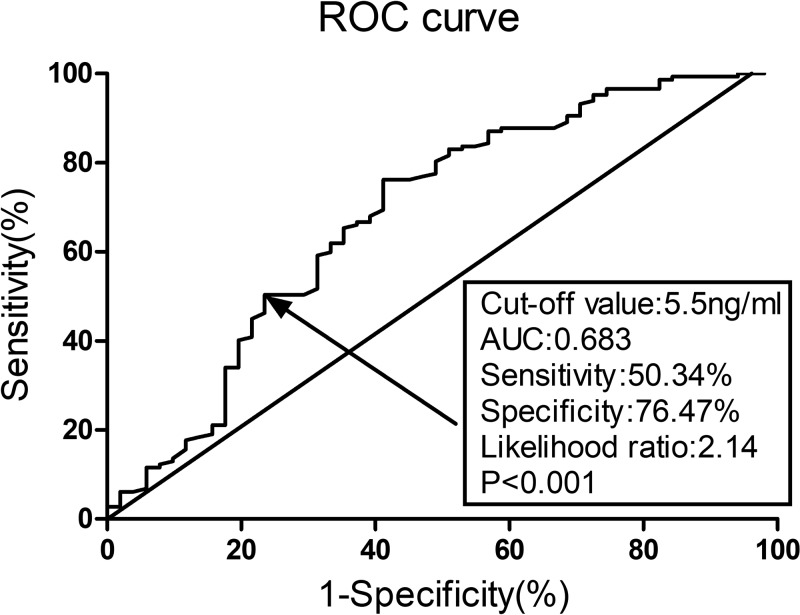
As shown in Figure 1, ROC curve analysis indicated that the MDA level on POD1 was a predictor for POCD, with an area under the curve (AUC) of 0.683, 95%CI of 0.590–0.775, a cut-off value of 5.5 nmol/ml, a sensitivity of 50.34%, and a specificity of 76.47% (P<0.001).
Figure 1. Predictive value of MDA on POD1 for POCD by ROC curve analysis.
MDA on POD1 was a predictor for POCD, with an AUC of 0.683, 95%CI of 0.590–0.775, a cut-off value of 5.5 nmol/ml, a sensitivity of 50.34%, and a specificity of 76.47%, respectively (P<0.001). POD, postoperative day; CI, confidence interval.
Discussion
In the present study, MDA expression on POD1 was an independent risk factor for POCD in elderly subjects undergoing surgery for hip fracture. The incidence rate of POCD within 7 postoperative days in our investigation was 25.8%, which was quite similar to the 29.9% reported by Ji et al. [17], 29.2% by Papadopoulos et al. [18], and 26.1% by Cheng et al. [19].
POCD is a neurological complication after surgery, and is very common in the elderly surgical patients [20]. POCD may lead to obstacles of visual memory, verbal memory, visuospatial abstraction, language comprehension, concentration, or attention [21]. To our knowledge, our understanding of the pathophysiology of POCD is relatively limited [22]. Researchers have performed numerous studies to investigate risk factors for POCD. Advanced age is universally identified as an important risk factor for POCD, indicating a basic role of brain tissue changes in POCD [23]. An increasing age is closely associated with an elevated incidence of endothelial dysfunction and cerebral atherosclerosis, which may lead to POCD [24]. However, our multivariate analysis did not support age as an independent risk factor for POCD. We considered that the relatively small range of age in the present study is the main explanation for this finding. The pathophysiology of POCD is generally agreed to be multifactorial, and whether POCD development is caused by general anesthesia remains unclear [25]. The traditional view holds that anesthesia is associated with an increased incidence of POCD. However, there were some studies that argued to the contrary [26]. A review by Rundshagen has indicated that general anesthesia does not play a causal role in POCD development [27]. A qualitative systematic review by Paredes et al. also indicated that general anesthesia is not significantly associated with the occurrence of POCD [28], which is in agreement with our results. It is well known that inflammatory response plays a critical role in activation of glial cell that leads to brain injury during POCD [29]. Accumulating evidence has disclosed that high levels of CRP are closely correlated with increased risks of POCD or postoperative delirium (POD) [30]. Some studies have revealed a significant association between serum CRP concentrations and POCD, indicating a critical role of the inflammatory response in POCD development [31,32]. However, some other researchers found that CRP was not an independent biomarker of cognitive impairment [33]. Indeed, our results did not support a predictive role of CRP for POCD.
The oxidative stress is associated increased proapoptotic proteins and decreased anti-apoptotic proteins in hippocampus and frontal cortex [34]. Furthermore, Zhang et al. has reported that neuronal apoptosis contributes to POCD, indicating the strong correlations between oxidative stress and POCD [35]. SOD is a vital enzyme that catalyzes the conversion of the superoxide radicals into hydrogen peroxide (H2O2) or oxygen (O2) [14]. The impairment of SOD has been reported to link with oxidative stress [36]. Some studies have reported that SOD is linked to neurocognitive deficits [37]. Alteration in SOD has been linked to the cellular apoptotic signaling introduced by mitochondrial factors or transcriptional factors [36]. However, no studies, including this present study, have reported the predictive role of SOD for POCD. MDA is a significant indicator of oxidative stress and can directly reflect plasma membrane damage induced by free radicals [38]. The overproduction of MDA is stimulated by increased free radicals [39], and MDA is used as an important biomarker for lipid peroxidation and peroxidative tissue injury [40]. MDA is widely used as a reliable and popular biomarker for oxidative stress assessment in clinical applications [41]. Our results indicated that MDA on POD1 was an independent risk factor for POCD, strongly suggesting a close association between oxidative stress and POCD. Excessive oxidative stress leads to lipid peroxidation of the cell membranes and the subsequent accumulation of MDA can damage brain cells, which may result in cognitive decline [13]. Pan et al. demonstrated that robustly increased hippocampal MDA after surgery was significantly associated with changes in postoperative cognition [42]. In addition to direct injury on brain cells, the complicated cross-talk between oxidative stress and the innate immune cells in the central nervous system is also a vital issue for the development of cognitive decline [42]. These points might at least partly explain the increased MDA levels in patients with POCD. A study conducted in an experimental model of tibial fracture in rats demonstrated that oxidative stress and mitochondrial dysfunction are two important contributors for POCD [12]. Notably, mitochondrial function can be impaired by oxidative stress via inducing structural changes [43]. Accumulated evidence has revealed that oxidative stress contributes to varied neurodegenerative disorders [44]. The vicious circle of a sustained oxidative environment and neuroinflammation in response to oxidative stress can damage healthy nerve cells, resulting in synapse dysfunction [45]. All these reports have indicated a close correlation between oxidative stress and POCD, which offers a possible explanation for the predictive role of MDA in POCD.
However, the present study has some great limitations. First, the inclusion of patients with oxidative stress-related diseases (e.g., diabetes and hypertension) and the medicines taken by the patients taken are factors that may have greatly influenced the results. Second, the determination of MDA by colorimetry or fluorimetry has historically relied on a reaction with TBA. However, this method of MDA measurement lacks sensitivity and specificity due to the possible reaction of TBA with some chemically reactive carbonyl group containing compounds (sugars, amino acids, etc.). Therefore, MDA may not be an ideal marker of lipid peroxidation as expected. Finally, only two biomarkers were measured for oxidative stress evaluation and they do not fully explain the redox homeostasis in POCD pathology.
Conclusions
In conclusion, we demonstrated that MDA on POD1 was an independent risk factor for POCD in elderly subjects undergoing hip fracture surgery. Furthermore, we suggest that oxidative stress intervention may be a promising strategy for POCD prevention.
Abbreviations
- ASA
American Society of Anesthesiologists
- AUC
area under the curve
- BMI
body mass index
- CRP
C-reactive protein
- MDA
malondialdehyde
- MMSE
Mini-Mental State Examination
- POCD
postoperative cognitive dysfunction
- POD1
postoperative day 1
- ROC
receiver operating characteristic
- SOD
superoxide dismutase
- TBA
thiobarbituric acid
Funding
This work was supported by the Clinical Research Funds Project of Zhejiang Medical Association [grant number 2018ZYC-A66].
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
B.G. and Y.G. participated in the conception and design, data collection, statistical analysis, and wrote the manuscript. C.X.W. participated in the conception and design and data collection.
References
- 1.Vutskits L. and Xie Z. (2016) Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat. Rev. Neurosci. 17, 705–717 10.1038/nrn.2016.128 [DOI] [PubMed] [Google Scholar]
- 2.Mason S.E., Noel-Storr A. and Ritchie C.W. (2010) The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J. Alzheimers Dis. 22, 67–79 10.3233/JAD-2010-101086 [DOI] [PubMed] [Google Scholar]
- 3.Androsova G., Krause R., Winterer G. and Schneider R. (2015) Biomarkers of postoperative delirium and cognitive dysfunction. Front. Aging Neurosci. 7, 112. 10.3389/fnagi.2015.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coburn M., Fahlenkamp A., Zoremba N. and Schaelte G. (2010) Postoperative cognitive dysfunction: incidence and prophylaxis. Anaesthesist 59, 177–184, quiz 85 10.1007/s00101-009-1657-2 [DOI] [PubMed] [Google Scholar]
- 5.Newman M.F., Kirchner J.L., Phillips-Bute B., Gaver V., Grocott H., Jones R.H.. et al. (2001) Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N. Engl. J. Med. 344, 395–402 10.1056/NEJM200102083440601 [DOI] [PubMed] [Google Scholar]
- 6.Nie H., Zhao B., Zhang Y.Q., Jiang Y.H. and Yang Y.X. (2012) Pain and cognitive dysfunction are the risk factors of delirium in elderly hip fracture Chinese patients. Arch. Gerontol. Geriatr. 54, e172–4 10.1016/j.archger.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 7.Kratz T., Heinrich M., Schlauss E. and Diefenbacher A. (2015) Preventing postoperative delirium. Dtsch. Arztebl. Int. 112, 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer S.T., Koenigsperger S., Olotu C. and Saller T. (2019) Biomarkers and postoperative cognitive function: could it be that easy? Curr. Opin. Anaesthesiol. 32, 92–100 10.1097/ACO.0000000000000676 [DOI] [PubMed] [Google Scholar]
- 9.Skvarc D.R., Berk M., Byrne L.K., Dean O.M., Dodd S., Lewis M.. et al. (2018) Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci. Biobehav. Rev. 84, 116–133 10.1016/j.neubiorev.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 10.Schrag M., Mueller C., Zabel M., Crofton A., Kirsch W.M., Ghribi O.. et al. (2013) Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: a meta-analysis. Neurobiol. Dis. 59, 100–110 10.1016/j.nbd.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 11.Kotekar N., Shenkar A. and Nagaraj R. (2018) Postoperative cognitive dysfunction – current preventive strategies. Clin. Interv. Aging 13, 2267–2273 10.2147/CIA.S133896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netto M.B., de Oliveira A.N. Jr, Goldim M., Mathias K., Fileti M.E., da Rosa N.. et al. (2018) Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav. Immun. 73, 661–669 10.1016/j.bbi.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 13.Ozacmak H.S., Ozacmak V.H. and Turan I. (2018) Ethyl pyruvate prevents from chronic cerebral hypoperfusion via preserving cognitive function and decreasing oxidative stress, caspase 3 activation and IL-1beta level. Bratisl. Lek. Listy. 119, 469–475 [DOI] [PubMed] [Google Scholar]
- 14.Keshavarzi S., Kermanshahi S., Karami L., Motaghinejad M., Motevalian M. and Sadr S. (2019) Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: the role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicology 72, 74–84 10.1016/j.neuro.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 15.Silbert B.S., Scott D.A., Evered L.A., Lewis M.S., Kalpokas M., Maruff P.. et al. (2006) A comparison of the effect of high- and low-dose fentanyl on the incidence of postoperative cognitive dysfunction after coronary artery bypass surgery in the elderly. Anesthesiology 104, 1137–1145 10.1097/00000542-200606000-00007 [DOI] [PubMed] [Google Scholar]
- 16.Moller J.T., Cluitmans P., Rasmussen L.S., Houx P., Rasmussen H., Canet J.. et al. (1998) Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 351, 857–861 [DOI] [PubMed] [Google Scholar]
- 17.Ji M.H., Shen J.C., Gao R., Liu X.Y., Yuan H.M., Dong L.. et al. (2013) Early postoperative cognitive dysfunction is associated with higher cortisol levels in aged patients following hip fracture surgery. J. Anesth. 27, 942–944 10.1007/s00540-013-1633-5 [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos G., Karanikolas M., Liarmakopoulou A., Papathanakos G., Korre M. and Beris A. (2012) Cerebral oximetry and cognitive dysfunction in elderly patients undergoing surgery for hip fractures: a prospective observational study. Open Orthop. J. 6, 400–405 10.2174/1874325001206010400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Q., Wang J., Wu A., Zhang R., Li L. and Yue Y. (2013) Can urinary excretion rate of 8-isoprostrane and malonaldehyde predict postoperative cognitive dysfunction in aging? Neurol. Sci. 34, 1665–1669 10.1007/s10072-013-1314-z [DOI] [PubMed] [Google Scholar]
- 20.Ni C., Xu T., Li N., Tian Y., Han Y., Xue Q.. et al. (2015) Cerebral oxygen saturation after multiple perioperative influential factors predicts the occurrence of postoperative cognitive dysfunction. BMC Anesthesiol. 15, 156. 10.1186/s12871-015-0117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W., Wang Y., Wu H., Lei L., Xu S., Shen X.. et al. (2014) Postoperative cognitive dysfunction: current developments in mechanism and prevention. Med. Sci. Monit. 20, 1908–1912 10.12659/MSM.892485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R., Wang G., Liu Y. and Zhang M. (2019) Preoperative smoking history is associated with decreased risk of early postoperative cognitive dysfunction in patients of advanced age after noncardiac surgery: a prospective observational cohort study. J. Int. Med. Res. 47, 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge Y., Ma Z., Shi H., Zhao Y., Gu X. and Wei H. (2014) Incidence and risk factors of postoperative cognitive dysfunction in patients underwent coronary artery bypass grafting surgery. Zhong Nan Da Xue Xue Bao Yi Xue Ban 39, 1049–1055 [DOI] [PubMed] [Google Scholar]
- 24.Rudolph J.L., Jones R.N., Levkoff S.E., Rockett C., Inouye S.K., Sellke F.W.. et al. (2009) Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation 119, 229–236 10.1161/CIRCULATIONAHA.108.795260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzimas P., Samara E., Petrou A., Korompilias A., Chalkias A. and Papadopoulos G. (2018) The influence of anesthetic techniques on postoperative cognitive function in elderly patients undergoing hip fracture surgery: general vs spinal anesthesia. Injury 49, 2221–2226 10.1016/j.injury.2018.09.023 [DOI] [PubMed] [Google Scholar]
- 26.Planel E., Richter K.E., Nolan C.E., Finley J.E., Liu L., Wen Y.. et al. (2007) Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J. Neurosci. 27, 3090–3097 10.1523/JNEUROSCI.4854-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rundshagen I. (2014) Postoperative cognitive dysfunction. Dtsch. Arztebl. Int. 111, 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paredes S., Cortinez L., Contreras V. and Silbert B. (2016) Post-operative cognitive dysfunction at 3 months in adults after non-cardiac surgery: a qualitative systematic review. Acta Anaesthesiol. Scand. 60, 1043–1058 10.1111/aas.12724 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., Li X., Li F. and An L. (2016) Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int. Immunopharmacol. 38, 426–433 10.1016/j.intimp.2016.06.031 [DOI] [PubMed] [Google Scholar]
- 30.Neerland B.E., Hall R.J., Seljeflot I., Frihagen F., MacLullich A.M., Raeder J.. et al. (2016) Associations between delirium and preoperative cerebrospinal fluid C-reactive protein, interleukin-6, and interleukin-6 receptor in individuals with acute hip fracture. J. Am. Geriatr. Soc. 64, 1456–1463 10.1111/jgs.14238 [DOI] [PubMed] [Google Scholar]
- 31.Liu X., Yu Y. and Zhu S. (2018) Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): a meta-analysis of observational studies. PLoS One 13, e0195659. 10.1371/journal.pone.0195659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y.H., Guo X.H., Zhang Q.M., Yan G.T. and Wang T.L. (2015) Serum CRP and urinary trypsin inhibitor implicate postoperative cognitive dysfunction especially in elderly patients. Int. J. Neurosci. 125, 501–506 10.3109/00207454.2014.949341 [DOI] [PubMed] [Google Scholar]
- 33.Wu C., Wang R., Li X. and Chen J. (2016) Preoperative serum microRNA-155 expression independently predicts postoperative cognitive dysfunction after laparoscopic surgery for colon cancer. Med. Sci. Monit. 22, 4503–4508 10.12659/MSM.898397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandravanshi L.P., Yadav R.S., Shukla R.K., Singh A., Sultana S., Pant A.B.. et al. (2014) Reversibility of changes in brain cholinergic receptors and acetylcholinesterase activity in rats following early life arsenic exposure. Int. J. Dev. Neurosci. 34, 60–75 10.1016/j.ijdevneu.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 35.Zhang X., Dong H., Li N., Zhang S., Sun J., Zhang S.. et al. (2016) Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J. Neuroinflammation 13, 127. 10.1186/s12974-016-0592-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maiti S., Acharyya N., Ghosh T.K., Ali S.S., Manna E., Nazmeen A.. et al. (2017) Green tea (Camellia sinensis) protects against arsenic neurotoxicity via antioxidative mechanism and activation of superoxide dismutase activity. Cent. Nerv. Syst. Agents Med. Chem. 17, 187–195 10.2174/1871524917666170201145102 [DOI] [PubMed] [Google Scholar]
- 37.Hardingham G.E. and Do K.Q. (2016) Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat. Rev. Neurosci. 17, 125–134 10.1038/nrn.2015.19 [DOI] [PubMed] [Google Scholar]
- 38.Abdel Fattah N.S., Ebrahim A.A. and El Okda E.S. (2011) Lipid peroxidation/antioxidant activity in patients with alopecia areata. J. Eur. Acad. Dermatol. Venereol. 25, 403–408 10.1111/j.1468-3083.2010.03799.x [DOI] [PubMed] [Google Scholar]
- 39.Rui B.R., Shibuya F.Y., Kawaoku A.J.T., Losano J.D.A., Angrimani D.S.R., Dalmazzo A.. et al. (2017) Impact of induced levels of specific free radicals and malondialdehyde on chicken semen quality and fertility. Theriogenology 90, 11–19 10.1016/j.theriogenology.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 40.Janero D.R. (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 9, 515–540 10.1016/0891-5849(90)90131-2 [DOI] [PubMed] [Google Scholar]
- 41.Cwynar A., Olszewska-Slonina D., Czajkowski R., Zegarska B., Bialecka A., Mecinska-Jundzill K.. et al. (2018) Investigation of oxidative stress in patients with alopecia areata by measuring the levels of malondialdehyde and ceruloplasmin in the blood. Postepy Dermatol. Alergol. 35, 572–576 10.5114/pdia.2017.68047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan K., Li X., Chen Y., Zhu D., Li Y., Tao G.. et al. (2016) Deferoxamine pre-treatment protects against postoperative cognitive dysfunction of aged rats by depressing microglial activation via ameliorating iron accumulation in hippocampus. Neuropharmacology 111, 180–194 10.1016/j.neuropharm.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 43.Berg R.M., Moller K. and Bailey D.M. (2011) Neuro-oxidative-nitrosative stress in sepsis. J. Cereb. Blood Flow Metab. 31, 1532–1544 10.1038/jcbfm.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gubandru M., Margina D., Tsitsimpikou C., Goutzourelas N., Tsarouhas K., Ilie M.. et al. (2013) Alzheimer’s disease treated patients showed different patterns for oxidative stress and inflammation markers. Food Chem. Toxicol. 61, 209–214 10.1016/j.fct.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 45.Verdile G., Keane K.N., Cruzat V.F., Medic S., Sabale M., Rowles J.. et al. (2015) Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators Inflamm. 2015, 105828. 10.1155/2015/105828 [DOI] [PMC free article] [PubMed] [Google Scholar]