Short abstract
Neuroimaging studies have demonstrated that reward system is associated with chronic pain diseases. In addition, previous studies have also demonstrated abnormal functional and structural brain regions in primary dysmenorrhea. However, the relation of reward system and primary dysmenorrhea is still unknown. Using the resting state functional magnetic resonance imaging, we aimed to investigate the functional connectivity changes of reward system during periovulatory phase in primary dysmenorrhea. Forty-one primary dysmenorrhea patients and 39 matched female healthy controls participated in this study. Compared to healthy controls, primary dysmenorrhea patients showed decreased connectivity of left nucleus accumbens with the bilateral anterior insula and the left amygdala and decreased connectivity of right nucleus accumbens with ventral tegmental area, the left hippocampus, the right orbital frontal cortex, and the right anterior insula. In addition, the decreased functional connectivity between the right nucleus accumbens-ventral tegmental area negatively correlated with the level of prostaglandin F2 alpha. Our findings provide neuroimaging evidence in support of the abnormal reward system connectivity in primary dysmenorrhea patients, which might contribute to a better understanding of the cerebral pathophysiology of primary dysmenorrhea.
Keywords: Primary dysmenorrhea, reward system, functional connectivity
Introduction
Primary dysmenorrhea (PDM) is a common gynecologic disease that often affects adolescents and women of reproductive age.1 It is characterized by a crampy pain located in the lower abdomen and may extend to the lumbar or thighs, which often occurs before or after the onset of the menstrual bleeding.1 The prevalence of PDM varies from 30% to 90%.2 The severe pain sometimes debilitating for brief periods of time and accompanied by associated symptoms (such as diarrhea, nausea, fatigue, headache, and dizziness), which has a great impact on the quality of life.3 Traditionally, uterine ischemia modulated by prostaglandin synthesis associated with other factors which affects the perception and the severity of the pain are considered to the main pathophysiology of PDM.1 Prostaglandin F2 alpha (PGF2α) level increased in women with PDM and was directly related to the pain.4 Recently, with advancing of neuroimaging techniques, more and more studies have confirmed that PDM might be associated with brain structural and functional.5–9
Previous studies have indicated that brain morphological changes exist in PDM. Gray matter changes were found in the hippocampus, anterior/dorsal posterior cingulate cortex (ACC/dPCC), periaqueductal gray (PAG), hypothalamus, left ventral portion of precuneus, left superior/middle temporal gyrus (STG/MTG), right cerebellar tonsil, and medial prefrontal cortex (mPFC).5,9 The brain functional abnormalities in PDM were found in default mode network (including the ventromedial prefrontal cortex, ACC, PCC, precuneus, inferior parietal cortex, inferior temporal cortex, hippocampus, and thalamus)6 and anterior insula (AI)10, and also in the hypoconnectivity between the PAG and the default mode network during painful menstruation and pain-free periovulatory phase.8,11 In addition, the altered functional connectivity in ACC was correlated with disease duration and severity.7 However, the existed neuroimaging studies mainly focused on the descending modulatory pain system (DMPS)12 that regulates the pain perception and endocrine function. Actually, pain is defined as an unpleasant experience for actual tissue damage.13 Previous evidences have manifested that the chronic pain was associated with anhedonia, the inability to experience pleasure.14 And abnormal dopamine receptor and response exist in chronic pain.15,16 Furthermore, studies of brain neural circuits have well documented that mesolimbic reward circuit is responsible for motivation and cognition behaviors, including pain.17,18 Therefore, the reward system might be involved in the pathophysiology of chronic pain, including PDM. However, few studies have attempted to explore the alteration of reward circuit network in PDM.
In this study, we aimed to investigate the potential functional alteration in reward network in patients with PDM at ovulatory period. As the nucleus accumbens (NAc) is the central node in reward system19, we constructed the reward network by NAc-based functional connectivity via the resting state functional magnetic resonance imaging (rs-fMRI) in PDM patients and matched healthy control (HC) subjects. Then, voxel-wise two-sample t-test was applied to evaluate the difference of reward network between PDM and HC groups. Furthermore, the correlation analysis between the abnormal brain reward network and PGF2α was also investigated, with the aim to explore whether the dysfunctional reward network at menstrual period is correlated with the plasma level of PGF2α in PDM.
Materials and methods
Subjects
A total of 80 right-handed subjects were recruited, including 41 PDM patients and 39 female HCs. All subjects were recruited from nearby university by advertisements. The study was approved by the Institutional Review Board of Affiliated Hospital of Chengdu University of Traditional Chinese Medicine. All participants signed the informed consent. All PDM patients received pelvic ultrasound to exclude secondary dysmenorrhea caused by organic pelvic disease (such as endometriosis or adenomyosis). The inclusion criteria for the PDM subjects were as follows: (1) 18–30 years old, no history of fertility; (2) a regular menstrual cycle of approximately 27 to 32 days; (3) a history of menstrual pain longer than 6 months; (4) an average menstrual pain score rated ≥ 4 on a visual analog scale (VAS, 0 = no pain sensation, 10 = the worst pain sensation) over the last 6 months; and (5) right-handedness. The inclusion criteria for HCs were similar to those for PDM subjects, but without pain during menses. The exclusion criteria for all subjects were as follows: (1) using oral contraceptives, hormonal supplements, Chinese medicine, or any centrally acting medication (e.g., opioid, antiepileptics) within the last 6 months; (2) organic pelvic disease; (3) psychiatric or neurologic diseases; and (4) any contraindications to magnetic resonance imaging (MRI). All subjects were instructed to refrain from ingesting caffeine and alcohol for 24 h before the MRI scan.
Experimental design
MRI scans were arranged at periovulatory phase (days 10–14 of the menstrual cycle), individually scheduled according to each subject’s menstrual cycle. Blood samples for prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) were collected at menstruation (days 1–3 of the menstrual cycle). Self-rating anxiety scale (SAS)20 and self-rating depression scale (SDS)21 were used to evaluate each subject’ s anxiety and depression level at periovulatory phase (days 10–14 of the menstrual cycle).
MRI data acquisition
All MRI images of subjects were acquired on a 3.0 Tesla MRI scanner (Siemens Allegra) at the Huaxi MR research Center, West China Hospital of Sichuan University. The subjects were instructed to remain awake during the scan (eyes closed, heads still but relaxed, without thinking about anything). Head cushions were used to minimize head motion, and earplugs were provided to reduce noise. High-resolution T1-weighted anatomical image was obtained with the following parameters: repetition time (TR) = 1900 ms; echo time (TE) =2.26 ms; flip angle = 9°; filed of view (FOV) = 256 mm × 256 mm; data matrix = 256 × 256; and in-plane resolution = 1 mm × 1 mm. Resting-state functional images were acquired using a gradient-echo echo-planar imaging sequence with the following parameters: TR = 2000 ms; TE = 30 ms; flip angle = 90°; FOV = 240 mm × 240 mm; matrix size = 64 × 64; and in-plane resolution = 3.75 mm × 3.75 mm.
Data preprocessing
The Data Processing Assistant for Resting-State fMRI (DPARSF), which is based on Statistical Parametrical Mapping software (SPM8, http://www.fil.ion.ucl.ac.uk/spm) in Matlab 8.0 (The MathWorks, Inc., Natick, MA, USA), was used to analyze image data. The first 10 time points were discarded for instability of the initial MRI signal. The remaining functional images preprocessing included slice timing, realignment for head motion correction, co-registration to each subject’s T1-weighted anatomical images, and normalization against the Montreal Neurological Institute (MNI) template. Subjects having head motion exceeded 2 mm or 2° were excluded from further analysis. The images were resliced with isotropic 3 mm3 voxel size when normalized to MNI space and spatially smoothed using a 6-mm full width at half-maximum Gaussian kernel. After linear trends had been removed, the images were filtered with a temporal band pass of 0.01–0.08 Hz. The noise of white matter, and cerebrospinal fluid and head motion-related covariates were regressed out.
Functional connectivity analysis
The bilateral NAc were applied as seed regions and selected from Harvard-Oxford subcortical atlas.22 For each subject, Pearson correlation analysis was utilized to calculate the correlation coefficients between the averaged seed region time course and time series of all brain voxels. Fisher’s r-to-z transformation was applied to improve the normality of the correlation coefficients.
Statistical analysis
To examine demographic and clinical symptoms between the PDM subjects and HCs, two-sample t-test was performed on SPSS Statistics, version 20. The threshold was set at a significant level of p < 0.05. For imaging analysis, the group difference on bilateral NAc functional connectivity networks was conducted using two-sample t-test (REST), controlling the effect of age, and 3dClustSim was used to correct the multiple comparisons (voxel level, p < 0.005; cluster level, α < 0.01; cluster sizes > 105; http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html).
To investigate the potential relationship between altered NAc functional connectivity and PGF2α in PDM patients, Pearson correlation was calculated. The significant level was set at p < 0.05.
Results
Demographics and clinical characteristic
The demographics and clinical characteristic of all participants are shown in Table 1. No significant difference was identified in age, SAS score, and SDS score between PDM patients and HCs (all p values > 0.05). The mean of PGF2α was 732.60, indicating the PGF2α level was elevated in PDM patients compared to the healthy women.23
Table 1.
Demographic and clinical traits for all participants.
| Characteristic | PDM (n = 41) | HC (n = 39) | T or χ2 | p |
|---|---|---|---|---|
| Age | 22.17 ± 2.73 | 22.92 ± 1.83 | −1.44 | 0.153 |
| VAS (days 10–14) | 0 | 0 | – | – |
| SAS (days 10–14) | 38.21 ± 6.39 | 35.85 ± 5.13 | 1.81 | 0.07 |
| SDS (days 10–14) | 39.54 ± 6.84 | 37.00 ± 6.28 | 1.72 | 0.09 |
| PGE2 (days 1–3) | 542.63 ± 225.34 | – | – | – |
| PGF2α (days 1–3) | 732.60 ± 121.10 | – | – | – |
PDM: primary dysmenorrhea; HC: healthy control; VAS: visual analog scale; SAS: self-rating anxiety scale; SDS: self-rating depression scale; PGE2: prostaglandin E2; PGF2α: prostaglandin F2 alpha.
Group difference on bilateral NAc functional connectivity networks
Significant group differences of the left NAc functional connectivity network between PDM patients and HCs are shown in Figure 1 and Table 2. Compared to HCs, PDM patients showed decreased left NAc functional connectivity in the bilateral AI and the left amygdala. As the result of left amygdala was not significant after strict whole brain 3dClustSim correction, we conducted the small volume 3dClustSim correction, and the result of left amygdala was significant after whole amygdala-based small volume correction. Significant group differences of the right NAc functional connectivity network between PDM patients and HCs are shown in Figure 2 and Table 2. Compared to HCs, PDM patients showed decreased right NAc functional connectivity in ventral tegmental area, left hippocampus (VTA/HIP), and the right orbital frontal cortex and AI (OFC/AI).
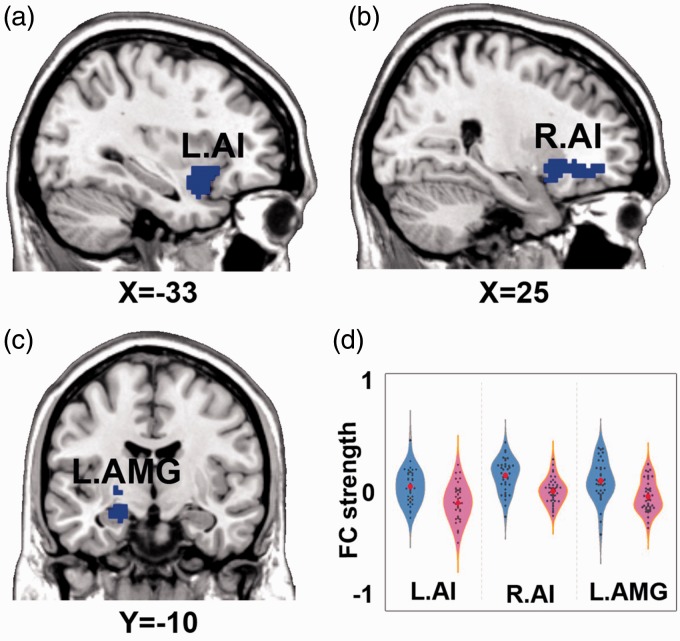
Figure 1.
Brain regions showing significant group difference on left NAFC network. Note: p < 0.005, 3dClustSim correction for bilateral AI, amygdala small volume based 3dClustSim correction for left amygdala. (a) the decreased NAFC in left AI; (b) the decreased NAFC in right AI; (c) the decreased NAFC in left amygdala; (d) the quantitative display of group difference of left NAFC network. Blue color indicates PDM < HC. The color bar presents with the z scores. The blue violin represents HC group, while pink violin represents PDM group. NAFC: nucleus accumbens functional connectivity; AMG: amygdala; AI: anterior insula.
Table 2.
Brain areas exhibiting significant group differences in the intrinsic NAFC network.
| Brain region | BA | Voxel size |
MNI coordinates (RAI) |
Peak Z score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Left NAFC network | ||||||
| R.AI | 48 | 149 | 24 | 27 | −9 | −3.92 |
| L.AI | 48 | 113 | −33 | 9 | −18 | −3.95 |
| L.AMG | 34 | 74 | −21 | −9 | −15 | −3.68 |
| Right NAFC network | ||||||
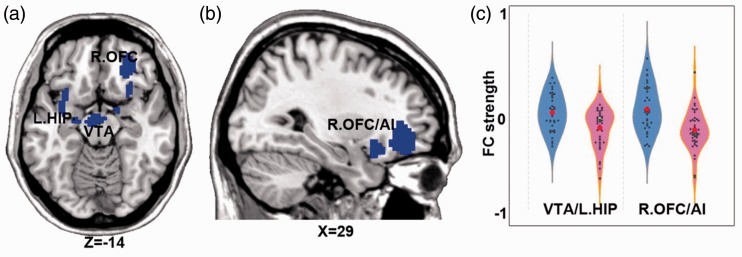
| VTA/L.HIP | 28 | 284 | −6 | −12 | −18 | −4.30 |
| R.OFC/AI | 11 | 236 | 30 | 36 | −6 | −4.21 |
NAFC: nucleus accumbens functional connectivity; BA, Broadmann area; RAI, Right anterior and inferior orientaion; MNI: Montreal Neurological Institute; VTA: ventral tegmental area; L.HIP: left hippocampus; R.OFC: right orbital frontal cortex; AI: anterior insula; L.AMG: left amygdala; L.AI: left anterior insula; R.AI: right anterior insula.
Figure 2.
Brain regions showing significant group differences on the right NAFC network. Note: p < 0.005, 3dClustSim correction. (a) the decreased right NAFC in VTA and left HIP; (b) the decreased right NAFC in right OFC and AI; (c) the quantitative display of group difference of right NAFC network; Blue color indicates PDM < HC. The color bar presents with the z scores. The blue violin represents HC group, while pink violin represents PDM group. OFC: orbital frontal cortex; VTA: ventral tegmental area; HIP: hippocampus; AI: anterior insula; NAFC: nucleus accumbens functional connectivity.
Correlation analysis results
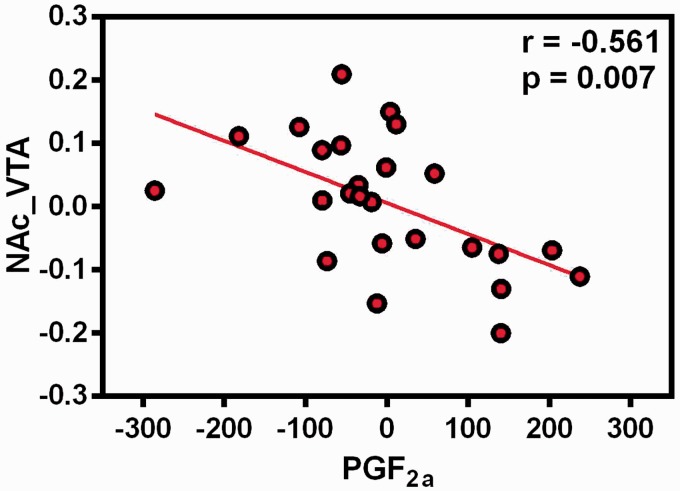
The correlation analysis revealed that the decreased functional connectivity between the right NAc and VTA was negatively correlated with level of PGF2α (r = −0.561, p = 0.007) (Figure 3). No other significant relationship was found (all p values > 0.05).
Figure 3.
The association between functional connectivity strength of right nucleus accumbens with VTA and PGF2α in the PDM group, controlling the age for covariate. NAc: nucleus accumbens; VTA: ventral tegmental area; PGF2a: prostaglandin F2 alpha.
Discussion
In this study, we investigated the potential alteration of intrinsic in reward network in patients with PDM. We found that the NAc functional connectivity was decreased in bilateral AI, amygdala, VTA, hippocampal, and the right OFC, during periovulatory phase. These abnormal functional connectivity reward network regions were located within reward circuit and DMPS. Moreover, we found that the decreased functional connectivity of the right NAc-VTA was associated with higher level of PGF2α at menstruation period in patients with PDM. Taken together, our findings suggested that the dysfunctional reward network would be a potential biomarker for the evaluation of PDM. This study would advance our understanding of the central nervous system mechanism of PDM.
The decreased NAc functional connectivity within the reward circuit
First, the decreased NAc functional connectivity in PDM patients was located within the reward circuit, including VTA, OFC, and hippocampal. The VTA-NAc dopaminergic transmitting is crucial for the reward processing, while the information delivery in the VTA-NAc modulates nociceptive and pain-related affective behaviors.24,25 The activation of NAc-projecting dopaminergic neurons in the VTA can suppress persistent pain.26 The decreased NAc-VTA functional connectivity in PDM patients would indicate that the reward network might be related to the modulation of pain. The OFC is a nexus for sensory integration.27 Neuroimaging studies found that the OFC participated in the conscious experience of pleasure and reward.28,29 Thus, it is involved in emotional and reward-related behaviors.27 Wu et al. reported that PDM patients had greater ReHo in the OFC and presented activated during menstrual phase of PDM.11 They also observed that increased activity in OFC at the onset of PDM was negatively related to subjective pain rating.30 The present findings of the NAc-OFC connectivity might be a reflection of the adaptive changes to the long-term menstrual pain state though emotional and reward feedback behaviors. The hippocampus is a part of the limbic system and plays a vital role in the processing of opiate-related reward memories. Reward-driven motivation can enhance long-term memory and makes it become adaptive.31 Hippocampus accepts the projections of dopamine neurons in the VTA and exists convergent connectivity between the NAc and VTA.31,32 In addition, structural and functional alters in hippocampus have been reported in the previous study of female menstrual cycle.9 Consequently, our finding may further verify that hippocampus is involved in the memory of pain and influence later pain adaptive behavior in PDM. Taken together, our finding indicated the abnormal reward circuit which related pain modulates as well as emotional and memory adaption mechanism in PDM patients.
The decreased NAc functional connectivity in the DMPS
Present results showed that the NAc FC with AI and amygdala were in PDM patients. The AI and amygdala are main part of the pain descending (top-down) pathway and engaged modulate transmission of ascending nociceptive signals.33 The AI receives afferent projections from thalamus and forms mutual connections with the amygdala, limbic system.34 Noxious stimuli and pain could activate the AI region. Previous studies have found that AI appears to be involved in sensorimotor processing, socio-emotional processing, and cognitive functions.35 The AI produces emotional awareness after receiving noxious stimulation from the thalamus, transmits this signal to the amygdala, which is sequentially attached to hypothalamus and PAG.36 Voxel-based morphometry studies found that gray volumes decreased at the AI cortex in patients with chronic back pain.37,38 In the previous study, it also found that the GM density of insula in patients with PDM was lower than HCs.5 Besides, the decreased FC between AI and mPFC was also observed,8,10 which suggested that abnormal structure and function of AI in patients with PDM. The amygdala, located in the medial temporal lobe, is important for emotional processing.39 As pain is a multidimensional experience, the amygdala has garnered much attention for involving the emotional-affective dimension of pain.40–43 Moreover, early evidence proved that the amygdala was involved in mediating the effects of stimulus-reward by projecting to the NAc.44 Altered amygdala functional connectivity has been observed in patients with chronic pain such as irritable syndrome,45,46 migraine,47 and urological chronic pelvic pain syndromes.48 Our study found that patients with PDM had decreased NAc FC between the AI and amygdala, suggested that the interaction between the descending modulate pain system and reward system was abnormal in patients with PDM.
The association of NAc-VTA FC and PGF2α level in PDM
PGF2α is an important regulator for menstruation. It constricts the endometrial vessels and the smooth muscle of the myometrium during menstruation.49 PGF2α excessively released generates an ischemia-reperfusion response causing painful menstruation.50 Therefore, it is generally considered to be the main reason for dysmenorrhea.51 Previous studies suggested that the increase of PGF2α was positively related to dysmenorrheic pain.52 This study found that the decreased FC of reward network NAc-VTA was related to the elevated level of PGF2α in the menstrual period, suggesting that the abnormalities of the intrinsic reward network in the pain-free period would be a potential predictor for the severity of pain in the menstrual period in PDM patients. Ovarian hormones act as critical neuroregulatory, neurotropic, and neuroprotective factors in brain, which affect on structure and function of the brain.53 And as a cyclic occurring pain conditions, patients with PDM accept the nociceptive input to the central nervous system and induce the alteration of structure and function, which further result in central sensitization.5 Therefore, PGF2α, as an important hormone involved in dysmenorrhea, has been directly and indirectly associated with brain “adaptive plasticity.” However, further longitudinal follow-up study is needed to verify our results. This correlation does not indicate the causal relationship between the ovulation reward network abnormality and the blood index, which needs to be further clarified through longitudinal research.
Limitations
There were several limitations in this study. First, we only focused on the NAc functional connectivity network in the pain-free state, not in the menstrual pain state. Future study should enroll patients with two periods of PDM and further investigate the dynamic variation of reward network in PDM. Second, the behavioral evaluation is limited in this study, and future studies should comprehensively assess the pain and reward system in the menstrual period and pain-free state. Third, we only investigated the functional alterations of reward system in PDM, and future studies should combine with multimode of neuroimaging data (e.g., structural and arterial spin labeling) to explore the association between abnormal reward system and PDM characteristics.
Conclusion
In summary, our study used NAc-based functional connectivity method to investigate the functional alteration in PDM. The results suggested that the PDM showed dysfunctional within the reward system and the connectivity between reward system and DMPS. In addition, the decreased functional connectivity of NAc-VTA was negatively correlated to the level of PGF2α. This study would advance our understanding of the central nervous system mechanism of PDM and provide a potential target for alleviating pain in the menstrual period.
Acknowledgment
The authors thank all the participants of this study.
Author Contributions
JY, FRL, and FZ contributed to the design of the study. YNW and YY participated in the collection of MRI data and clinical estimate. MYW, WW, and XLG recruited participants. SYY performed imaging data analysis. QZ and SYY wrote the manuscript. All authors have read and approved the final manuscript.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with Helsinki Declaration and its later amendments or comparable ethical standards.
Ethical Statements
Informed consent was obtained from all individual participants included in the study.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the programs of the National Natural Science Foundation of China (No. 81574089 and No. 81590951) and the Fok Ying-Tong Education Foundation, China (No. 151043), Fund of Science and Technology Department of Sichuan Province, China(No. 2018JY0249), Initiative Postdocs Supporting Program (No. BX20190046).
References
- 1.Burnett M, Lemyre M. No. 345-primary dysmenorrhea consensus guideline. J Obstet Gynaecol Can 2017; 39: 585–595. [DOI] [PubMed] [Google Scholar]
- 2.Hong J, Mark J, Gita M. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev 2014; 36: 104–2. [DOI] [PubMed] [Google Scholar]
- 3.Unsal A, Ayranci U, Tozun M, Arslan G, Calik E. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci 2010; 115: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenwaks Z, Jones GS, Henzl MR, Dubin NH, Ghodgaonkar RB, Hoffman S. Naproxen sodium, aspirin, and placebo in primary dysmenorrhea. Reduction of pain and blood levels of prostaglandin F2-alpha metabolite. Am J Obstet Gynecol 1981; 140: 592–598. [DOI] [PubMed] [Google Scholar]
- 5.Cheng-Hao T, Niddam DM, Hsiang-Tai C, Li-Fen C, Yong-Sheng C, Yu-Te W, Tzu-Chen Y, Jiing-Feng L, Jen-Chuen H. Brain morphological changes associated with cyclic menstrual pain. Pain 2010; 150: 462–468. [DOI] [PubMed] [Google Scholar]
- 6.Liu P, Liu Y, Wang G, Yang X, Jin L, Sun J, Qin W. Aberrant default mode network in patients with primary dysmenorrhea: a fMRI study. Brain Imaging Behav 2017; 11: 1479–1485. [DOI] [PubMed] [Google Scholar]
- 7.Peng L, Liu Y, Wang G, Ru L, Ying W, Fan Y, Yang Y, Deng D, Wei Q. Changes of functional connectivity of the anterior cingulate cortex in women with primary dysmenorrhea. Brain Imaging Behav 2017; 12: 710–717. [DOI] [PubMed] [Google Scholar]
- 8.Wei SY, Chao HT, Tu CH, Li WC, Low I, Chuang CY, Chen LF, Hsieh JC. Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain 2016; 157: 92–2. [DOI] [PubMed] [Google Scholar]
- 9.Lisofsky N, Mårtensson J, Eckert A, Lindenberger U, Gallinat J, Kühn S. Hippocampal volume and functional connectivity changes during the female menstrual cycle. Neuroimage 2015; 118: 154–162. [DOI] [PubMed] [Google Scholar]
- 10.Dun WH, Yang J, Yang L, Ding D, Ma XY, Liang FL, von Deneen KM, Ma SH, Xu XL, Liu J. Abnormal structure and functional connectivity of the anterior insula at pain-free periovulation is associated with perceived pain during menstruation. Brain Imaging Behav 2017; 11: 1787–1795. [DOI] [PubMed] [Google Scholar]
- 11.Wu T-H, Tu C-H, Chao H-T, Li W-C, Low I, Chuang C-Y, Yeh T-C, Cheng C-M, Chou C-C, Chen L-F, Hsieh J-C. Dynamic changes of functional pain connectome in women with primary dysmenorrhea. Sci Rep 2016; 6: 24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology 2008; 23: 371–380. [DOI] [PubMed] [Google Scholar]
- 13.Merskey H, Bogduk N. Part III: pain terms, a current list with definitions and notes on usage. Pain 1994; 24: S215–S221. [Google Scholar]
- 14.Michael K N, Carissa M C, Ali A, Gin Singh M. Depressive symptoms in patients with chronic pain. Med J Aust 2009; 190: 66–70. [DOI] [PubMed] [Google Scholar]
- 15.Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, Luutonen S, Någren K, Jääskeläinen S. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain 2003; 101: 149–154. [DOI] [PubMed] [Google Scholar]
- 16.Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci 2007; 25: 3576–2. [DOI] [PubMed] [Google Scholar]
- 17.Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron 2016; 89: 11–36. [DOI] [PubMed] [Google Scholar]
- 18.Saadé NE, Atweh SF, Bahuth NB, Jabbur SJ. Augmentation of nociceptive reflexes and chronic deafferentation pain by chemical lesions of either dopaminergic terminals or midbrain dopaminergic neurons. Brain Res 1997; 751: 1–12. [DOI] [PubMed] [Google Scholar]
- 19.Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev 2016; 68: 282–2. [DOI] [PubMed] [Google Scholar]
- 20.Zung WW. A rating instrument for anxiety disorders. Psychosomatics 1971; 12: 371–379. [DOI] [PubMed] [Google Scholar]
- 21.Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry 1965; 13: 508–515. [DOI] [PubMed] [Google Scholar]
- 22.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–980. [DOI] [PubMed] [Google Scholar]
- 23.Lumsden MA, Kelly RW, Baird DT. Primary dysmenorrhoea: the importance of both prostaglandins E2 and F2 alpha. Br J Obstet Gynaecol 1983; 90: 1135–1140. [DOI] [PubMed] [Google Scholar]
- 24.Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci 2016; 19: 220–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi C, Guo B, Ren K, Yao H, Wang M, Sun T, Cai G, Liu H, Li R, Luo C, Wang W, Wu S. Chronic inflammatory pain decreases the glutamate vesicles in presynaptic terminals of the nucleus accumbens. Mol Pain 2018; 14: 174480691878125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altier NG, Stewart J. Intra-VTA infusions of the substance P analogue, DiMe-C7, and intra-accumbens infusions of amphetamine induce analgesia in the formalin test for tonic pain. Brain Res 1993; 628: 279–285. [DOI] [PubMed] [Google Scholar]
- 27.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005; 6: 691–702. [DOI] [PubMed] [Google Scholar]
- 28.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 2003; 301: 1104–1107. [DOI] [PubMed] [Google Scholar]
- 29.Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex 2003; 13: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 30.Tu CH, Niddam DM, Chao HT, Liu RS, Hwang RJ, Yeh TC, Hsieh JC. Abnormal cerebral metabolism during menstrual pain in primary dysmenorrhea. Neuroimage 2009; 47: 28–35. [DOI] [PubMed] [Google Scholar]
- 31.Kahn I, Shohamy D. Intrinsic connectivity between the hippocampus, nucleus accumbens and ventral tegmental area in humans. Hippocampus 2013; 23: 187–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull 1994; 33: 445–2. [DOI] [PubMed] [Google Scholar]
- 33.Davis KD, Flor H, Greely HT, Iannetti GD, Mackey S, Ploner M, Pustilnik A, Tracey I, Treede RD, Wager TD. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol 2017; 13: 624–2. [DOI] [PubMed] [Google Scholar]
- 34.Zhuo M. Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience 2016; 338: 220–229. [DOI] [PubMed] [Google Scholar]
- 35.Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol 2017; 34: 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu C, Tao Y, Zhao H, Ming Z, Meng F, Hao F, Xie Y, Hui X. Insular cortex is critical for the perception, modulation, and chronification of pain. Neurosci Bull 2016; 32: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fritz HC, McAuley JH, Wittfeld K, Hegenscheid K, Schmidt CO, Langner S, Lotze M. Chronic back pain is associated with decreased prefrontal and anterior insular gray matter: results from a population-based cohort study. J Pain 2016; 17: 111–118. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda E, Li T, Kobinata H, Zhang S, Kurata J. Anterior insular volume decrease is associated with dysfunction of the reward system in patients with chronic pain. Eur J Pain 2018; 22: 1170–2. [DOI] [PubMed] [Google Scholar]
- 39.Thompson JM, Neugebauer V. Amygdala plasticity and pain. Pain Res Manag 2017; 2017: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp 2014; 35: 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev 2009; 60: 226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol 2015; 227: 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature 2015; 517: 284–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience 1989; 30: 77–75. [DOI] [PubMed] [Google Scholar]
- 45.Qi R, Liu C, Ke J, Xu Q, Ye Y, Jia L, Wang F, Zhang LJ, Lu GM. Abnormal amygdala resting-state functional connectivity in Irritable Bowel Syndrome. AJNR Am J Neuroradiol 2016; 37: 1139–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Icenhour A, Witt ST, Elsenbruch S, Lowén M, Engström M, Tillisch K, Mayer EA, Walter S. Brain functional connectivity is associated with visceral sensitivity in women with Irritable Bowel Syndrome. Neuroimage Clin 2017; 15: 449–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 2011; 70: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinhans NM, Yang CC, Strachan ED, Buchwald DS, Maravilla KR. Alterations in connectivity on functional magnetic resonance imaging with provocation of lower urinary tract symptoms: a MAPP research network feasibility study of urological chronic pelvic pain syndromes. J Urol 2016; 195: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen DV, Andersen KB, Wagner G. Prostaglandins in the menstrual cycle of women. A review. Dan Med Bull 1987; 34: 178–182. [PubMed] [Google Scholar]
- 50.Eva B, Sofia BK, Pierre C, Eric M, Nathalie H, Laberge PY, Mathieu L, Fortier MA. The human aldose reductase AKR1B1 qualifies as the primary prostaglandin F synthase in the endometrium. J Clin Endocrinol Metab 2011; 96: 210–219. [DOI] [PubMed] [Google Scholar]
- 51.Ma Y-X, Ma L-X, Liu X-L, Ma Y-X, Lv K, Wang D, Liu J-P, Xing J-M, Cao H-J, Gao S-Z, Zhu J. A comparative study on the immediate effects of electroacupuncture at Sanyinjiao (SP6), Xuanzhong (GB39) and a non-meridian point, on menstrual pain and uterine arterial blood flow, in primary dysmenorrhea patients. Pain Med 2010; 11: 1564–1575. [DOI] [PubMed] [Google Scholar]
- 52.Powell AM, Chan WY, Alvin P, Litt IF. Menstrual-PGF2 alpha, PGE2 and TXA2 in normal and dysmenorrheic women and their temporal relationship to dysmenorrhea. Prostaglandins 1985; 29: 273–290. [DOI] [PubMed] [Google Scholar]
- 53.Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci 2015; 9: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]