Abstract
Tyrosol is extensively used in the pharmaceutical industry as an important natural product from plants. In this study, an exogenous pathway involved in catalyzing tyrosine to tyrosol was introduced into Saccharomyces cerevisiae. Furthermore, The pyruvate decarboxylase gene pdc1 was deleted to redirect the flux distribution at the pyruvate node, and a bifunctional NAD+-dependent fused chorismate mutase/prephenate dehydrogenase from E. coli (EcTyrA) and its' tyrosine inhibition resistant mutant (EcTyrAM53I/A354V) were heterologously expression in S. cerevisiae to tuning up the chorismate metabolism effectively directed the metabolic flux toward tyrosol production. Finally, the tyrosol yield of the engineered strain GFT-4 was improved to 126.74 ± 6.70 mg/g DCW at 48 h, increased 440 times compared with that of the control strain GFT-0 (0.28 ± 0.01 mg/g DCW). The new synergetic engineering strategy developed in this study can be further applied to increase the production of high value-added aromatic compounds derived from aromatic amino acid or shikimate in S. cerevisiae.
Keywords: tyrosol, Ehrlich pathway, shikimate pathway, chorismate, Saccharomyces cerevisiae
Introduction
Tyrosol is a phenethyl alcohol derivative known to have antioxidant and anti-inflammatory effects (Kim et al., 2017). Tyrosol is widely found in many traditional fermented foods such as wines (Lafka et al., 2007) and virgin olive oil (Celano et al., 2018). Tyrosol is an important pharmaceutical intermediate and can be used as a precursor of salidroside (Ma et al., 2012), icariside D2 (Liao et al., 2018; Torrens-Spence et al., 2018), and hydroxytyrosol (Allouche and Sayadi, 2005; Li et al., 2018) in the pharmaceutical industry. These drugs have activities against cardiovascular disease (Granados-Principal et al., 2010; Hu et al., 2014), cancer (Hu et al., 2010; Liu et al., 2012), and viruses (Wang et al., 2009). There are three industrial methods used to produce tyrosol. The first method is extracting tyrosol from a variety of natural plants such as olive and Rhodiola rosea. The second method is chemical synthesis, such as producing tyrosol with the toxic phenylethyl alcohol and aromatic amine that is high-cost and not environmentally friendly (Henry et al., 1950; Yamada and Fujii, 1963). The third method is biosynthesizing tyrosol in microorganisms from sugars (Bai et al., 2014; Jiang et al., 2018). In recent years, the biosynthesis of tyrosol has received increasing attention attributed to it being low-cost, effective for production, and environmentally friendly. Thus, producing tyrosol via biosynthetic pathways in microorganisms is sustainable and has good prospects for development.
There are two main natural pathways to biosynthesize tyrosol: the Ehrlich pathway in microbes and the tyrosol synthesis pathway in plants (Jiang et al., 2018; Torrens-Spence et al., 2018). In the Ehrlich pathway of Saccharomyces cerevisiae, phenylpyruvate decarboxylase ARO10 catalyzes the conversion of 4-hydroxyphenylpyruvate (4HPP) to 4-hydroxyphenylacetaldehyde (4HPAA), followed by the reduction of 4HPAA to tyrosol by alcohol dehydrogenases (ADHs) (Sentheshanmuganathan and Elsden, 1958). In the plant pathway, tyrosine is converted into 4HPAA by aromatic aldehyde synthase (AAS) (Kaminaga et al., 2006; Torrens-Spence et al., 2012, 2013), followed by the reduction of 4HPAA to tyrosol catalyzed by ADHs.
Compared to plants, producing tyrosol by microorganisms is preferable, as microorganisms are fast-growing and utilize cheap and widely available carbon sources. Recently, scientists have attempted to biosynthesize tyrosol in E. coli (Satoh et al., 2012; Bai et al., 2014; Chung et al., 2017; Xue et al., 2017). However, compared to E. coli, S. cerevisiae is a robust, endotoxin-free microbial host strain with a long history of use in food and pharmaceutical fermentation, and has a Generally Recognized as Safe (GRAS) designation from the U.S. Food and Drug Administration (FDA). Furthermore, tyrosol is a natural substance occurring in S. cerevisiae fermentation, which can be biosynthesized via the Ehrlich pathway. In S. cerevisiae, phosphoenolpyruvate (PEP) derived from glycolysis and erythrose-4-phosphate (E4P) derived from the pentose phosphate pathway (PPP) are two substrates of shikimate pathway (Suástegui et al., 2016). Chorismate is the common metabolic precursor for the production of aromatic amino acids and their derivatives, and can be further converted into tyrosol via chorismate metabolism and Ehrlich pathway. It has been reported that overexpression of the prephenate dehydrogenase TYR1, the tyrosine feedback-resistant 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) synthase ARO4K229L, the tyrosine feedback-resistant chorismate mutase ARO7G141S and shikimate kinase AROL involved in the catalytic reactions of shikimate improve the yields of tyrosine and tyrosol (Gold et al., 2015; Jiang et al., 2018).
Currently, there are only a few reports of biosynthesizing tyrosol in S. cerevisiae (Jiang et al., 2018; Torrens-Spence et al., 2018). It is necessary to develop more engineering strategies to further improve the production of tyrosol in S. cerevisiae and expand the biosynthesis of tyrosol from the lab-scale to the industrial scale.
In this study, we modified three modules on the pathway of tyrosol biosynthesis in S. cerevisiae. First, the Ehrlich Pathway was rewired by introducing the exogenous pathway for directly converting tyrosine to tyrosol; second, pdc1 was disrupted to reduce the carbon flux below the pyruvate node; third, the upstream pathway of the chorismate metabolism was enhanced. Ultimately, a 440-fold increase in tyrosol yield was obtained compared to the strain harboring empty vector.
Materials and Methods
Strains and Culture Conditions
The S. cerevisiae laboratory strain BY4741 was used as a parent strain in this study. E. coli strain GB05-dir, a CcdB-sensitive strain, was used to construct the recombinant plasmids by Red-ET recombineering (Fu et al., 2012). The wild type S. cerevisiae strain BY4741 was incubated in YPD medium containing 10 g/L yeast extract, 20 g/L tryptone, and 20 g/L glucose. All strains engineered from the parent strain BY4741 were incubated in synthetic complete drop-out medium lacking uracil (SC-Ura) containing 1.77 g/L yeast nitrogen base without amino acids, 1.29 g/L DO supplement-Ura, and 20 g/L glucose as the sole carbon source. Yeast nitrogen base without amino acids was purchased from Solarbio (Beijing, China), DO supplement-Ura was purchased from Coolaber (Beijing, China), and other chemicals were purchased from Sinopharm Chemical Reagent Co, Ltd (Shanghai, China). All engineered S. cerevisiae strains were cultured in 300 mL shaking flasks containing 50 mL SC-Ura medium at 30°C with constant orbital shaking at 200 rpm.
Construction and Transformation of Plasmids and Linearized Expression Cassettes
The primers used in this study were listed in Supplementary Table 3. All the promoters and terminators were amplified from S. cerevisiae BY4741 genomic DNA. The codon-optimized alcohol dehydrogenase (GenBank: WP_015958513.1) gene from E. coli (EcADHsyn) was synthesized and ligated with the TEF1 promoter and PGK1 terminator via fusion PCR, resulting in the fragment PTEF1-EcADHsyn-TPGK1. The codon-optimized AAS (GenBank: AAA33860.1) gene from Petroselinum crispum (PcAASsyn) was synthesized and ligated with the TDH3 promoter and CYC1 terminator via fusion PCR, resulting in the fragment PTDH3-PcAASsyn-TCYC1. The fragments PTDH3-PcAASsyn-TCYC1 and PTEF1-EcADHsyn- TPGK1 were inserted into plasmid pJFE3 by Red-ET recombineering, generating the recombinant plasmid pT1. The pdc1 upstream and pdc1 downstream fragments were amplified using the genomic DNA of S. cerevisiae BY4741 as a template. The linearized expression cassette pT2 was constructed by ligating the fragments pdc1 upstream (500 bp), kanMX, and pdc1 downstream (500 bp) via fusion PCR. The fragment EcTyrA-TCYC1was constructed by ligating chorismate mutase/prephenate dehydrogenase (Genbank: WP_103849370.1) gene sequence amplified from the E. coli genome and CYC1 terminator via fusion PCR. The fragment EcTyrA-TCYC1 was inserted into pT2 under the PDC1 promoter, resulting in the EcTyrA expression cassette pT3. The EcTyrAM53I/A354V expression cassette pT4 was constructed based on pT3 using PCR-based site-directed mutagenesis. All the plasmids and linearized expression cassettes used or constructed in this study were listed in Supplementary Table 1. The plasmids and linearized expression cassettes were sequenced before transforming them into S. cerevisiae BY4741 via lithium acetate-mediated transformation (Schiestl and Gietz, 1989; Gietz et al., 1995). All of the strains used and constructed in this study were listed in Supplementary Table 2.
Measurement of Cell Growth by Optical Density and Dry Cell Weight
The cell growth of S. cerevisiae was determined by measuring the OD600 value with a UV/VIS spectrophotometer (2802 UV/VIS Spectrophotometer, Unico Inc., USA). Cell concentration was calculated from a standard curve relating OD600 to dry cell weight (1 OD600 = 0.2959 g DCW/L).
The Identification and Quantification of Tyrosol
The fermentation broth of S. cerevisiae was centrifuged at 10,732.8 × g for 2 min at 4°C, and then filtered with a 0.22 μm organic phase membrane. The standard tyrosol was purchased from Sigma-Aldrich (St. Louis, USA). Tyrosol concentration in the supernatant and standard tyrosol were analyzed and quantified by the Hitachi Elite LaChrom HPLC System (HITACHI, Japan) equipped with a C18 reverse-phase chromatography column (InertSustain C18 column, 250 × 4.6 mm, 5 μm) and Diode Array Detector. The HPLC conditions were according to a previous study (Satoh et al., 2012). The retention time of tyrosol was 19.05 min in the HPLC chromatogram. High-performance liquid chromatography-mass spectrometry (HPLC-MS) was performed on a Thermo Scientific Dionex UltiMate 3000 LC system and a Maxis Impact HD Mass Spectrometer with an ESI ionization probe. The positive mode was used to detect tyrosol in the mass range of 50–700 m/z.
Results
Design of Rational Metabolic Engineering to Increase the Tyrosol Production in S. cerevisiae
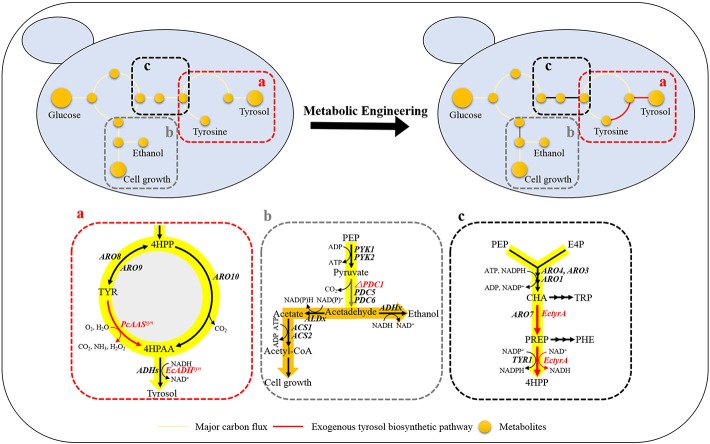
Tyrosol can be biosynthesized natively in S. cerevisiae via the endogenous pathway. The laboratory S. cerevisiae strain BY4741 was transformed with empty plasmid pJFE3, generating the control strain GFT-0. After 48 h of cultivation of GFT-0, 0.28 ± 0.01 mg/g DCW of tyrosol was detected in the medium (Figure 2). To improve the tyrosol yield of S. cerevisiae, we modified three modules to reprogram S. cerevisiae (Figure 1). First, the Ehrlich pathway was rewired (Figure 1a). Second, pdc1 in S. cerevisiae was disrupted to redirect the flux distribution at the pyruvate node (Figure 1b). Finally, the upstream pathway of the chorismate metabolism was engineered (Figure 1c).
Figure 2.
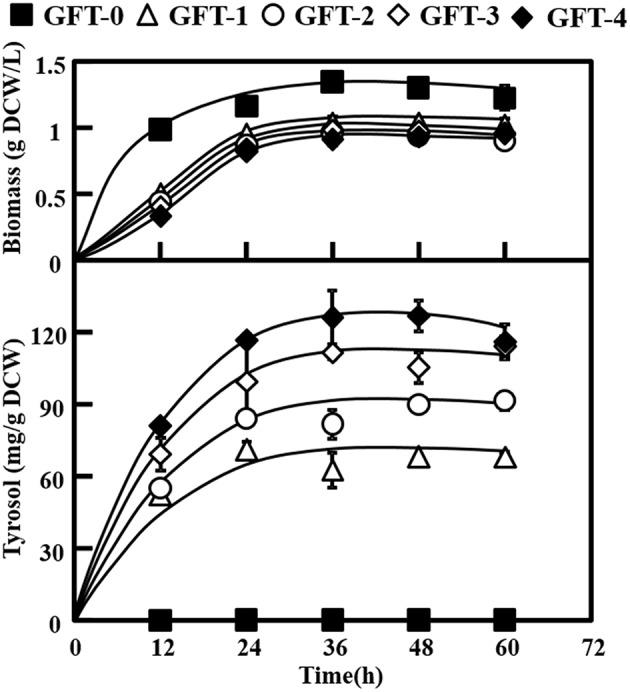
Effects of metabolic engineering of Saccharomyces cerevisiae on tyrosol and biomass. The engineered strains (GFT-1, GFT-2, GFT-3, and GFT-4) and control strain harboring the empty vector pJFE3 (GFT-0) were cultivated in SC-Ura medium with 2% glucose for 48 h. All experiments were performed using three biological replicates.
Figure 1.
Design of rational metabolic engineering to enhance tyrosol production in S. cerevisiae. Metabolites: 4HPP, 4-hydroxyphenylpyruvate; TYR, tyrosine; 4HPAA, 4-hydroxyphenylacetaldehyde; PEP, phosphoenolpyruvate; Acetyl-CoA, acetyl coenzyme A; E4P, erythrose-4-phosphate; CHA, chorismate; PREP, prephenate; TRP, tryptophan; and PHE, phenylalanine. Genes: aminotransferase gene (ARO8 and ARO9); phenylpyruvate decarboxylase gene(ARO10); pyruvate kinase gene (PYK1 and PYK2); pyruvate decarboxylase genes (PDC1, PDC5, and PDC6); chorismate mutase gene (ARO7); 3-deoxy-7-phosphoheptulonate synthase (ARO4 and ARO3); pentafunctional AROM polypeptide (ARO1); the NADP+-dependent prephenate dehydrogenase gene (TYR1); alcohol dehydrogenase genes (ADHs). The codon-optimized aromatic aldehyde synthase gene from Petroselinum crispum (PcAASsyn) and the NADH-dependent aryl-alcohol dehydrogenase gene from E. coli (EcADHsyn) were heterologously expressed in S. cerevisiae to convert tyrosine to 4HPAA (a), the native pyruvate decarboxylase gene PDC1 was knocked out for tuning down the carbon flux below the pyruvate node (b), the NAD+-dependent fused chorismate mutase/prephenate dehydrogenase gene from Escherichia coli (EctyrA) was expressed heterologously in S. cerevisiae to enhance choristmate metabolism (c).
Rewiring the Ehrlich Pathway in S. cerevisiae
The plasmid pT1 was transformed into S. cerevisiae BY4741 strain, resulting in strain GFT-1. Differing from the control strain GFT-0 with an empty plasmid pJFE3, tyrosine was converted into tyrosol directly catalyzed by PcAAS and the step from 4HPAA into tyrosol was strengthened in strain GFT-1 (Figure 1a).
As shown in Figure 2, strain GFT-1 produced 67.87 ± 0.60 mg/g DCW tyrosol after 48 h of shake flask cultivation, a 236-fold improvement compared to strain GFT-0. Thus, the introduction of the artificial PcAASsyn-EcADHsyn pathway greatly increased the tyrosol yield of S. cerevisiae.
Disrupting pdc1 in S. cerevisiae
We aimed to disrupt the pyruvate decarboxylase gene pdc1 to reduce the flux distribution at the pyruvate node and redirect the metabolic flux from PEP to the desired product tyrosol rather than toward ethanol and cell growth (Figure 1b). PDC1 was one of the main enzymes of the three pyruvate decarboxylases (PDC1, PDC5, and PDC6) (Wang et al., 2015). Therefore, pdc1 in strain GFT-1 was disrupted, generating strain GFT-2. The tyrosol yield of strain GFT-2 reached 89.92 ± 1.48 mg/g DCW after 48 h of shake flask cultivation, and was significantly improved by 32.49%, compared to that of strain GFT-1 (Figure 2). Meanwhile, there was no obvious change between the biomass of strains GFT-1 and GFT-2 (Figure 2) and no changes was observed on ethanol production (Data not shown). These results proved that disruption of pdc1 in S. cerevisiae increased the tyrosol production and has no significant influence on the ethanol fermentation and the cell growth.
Engineering the Upstream Pathway of the Chorismate Metabolism in S. cerevisiae
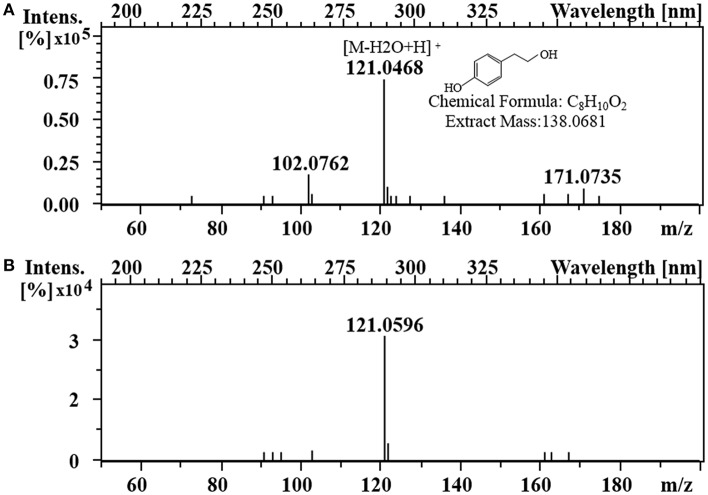
To enhance the upstream pathway of chorismate metabolism, a bifunctional chorismate mutase/ prephenate dehydrogenase gene from E. coli (EcTyrA) was heterologously expressed in S. cerevisiae (Figure 1c). The NAD+-dependent EcTyrA catalyzes the rearrangement of chorismate to prephenate (PREP) as well as the oxidative decarboxylation of PREP to 4 HPP, and EcTyrAM53I/A354V was resistant to tyrosine inhibition (Lütke-Eversloh and Stephanopoulos, 2005; Lutke-Eversloh and Stephanopoulos, 2007). Therefore, the TyrA from E. coli (EcTyrA) or its' tyrosine-insensitive variant (EcTyrAM53I/A354V) was inserted into the pdc1 site in strain GFT-1 to enhance chorismate metabolism, generating the strain GFT-3 and GFT-4. The tyrosol yield of the strain GFT-3 and GFT-4 reached 105.13 ± 6.28 mg/g DCW and 126.74 ± 6.70 mg/g DCW after 48 h of shake flask cultivation, and were improved by 16.91 and 40.94%, respectively, compared to that of strain GFT-2 (Figure 2). However, there was no significant change in biomass among strains GFT-2, GFT-3, and GFT-4 (Figure 2). These results suggested that heterologously expressing EcTyrA or EcTyrAM53I/A354V in S. cerevisiae could enhance the upstream of chorismate metabolism and improve the tyrosol production. Finally, HPLC-MS was applied to identify the tyrosol in the fermentation broth, and the peak had a molecular ion at 121.0468 ([M-H2O+H]+) was corresponding to the standard tyrosol (m/z = 121.0596) (Figure 3).
Figure 3.
Identification of tyrosol. LC-MS analysis of standard tyrosol (A) and fermentation supernatant extracts (B).
Discussion
S. cerevisiae is a commonly used industrial microorganism and a promising cell factory for the production of valuable natural products. Though S. cerevisiae produces tyrosol naturally with the Ehrlich pathway, the yield of tyrosol is rather low. In this study, we modified three modules involved in tyrosol biosynthesis to effectively improve the tyrosol production of S. cerevisiae (Figure 1).
In the Ehrlich pathway of S. cerevisiae, 4-hydroxyphenylpyruvate (4HPP) is an important intermediate, which is not only directly converted into 4HPAA but also is reversibly converted into tyrosine, and the flux distribution at the 4HPP node is not beneficial for the production of tyrosol. The competitive byproduct tyrosine from the 4HPP node could not be converted into tyrosol with the endogenous Ehrlich pathway in S. cerevisiae. Moreover, DAHP synthase ARO4 and chorismate mutase ARO7 play important roles in amino acid metabolism and both are strongly feedback inhibited by tyrosine accumulated in S. cerevisiae (Gold et al., 2015). In this study, the Ehrlich pathway in GFT-1 was rewired by introducing PcAAS that could convert tyrosine into 4HPAA, and then 4HPAA was synthesized via the artificial pathway and native tyrosol biosynthetic pathway simultaneously (Figure 1a). As shown in Figure 2, tyrosol yield of GFT-1 was greatly improved, compared to strain GFT-0 with empty pJFE3 vector. The result are consistent with those of others (Jiang et al., 2018; Torrens-Spence et al., 2018).
It was pointed out that competing fermentation pathways in engineered hosts should be eliminated to increase the conversion rate of feedstock to product (Aslan et al., 2017). PEP is a key central metabolism intermediate involved in glycolysis in S. cerevisiae and one of the precursors of shikimate pathway and pyruvate metabolism. The major carbon flux from the PEP node was directed to pyruvate and then used for cell growth and ethanol fermentation, whereas the minor proportion went to the shikimate pathway followed by the aromatic acids biosynthesis pathway (Gottardi et al., 2017). In S. cerevisiae, there are three main pyruvate decarboxylase genes (pdc1, pdc5, and pdc6). It was proved that the yeast cannot grow on medium with glucose as the single carbon source after disrupting all pdc1, pdc5, and pdc6 (Flikweert et al., 1996). It was reported that disruption of pdc1 caused a 30% decrease in pyruvate decarboxylase activity (Wang et al., 2015), and, on the contrary, enhanced the expression level of pdc5 and respiratory metabolism (Eberhardt et al., 1999; Gottardi et al., 2017). In this study, pdc1 in S. cerevisiae was disrupted and the tyrosol production of strain GFT-2 was significantly improved by 32.50% (Figure 2). We speculated that two reasons contribute to the improvement of tyrosol production in S. cerevisiae: (1) Disruption of pdc1 led to a stronger respiratory metabolism that improved the ATP amount. Finally, the availability of PEP to shikimate pathway was increased owe to the increase of the ATP amount (Gottardi et al., 2017). (2) Disruption of pdc1 resulted in the increase of expression level of pdc5, and PDC5 could compensate for the PDC activity of S. cerevisiae pdc1Δ strain (Wang et al., 2015). Differed from PDC1 and PDC6, PDC5 had the same catalytic ability as well as ARO10 that decarboxylated the aromatic substrate phenylpyruvate into phenylethanol (Romagnoli et al., 2012). Thus, we speculated that higher expression of pdc5 might improve the conversion from 4HPP to 4HPAA. Our work suggested that disruption of pdc1 in S. cerevisiae was a suitable strategy to improve tyrosol production.
Chorismate, which is a major branch point intermediate metabolite in biosynthesis pathways of aromatic acids and phenolic compounds, is synthesized by the shikimate pathway (Thompson et al., 2015). In S. cerevisiae, chorismate is consecutively converted by chorismate mutase ARO7 and prephenate dehydrogenase TYR1 to 4HPP via PREP (Rodriguez et al., 2015). The activity of ARO7 was allosterically inhibited by tyrosine and the conversion from PREP to 4HPP catalyzed by TYR1, which was reported as a bottleneck of the L-tyrosine synthetic pathway in S. cerevisiae (Mao et al., 2017). TyrA from E. coli (EcTyrA) was a bifunctional NAD+-dependent fused chorismate mutase/prephenate dehydrogenase (Bhosale et al., 1982) and overexpression of TyrA improved tyrosine production in E. coli (Lutke-Eversloh and Stephanopoulos, 2007). Furthermore, EcTyrA was reported to be a homodimer consisted of CM domain and PDH domain, and was regulated by the feedback inhibition of tyrosine. The variant EcTyrAM53I/A354V was resistant to the inhibition of tyrosine (Lütke-Eversloh and Stephanopoulos, 2005). In this study, the genes EcTyrA or its' variant—EcTyrAM53I/A354V was inserted into the pdc1 site in strain GFT-2 and tyrosol production of strain GFT-3 and GFT-4 were improved by 16.91 and 40.94%, respectively, compared to that of strain GFT-2. Moreover, the improvement of tyrosol yield with heterologously expressing EcTyrAM53I/A354V in S. cerevisiae was higher than that of EcTyrA (Figure 2). We hypothesized that chorismate metabolism followed by the Ehrlich pathway might be strengthened and the inhibition from tyrosine was alleviated by expressing EcTyrAM53I/A354V in S. cerevisiae.
Differing from previous work by Jiang et al. (2018), our study showed that the tyrosol yield was significantly improved up to 86.74% owe to the introduction of EcTyrAM53I/A354V and the disruption of pdc1 simultaneously based on the expression of PcAASsyn (Figure 2).
In this study, it was proved that the engineering S. cerevisiae with the modification of three modules was more suitable for tyrosol production. The new engineering strategy could also be further applied to enhance the production of aromatic amino acids and their derivatives in S. cerevisiae.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
WG, QH, SH, and SN performed experiments. WG and XF wrote the manuscript and conceived the study. WG, HL, YJ, XB, YS, and XF were involved in analysis and interpretation of experimental data. XF and HL coordinated the project.
Conflict of Interest Statement
SH was employed by company Shandong Henglu Biological Technology Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was financially supported by the Major Program of Shandong Province Natural Science Foundation (No. ZR2018ZB0209), the Foundation of Key Laboratory of Industrial Fermentation Microbiology of the Ministry of Education and the Tianjin Key Lab of Industrial Microbiology (Tianjin University of Science & Technology) (No. 2018KF003), the Key Technologies R&D Program of Shandong Province (No. 2018GSF121021), the 111 Project (No. B16030), and the National Natural Science Foundation of China (No. 31570040 and No. 31870785).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2019.00152/full#supplementary-material
References
- Allouche N., Sayadi S. (2005). Synthesis of hydroxytyrosol, 2-hydroxyphenylacetic acid, and 3-hydroxyphenylacetic acid by differential conversion of tyrosol isomers using Serratia marcescens strain. J. Agric. Food Chem. 53, 6525–6530. 10.1021/jf050972w [DOI] [PubMed] [Google Scholar]
- Aslan S., Noor E., Bar-Even A. (2017). Holistic bioengineering: rewiring central metabolism for enhanced bioproduction. Biochem. J. 474, 3935–3950. 10.1042/BCJ20170377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Bi H., Zhuang Y., Liu C., Cai T., Liu X., et al. (2014). Production of salidroside in metabolically engineered Escherichia coli. Sci. Rep. 4:6640. 10.1038/srep06640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale S. B., Rood J. I., Sneddon M. K., Morrison J. F. (1982). Production of chorismate mutase-prephenate dehydrogenase by a strain of Escherichia coli carrying a multicopy, TyrA plasmid. Isolation and properties of the enzyme. Biochim. Biophys. Acta 717, 6–11. 10.1016/0304-4165(82)90372-5 [DOI] [PubMed] [Google Scholar]
- Celano R., Piccinelli A. L., Pugliese A., Carabetta S., di Sanzo R., Rastrelli L., et al. (2018). Insights into the analysis of phenolic secoiridoids in extra virgin olive oil. J. Agric. Food. Chem. 66, 6053–6063. 10.1021/acs.jafc.8b01751 [DOI] [PubMed] [Google Scholar]
- Chung D., Kim S. Y., Ahn J. H. (2017). Production of three phenylethanoids, tyrosol, hydroxytyrosol, and salidroside, using plant genes expressing in Escherichia coli. Sci. Rep. 7:2578. 10.1038/s41598-017-02042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt I., Cederberg H., Li H., König S., Jordan F., Hohmann S. (1999). Autoregulation of yeast pyruvate decarboxylase gene expression requires the enzyme but not its catalytic activity. Eur. J. Biochem. 262, 191–201. 10.1046/j.1432-1327.1999.00370.x [DOI] [PubMed] [Google Scholar]
- Flikweert M. T., Van Der Zanden L., Janssen W. M., Steensma H. Y., Van Dijken J. P., Pronk J. T. (1996). Pyruvate decarboxylase: an indispensable enzyme for growth of Saccharomyces cerevisiae on glucose. Yeast 12, 247–257. [DOI] [PubMed] [Google Scholar]
- Fu J., Bian X. Y., Hu S. B., Wang H. L., Huang F., Seibert P. M., et al. (2012). Full-length RecE enhances linear-linear homologous recombination and facilitates direct cloning for bioprospecting. Nat. Biotechnol. 30, 440–446. 10.1038/nbt.2183 [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Schiestl R. H., Willems A. R., Woods R. A. (1995). Studies on the transformation of intact yeast-cells by the Liac/S-DNA/Peg procedure. Yeast 11, 355–360. 10.1002/yea.320110408 [DOI] [PubMed] [Google Scholar]
- Gold N. D., Gowen C. M., Lussier F. X., Cautha S. C., Mahadevan R., Martin V. J. (2015). Metabolic engineering of a tyrosine-overproducing yeast platform using targeted metabolomics. Microb. Cell. Fact. 14:73. 10.1186/s12934-015-0252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi M., Reifenrath M., Boles E., Tripp J. (2017). Pathway engineering for the production of heterologous aromatic chemicals and their derivatives in Saccharomyces cerevisiae: bioconversion from glucose. FEMS Yeast Res. 17:fox035. 10.1093/femsyr/fox035 [DOI] [PubMed] [Google Scholar]
- Granados-Principal S., Quiles J. L., Ramirez-Tortosa C. L., Sanchez-Rovira P., Ramirez-Tortosa M. C. (2010). Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr. Rev. 68, 191–206. 10.1111/j.1753-4887.2010.00278.x [DOI] [PubMed] [Google Scholar]
- Henry M., Calvin W., Stuntz F. (1950). The synthesis of β-(3-amino-4-hydroxyphenyl)-ethanol; 3-aminotyrosol. J. Am. Chem. Soc. 72, 1361–1364. 10.1021/ja01159a077 [DOI] [Google Scholar]
- Hu T., He X. W., Jiang J. G., Xu X. L. (2014). Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food. Chem. 62, 1449–1455. 10.1021/jf405820v [DOI] [PubMed] [Google Scholar]
- Hu X. L., Lin S. X., Yu D. H., Qiu S. F., Zhang X. Q., Mei R. H. (2010). A preliminary study: the anti-proliferation effect of salidroside on different human cancer cell lines. Cell. Biol. Toxicol. 26, 499–507. 10.1007/s10565-010-9159-1 [DOI] [PubMed] [Google Scholar]
- Jiang J. J., Yin H., Wang S., Zhuang Y., Liu S. W., Liu T., et al. (2018). Metabolic engineering of Saccharomyces cerevisiae for high-level production of salidroside from glucose. J. Agric. Food. Chem. 66, 4431–4438. 10.1021/acs.jafc.8b01272 [DOI] [PubMed] [Google Scholar]
- Kaminaga Y., Schnepp J., Peel G., Kish C. M., Ben-Nissan G., Weiss D., et al. (2006). Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation. J. Biol. Chem. 281, 23357–23366. 10.1074/jbc.M602708200 [DOI] [PubMed] [Google Scholar]
- Kim Y. Y., Lee S., Kim M. J., Kang B. C., Dhakal H., Choi Y. A., et al. (2017). Tyrosol attenuates lipopolysaccharide-induced acute lung injury by inhibiting the inflammatory response and maintaining the alveolar capillary barrier. Food. Chem. Toxicol. 109, 526–533. 10.1016/j.fct.2017.09.053 [DOI] [PubMed] [Google Scholar]
- Lafka T. I., Sinanoglou V., Lazos E. S. (2007). On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food. Chem. 104, 1206–1214. 10.1016/j.foodchem.2007.01.068 [DOI] [Google Scholar]
- Li X. L., Chen Z. Y., Wu Y. F., Yan Y. J, Sun X. X., Yuan Q. P. (2018). Establishing an artificial pathway for efficient biosynthesis of hydroxytyrosol. ACS. Synth. Biol. 7, 647–654. 10.1021/acssynbio.7b00385 [DOI] [PubMed] [Google Scholar]
- Liao Z. H., Qiu F., Zeng J. L., Gu L., Wang B. J., Lan X. Z., et al. (2018). A novel UDP-glycosyltransferase of Rhodiola crenulata converts tyrosol to specifically produce icariside D2. Biomed. Res. Int. 2018:7970590. 10.1155/2018/7970590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. B., Li X. S., Simoneau A. R., Jafari M., Zi X. L. (2012). Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol. Carcinog. 51, 257–267. 10.1002/mc.20780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütke-Eversloh T., Stephanopoulos G. (2005). Feedback inhibition of chorismate mutase/prephenate dehydrogenase (TyrA) of Escherichia coli: generation and characterization of tyrosine-insensitive mutants. Appl. Environ. Microbiol. 71, 7224–7228. 10.1128/AEM.71.11.7224-7228.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutke-Eversloh T., Stephanopoulos G. (2007). L-tyrosine production by deregulated strains of Escherichia coli. Appl. Microbiol. Biotechnol. 75, 103–110. 10.1007/s00253-006-0792-9 [DOI] [PubMed] [Google Scholar]
- Ma L. Q., Liu C. M., Yu H. S., Zhang J. X., Gao D. Y., Li Y. F., et al. (2012). Salidroside biosynthesis pathway: the initial reaction and glycosylation of tyrosol. Sheng Wu Gong Cheng Xue Bao 28, 282–294. [PubMed] [Google Scholar]
- Mao J. W., Liu Q. L., Song X. F., Wang H. S., Feng Y. H., Xu H. J., et al. (2017). Combinatorial analysis of enzymatic bottlenecks of L-tyrosine pathway by p-coumaric acid production in Saccharomyces cerevisiae. Biotechnol. Lett. 39, 977–982. 10.1007/s10529-017-2322-5 [DOI] [PubMed] [Google Scholar]
- Rodriguez A., Kildegaard K. R., Li M. J., Borodina I., Nielsen J. (2015). Establishment of a yeast platform strain for production of p-coumaric acid through metabolic engineering of aromatic amino acid biosynthesis. Metab. Eng. 31, 181–188. 10.1016/j.ymben.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Romagnoli G., Luttik M. A., Kötter P., Pronk J. T., Daran J. M. (2012). Substrate specificity of thiamine pyrophosphate-dependent 2-Oxo-Acid decarboxylases in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 78, 7538–7548. 10.1128/AEM.01675-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh Y., Tajima K., Munekata M., Keasling J. D., Lee T. S. (2012). Engineering of a tyrosol-producing pathway, utilizing simple sugar and the central metabolic tyrosine, in Escherichia coli. J. Agric. Food. Chem. 60, 979–984. 10.1021/jf203256f [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. (1989). High-efficiency transformation of intact yeast-cells using single stranded nucleic-acids as a carrier. Curr. Genet. 16, 339–346. 10.1007/BF00340712 [DOI] [PubMed] [Google Scholar]
- Sentheshanmuganathan S., Elsden S. R. (1958). The mechanism of the formation of tyrosol by Saccharomyces cerevisiae. Biochem. J. 69, 210–218. 10.1042/bj0690210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Chen X., Peng B. Y., Chen L. Y., Hou J., Bao X. M. (2012). An efficient xylose-fermenting recombinant Saccharomyces cerevisiae strain obtained through adaptive evolution and its global transcription profile. Appl. Microbiol. Biotechnol. 96, 1079–1091. 10.1007/s00253-012-4418-0 [DOI] [PubMed] [Google Scholar]
- Suástegui M., Guo W., Feng X., Shao Z. (2016). Investigating strain dependency in the production of aromatic compounds in Saccharomyces cerevisiae. Biotechnol. Bioeng. 113, 2676–2685. 10.1002/bit.26037 [DOI] [PubMed] [Google Scholar]
- Thompson B., Machas M., Nielsen D. R. (2015). Creating pathways towards aromatic building blocks and fine chemicals. Curr. Opin. Biotechnol. 36, 1–7. 10.1016/j.copbio.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Torrens-Spence M. P., Gillaspy G., Zhao B., Harich K., White R. H., Li J. (2012). Biochemical evaluation of a parsley tyrosine decarboxylase results in a novel 4-hydroxyphenylacetaldehyde synthase enzyme. Biochem. Biophys. Res. Commun. 418, 211–216. 10.1016/j.bbrc.2011.12.124 [DOI] [PubMed] [Google Scholar]
- Torrens-Spence M. P., Liu P., Ding H., Harich K., Gillaspy G., Li J. (2013). Biochemical evaluation of the decarboxylation and decarboxylation-deamination activities of plant aromatic amino acid decarboxylases. J. Biol. Chem. 288, 2376–2387. 10.1074/jbc.M112.401752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens-Spence M. P., Pluskal T., Li F. S., Carballo V., Weng J. K. (2018). Complete pathway elucidation and heterologous reconstitution of Rhodiola salidroside biosynthesis. Mol. Plant. 11, 205–217. 10.1016/j.molp.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Wang D. P., Wang L., Hou L., Deng X. H., Gao Q., Gao N. F. (2015). Metabolic engineering of Saccharomyces cerevisiae for accumulating pyruvic acid. Ann. Microbiol. 65, 2323–2331. 10.1007/s13213-015-1074-5 [DOI] [Google Scholar]
- Wang H. B., Ding Y. Y., Zhou J., Sun X. L., Wang S. W. (2009). The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomedicine 16, 146–155. 10.1016/j.phymed.2008.07.013 [DOI] [PubMed] [Google Scholar]
- Xue Y. X., Chen X. Z., Yang C., Chang J. Z., Shen W., Fan Y. (2017). Engineering Eschericha coli for enhanced tyrosol production. J. Agric. Food. Chem. 65, 4708–4714. 10.1021/acs.jafc.7b01369 [DOI] [PubMed] [Google Scholar]
- Yamada S., Fujii T. (1963). Preperation of tyrosol and 4-methoxyphenyl alcohol. Chem. Pharm. Bull. 11, 258–260. 10.1248/cpb.11.258 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.