Abstract
Late recurrence of estrogen receptor (ER) positive breast cancer is common. When tissues from a recurrent or metastatic focus are available, re-evaluation of ER, progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) status is recommended for treatment selection. This case report describes a 59-year-old woman who underwent surgery for left breast cancer, with a histopathological diagnosis of invasive ductal carcinoma (pathological stage T2N1aM0 Stage IIB, ER positive, PgR positive and HER2 negative). A health check-up 16 years after surgery revealed multiple hepatic mass lesions, and the patient was referred to our hospital for tests. Based on computed tomography, intrahepatic bile duct cancer or metastatic hepatic tumors were suspected, and a liver biopsy was performed. The histopathological diagnosis was a poorly differentiated adenocarcinoma (ER negative, PgR negative and HER2 positive), and the distinction from poorly differentiated intrahepatic bile duct cancer was difficult. Fluorodeoxyglucose (FDG)-positron emission tomography revealed FDG accumulation in the patient's bones and soft tissues, in addition to the hepatic tumors. The patterns and finding of metastasis were compatible with breast cancer recurrence, and the patient was diagnosed with postoperative recurrence of left breast cancer. Pertuzumab, trastuzumab, and docetaxel were started, and the therapeutic effect was assessed as a partial response. It was evident that in this case, the expression of hormone receptors and HER2 differed between the primary focus and the recurrence foci, and this contributed to the treatment strategy. Whenever possible, a biopsy should be performed for lesions that are suspected to be distal metastases.
Keywords: Breast cancer, Late recurrence, Heterogeneity, HER2
Introduction
According to the Early Breast Cancer Trialists' Collaborative Group's (EBCTCG) data regarding patients who are lymph node positive (N1–3), the late recurrence of estrogen receptor (ER) positive breast cancer is not rare [1]. The National Comprehensive Cancer Network (NCCN) guidelines [2] and Advanced Breast Cancer (ABC) guidelines [3] recommend the re-evaluation of ER, progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) expression when tissues from the foci of recurrent or metastatic breast cancer are accessible. Receptor conversion is a common finding in breast cancer. However, the positive conversion of HER2 in metastatic foci occurs at comparatively low rates [4]. Here we report the case of a patient with late recurrent breast cancer, in which biopsy of a hepatic tumor revealed liver metastasis with HER2-positive conversion. These findings contributed to the treatment strategy.
Case Report
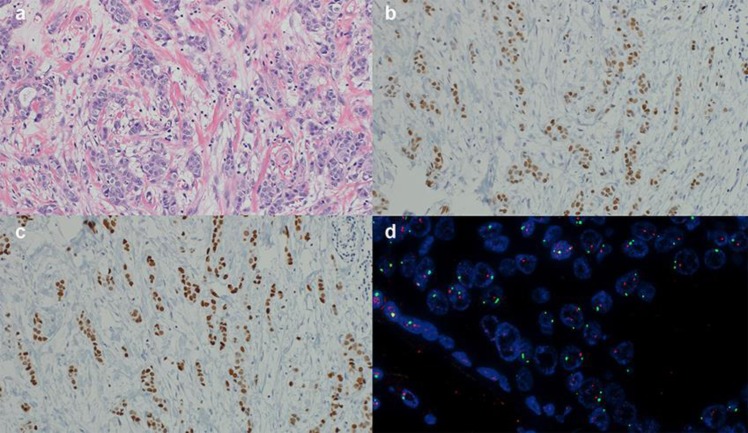
The patient was a 59-year-old woman in whom multiple hepatic masses with a maximum size of 70 mm were identified by abdominal ultrasonography during a health check-up, after which she was referred to our hospital for tests. The patient was diagnosed with left breast cancer 16 years previously at a different hospital. At that time, she had undergone a left mastectomy and axillary lymph node dissection, and her histopathological examination resulted in a diagnosis of invasive ductal carcinoma (ER positive, PgR positive, and HER2 negative; Fig. 1). Subsequently, the patient was administered tegafur plus uracil for two years and tamoxifen for five years.
Fig. 1.
Histopathological findings in the primary focus in the left breast. a: Invasive ductal carcinoma (×20), b: ER positive: ≥90% (×20), c: PgR positive: ≥90% (×20), d: HER2 (FISH) negative: amplification ratio 1.07 (×40). ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; FISH, fluorescence in situhybridization.
When the patient was initially examined at this hospital, her blood biochemistry tests showed elevated levels of hepatobiliary enzymes, with aspartate aminotransferase (AST) at 70 U/L, lactate dehydrogenase (LDH) at 494 U/L, alkaline phosphatase (ALP) at 341 U/L, and gamma-glutamyl transferase (GGT) at 124 U/L and elevated levels of carcinoembryonic antigen (CEA) at 313.2 ng/mL, cancer antigen 15–3 (CA15–3) at 199.8 U/mL, and Nation Cancer Center-Stomach-439 (NCC-ST-439) at 11.4 U/mL.
The computed tomography (CT) scan revealed a poorly defined mass, 70 mm in diameter, in the liver at S5/6. Several peripheral masses that were 5–10 mm in diameter were also observed, without clear biliary dilatation. A metastatic hepatic tumor or intrahepatic bile duct cancer was suspected, and a liver biopsy was performed to make a definitive diagnosis.
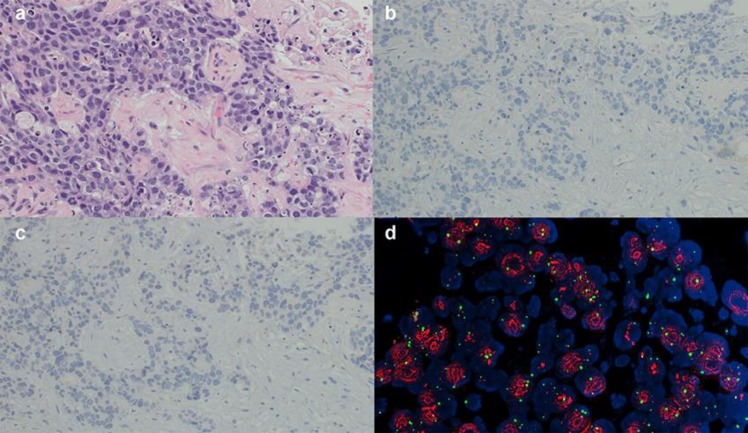
The liver biopsy specimen indicated the presence of solid proliferating tumor cells that formed a glandular structure, resulting in the diagnosis of poorly differentiated adenocarcinoma (Fig. 2a). Immunostaining revealed that the specimen was cytokeratin 7 positive, cytokeratin 20 negative, ER negative, PgR negative, gross cystic disease fluid protein-15 negative, and HER2 (fluorescence in situ hybridization [FISH]) positive (Fig. 2b, c, d). Although there was some resemblance to the previous breast cancer surgical specimen, the distinction of this tumor from poorly differentiated intrahepatic bile duct cancer was difficult.
Fig. 2.
Histopathological findings upon liver biopsy. a: Poorly differentiated adenocarcinoma (×20), b: ER negative (×20), c: PgR negative (×20), d: HER2 (FISH) positive: amplification ratio 4.76 (×40). ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; FISH, fluorescence in situhybridization.
Fluorodeoxyglucose (FDG)-positron emission tomography was performed, revealing an accumulation of FDG in the cervical vertebrae, pectoralis major muscle, internal thoracic lymph nodes, multiple hepatic masses, iliac bone, and femur (Fig. 3a). The pattern of metastases and imaging findings were consistent with breast cancer recurrence and resulted in the diagnosis of post-surgical left breast cancer recurrence, liver metastasis, lymph node metastasis, and bone metastasis.
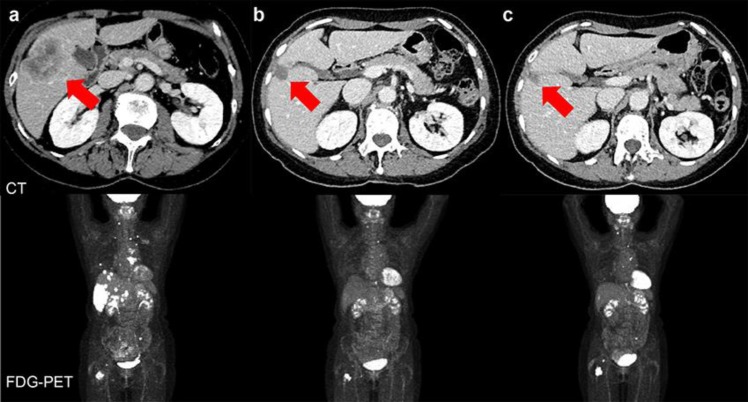
Fig. 3.
CT and FDG-PET findings before and after the start of treatment. a: Before treatment, b: After 6 cycles of treatment, c: After 13 cycles of treatment: Red arrows indicate the tumor in the liver. CT, computed tomography; FDG. PET, fluorodeoxyglucose-positron emission tomography.
A biopsy of the metastatic focus in the liver showed that it was ER negative, PgR negative, and HER2 positive; therefore, combination therapy comprising pertuzumab, trastuzumab, and docetaxel was initiated, which is the recommended first-line therapy for HER2-positive metastases and recurrent breast cancer. After 6 cycles, the CT scan showed that the hepatic tumor had shrunk to 20 mm (71% reduction), which was considered a partial response (PR). The CT after 13 cycles did not show any growth in the metastatic focus in the liver, which was judged to indicate continuing PR (Fig. 3b, c). Tumor markers steadily decreased to normal values during treatment, and although pertuzumab and trastuzumab were continued as maintenance therapy from the seventh cycle, normal values were maintained even after 17 cycles. The adverse events have been mild, and the patient's overall physical condition during treatment has remained good. After 15 months from the start of treatment, her treatment is continuing.
Discussion
According to the EBCTCG's information for patients who are lymph node positive (N1–3), the distant recurrence rate is 10% at 5 years, 19% at 10 years, 25% at 15 years, and 31% at 20 years [1]. The late recurrence of ER positive breast cancer is not rare, and the characteristics of late recurrence are early clinical stage, minimal lymph node metastasis, and predominantly ER-positive tissue. Late recurrence most often occurs as a soft tissue or bone metastasis, and is associated with significantly longer survival than early recurrence [5]. In the present case, the primary focus was hormone receptor-positive (ER and PgR), with metastasis in one axillary lymph node, and the recurrence was also observed in bone and soft tissue, including the pectoralis major muscle and liver, in line with the reported characteristics of late recurrence.
Two hypotheses have been proposed to explain the mechanism of late recurrence. The first is a tumor dormancy theory [6], and the second is a slow growing tumor theory [7]. The fact that patients showed good overall survival rates after late recurrence suggests that both theories are probably true.
Tumor, treatment, and measurement factors can be considered to explain the discordance of ER, PgR, and HER2 statuses between primary and metastatic foci [8, 9, 10, 11, 12]. One tumor factor is the heterogeneity of cancer tissue: the genetic instability of cancer cells or cancer stem cells makes them prone to genetic and epigenetic alterations, which generates heterogeneity. Cancer tissue also contains several genetically different clones, and it has been suggested that the formation of metastatic foci involves clonal selection [8, 11]. Other contributory tumor factors may include the effects of genetic and epigenetic modifications in bone marrow, changes in immunological defenses, the microenvironment, and genetic drift as the result of cancer cell proliferation. Modifications that result from treatment can include changes in protein levels due to chemotherapy, which can decrease HER2, cleave extracellular domains, and eliminate chemotherapy-sensitive tumor cells. Similarly, the reversal of a positive ER status could result from hormone therapy (through phosphatidylinositol-3 kinase/Akt/mammalian target of rapamycin or mitogen- activated protein kinase pathways) or receptor activation (epidermal growth factor receptor, insulin-like growth factor 1 receptor, or functional roles of fibroblast growth factor receptors). Measurement factors could include the assessment of small samples that do not reflect the tissue as a whole (sampling heterogeneity), decreased staining due to poor fixation conditions, instability of immunohistochemical procedures, and measurement bias due to analyses by different pathologists.
According to studies on ER, PgR, and HER2 discordance between the primary focus and metastatic foci, the discordance rate has been reported as 10–18% for ER, 25–40% for PgR [10, 13, 14], and 3–24% for HER2 [10, 13, 14, 15]. In 14–64% of these cases, the treatment strategy was modified based on the discordance [13, 14, 15].
According to a systematic review and meta-analysis for ER, PgR, and HER2, the positive to negative conversion percentages were of 22.5% (95% confidence interval [CI] = 16.4–30%), 49.4% (95% CI = 40.5–58.2%), and 21.3% (95% CI = 14.3–30.5%), respectively. The negative to positive conversion percentages were 21.5% (95% CI = 18.1–25.5%), 15.9% (95% CI = 11.3–22%), and 9.5% (95% CI = 7.4–12.1%), respectively [4]. Receptor conversion is a common finding in breast cancer; however, the positive conversion of HER2 in metastatic foci occurs at comparatively low rates.
Patients in whom ER and HER2 become negative are suggested to have a poorer prognosis than those in whom ER and HER2 become positive [15], implying that a positive conversion to HER2 may be associated with better prognosis. The NCCN [2] and ABC [3] guidelines recommend that ER, PgR, and HER2 should be evaluated and considered during therapy selection. Considering the reproducibility of tests, the heterogeneity of tumors, and the changes in the nature of tumors, and metastatic foci biopsies are also recommended when a long time has passed before the metastasis or recurrence, when ER, PgR, and HER2 were originally tested by another institution, or when the treatment effects are contrary to expectations.
In the present case, a liver biopsy of a hepatic tumor in a patient with a history of breast cancer showed that the expression of hormone receptors and HER2 in the liver metastasis differed from those in the primary tumor, and this contributed to the successful treatment strategy. Confirmation of hormone receptors and HER2 expression in metastatic foci is useful for modifying patient treatment strategies and predicting prognoses; therefore, it is important to biopsy lesions that are suspected to be distal metastases, whenever possible.
Statement of Ethics
Subjects have given their written informed consent to publish their case (including publication of images).
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
Not applicable.
Author Contributions
KM drafted the work. HY, NA, KT, SH and IT substantively revised it. All authors read and approved the final manuscript.
References
- 1.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. EBCTCG 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017 Nov;377((19)):1836–46. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network [Internet] NCCN Guidelines: NCCN Clinical Practice Guidelines in Oncology v4. Breast Cancer. 2018 [cited 2019 Feb 8]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx#site. [Google Scholar]
- 3.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) Ann Oncol. 2018 Aug;29((8)):1634–57. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrijver WA, Suijkerbuijk KP, van Gils CH, van der Wall E, Moelans CB, van Diest PJ. Receptor conversion in distant breast cancer metastases: A systemic review and meta-analysis. J Natl Cancer Inst. 2018 Jun;110((6)):568–80. doi: 10.1093/jnci/djx273. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura R, Osako T, Nishiyama Y, Tashima R, Nakano M, Fujisue M, et al. Evaluation of factors related to late recurrence—later than 10 years after the initial treatment—in primary breast cancer. Oncology. 2013;85((2)):100–10. doi: 10.1159/000353099. [DOI] [PubMed] [Google Scholar]
- 6.Demicheli R, Terenziani M, Valagussa P, Moliterni A, Zambetti M, Bonadonna G. Local recurrences following mastectomy: support for the concept of tumor dormancy. J Natl Cancer Inst. 1994 Jan;86((1)):45–8. doi: 10.1093/jnci/86.1.45. [DOI] [PubMed] [Google Scholar]
- 7.Henneford J, Baserga R, Wartman WB. The time of appearace of metastases after surgical removal of the primary tumor. Br J Cancer. 1962 Dec;16((4)):599–607. doi: 10.1038/bjc.1962.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuukasjärvi T, Karhu R, Tanner M, Kähkönen M, Schäffer A, Nupponen N, et al. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 1997 Apr;57((8)):1597–604. [PubMed] [Google Scholar]
- 9.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. American Society of Clinical Oncology. College of American Pathologists Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013 Nov;31((31)):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 10.Liedtke C, Broglio K, Moulder S, Hsu L, Kau SW, Symmans WF, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009 Dec;20((12)):1953–8. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanton C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 2012 Oct;72((19)):4875–82. doi: 10.1158/0008-5472.CAN-12-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleppe M, Levine RL. Tumor heterogeneity confounds and illuminates: assessing the implications. Nat Med. 2014 Apr;20((4)):342–4. doi: 10.1038/nm.3522. [DOI] [PubMed] [Google Scholar]
- 13.Amir E, Miller N, Geddie W, Freedman O, Kassam F, Simmons C, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012 Feb;30((6)):587–92. doi: 10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto A, Jinno H, Murata T, Seki T, Takahashi M, Hayashida T, et al. Prognostic implications of receptor discordance between primary and recurrent breast cancer. Int J Clin Oncol. 2015 Aug;20((4)):701–8. doi: 10.1007/s10147-014-0759-2. [DOI] [PubMed] [Google Scholar]
- 15.Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012 Feb;30((6)):593–9. doi: 10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]