Abstract
The temporomandibular joint (TMJ) disc nutrient environment profoundly affects cell energy metabolism, proliferation, and biosynthesis. Due to technical challenges of in vivo measurements, the human TMJ disc extracellular nutrient environment under load, which depends on metabolic rates, solute diffusion, and disc morphometry, remains unknown. Therefore, the study objective was to predict the TMJ disc nutrient environment under loading conditions using combined experimental and computational modeling approaches. Specifically, glucose consumption and lactate production rates of porcine TMJ discs were measured under varying tissue culture conditions (n = 40 discs), and mechanical strain-dependent glucose and lactate diffusivities were measured using a custom diffusion chamber (n = 6 discs). TMJ anatomy and loading area were obtained from magnetic resonance imaging of healthy human volunteers (n = 11, male, 30 ± 9 y). Using experimentally determined nutrient metabolic rates, solute diffusivities, TMJ anatomy, and loading areas, subject-specific finite element (FE) models were developed to predict the 3-dimensional nutrient profiles in unloaded and loaded TMJ discs (unloaded, 0% strain, 20% strain). From the FE models, glucose, lactate, and oxygen concentration ranges for unloaded healthy human TMJ discs were 0.6 to 4.0 mM, 0.9 to 5.0 mM, and 0% to 6%, respectively, with steep gradients in the anterior and posterior bands. Sustained mechanical loading significantly reduced nutrient levels (P < 0.001), with a critical zone in which cells may die representing approximately 13.5% of the total disc volume. In conclusion, this study experimentally determined TMJ disc metabolic rates, solute diffusivities, and disc morphometry, and through subject-specific FE modeling, revealed critical interactions between mechanical loading and nutrient supply and metabolism for the in vivo human TMJ disc. The results suggest that TMJ disc homeostasis may be vulnerable to pathological loading (e.g., clenching, bruxism), which impedes nutrient supply. Given difficulties associated with direct in vivo measurements, this study provides a new approach to systematically investigate homeostatic and degenerative mechanisms associated with the TMJ disc.
Keywords: temporomandibular joint, disc nutrition, compressive loading, solute diffusion, cellular energy metabolism, finite element analysis
Introduction
Approximately 35 million people in the United States are affected by temporomandibular joint (TMJ) disorders (TMDs), with significant morbidity and financial burden (Stowell et al. 2007). Although TMD etiology remains unclear, TMJ disc degeneration and mechanical dysfunction are found in approximately 30% of TMD patients (Schiffman et al. 2014). Significant changes in disc morphology, biochemistry, material properties, and function accompany disc degeneration (Stegenga 2001), with degenerative processes occurring when TMJ disc cells fail to maintain homeostasis between matrix synthesis and degradation, potentially as the result of imbalances between nutrient supply and nutrient demand (Nickel et al. 2018).
Previous studies found that nutrients (i.e., glucose, lactate, and oxygen) play crucial roles in fibrocartilaginous tissue homeostasis by affecting cell viability, matrix synthesis, and inflammatory factor response (Bibby and Urban 2004; Martin et al. 2004). In addition, fibrocartilage cell metabolic rates show strong interactions between energy metabolism and oxygen and glucose concentration levels (Heywood et al. 2006). In TMJ disc cells, hypoxia with inflammation has been found to modulate gene expression of matrix metalloproteinases and tenascin-C (Yamaguchi et al. 2005; Tojyo et al. 2008). Our recent studies on TMJ disc cells further demonstrate while hypoxic conditions increase cell proliferation, decrease oxygen consumption, decrease adenosine triphosphate (ATP) production, and decrease matrix synthesis, glucose is the limiting nutrient for TMJ disc cell viability (Cisewski et al. 2015).
Similar to other avascular tissues, critical nutrients required for TMJ disc cell metabolism and viability are supplied by blood vessels and synovial fluid at the disc margins (Piette 1993). Within the disc, small nutrient solute (glucose and oxygen) transport is mainly regulated by diffusion, since the convective contribution of “pumping” small solutes is minimal (Selard et al. 2003; Ferguson et al. 2004; Yao and Gu 2007). Thus, the balance between nutrient/metabolite diffusion through the matrix and cell nutrient metabolism establishes a concentration gradient across the disc. Furthermore, previous studies demonstrated the diffusion of glucose and ions decreased with increased compressive strain in fibrocartilage tissues (Maroudas et al. 1975; Jackson and Gu 2009; Wu et al. 2016). Therefore, by impeding solute diffusion, mechanical loading may further regulate the nutrient gradient across the TMJ disc (Kuo, Wright, et al. 2011; Wright et al. 2013).
Due to difficulties associated with direct in vivo measurements (Urban et al. 2004), computational finite element (FE) models have been employed to predict the extracellular mechanochemical environment inside fibrocartilaginous tissues under load (Selard et al. 2003; Ferguson et al. 2004). For the TMJ disc, previous FE analyses predicted mechanical stress distribution, fluid pressurization, and disc lubrication using elastic, nonlinear viscoelastic models (Koolstra and van Eijden 2007; Tanaka et al. 2007; del Palomar et al. 2008) or poroelastic or biphasic mixture models (Beek et al. 2003; Spilker et al. 2009). However, the impact of joint loading on the TMJ disc nutrient environment remains unknown. As such, the study objective was to predict the extracellular nutrient environment in unloaded and loaded TMJ discs using combined experimental and computational modeling approaches. Specifically, nutrient metabolic rates, solute diffusivities, and TMJ anatomy and loading area were experimentally determined, with subject-specific FE models developed to predict 3-dimensional (3D) nutrient profiles in unloaded and loaded human TMJ discs. Given its high cell density and oxygen consumption rate (OCR) (Kuo, Shi, et al. 2011) and low solute diffusivity (Wright et al. 2013), we hypothesized that the TMJ disc has a sensitive extracellular nutrient environment with steep nutrient gradients. Sustained mechanical loading could further impede TMJ disc nutrient transport, leading to steeper nutrient gradients and increased cellular susceptibility to pathological conditions such as clenching or bruxism.
Materials and Methods
Specimen Preparation
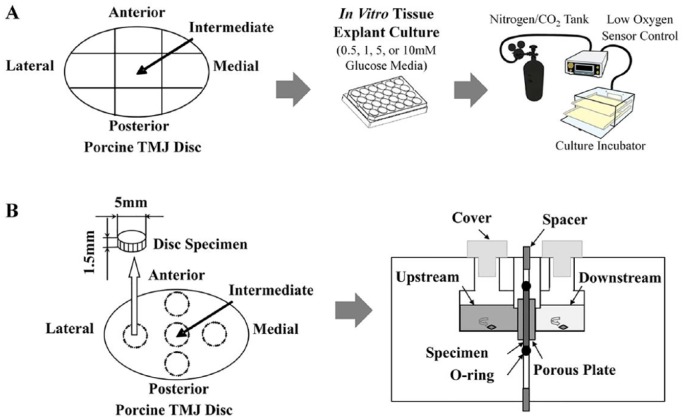
Forty-six porcine heads (American Yorkshire, male, 6–8 mo) were collected from a local abattoir within 2 h of slaughter, and single TMJs were removed from each porcine head. TMJ discs were extracted and washed with 5 changes of phosphate-buffered saline (PBS). Specimen explants were harvested from each TMJ disc in 5 regions (anterior, intermediate, lateral, medial, and posterior) for cellular metabolic rate (n = 40 discs) and solute diffusion (n = 6 discs) experiments. Due to the scarcity of fresh healthy human TMJ disc tissue and similarities between porcine and human TMJ disc biomechanics and biology (Kalpakci et al. 2011; Stembirek et al. 2012), porcine TMJ disc cellular nutrient metabolic rates and nutrient solute diffusion properties are considered acceptable substitutes for the finite element modeling.
Cellular Metabolic Rates
After tissue dissection, TMJ disc explant volume was immediately measured in PBS following Archimedes’ principle (Kuo, Wright, et al. 2011). TMJ explants from each disc region were then minced and placed into 24-well plates (Fig. 1A). Wells were filled with fetal bovine serum–free Dulbecco’s modified Eagle’s medium (DMEM) (HyClone, Fisher Scientific) solutions with 0.5, 1, 5, or 10 mM glucose. Prior to explant plating, the tissue culture incubator was preset to 2.5% or 5% oxygen, within the physiologic range for nondegenerate fibrocartilage (Heywood and Lee 2010). Glucose and lactate concentrations in each culture well were measured after 4 and 18 h (YSI 2700 Select Biochemistry Analyzer; YSI, Inc.). Following each experiment, TMJ disc explants were digested and cell viabilities were determined via trypan blue exclusion to confirm >90% viability. Cell counts were compared to reported porcine TMJ disc in situ cell density values (Kuo, Shi, et al. 2011). From the concentration change of culture medium, time interval, and cell numbers (cell density × tissue volume), TMJ disc cell glucose consumption (GCR) and lactate production (LPR) rates were calculated under the varying oxygen (2.5% and 5%) and glucose conditions (0.5, 1, 5, or 10 mM) (Bibby et al. 2005).
Figure 1.
Experimental designs to measure cellular metabolic rates and nutrient solute diffusivities. (A) Schematic of porcine temporomandibular joint (TMJ) disc explant harvest cultured in a 24-well culture dish with 0.5, 1, 5, or 10 mM glucose media in a low-oxygen (2.5% or 5% oxygen level) incubator. (B) Schematic of TMJ disc explant harvest and custom solute diffusion chamber.
Nutrient Solute Diffusivities
Cylindrical TMJ explants were punched from each disc region using a 5-mm corneal trephine (Fig. 1B). Superior and inferior TMJ disc explant surfaces were removed via sledge microtome to eliminate biconcavity (Wu et al. 2016). TMJ explant initial height was measured using a current-sensing micrometer (Kuo, Wright, et al. 2011; Wright et al. 2013), with each explant held at this initial height in the diffusion chamber to measure diffusivity under 0% strain. Then, 500 µL of 20 mg/mL glucose with 10 mg/mL lactate mixed into normal PBS was pipetted into the upstream chamber, while 200 µL of concentrated PBS solution was pipetted into the downstream chamber to balance upstream osmotic pressure. The diffusion chamber was placed on a stir plate in an incubator to maintain 37°C, with active stir bars to prevent stagnant boundary layers. Glucose and lactate concentrations in the downstream chamber (Fig. 1B) were measured by YSI 2700 Select Biochemistry Analyzer every 15 min until steady state was reached (same concentration within 5% measured 3 times concurrently), with each test lasting approximately 2 h. Following the final 15-min interval, TMJ disc explants were reequilibrated in PBS (~1 h) to wash out residual glucose and lactate. Following reequilibration, the spacer in the diffusion chamber was adjusted to repeat experimental measurements at 10% and 20% strain. Glucose and lactate diffusivities were calculated at steady state for each strain increment (0%, 10%, and 20%) (Jackson and Gu 2009; Wu et al. 2016).
Subject-Specific FE Modeling
Eleven healthy human male volunteers (30 ± 9 y) underwent magnetic resonance imaging (MRI) (Echelon 1.5T; Hitachi America) under institutional approval, and 3D models of the condyle, fossa, and disc were reconstructed (Amira v6.5; Thermo Fisher Scientific). To predict TMJ disc nutrient environment, subject-specific FE models were built from MRI-based 3D condyle, disc, and fossa geometries (COMSOL), modeled with 0.4 to 0.6 million second-order tetrahedral Lagrange elements.
Fick’s diffusion equation was used to model TMJ disc nutrient metabolism and solute transport (Selard et al. 2003), with nutrient metabolic rates under the varying nutrient levels determined from the study in vitro cellular metabolic measurements. Strain-dependent glucose and lactate diffusivities in the FE model were collected from the study solute diffusion experiments, and oxygen diffusivities were calculated using the empirical constitutive relation reported in the literature (Urban et al. 1977) (see Appendix). By correlating cell viability with glucose level, TMJ disc cell viability was predicted following the theoretical framework from fibrocartilage cells (Zhu et al. 2012). A linear relationship between pH and lactate concentration was used to calculate TMJ disc pH values (Selard et al. 2003).
Previous FE analysis and MRI imaging data have shown that changes in human TMJ disc thickness under physiologic joint loading is 15% to 20% (Koolstra and van Eijden 2005). In this study, the effect of TMJ disc compression under static biting on disc nutrient environment was simulated by mimicking the attenuated localized solute exchange through the loading surfaces and considering strain-dependent solute diffusion in the loading region of the disc. Specifically, the contact areas between the mandibular condyle, disc, and fossa were approximated using a previously reported minimal distance method (Gallo et al. 2000). Within the disc loading region between the superior and inferior contact areas, uniform strain distributions (i.e., nonloaded, 0% strain, 20% strain) and a 4-h loading period were assumed to predict the envelope of the effect of sustained mechanical loading on the TMJ disc nutrient environment. Accordingly, superior and inferior TMJ disc contact areas were defined as impermeable in the FE model to mimic localized blocking of solute exchange. Effect of compressive strain on solute diffusion was incorporated using the strain-dependent diffusivities from the study solute diffusion experiments. The detailed derivation of the diffusion governing equations and constitutive relationships for the FE modeling are fully described in the Appendix.
Statistical Analysis
TMJ disc explant metabolic rates were analyzed for significant differences by oxygen level, glucose concentration, and disc region using a 3-way analysis of variance (ANOVA), including a random effect for TMJ disc to accommodate within-disc correlation. Where significant differences among nutrient conditions were detected, t tests (2.5% vs. 5% oxygen) or 1-way ANOVA with Tukey’s post hoc (among 4 glucose levels: 0.5, 1, 5, or 10 mM) were used for pairwise comparisons. Differences in TMJ explant solute diffusivities by disc region and strain increment were determined by 2-way ANOVA, including a random effect for disc, with Tukey’s post hoc test to determine pairwise differences. Similarly, differences in minimum glucose and oxygen levels, maximum lactate level, minimum cell viability, and volume of critical zone (glucose level below 0.5 mM) from the FE simulations were determined by loading condition using 1-way ANOVA with Tukey’s post hoc. All statistical analyses were conducted using SPSS (version 23.0; SPSS, Inc.). Statistical significance was determined at P < 0.05, with descriptive statistics reported as mean ± standard deviation.
Results
Cellular Metabolic Rates
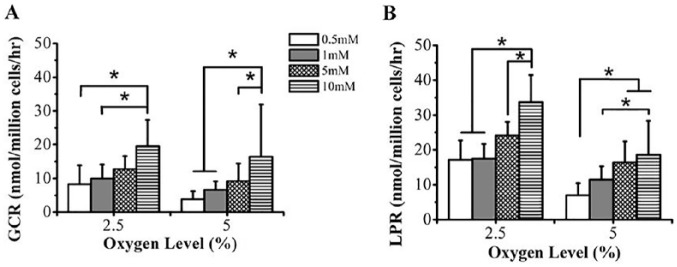
Decreasing oxygen level and increasing glucose concentration increased GCR (P = 0.033; Fig. 2A). GCR significantly increased with a decrease in oxygen level at 0.5 mM glucose (P = 0.031; 2.5% oxygen: 8.3 ± 5.5 nmol/million cells/h; 5% oxygen: 3.8 ± 2.4 nmol/million cells/h) and 1 mM glucose (P = 0.028; 2.5% oxygen: 9.9 ± 4.4 nmol/million cells/h; 5% oxygen: 6.6 ± 2.5 nmol/million cells/h) but not at 5 mM (P = 0.063) or 10 mM glucose (P = 0.088). Under 2.5% and 5% oxygen conditions, significant glucose effects were observed, with highest GCR at 10 mM glucose (2.5% oxygen: P < 0.001; 19.6 ± 7.8 nmol/million cells/h; 5% oxygen: P < 0.001; 16.5 ± 15.5 nmol/million cells/h) and lowest GCR at 0.5 mM glucose.
Figure 2.
Effect of nutrient levels (glucose and oxygen) on glucose consumption rate (GCR) and lactate production rate (LPR) of porcine temporomandibular joint (TMJ) disc cells. (A) GCR and (B) LPR (nmol/million cells/h) of TMJ disc explants versus glucose media concentration (0.5, 1, 5, and 10 mM) and oxygen level (2.5 and 5%). *P < 0.05. Sample size n = 40 porcine TMJ discs (n = 5 discs for GCR and LPR experimental runs under each nutrient condition).
Similarly, LPR increased with decreasing oxygen level and increasing glucose concentration (P = 0.031; Fig. 2B). LPR significantly increased with a decrease in oxygen level at 0.5 mM glucose (P = 0.001; 2.5% oxygen: 17.2 ± 5.5 nmol/million cells/h; 5% oxygen: 7.0 ± 3.4 nmol/million cells/h), 1 mM glucose (P = 0.031; 2.5% oxygen: 17.5 ± 4.2 nmol/million cells/h; 5% oxygen: 11.5 ± 3.8 nmol/million cells/h), 5 mM glucose (P = 0.004; 2.5% oxygen: 24.2 ± 3.9 nmol/million cells/h; 5% oxygen: 16.4 ± 6.0 nmol/million cells/h), and 10 mM glucose (P < 0.001; 2.5% oxygen: 33.7 ± 7.8 nmol/million cells/h; 5% oxygen: 18.6 ± 9.8 nmol/million cells/h). Furthermore, a significant glucose effect was observed, with highest LPR at 10 mM glucose under 2.5% (P < 0.001) and 5% oxygen conditions (P < 0.001).
No significant regional differences were observed for GCR and LPR under varying oxygen (P = 1.0) or glucose levels (P = 0.983), and results are reported as averages for each disc across all regions.
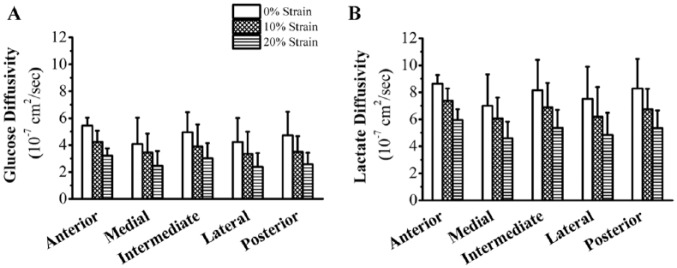
Glucose and Lactate Diffusivities
A significant strain effect was found for glucose and lactate diffusivities (P < 0.001; Fig. 3). Cross-region glucose diffusivity at 0% strain was 4.7 ± 1.6 × 10−7 cm2/s, decreasing at 10% strain to 3.7 ± 1.3 × 10−7 cm2/s (−21%) and at 20% strain to 2.7 ± 1.0 × 10−7 cm2/s (−27%). Lactate diffusivity was 7.9 ± 2.0 × 10−7 cm2/s, 6.6 ± 1.6 × 10−7 cm2/s (−16%), and 5.2 ± 1.3 × 10−7 cm2/s (−21%) for 0%, 10%, and 20% strains, respectively. No significant regional effect was found for strain-dependent glucose (P = 0.088) or lactate (P = 0.075) diffusivities, and results are reported as averages for each disc across all regions.
Figure 3.
Effect of compressive strain on regional distribution of glucose and lactate diffusivities in porcine temporomandibular joint (TMJ) discs. (A) Glucose and (B) lactate diffusivities of TMJ discs versus mechanical strain (0%, 10%, and 20%) and disc region (anterior, intermediate, lateral, medial, and posterior). Sample size n = 6 porcine TMJ discs: 6 (disc) × 3 (strain) × 5 (region) = 90 diffusivity measurements.
Human TMJ Disc Nutrient Environment Prediction
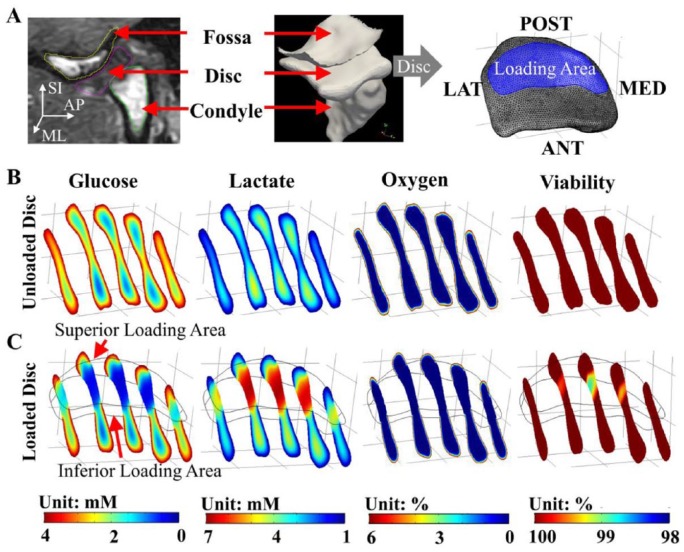
From healthy TMJ disc nutrient environment FE models, physiologic ranges of glucose, lactate, and oxygen concentrations without loading were 0.6 to 4.0 mM, 0.9 to 5.0 mM, and 0% to 6%, respectively (Fig. 4). In the anterior and posterior bands, steep glucose and lactate concentration gradients were found. Except for disc surfaces, oxygen concentrations were lower than 2.5%. TMJ disc pH values ranged from 7.0 to 7.4 without loading. A significant loading effect was found for minimum glucose and maximum lactate levels (P < 0.001) in the TMJ disc (Fig. 5A, B). The minimum glucose level dropped more than 50% in the loaded disc after 4 h (0% strain: 0.3 ± 0.1 mM; 20% strain: 0.2 ± 0.1 mM) compared to the unloaded disc (0.6 ± 0.3 mM). By comparison, maximum lactate level increased more than 32% in the loaded disc (0% strain: 6.6 ± 0.2 mM; 20% strain: 6.7 ± 0.1 mM) compared to unloaded disc (5.0 ± 0.3 mM).
Figure 4.
Finite element (FE) analysis of nutrient environment and cell viability in unloaded and loaded temporomandibular joint (TMJ) discs from 1 representative human subject. (A) Magnetic resonance imaging–based subject-specific TMJ FE model. Subject-specific nutrient profiles (glucose, lactate, and oxygen) and cell viability distributions in (B) unloaded and (C) loaded TMJ discs.
Figure 5.
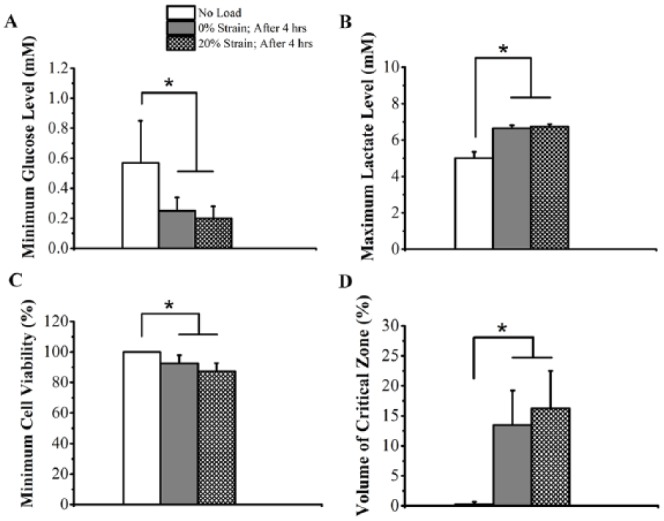
Effect of mechanical loading on (A) minimum glucose level, (B) maximum lactate level, (C) minimum cell viability, and (D) volume of critical zone in temporomandibular joint (TMJ) discs from 11 human subjects. “No load”: unloaded TMJ disc; “0% Strain; After 4 h”: solute diffusivities collected under 0% strain level were used for the whole TMJ disc; “20% Strain; After 4 h”: solute diffusivities collected under 20% strain level were used for the loaded disc region. The solute diffusion through the superior and inferior loading surfaces of the TMJ disc was blocked for 4 h. *P < 0.05. Sample size: n = 11 human TMJ discs in 11 healthy human male volunteers.
Given fibrocartilaginous cells start to die when glucose levels fall below 0.5 mM (Bibby and Urban 2004; Cisewski et al. 2015), glucose levels were correlated with cell viability, allowing cell death due to nutrient deficiency to be predicted. A significant loading effect was found for minimum cell viability (P < 0.001) and critical zone volume where glucose concentrations were less than 0.5 mM (P < 0.001) after 4 h of loading. Minimum cell viabilities dropped to 92.5% ± 5.4% and 87.4% ± 5.2% under 0% and 20% strain loading conditions, respectively (Fig. 5C). Critical zone volume increased from 0% of total disc volume under no loading to more than 13.5% of total disc volume under loading conditions (0% strain: 13.5% ± 5.8%; 20% strain: 16.3% ± 6.3%) (Fig. 5D). In addition, correlating lactate levels with pH values, an acidic extracellular environment was predicted inside the loading region (minimum pH value: ~6.8 for both 0% and 20% strains).
Discussion
It has been postulated that alterations to TMJ disc nutrient supply trigger a cascade of events leading to disc degeneration (Yamaguchi et al. 2005; Tojyo et al. 2008; Kuo, Wright, et al. 2011; Wright et al. 2013; Cisewski et al. 2015). However, given difficulties associated with in vivo measurements of the nutrient environment of fibrocartilaginous tissues, little is known about the nutrient profile within the human TMJ disc or the effect of loading on the extracellular nutrient environment. As such, the study objective was to predict the nutrient environment in unloaded and loaded TMJ discs using combined experimental and computational modeling approaches.
Study results showed unloaded human TMJ disc has a sensitive extracellular nutrient environment with steep nutrient gradients in the anterior and posterior bands. The minimum glucose level in the disc was ~0.6 mM, near the critical glucose level for fibrocartilage cell viability (0.5 mM) (Bibby and Urban 2004; Cisewski et al. 2015). Except for disc surfaces, the TMJ disc was predicted to be hypoxic (oxygen concentration lower than 1% in 90% of the disc volume) (Fig. 4B), unique from other fibrocartilage tissues (Zhou et al. 2004; Travascio and Jackson 2017). The delicate TMJ disc environment could be due to its unique tissue and cellular properties, with nutrient solute diffusivities 30% to 70% lower, oxygen consumption rates 2 to 10 times higher, and cell densities 2 to 13 times higher than other fibrocartilages (Maroudas et al. 1975; Jackson and Gu 2009; Kuo, Shi, et al. 2011).
Study results demonstrated sustained mechanical loading significantly alters the nutrient distribution inside the TMJ disc by impeding solute transport, leading to steeper glucose and lactate gradients (Fig. 4C). Restriction of nutrient supply and waste removal in the loaded disc were due to localized blocking of solute exchange through the loading surfaces and the impedance effect of compressive strain on diffusion. As shown in the in vitro diffusion experiments, glucose and lactate diffusivities decreased with increasing compressive strain, with significant differences at 20% compression. Strain effects on diffusivity are likely associated with fluid exudation and decreased tissue porosity under load, consistent with other fibrocartilaginous tissues (Maroudas et al. 1975; Jackson and Gu 2009; Wu et al. 2016).
Study results further indicated nutrient deficiency due to abnormal sustained loading (e.g., clenching or bruxism) may result in homeostasis disruption and initiate cell death, potentially associated with TMJ pathologies (Kuo, Wright, et al. 2011; Wright et al. 2013). Energy metabolic experiments determined that TMJ disc cell GCR and LPR increased with decreasing oxygen level or increasing glucose concentration, indicating TMJ cells are sensitive to oxygen availability and could switch to glycolysis under hypoxic conditions (Cisewski et al. 2015). FE analysis demonstrated that, in the hypoxic TMJ disc region, increased cell glycolytic rate could increase glucose demand, leading to steeper glucose gradients across the disc, and increase risks to cell viability under pathologic loading conditions. By correlating critical glucose level with cell viability, sustained mechanical loading results in cell death in a critical zone greater than 13.5% of the total disc volume (Fig. 5C, D). Furthermore, while glucose may be the critical nutrient for TMJ disc cell survival, the production of ATP, collagen, and proteoglycan was severely inhibited under hypoxic conditions (Cisewski et al. 2015), indicating TMJ disc matrix synthesis homeostasis may be vulnerable to pathologic loading conditions.
Study limitations include the collection of cellular nutrient metabolic rates and nutrient solute diffusion properties from healthy male porcine TMJ disc tissue due to scarcity of fresh healthy human TMJ disc tissue. These properties from porcine TMJ discs are expected to be similar to human characteristics, given similarities in biomechanics and biology (Kalpakci et al. 2011; Stembirek et al. 2012). Further validation is warranted once fresh healthy human TMJ disc tissue is available. FE model loading conditions were simplified to 4-h static compression on the disc (Koolstra and van Eijden 2005), which rarely occurs in vivo (Raphael et al. 2012). Physiological loading conditions (predominately dynamic) are anticipated to be more complex (Iwasaki et al. 2017; Wei et al. 2017), with the results from this study anticipated to capture the envelope of effect of joint loading on mechanobiological environment. Other factors such as sex, age, or disc displacement affecting TMJ disc nutrient environment (e.g., morphology, loading conditions, hormone levels, synovial environment, and cell metabolic behaviors) were not included, so further studies are highly necessary. As suggested by the clinical study Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA), TMD is a complex “multifactorial” disease: a number of risk factors in many domains (e.g., genetic, environmental, behavioral) may contribute to TMD development (Slade et al. 2013). Further systematic investigations of the risk factor interactions may help to better elucidate TMJ disc degeneration pathophysiology and TMD etiological mechanisms.
In conclusion, this study experimentally determined porcine TMJ disc metabolic rates, solute diffusivities, and human TMJ disc morphometry, and through subject-specific FE modeling, it predicted the sensitive nutrient environment with steep nutrient gradients in healthy human TMJ disc and identified that TMJ disc homeostasis may be vulnerable to pathological sustained loading (e.g., clenching, bruxism), which impedes nutrient supply. Given difficulties associated with direct in vivo measurements, this study provides a new approach to further investigate systematically homeostatic and degenerative mechanisms associated with the TMJ disc.
Author Contributions
Y. Wu, H. Yao, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; S.E. Cisewski, contributed to design, data acquisition, and analysis, drafted and critically revised manuscript; M.C. Coombs, contributed to conception, data analysis, and interpretation, drafted and critically revised the manuscript; M.H. Brown, F. Wei, X. She, contributed to design, data acquisition, and analysis, drafted the manuscript; M.J. Kern, contributed to conception, design, and data interpretation, critically revised the manuscript; Y.M. Gonzalez, L.M. Gallo, V. Colombo, L.R. Iwasaki, J.C. Nickel, contributed to data acquisition and analysis, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519851044 for Effect of Sustained Joint Loading on TMJ Disc Nutrient Environment by Y. Wu, S.E. Cisewski, M.C. Coombs, M.H. Brown, F. Wei, X. She, M.J. Kern, Y.M. Gonzalez, L.M. Gallo, V. Colombo, L.R. Iwasaki, J.C. Nickel and H. Yao in Journal of Dental Research
Acknowledgments
We thank Dr. Elizabeth H. Slate for her valuable assistance in statistical analysis.
Footnotes
A supplemental appendix to this article is available online.
This project was supported by National Institutes of Health (NIH) grants P20GM121342, R03DE018741, and R01DE021134 to H. Yao; R01DE016417 to J.C. Nickel; and NIH T32 and F32 postdoctoral fellowships DE017551 and DE027864 to M.C. Coombs.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: V. Colombo  https://orcid.org/0000-0001-9612-5294
https://orcid.org/0000-0001-9612-5294
References
- Beek M, Koolstra JH, van Eijden TM. 2003. Human temporomandibular joint disc cartilage as a poroelastic material. Clin Biomech. 18(1):69–76. [DOI] [PubMed] [Google Scholar]
- Bibby SR, Jones DA, Ripley RM, Urban JP. 2005. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine. 30(5):487–496. [DOI] [PubMed] [Google Scholar]
- Bibby SR, Urban JP. 2004. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 13(8):695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisewski SE, Zhang L, Kuo J, Wright GJ, Wu Y, Kern MJ, Yao H. 2015. The effects of oxygen level and glucose concentration on the metabolism of porcine TMJ disc cells. Osteoarthritis Cartilage. 23(10):1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Palomar AP, Santana-Penin U, Mora-Bermudez MJ, Doblare M. 2008. Clenching TMJs-loads increases in partial edentates: a 3D finite element study. Ann Biomed Eng. 36(6):1014–1023. [DOI] [PubMed] [Google Scholar]
- Ferguson SJ, Ito K, Nolte LP. 2004. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech. 37(2):213–221. [DOI] [PubMed] [Google Scholar]
- Gallo LM, Nickel JC, Iwasaki LR, Palla S. 2000. Stress-field translation in the healthy human temporomandibular joint. J Dent Res. 79(10):1740–1746. [DOI] [PubMed] [Google Scholar]
- Heywood HK, Bader DL, Lee DA. 2006. Rate of oxygen consumption by isolated articular chondrocytes is sensitive to medium glucose concentration. J Cell Physiol. 206(2):402–410. [DOI] [PubMed] [Google Scholar]
- Heywood HK, Lee DA. 2010. Low oxygen reduces the modulation to an oxidative phenotype in monolayer-expanded chondrocytes. J Cell Physiol. 222(1):248–253. [DOI] [PubMed] [Google Scholar]
- Iwasaki LR, Gonzalez YM, Liu Y, Liu H, Markova M, Gallo LM, Nickel JC. 2017. Mechanobehavioral scores in women with and without TMJ disc displacement. J Dent Res. 96(8):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Gu W. 2009. Transport properties of cartilaginous tissues. Curr Rheumatol Rev. 5(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpakci KN, Willard VP, Wong ME, Athanasiou KA. 2011. An interspecies comparison of the temporomandibular joint disc. J Dent Res. 90(2):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolstra JH, van Eijden TM. 2005. Combined finite-element and rigid-body analysis of human jaw joint dynamics. J Biomech. 38(12):2431–2439. [DOI] [PubMed] [Google Scholar]
- Koolstra JH, van Eijden TM. 2007. Consequences of viscoelastic behavior in the human temporomandibular joint disc. J Dent Res. 86(12):1198–1202. [DOI] [PubMed] [Google Scholar]
- Kuo J, Shi C, Cisewski S, Zhang L, Kern MJ, Yao H. 2011. Regional cell density distribution and oxygen consumption rates in porcine TMJ discs: an explant study. Osteoarthritis Cartilage. 19(7):911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Wright GJ, Bach DE, Slate EH, Yao H. 2011. Effect of mechanical loading on electrical conductivity in porcine TMJ discs. J Dent Res. 90(10):1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A, Stockwell RA, Nachemson A, Urban J. 1975. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 120:113–130. [PMC free article] [PubMed] [Google Scholar]
- Martin G, Andriamanalijaona R, Grassel S, Dreier R, Mathy-Hartert M, Bogdanowicz P, Boumediene K, Henrotin Y, Bruckner P, Pujol JP. 2004. Effect of hypoxia and reoxygenation on gene expression and response to interleukin-1 in cultured articular chondrocytes. Arthritis Rheum. 50(11):3549–3560. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Gonzalez YM, Gallo LM, Yao H. 2018. Mechanobehavior and ontogenesis of the temporomandibular joint. J Dent Res. 97(11):1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette E. 1993. Anatomy of the human temporomandibular joint: an updated comprehensive review. Acta Stomatol Belg. 90(2):103–127. [PubMed] [Google Scholar]
- Raphael KG, Sirois DA, Janal MN, Wigren PE, Dubrovsky B, Nemelivsky LV, Klausner JJ, Krieger AC, Lavigne GJ. 2012. Sleep bruxism and myofascial temporomandibular disorders: a laboratory-based polysomnographic investigation. J Am Dent Assoc. 143(11):1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, et al. ; International RDC/TMD Consortium Network, International Association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. 2014. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 28(1):6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selard E, Shirazi-Adl A, Urban JP. 2003. Finite element study of nutrient diffusion in the human intervertebral disc. Spine. 28(17):1945–1953. [DOI] [PubMed] [Google Scholar]
- Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, Dubner R, Diatchenko L, Smith SB, Knott C, et al. 2013. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: implications and future directions. J Pain. 14(12 Suppl):T116–T124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilker RL, Nickel JC, Iwasaki LR. 2009. A biphasic finite element model of in vitro plowing tests of the temporomandibular joint disc. Ann Biomed Eng. 37(6):1152–1164. [DOI] [PubMed] [Google Scholar]
- Stegenga B. 2001. Osteoarthritis of the temporomandibular joint organ and its relationship to disc displacement. J Orofac Pain. 15(3):193–205. [PubMed] [Google Scholar]
- Stembirek J, Kyllar M, Putnova I, Stehlik L, Buchtova M. 2012. The pig as an experimental model for clinical craniofacial research. Lab Anim. 46(4):269–279. [DOI] [PubMed] [Google Scholar]
- Stowell AW, Gatchel RJ, Wildenstein L. 2007. Cost-effectiveness of treatments for temporomandibular disorders: biopsychosocial intervention versus treatment as usual. J Am Dent Assoc. 138(2):202–208. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Hirose M, Inubushi T, Koolstra JH, van Eijden TM, Suekawa Y, Fujita R, Tanaka M, Tanne K. 2007. Effect of hyperactivity of the lateral pterygoid muscle on the temporomandibular joint disk. J Biomech Eng. 129(6):890–897. [DOI] [PubMed] [Google Scholar]
- Tojyo I, Yamaguchi A, Nitta T, Yoshida H, Fujita S, Yoshida T. 2008. Effect of hypoxia and interleukin-1beta on expression of tenascin-C in temporomandibular joint. Oral Dis. 14(1):45–50. [DOI] [PubMed] [Google Scholar]
- Travascio F, Jackson AR. 2017. The nutrition of the human meniscus: a computational analysis investigating the effect of vascular recession on tissue homeostasis. J Biomech. 61:151–159. [DOI] [PubMed] [Google Scholar]
- Urban JP, Holm S, Maroudas A, Nachemson A. 1977. Nutrition of the intervertebral disk: an in vivo study of solute transport. Clin Orthop Relat Res. 129:101–114. [PubMed] [Google Scholar]
- Urban JP, Smith S, Fairbank JC. 2004. Nutrition of the intervertebral disc. Spine. 29(23):2700–2709. [DOI] [PubMed] [Google Scholar]
- Wei F, Van Horn MH, Coombs MC, She X, Gonzales TS, Gonzalez YM, Scott JM, Iwasaki LR, Nickel JC, Yao H. 2017. A pilot study of nocturnal temporalis muscle activity in TMD diagnostic groups of women. J Oral Rehabil. 44(7):517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GJ, Kuo J, Shi C, Bacro TR, Slate EH, Yao H. 2013. Effect of mechanical strain on solute diffusion in human TMJ discs: an electrical conductivity study. Ann Biomed Eng. 41(11):2349–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cisewski SE, Wegner N, Zhao S, Pellegrini VD, Jr, Slate EH, Yao H. 2016. Region and strain-dependent diffusivities of glucose and lactate in healthy human cartilage endplate. J Biomech. 49(13):2756–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Tojyo I, Yoshida H, Fujita S. 2005. Role of hypoxia and interleukin-1beta in gene expressions of matrix metalloproteinases in temporomandibular joint disc cells. Arch Oral Biol. 50(1):81–87. [DOI] [PubMed] [Google Scholar]
- Yao H, Gu WY. 2007. Convection and diffusion in charged hydrated soft tissues: a mixture theory approach. Biomech Model Mechanobiol. 6(1–2):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Cui Z, Urban JP. 2004. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 50(12):3915–3924. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Jackson AR, Gu WY. 2012. Cell viability in intervertebral disc under various nutritional and dynamic loading conditions: 3D finite element analysis. J Biomech. 45(16):2769–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519851044 for Effect of Sustained Joint Loading on TMJ Disc Nutrient Environment by Y. Wu, S.E. Cisewski, M.C. Coombs, M.H. Brown, F. Wei, X. She, M.J. Kern, Y.M. Gonzalez, L.M. Gallo, V. Colombo, L.R. Iwasaki, J.C. Nickel and H. Yao in Journal of Dental Research