Abstract
Bone morphogenetic protein (BMP) signaling performs multiple essential functions during craniofacial development. In this study, we used the adult mouse incisor as a model to uncover how BMP signaling maintains tissue homeostasis and regulates mesenchymal stem cell (MSC) fate by mediating WNT and FGF signaling. We observed a severe defect in the proximal region of the adult mouse incisor after loss of BMP signaling in the Gli1+ cell lineage, indicating that BMP signaling is required for cell proliferation and odontoblast differentiation. Our study demonstrates that BMP signaling serves as a key regulator that antagonizes WNT and FGF signaling to regulate MSC lineage commitment. In addition, BMP signaling in the Gli1+ cell lineage is also required for the maintenance of quiescent MSCs, suggesting that BMP signaling not only is important for odontoblast differentiation but also plays a crucial role in providing feedback to the MSC population. This study highlights multiple important roles of BMP signaling in regulating tissue homeostasis.
Keywords: cell proliferation, differentiation, stem cells, tooth, odontoblast, growth factor signaling
Introduction
Mesenchymal stem cells (MSCs) are critical for tissue regeneration due to their function in maintaining tissue homeostasis. MSCs were first identified in the bone marrow and also reside in a large variety of tissues, including blood, placenta, tooth, and adipose tissue. MSCs have the capacity to self-renew continuously and the potential to differentiate into multiple cell lineages (Valtieri and Sorrentino 2008; Simons and Clevers 2011; Kfoury and Scadden 2015; Zhao et al. 2015). Previous studies of MSCs mainly focused on their trilineage differentiation ability and their expression of cell surface markers in vitro (Bianco et al. 2013). Recent studies using in vivo cell lineage analysis significantly improved our understanding of the niche environment where these MSCs reside and how they are regulated in vivo (Michelozzi et al. 2017). These studies helped us gain a better understanding of the in vivo molecular regulatory network involved in regulating MSC fate to support tissue homeostasis.
The mouse incisor provides an excellent model for studying MSCs because the incisor grows continuously throughout the animal’s lifetime. This continuous growth is enabled by epithelial stem cells that give rise to enamel-forming ameloblasts and MSCs whose derivatives form dentin and pulp (Wang et al. 2007; Mitsiadis et al. 2011; Cao et al. 2013; Kuang-Hsien Hu et al. 2014). We recently showed that quiescent Gli1+ cells near the neurovascular bundle are typical MSCs. These Gli1+ incisor MSCs exit from their niche and become transit-amplifying cells (TACs). These TACs can be identified based on their active proliferation and give rise to more committed preodontoblasts, terminally differentiated odontoblasts, and dental pulp cells (Feng et al. 2011; Zhao et al. 2014).
Bone morphogenetic proteins (BMPs) are a group of signaling molecules that belong to the transforming growth factor–β (TGF-β) superfamily of proteins. BMP signaling is indispensable for embryonic development and tissue homeostasis (Wang et al. 2014). During craniofacial development, altered BMP signaling can affect the size, shape, and position of teeth (Plikus et al. 2005). We also recently showed that BMP signaling controls a transcriptional network to regulate the fate of MSCs during molar root development (Feng et al. 2017). In addition, suture MSCs also depend on the BMP signaling pathway to regulate suture homeostasis via balancing osteogenesis and osteoclastogenesis activity (Guo et al. 2018). BMP signaling interacts with other signaling pathways to exert its activity. For example, the BMP-WNT signaling cascade plays an important role in regulating cranial neural crest cell (CNCC)–derived dental mesenchymal cell fate during tooth development (Kleber et al. 2005; Li et al. 2011; Zhang et al. 2015). Also, interaction between BMP and FGF signaling pathways is required for specifying sites of tooth development (Neubuser et al. 1997; Tucker and Sharpe 2004; Mason 2007). However, the functional significance of BMP signaling and its interaction with other signaling molecules in regulating the fate of MSCs in adult mouse incisors are still unknown.
In this study, we sought to investigate the functional significance of BMP signaling in regulating the fate of MSCs in adult mouse incisors. Our results show that activated BMP signaling is associated with preodontoblasts/odontoblasts and dental pulp cells. Loss of Bmpr1a in the lineage derived from Gli1+ cells led to compromised odontoblast differentiation and incisor growth defects. Furthermore, our study demonstrates that BMP signaling serves as a key regulator that antagonizes WNT and FGF signaling to regulate the fate of MSCs. Importantly, we found that compromised BMP signaling in the Gli1+ lineage also led to a diminished Gli1+ MSC population, suggesting that BMP not only is important for odontoblast differentiation but also plays a crucial role in providing feedback to maintain the MSC population. This study highlights the essential role of BMP signaling in the molecular network that regulates adult mouse incisor tissue homeostasis.
Materials and Methods
For details, see the supplemental appendix.
Results
BMP Signaling Is Active in Preodontoblasts/Odontoblasts in the Adult Mouse Incisor
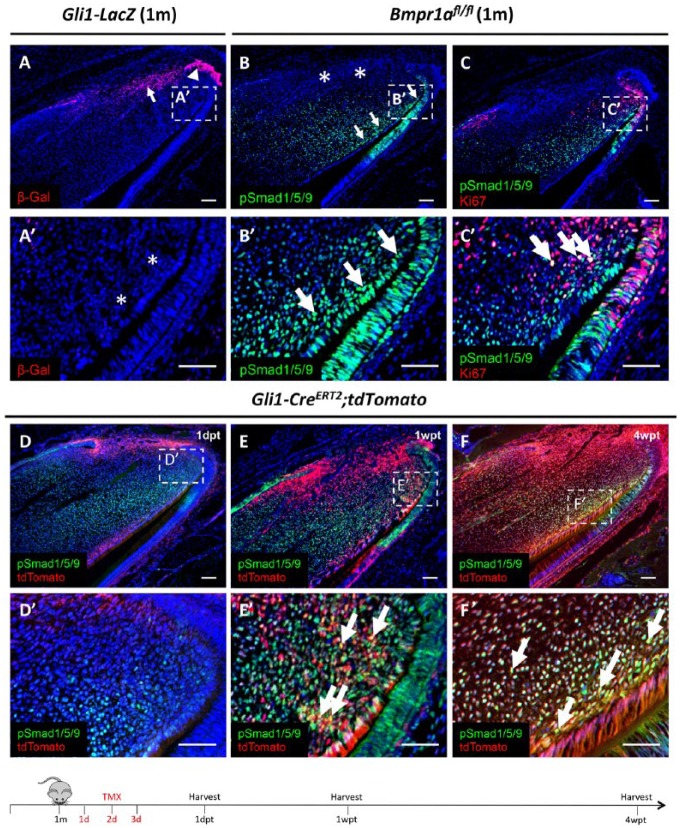
Bmp2, 4, 7, and their antagonist, Follistatin, have been reported to be expressed in the mesenchyme of mouse incisors (Wang et al. 2007). To determine whether BMP signaling is activated in Gli1+ MSCs, we first examined Gli1 expression in incisors using Gli1-LacZ mice. We found that Gli1 expression was detectable in both the epithelium and mesenchyme near the cervical loop but was absent from the preodontoblast region (Fig. 1A, A′), consistent with previous results (Zhao et al. 2014). Next, to test for BMP signaling activity, we examined the expression of phosphorylated Smad1/5/9 (pSmad1/5/9), a readout of activated BMP signaling. We found that BMP signaling was active in the preodontoblasts/odontoblasts, dental pulp, and a small number of TACs in 1-mo-old control mice but was not detectable in the MSC region (Fig. 1B, B′). In addition, we sought to determine whether BMP signaling was active in TACs, which are derived from MSCs and identifiable based on their active proliferation status. We performed double staining of Ki-67 and pSmad1/5/9 in 1-mo-old control mice and found that BMP signaling activity was detected adjacent to but not overlapping with the majority of TACs, except for a few TACs in the most distal region bordering pulp cells and preodontoblasts (Fig. 1C, C′).
Figure 1.
Activation of bone morphogenetic protein (BMP) signaling in Gli1+ mesenchymal stem cell (MSC)–derived preodontoblasts/odontoblasts in adult mouse incisors. (A) β-Gal immunostaining (red) of sagittal sections of incisors from 1-mo-old (1m) Gli1-LacZ mice. Arrow and arrowhead indicate Gli1+ cells in the proximal end of the incisor epithelium and mesenchyme, respectively. (A′) Higher magnification of boxed region in A shows absence of Gli1 expression in the preodontoblast/odontoblast region indicated by asterisks. (B, B′) pSmad1/5/9 immunostaining (green) of sagittal sections of incisors from 1-mo-old (1m) Bmpr1afl/fl mice. Arrows indicate pSmad1/5/9 signaling in the preodontoblast/odontoblast region, and asterisks indicate absence of pSmad1/5/9 signaling in the Gli1+ MSC region. Boxed area in B is shown magnified in B′. (C) pSmad1/5/9 (green) and Ki-67 (red) double immunostaining of sagittal sections of incisors from 1-mo-old (1m) Bmpr1afl/fl mice. Boxed area in C is shown magnified in C′. Arrows indicate colocalization of Ki-67+ cells and BMP signaling activity (yellow). (D–F′) Lineage tracing of sagittal sections of incisors from 1-mo-old Gli1-CreERT2;tdTomato mice 1 d (1dpt), 1 wk (1wpt), and 4 wk (4wpt) posttamoxifen induction. Red indicates Gli1-derived cells; green indicates BMP signaling activity; yellow indicates colocalization of fluorescent staining (arrows). Boxes in D to F are shown magnified in D′ to F′, respectively. Schematic diagram at the bottom indicates induction protocol. Scale bars, 100 µm.
To analyze the role of BMP signaling in maintaining incisor homeostasis, we investigated colocalization of active BMP signaling and the progeny of Gli1+ cells using lineage tracing. One day after tamoxifen induction of 1-mo-old Gli1-CreERT2;tdTomato mice, Gli1+ (tdTomato+) cells were located in the proximal region, where BMP activity was not detectable (Fig. 1D, D′). One week after tamoxifen induction, as Gli1+ MSCs began to exit their niche and migrate to the TAC region, Gli1+ progeny colocalized with BMP signaling activity in the transition zone between TACs and preodontoblasts (Fig. 1E, E′). Four weeks after induction, as progeny of Gli1+ cells differentiated into odontoblasts and dental pulp cells, we found that they colocalized extensively with activated BMP signaling in the preodontoblast region and dental pulp cells in close proximity to this region (Fig. 1F, F′). Thus, the activation of BMP signaling in the Gli1+ progeny suggests that it may play an important role in the TAC-preodontoblast/odontoblast transition and odontoblast differentiation process.
Loss of BMP Signaling in Gli1+ Derived Cells Leads to an Arrest of Incisor Growth
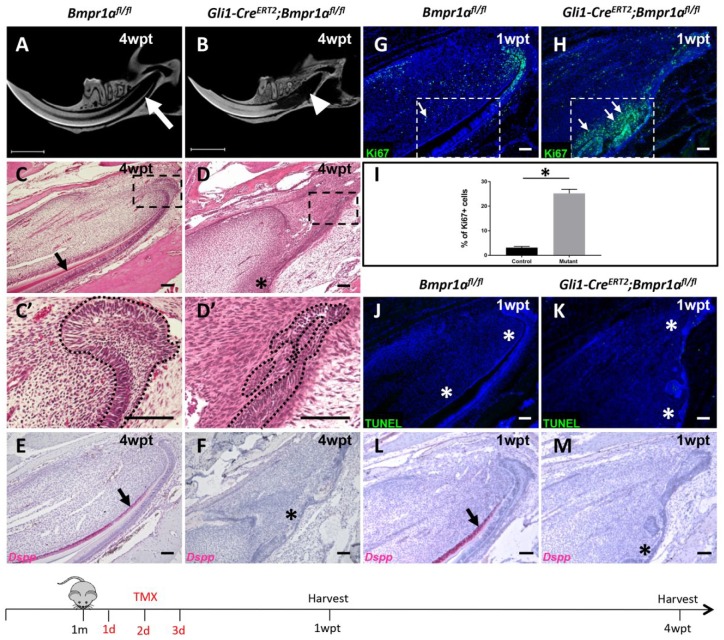
To test our hypothesis that BMP signaling is essential for maintaining mesenchymal tissue homeostasis and continued growth of the adult mouse incisor, we generated Gli1-CreERT2;Bmpr1afl/fl mice, in which Bmpr1a was lost in Gli1+-derived cells. We confirmed that BMP signaling was efficiently deleted after injection of tamoxifen based on a lack of pSmad1/5/9 expression in the dental pulp of Gli1-CreERT2;Bmpr1afl/fl incisors (Appendix Fig. 1A, B). After loss of Bmpr1a in Gli1+-derived cells, we observed significantly shorter incisor dentin and a severe defect of the proximal region of the incisor 4 wk after tamoxifen induction in adult Gli1-CreERT2;Bmpr1afl/fl mice using micro–computed tomography (CT) analysis (Fig. 2A, B). Eight and 12 wk after induction, when the contribution of the targeted Gli1 cells had expanded to reach the distal end of the incisor, we observed more significant shortening of the distal region of the incisor dentin compared to control mice (Appendix Fig. 2C–F), suggesting that loss of BMP signaling affects turnover and tissue homeostasis, eventually disrupting incisor growth in Gli1-CreERT2;Bmpr1afl/fl mice. Histological analysis further revealed that the cervical loop was disorganized and dentin in the proximal region was not detectable 4 wk after tamoxifen induction in Gli1-CreERT2;Bmpr1afl/fl incisors (Fig. 2C, C′, D, D′). Moreover, expression of dentin sialophosphoprotein (Dspp), an odontoblast differentiation marker, was undetectable in the preodontoblast region even though Dspp expression was observed in the distal region (Fig. 2E, F, Appendix Fig. 3A, C). These results suggest that there is a functional requirement for BMP signaling to support the differentiation of Gli1+ cells. To analyze the cellular mechanism of incisor growth defects, we examined proliferative and apoptotic activity in the incisor mesenchyme 1 wk after tamoxifen induction. We detected ectopic proliferating cells in the preodontoblast region in Gli1-CreERT2;Bmpr1afl/fl mice, indicated by Ki-67 immunostaining (Fig. 2G–I). In contrast, apoptosis appeared unaffected 1 wk after induction in Gli1-CreERT2;Bmpr1afl/fl mouse incisors, as assessed by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay (Fig. 2J, K). In the putative preodontoblast region where ectopic proliferative cells were detected, expression of odontoblast marker Dspp was completely absent (Fig. 2L, M), while the odontoblasts in the distal region retained Dspp expression (Appendix Fig. 3B), indicating that ectopic proliferation may contribute to differentiation defects. In addition, we analyzed the expression change of amelogenin (Amelx), a marker of ameloblasts, and observed a reduction in its expression from 1 wk to 4 wk after induction that proceeded in a proximal-to-distal direction in Gli1-CreERT2;Bmpr1afl/fl mouse incisors (Appendix Fig. 3D–F). Eight weeks after tamoxifen induction, Gli1-CreERT2;Bmpr1afl/fl mice exhibited severely disorganized dental pulp tissue and abnormal epithelial structures that morphologically did not resemble the normal cervical loop. In addition, some ectopic cartilage-like structures that were positive for both collagen II and tdTomato were present in the dental pulp cavity in Gli1-CreERT2;Bmpr1afl/fl;tdTomato mouse incisors, suggesting that the Gli1+ progeny switched to a chondrogenic fate following loss of BMP signaling (Appendix Fig. 4A, A′, A′′, B, B′, C, C′, C′′). Our results indicate that BMP signaling is specifically required for continued incisor growth and cell fate determination in the adult mouse incisor.
Figure 2.
Loss of bone morphogenetic protein (BMP) signaling in Gli1+ cells arrests incisor growth. (A, B) Micro–computed tomography analysis of incisors from 1-mo-old Bmpr1afl/fl (A) and Gli1-CreERT2;Bmpr1afl/fl (B) mice 4 wk after tamoxifen induction (4wpt). Arrow indicates proximal end of Bmpr1afl/fl incisors and arrowhead indicates lack of proximal end of Gli1-CreERT2;Bmpr1afl/fl incisors. (C, D′) Hematoxylin and eosin staining of sagittal sections of mandibular incisors from 1-mo-old Bmpr1afl/fl (C, C′) and Gli1-CreERT2;Bmpr1afl/fl (D, D′) mice 4 wk after tamoxifen induction (4wpt). Boxes in C and D are magnified in C′ and D′, respectively. Arrow in C indicates dentin formation and asterisk in D indicates absence of dentin formation. Dotted lines in C′ and D′ indicate the cervical loop’s outline. (E, F) Dspp RNAscope in situ hybridization (red) of sagittal sections of mandibular incisors from control (E) and Gli1-CreERT2;Bmpr1afl/fl (F) mice 4 wk after tamoxifen (4wpt) induction at 1 mo of age. Arrow indicates Dspp+ odontoblasts and asterisk indicates absence of Dspp expression. (G, H) Ki-67 immunostaining (green) of sagittal sections of mandibular incisors from 1-mo-old Bmpr1afl/fl (G) and Gli1-CreERT2;Bmpr1afl/fl (H) mice 1 wk after tamoxifen induction (1wpt). Arrows indicate Ki-67 expression in the preodontoblast/odontoblast region (boxed area) of the incisor. (I) Quantitation of Ki-67+ cells in Bmpr1afl/fl (control) and Gli1-CreERT2;Bmpr1afl/fl (mutant) incisor odontogenic corresponding to the boxed areas in G and H, respectively. Quantitation was performed by calculating the percentage of Ki-67+ cells per section (n = 4). *P < 0.05. (J, K) Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining (green) of sagittal sections of mandibular incisors from 1-mo-old Bmpr1αfl/fl control (J) and Gli1-CreERT2;Bmpr1afl/fl (K) mice 1 wk after tamoxifen induction (1wpt). Asterisks in J and K indicate absence of apoptotic activities in the entire proximal ends of incisors. (L, M) Dspp RNAscope in situ hybridization (red) of sagittal sections of mandibular incisors from 1-mo-old Bmpr1αfl/fl control (L) and Gli1-CreER;Bmpr1αfl/fl (M) mice 1 wk after tamoxifen induction (1wpt). Arrow in L indicates Dspp+ odontoblasts in the control odontogenic region, and asterisk in M indicates absence of signaling in the Gli1-CreERT2;Bmpr1αfl/fl odontogenic region. Schematic diagram at the bottom indicates induction protocol. Scale bars (A, B) 150μm; (C–H, J–M), 100 µm.
Previous studies showed that Gli1+ cells in adult mouse incisors contribute to mesenchymal as well as epithelial cell lineages (Zhao et al. 2014). To rule out the possibility that the odontoblast defect in Gli1-CreERT2; Bmpr1afl/fl mice was a secondary effect caused by loss of BMP signaling in the dental epithelium, we generated K14-rtTA;tetO-Cre;Bmpr1afl/fl mice, in which BMP signaling was specifically ablated in Gli1+-derived dental epithelial cells. Four weeks after doxycycline induction at 1 mo of age, dentin formation was unaffected in the proximal end of incisors of K14-rtTA;tetO-Cre;Bmpr1afl/fl mice based on micro-CT analysis (Appendix Fig. 5A, B). As expected, pSmad1/5/9 expression was lost only in the epithelial tissue and not in the mesenchyme of K14-rtTA;tetO-Cre;Bmpr1afl/fl mice (Appendix Fig. 5C, D). More important, expression of odontoblast differentiation marker Dspp in K14-rtTA;tetO-Cre;Bmpr1afl/fl mice was indistinguishable compared to Bmpr1afl/fl control mice (Appendix Fig. 5E, F). The results demonstrate that BMP signaling in the dental mesenchyme, rather than in the dental epithelium, is specifically required to regulate odontoblast differentiation and dentin formation in adult mouse incisors, consistent with our previous study on molar root dentinogenesis (Feng et al. 2017).
Loss of BMP Signaling in the Adult Mouse Incisor Results in Upregulation of WNT and FGF Signaling Pathways
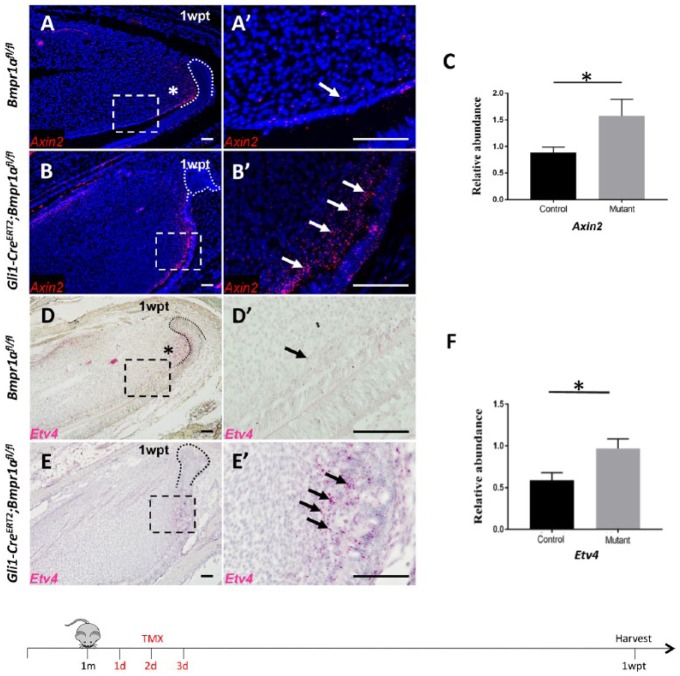
To investigate how BMP signaling regulates the balance between proliferation and differentiation, we explored signaling networks related to proliferation of TACs in incisors. Previous studies demonstrated that WNT signaling activity (An et al. 2018) and FGF ligands (Fgf 3 and Fgf10) expression are typically detectable in the TAC region (Harada et al. 2002; Wang et al. 2007). We analyzed activities of WNT and FGF signaling in Gli1-CreERT2;Bmpr1afl/fl incisors using Axin2 and Etv4 as readouts, respectively. In control incisors, WNT and FGF signaling was highly active in the TAC region, proximal to the region where BMP signaling was activated (Fig. 3A, D). One week after tamoxifen induction, in Gli1-CreERT2;Bmpr1afl/fl mice, WNT signaling was increased in dental pulp cells close to the preodontoblast region compared to controls (Fig. 3A, A′, B, B′). Similarly, we found that Etv4 expression was upregulated in the same region (Fig. 3D, D′, E, E′). We confirmed the upregulation of WNT and FGF signaling by quantitative polymerase chain reaction (qPCR) analysis (Fig. 3C, F). In addition, we found that ectopic WNT and FGF signaling activity overlapped with the region where proliferation was upregulated (Fig. 2H), suggesting that increased WNT and FGF signaling may be responsible for ectopic proliferation and odontoblast differentiation defects in Gli1-CreERT2;Bmpr1afl/fl mice.
Figure 3.
Loss of bone morphogenetic protein (BMP) signaling in the adult mouse incisor upregulates WNT and FGF signaling pathways in the odontogenic region. (A–B′) RNAscope in situ hybridization (red) of Axin2 in sagittal sections of mandibular incisors from 1-mo-old Bmpr1αfl/fl and Gli1-CreERT2;Bmpr1afl/fl mice 1 wk after tamoxifen induction (1wpt). Boxes in A and B outline the preodontoblast/odontoblast region and are shown magnified in A′ and B′, respectively. Asterisk indicates Axin2 expression in the transit-amplifying cell (TAC) region. Arrows indicate Axin2 expression in the preodontoblast/odontoblast region. Dotted lines in A and B indicate the cervical loop’s outline. (C) Quantitative polymerase chain reaction (PCR) analysis of Axin2 in 1-mo-old Bmpr1αfl/fl (control) and Gli1-CreERT2;Bmpr1afl/fl (mutant) incisors 1 wk after tamoxifen induction. n = 4, *P < 0.05. (D, E′) Etv4 RNAscope in situ hybridization (red) of sagittal sections of mandibular incisors from 1-mo-old Bmpr1αfl/fl and Gli1-CreER;Bmpr1αfl/fl mice 1 wk after tamoxifen induction (1wpt). Boxes in D and E outline preodontoblast/odontoblast region and are shown magnified in D′ and E′, respectively. Asterisk indicates Etv4 expression in the TAC region. Arrows indicate Etv4 expression in the preodontoblast/odontoblast region. Dotted lines in D and E indicate the cervical loop’s outline. (F) qPCR analysis of Etv4 in 4-wk-old Bmpr1αfl/fl (control) and Gli1-CreERT2;Bmpr1afl/fl (mutant) incisors 1 wk after tamoxifen induction. n = 4, *P < 0.05. Schematic diagram at the bottom indicates induction protocol. Scale bars, 100 µm.
Compromised BMP Signaling in Preodontoblasts Leads to a Diminished Gli1+ MSC Population
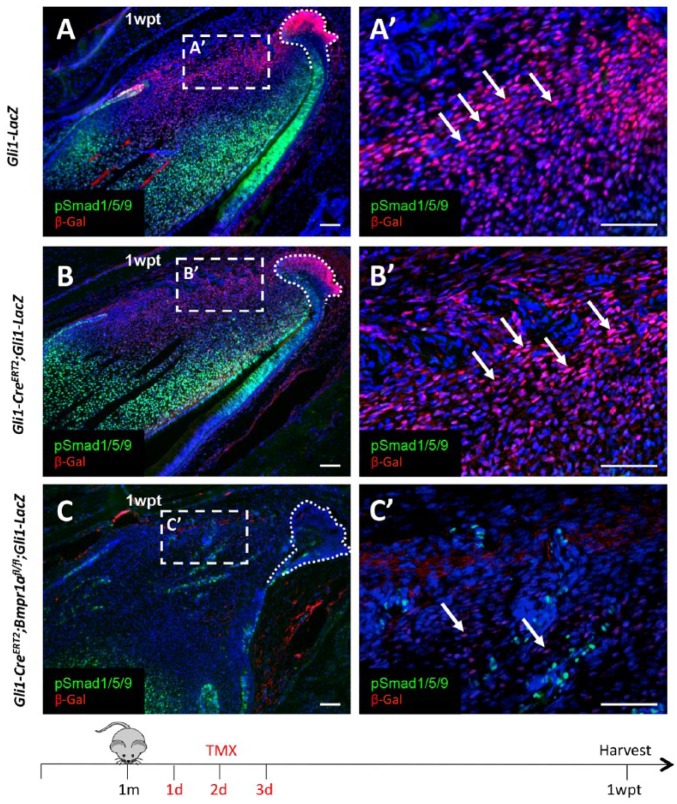
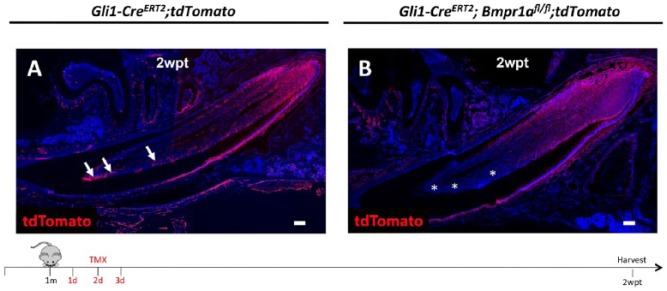
Based on previous studies showing that stem cell progeny residing in close proximity to stem cells can also regulate stem cell homeostasis (Hsu et al. 2014), we hypothesized that the MSC population is affected after loss of BMP signaling in Gli1+ progeny in mouse incisors. To rule out the possibility that Gli1 deficiency contributes to a diminished Gli1+ cell population, we compared Gli1-LacZ and Gli1-CreERT2;Gli1-LacZ mouse incisors and found that distribution patterns of Gli1+ MSCs in these groups were similar. Next, we examined Gli1 expression in Gli1-CreERT2;Bmpr1afl/fl;Gli1-LacZ mice and found that Gli1+ MSCs were greatly reduced in number compared to the controls (Fig. 4A, A′, B, B′, C, C′). To further investigate whether the loss of Gli1+ MSCs was due to accelerated differentiation, we compared the MSCs’ contribution to their progeny in control (Gli1-CreERT2;tdTomato) and mutant (Gli1-CreERT2;Bmpr1afl/fl;tdTomato) incisors 2 wk after tamoxifen induction. We observed a reduced number of Gli1+ progeny in mutant incisors, particularly in the more distal region, suggesting that the differentiation rate of Gli1+ MSCs was slower, rather than faster, in the mutant incisors (Fig. 5A, B). Since we did not find increased apoptosis in mutant incisors, reduction of the MSC population was likely due to impaired self-renewal of Gli1+ MSCs. Considering these findings, BMP signaling in the preodontoblast/odontoblast region may provide feedback to MSCs in the mouse incisor to sustain the MSC population and maintain tissue homeostasis.
Figure 4.
Compromised bone morphogenetic protein (BMP) signaling in Gli1-derived progeny leads to diminished Gli1+ mesenchymal stem cells (MSCs). (A–C′) β-Gal (red) and pSmad1/5/9 (green) double immunostaining of sagittal sections of incisors from Gli1-LacZ (A), Gli1-CreERT2;Gli1-LacZ (B), and Gli1-CreERT2;Bmpr1αfl/fl;Gli1-LacZ (C) mice 1 wk posttamoxifen induction (1wpt) at 1 mo of age. Boxes in A, B, and C are shown magnified in A′, B′, and C′, respectively. Arrows indicate Gli1+ cells in the proximal region of the incisor. Dotted lines in A, B, and C indicate the cervical loop’s outline. Schematic diagram at the bottom indicates induction protocol. Scale bars, 100 µm.
Figure 5.
Differentiation rate of Gli1+ mesenchymal stem cells (MSCs). (A, B) tdTomato (red) immunostaining of sagittal sections of incisors from Gli1-CreERT2;tdTomato (A) and Gli1-CreERT2; Bmpr1afl/fl;tdTomato (B) mice 2 wk posttamoxifen induction (2wpt) at 1 mo of age. Arrows indicate Gli1+ cells in the distal region of the incisor. Asterisks indicate absence of Gli1+ cells in the distal region of the incisor. Schematic diagram at the bottom indicates induction protocol. Scale bars, 100 µm.
Discussion
Homeostasis is a dynamic state of equilibrium that serves to maintain steady internal conditions for optimal function in an organism. Our investigation of incisor homeostasis focuses on the MSC population residing in the proximal region of the mouse incisor that continuously gives rise to TACs and odontoblasts, thus ultimately contributing to dentin formation to sustain continuous and lifelong incisor growth. This replenishment from the proximal end of the incisor balances out the continual loss at the distal tip due to gnawing. In this study, we found that loss of function of BMP signaling in Gli1-derived dental mesenchymal cells had unexpected effects on tissue homeostasis in adult incisors—increased proliferation and defective odontoblast differentiation associated with upregulated WNT and FGF signaling. More important, our study reveals that ablation of BMP signaling in Gli1-derived dental mesenchymal cells sends feedback to Gli1+ MSCs and provides new insights into the biological function of BMP signaling in regulating MSC fate during tissue homeostasis.
BMP signaling functions as a key regulator for multiple developmental events as well as for maintenance of adult tissue homeostasis (Wang et al. 2014). During tooth development, BMP signaling controls tooth crown patterning and morphogenesis (Vainio et al. 1993; Andl et al. 2004; Kassai et al. 2005). It is also indispensable for odontoblast differentiation during molar root elongation (Feng et al. 2017). Similarly, our results demonstrate that BMP signaling is specifically activated when Gli1+ MSC progeny undergo odontogenic differentiation in the mouse incisor. Moreover, when BMP is ablated from the Gli1+ MSC lineage, these cells fail to differentiate into postmitotic odontoblasts and instead maintain their proliferative status. During lineage commitment, this may reflect that distinct signaling pathways are required to regulate the MSC differentiation hierarchy. We observed that FGF and WNT signaling pathways were activated when MSCs transited into highly proliferative TACs prior to odontoblast differentiation in the mouse incisor, then became repressed when TACs started to differentiate into preodontoblasts/odontoblasts. The reduction of FGF and WNT signaling coincided with the activation of BMP signaling during this proliferation-to-differentiation switch, suggesting that BMP signaling antagonizes WNT/FGF signaling to facilitate this event. Furthermore, absence of BMP signaling in the preodontoblasts/odontoblasts led to ectopic activation of WNT and FGF signaling pathways, which may contribute to abnormal maintenance of their proliferative status, consistent with the result of an increased Ki-67 signal in the ectopic site. Collectively, the tight regulation of various signaling pathways may play an essential role in cell dynamics during incisor homeostasis.
BMP signaling mediates diverse signaling pathways to regulate the balance between MSC-derived cell proliferation and differentiation during tissue homeostasis. In our Bmpr1a mutant model, multiple cell types were affected by the loss of BMP signaling. The MSC population was reduced, ectopic proliferative cells were found in the preodontoblast region, odontoblast differentiation was impaired, and ectopic chondrocytes were found in the dental pulp cavity at later time points after tamoxifen induction. In addition, we noticed that when BMP signaling is abrogated in the mesenchyme, it can cause the epithelium to collapse but not vice versa, indicating the mesenchyme’s key role in epithelial-mesenchymal interaction within incisor homeostasis. Our findings are consistent with a previous report that mesenchymal-derived signals such as WNT and FGF regulate cell survival and proliferation in the cervical loop epithelium (Harada et al. 2002; Yang et al. 2015).
In non-self-renewing organs, such as mouse molars, previous studies demonstrated that BMP signaling coordinates a transcriptional network to regulate the fate of MSCs (Feng et al. 2017). Specifically, loss of BMP signaling in Gli1-derived dental MSCs results in defective odontoblast differentiation during the limited growth of molar roots (Feng et al. 2017). However, compromised BMP signaling in self-renewing organs, such as mouse incisors, leads to different outcomes. In this study, we found that ablation of BMP signaling in Gli1-derived dental pulp MSCs in mouse incisors affected odontoblast differentiation, consistent with the function of BMP signaling in non-self-renewing mouse molars (Feng et al. 2017). We further found that BMP also signals back to MSCs, leading to the diminution of the Gli1+ MSC population and arrested incisor growth. Because activated BMP signaling is present in preodontoblasts/odontoblasts as well as in a few TACs in mouse incisors, we suggest that loss of BMP signaling in these cell populations likely has dual effects—inhibiting odontoblast differentiation in a forward manner, similar to what was reported for mouse molars (Feng et al. 2017), and affecting MSC maintenance in a feedback manner, similar to what was observed in hair follicles (Hsu et al. 2014). However, loss of BMP signaling in preodontoblasts and odontoblasts may not have a direct effect on MSCs. Further study is required to investigate molecular and cellular mechanisms through which preodontoblasts and odontoblasts provide feedback to MSCs. Significantly, our studies shed light on how BMP signaling plays dual roles in regulating the fate of MSCs during tissue homeostasis.
In conclusion, our study highlights multiple roles of BMP signaling in regulating tissue homeostasis—balancing proliferation and differentiation, as well as maintaining the MSC population. This study expands our understanding of how BMP signaling tightly regulates the MSC progeny hierarchy during lineage commitment and provides insight into how BMP antagonizes WNT and FGF signaling pathways to regulate tissue homeostasis. The implications of these findings for our understanding of the molecular mechanisms of lineage commitment and maintenance of dental pulp MSCs are significant and can be applied to novel biological approaches for tooth regeneration.
Author Contributions
C. Shi, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript; Y. Yuan, Y. Guo, J. Jing, T.V. Ho, X. Han, contributed to analysis, critically revised the manuscript; J. Li, contributed to data analysis, drafted and critically revised the manuscript; J. Feng, contributed to data interpretation, critically revised the manuscript; Y. Chai, contributed to conception and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519850812 for BMP Signaling in Regulating Mesenchymal Stem Cells in Incisor Homeostasis by C. Shi, Y. Yuan, Y. Guo, J. Jing, T.V. Ho, X. Han, J. Li, J. Feng and Y. Chai in Journal of Dental Research
Acknowledgments
We thank Sarah E. Millar for Bmpr1afl/fl mice. We also thank Julie Mayo, Bridget Samuels, and Linda Hattemer for their critical reading of the manuscript.
Footnotes
This work was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health (R01 DE025221 and R01 DE026339 to Y. Chai).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is available online.
References
- An Z, Akily B, Sabalic M, Zong G, Chai Y, Sharpe PT. 2018. Regulation of mesenchymal stem to transit-amplifying cell transition in the continuously growing mouse incisor. Cell Rep. 23(10):3102–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. 2004. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 131(10):2257–2268. [DOI] [PubMed] [Google Scholar]
- Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. 2013. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 19(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Jheon A, Li X, Sun Z, Wang J, Florez S, Zhang Z, McManus MT, Klein OD, Amendt BA. 2013. The Pitx2:miR-200c/141:noggin pathway regulates Bmp signaling and ameloblast differentiation. Development. 140(16):3348–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Jing J, Li J, Zhao H, Punj V, Zhang T, Xu J, Chai Y. 2017. BMP signaling orchestrates a transcriptional network to control the fate of mesenchymal stem cells in mice. Development. 144(14):2560–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. 2011. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci USA. 108(16):6503–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Yuan Y, Wu L, Ho TV, Jing J, Sugii H, Li J, Han X, Feng J, Guo C, et al. 2018. BMP-IHH-mediated interplay between mesenchymal stem cells and osteoclasts supports calvarial bone homeostasis and repair. Bone Res. 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, Sekine K, Ohuchi H. 2002. FGF10 maintains stem cell compartment in developing mouse incisors. Development. 129(6):1533–1541. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. 2014. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 157(4):935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassai Y, Munne P, Hotta Y, Penttila E, Kavanagh K, Ohbayashi N, Takada S, Thesleff I, Jernvall J, Itoh N. 2005. Regulation of mammalian tooth cusp patterning by ectodin. Science. 309(5743):2067–2070. [DOI] [PubMed] [Google Scholar]
- Kfoury Y, Scadden DT. 2015. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 16(3):239–253. [DOI] [PubMed] [Google Scholar]
- Kleber M, Lee HY, Wurdak H, Buchstaller J, Riccomagno MM, Ittner LM, Suter U, Epstein DJ, Sommer L. 2005. Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J Cell Biol. 169(2):309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang-Hsien Hu J, Mushegyan V, Klein OD. 2014. On the cutting edge of organ renewal: identification, regulation, and evolution of incisor stem cells. Genesis. 52(2):79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Huang X, Xu X, Mayo J, Bringas P, Jr, Jiang R, Wang S, Chai Y. 2011. SMAD4-mediated WNT signaling controls the fate of cranial neural crest cells during tooth morphogenesis. Development. 138(10):1977–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I. 2007. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 8(8):583–596. [DOI] [PubMed] [Google Scholar]
- Michelozzi IM, Pievani A, Pagni F, Antolini L, Verna M, Corti P, Rovelli A, Riminucci M, Dazzi F, Biondi A, et al. 2017. Human aplastic anaemia-derived mesenchymal stromal cells form functional haematopoietic stem cell niche in vivo. Br J Haematol. 179(4):669–673. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Feki A, Papaccio G, Catón J. 2011. Dental pulp stem cells, niches, and notch signaling in tooth injury. Adv Dent Res. 23(3):275–279. [DOI] [PubMed] [Google Scholar]
- Neubuser A, Peters H, Balling R, Martin GR. 1997. Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell. 90(2):247–255. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Zeichner-David M, Mayer JA, Reyna J, Bringas P, Thewissen JG, Snead ML, Chai Y, Chuong CM. 2005. Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity. Evol Dev. 7(5):440–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons BD, Clevers H. 2011. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 145(6):851–862. [DOI] [PubMed] [Google Scholar]
- Tucker A, Sharpe P. 2004. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 5(7):499–508. [DOI] [PubMed] [Google Scholar]
- Vainio S, Karavanova I, Jowett A, Thesleff I. 1993. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 75(1):45–58. [PubMed] [Google Scholar]
- Valtieri M, Sorrentino A. 2008. The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 217(2):296–300. [DOI] [PubMed] [Google Scholar]
- Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, et al. 2014. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 1(1):87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, Maas RL, Chuong CM, Schimmang T, Thesleff I. 2007. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 5(6):e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Balic A, Michon F, Juuri E, Thesleff I. 2015. Mesenchymal Wnt/β-catenin signaling controls epithelial stem cell homeostasis in teeth by inhibiting the antiapoptotic effect of Fgf10. Stem Cells. 33(5):1670–1681. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang J, Deng F, Huang E, Yan Z, Wang Z, Deng Y, Zhang Q, Zhang Z, Ye J, et al. 2015. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs). Biomaterials. 39:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Feng J, Ho TV, Grimes W, Urata M, Chai Y. 2015. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 17(4):386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, Chai Y. 2014. Secretion of Shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 14(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519850812 for BMP Signaling in Regulating Mesenchymal Stem Cells in Incisor Homeostasis by C. Shi, Y. Yuan, Y. Guo, J. Jing, T.V. Ho, X. Han, J. Li, J. Feng and Y. Chai in Journal of Dental Research