Abstract
Background.
Adult kidney transplantation is most commonly into an extraperitoneal potential space, and surgically placed drains are used routinely in many centers. There is limited evidence of clinical benefit for prophylactic drainage in other major abdominal and vascular surgery. Transplantation is, however, a unique setting combining organ dysfunction and immunosuppression, and the risks and benefits of prophylactic drain placement are not known. This study attempts to examine existing literature to determine whether prophylactic intraoperative drains have an impact on the likelihood of perigraft fluid collections and other wound-related complications following kidney transplantation.
Methods.
A literature search of MEDLINE and EMBASE was conducted to identify published comparative studies, including recipients receiving prophylactic drains to recipients in whom drains were omitted. The main outcomes were the incidence of peritransplant fluid collections and wound-related complications. Meta-analysis was performed on these data.
Results.
Four retrospective cohort studies were deemed eligible for quantitative analysis and 1 additional conference abstract was included in qualitative discussion. A total of 1640 patients, 1023 with drains and 617 without, were included in the meta-analysis. There was a lower rate of peritransplant collections associated with the drain group (RR 0.62; 95% confidence interval, 0.42-0.90). There was no significant difference in the incidence of wound-related complications between the groups (RR 0.85; 95% confidence interval, 0.34-2.11).
Conclusions.
These data associate a higher rate of peritransplant fluid collections with omission of prophylactic drainage, without a difference in the incidence of wound-related complications. Further research is required to definitively determine the impact of drains in this patient group.
INTRODUCTION
With improving perioperative care, recipient and donor selection, and changes to immunosuppressive regimens, kidney transplantation is increasingly being performed in older and more comorbid recipients, while outcomes remain stable or have improved.1 Although wound complications do not often impact on long-term graft or patient outcomes, they contribute significantly to postoperative morbidity, cost, and the quality of patient-reported outcomes. These complications are most often wound infections, superficial dehiscence, fascial dehiscence, evisceration, or perigraft fluid collections.1-4
In the context of kidney transplantation, perigraft fluid collections generally occur secondary to lymphatic leak from unsealed lymph vessels at the donor kidney hilum and/or the recipient’s own lymphatics within the extraperitoneal space.5-9 The reported incidence of perigraft fluid collections is highly variable, ranging between 0.6% and 51%.5-13 Most of these are small, not associated with symptoms or graft dysfunction, and incidentally detected on routine ultrasound scanning.14 Although the peak incidence is 2–6 weeks posttransplant, these collections have been known to occur 6 months following surgery.15,16 The significant variation of the reported incidence in the literature is likely due to variable utilization of posttransplant surveillance imaging and the lack of a precise definition of what is considered a significant collection, particularly in terms of volume. In this study, we shall use the term perigraft fluid collections to encompass all fluid collections adjacent to a kidney transplant. Other terms used interchangeably in the literature include lymphocele or seroma but lack any universally accepted definition.
Prophylactic drains are often inserted by surgeons in the extra peritoneal space surrounding the graft, primarily to prevent the accumulation of these fluid collections.17 However, in most cases, these drains are removed weeks before the peak incidence of fluid accumulation. Commonly accepted risk factors for the development of symptomatic collections are obesity, diabetes, age, smoking, delayed graft function, poor nutrition, and surgical technique.3,18-22 However, few studies have examined whether the presence of a prophylactic drain inserted at the termination of surgery reduces the incidence of complications, particularly the development of perigraft collections, symptomatic or otherwise.1,17,23,24
Previous research into the effectiveness of prophylactic drainage in reducing the incidence of complications following gastrointestinal,25 vascular,26 thyroid,27 and breast cancer surgery28,29 have not universally substantiated a benefit to drainage. Additionally, the presence of a prophylactic drain remaining in a postoperative wound may increase the risk of infection of the surgical site and be a cause of postoperative pain.28–31 Notwithstanding these data on the use of prophylactic drains in other contexts, the immunosuppressed state in transplantation may promote the incidence of surgical site complications, such as fluid accumulation,1,3,4,20,32-35 and hence, extrapolating data from other surgery to the kidney transplant population may be problematic. The aim of this review was to assess the evidence for prophylactic drain insertion in kidney transplantation, specifically with respect to the incidence of posttransplant perigraft fluid collections.
MATERIALS AND METHODS
The study protocol for this systematic review was prospectively registered with PROSPERO (CRD42017058451) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.36
Search
A systematic search was applied to MEDLINE and EMBASE to identify all publications that reported on the presence of a perigraft fluid collection following renal transplantation when comparing patient groups with and without intraoperative prophylactic drains. Search keywords were: “Lymphocele,” “Seroma,” “Perigraft fluid collection,” “Drainage,” “prophylactic drain,” “intraoperative drain,” and “Kidney transplantation.” Subject headings were adjusted to comply with the specific indexing system of each database. All articles were vetted sequentially by title, abstract, and full text by 2 independent reviewers at each step. All disagreements were resolved by consensus after discussion between the reviewers. Reference lists of all included articles were searched manually for further studies also meeting inclusion criteria.
Study Selection and Inclusion Criteria
Randomized controlled trials and observational articles were considered appropriate for this review, including only studies in English. Articles were included only if they presented data for at least 10 patients per group and included information regarding use of an intraoperative prophylactic drain and absence or presence of a fluid collection following renal transplant. Pediatric and animal studies, and studies without comparator groups, were excluded. Case reports, systematic reviews, meta-analyses, and editorials were also excluded.
Data Extraction
Two independent researchers (K.D. and S.P.C.) extracted the data into a template consisting the following: authors, study date, recipient age, diabetes status, body mass index, use of an intraoperative drain, follow-up period, molecular target of rapamycin (mTOR) inhibitor use, and rates of perigraft fluid collection. In case of disagreement, a third reviewer (J.M.L.) was consulted.
Study characteristics including definitions used for perigraft fluid collections and the diagnostic criteria used to identify the presence of a fluid collection and the protocol for the removal of drains were also extracted by 2 independent authors, and disagreements were resolved by consensus.
Outcomes
Primary study outcome included the presence of a perigraft fluid collection as defined by the authors of the respective studies. Secondary outcomes included wound complications, which were defined as evisceration, dehiscence, and/or infection.
Statistical Analysis
Only observational studies were included in the meta-analysis as no randomized controlled trials were found. Risk ratios between comparable groups were estimated using DerSimonian and Laird random-effects models. Random-effects models were used as they are more conservative in their effect estimation in the presence of potential heterogeneity. Where the authors distinguished between lymphoceles, seromas, or perigraft fluid collections, these outcomes were combined into 1 outcome, perigraft fluid collections, for the purposes of meta-analysis. Data were entered and analyzed using REVMAN 5.3 software. For the purposes of assessing wound complication rates, including evisceration, dehiscence, or infection, cumulative rates were combined into 1 variable for the purposes of meta-analysis.
Risk of Bias
Two authors independently ascertained risk of bias using the Newcastle-Ottawa quality assessment tool for Cohort studies.37
Missing Data
Where the studies did not specify the number of patients in each subgroup, the results were examined to determine if the numbers in each group could be calculated using the odds ratio or risk ratios given in the respective articles.
Assessing Heterogeneity
Heterogeneity was assessed using the Q test and I2 index. Degree of heterogeneity was classified as none (I2 <25%); low (I2 25–49%); moderate (I2 50–74%); and high (I2 75–100%).
Assessing Reporting Bias
Formal assessment of reporting bias using funnel plots was not possible due to the limited number of studies.
RESULTS
Overall Study Selection and Data Extraction
Articles comparing fluid collection incidence with and without drains following renal transplantation were analyzed. The study selection is summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram (Figure 1). Ninety-six studies were identified, and following screening, 5 studies were deemed eligible for analysis. Of these 5, 4 were retrospective single-center studies and the remaining was a conference abstract detailing a prospective randomized trial. The conference abstract did not provide sufficient data to include in meta-analysis and so was excluded in statistical analysis.
FIGURE 1.
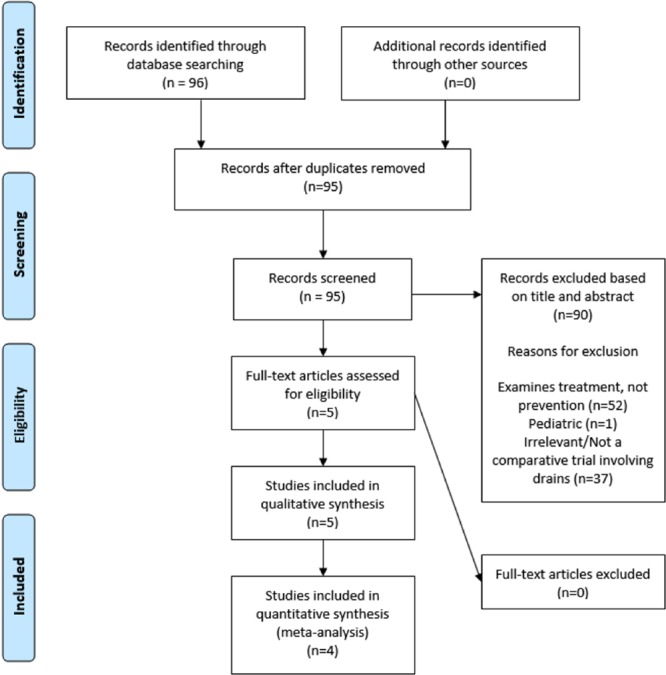
PRISMA flow diagram for studies including qualitative and quantitative analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study Characteristics
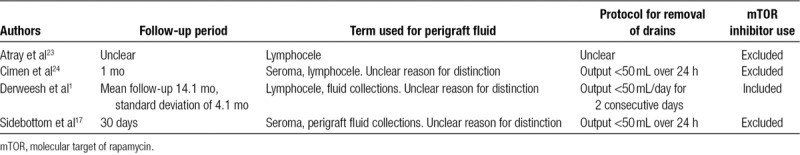
Of the 4 cohort studies included, total number of participants varied from 13823 to 680.17 Follow-up duration, the terms used for fluid collections (eg, lymphocele, perigraft fluid collections etc.), protocol for the removal of the drains, and use of mTOR inhibitors in each of the studies is summarized in Table 1.
TABLE 1.
Study characteristics of the 4 studies included in meta-analysis
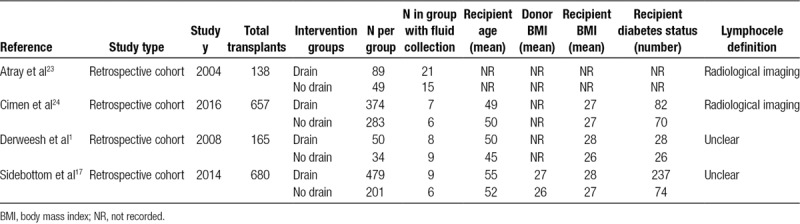
Baseline patient characteristics, including comparator groups and donor and recipient information, as well as fluid collection rates are summarized in Table 2. A total of 1640 kidney transplants, outlined in the 4 retrospective cohort studies1,17,23,24 were included in the analysis. Of these patients, 1023 patients had prophylactic drains inserted and 617 had no drains.
TABLE 2.
Baseline characteristics and incidence of lymphoceles in drain and no drain groups for included studies
The prospective Randomized Controlled Trial as described by Fahmy et al38 includes 315 patients in total, randomly assigned into drain and no drain groups (203 patients and 112 patients, respectively). This abstract did not state mTOR inhibitor use, and furthermore, it was not possible to determine what definition was used to classify the presence of a lymphocele. This study also did not report rates of fluid collection in drain and no drain groups and so was not included in the quantitative analysis due to lack of specific information.
Comparison of Outcomes in Drain and No Drain Groups
The impact of drains on the incidence of fluid collections was examined using a DerSimonian and Laird random-effects model. Results are shown in Figure 2. These data show a statistically significant reduction in the risk of perigraft fluid collections attributed to the use of prophylactic drain insertion (relative risk 0.62; 95% confidence interval, 0.42-0.90, P value = 0.01). The impact of drains on the incidence of wound complications was examined in a similar manner. The wound complications of interest and their reported incidence in each individual study is shown in Table 3, and the meta-analysis results are shown in Figure 3. These data do not show a statistically significant difference in terms of outcomes between drain and no drain groups (relative risk 0.88; 95% confidence interval, 0.41-1.87, P value = 0.73).
FIGURE 2.
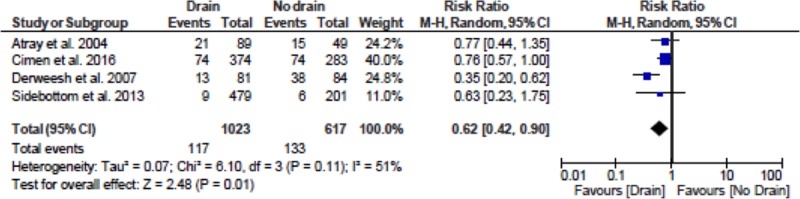
Meta-analysis for rate of incidence of perigraft fluid collections in drain and no drain groups. CI, confidence interval.
TABLE 3.
Wound complications as reported by the authors of the respective studies

FIGURE 3.
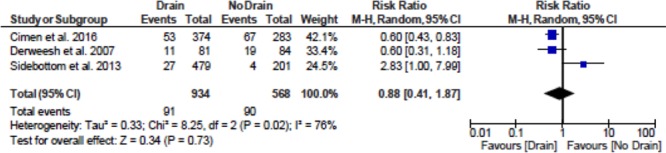
Meta-analysis for rate of incidence of wound complications in drain and no drain groups. CI, confidence interval.
Risk of Bias
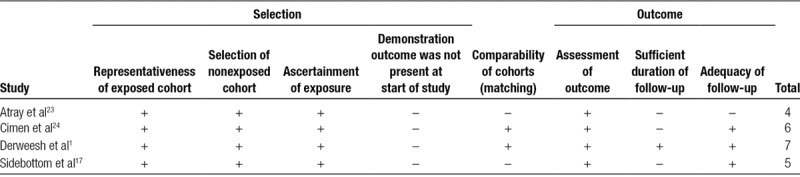
The risk of bias for included studies using the Newcastle-Ottawa tool for cohort studies is shown in Table 4. Overall, the representativeness and selection of the exposed cohort, ascertainment of exposure to the intervention, and assessment of the outcome scored highly in all the included articles. Demonstration that the outcome of interest was not present at the start of the study scored low in all included articles. The remaining domains showed mixed results.
TABLE 4.
Risk of bias table for included studies using the Newcastle-Ottawa scale for cohort studies
DISCUSSION
This study has attempted to describe the relationship between insertion of prophylactic drain postrenal transplantation and its effect on the development of postoperative peritransplant fluid collections. Drain insertion reduced the risk of subsequent perigraft fluid collections but this did not translate into an increased risk of wound complications.
This study, however, suffers from a number of significant limitations. Foremost, there is the issue of a lack of precise definition as to what constitutes a clinically significant perigraft fluid collection. The terms “lymphocele” and “seroma” are often used interchangeably in the literature, but these terms are not clearly defined. Recently, the term “symptomatic lymphocele” has been introduced into the literature which implies that the fluid collection “required” intervention. This term, however, is not reproducible, as while it does suggest the fluid collection is more clinically significant, the need for intervention is subjective and dependent on the supervising physician’s judgement. Some studies in this analysis only included symptomatic lymphocele, while some did not specify. We would have preferred to include only “symptomatic lymphocele” in this analysis, however, the term is not universally accepted in the literature and exclusion of all data where this was not made explicit would have excluded most of the study data.
Another important issue is that all studies included in meta-analysis were vulnerable to selection bias as they were nonrandomized. Typically, these studies generated 2 dichotomous groups based on surgeons’ preference for drain insertion or practice era effects. In the case where the surgeon’s preference is to omit drain insertion, this may correlate with a preference towards routine imaging of these patients and thus coincide with a higher likelihood of diagnosing fluid collections. With era-based selection, changes in immunosuppression regimes could have a significant effect on rates of collection development, as can era-based changes in practice with respect to timing and frequency of imaging and protocol biopsy. It is equally possible that selection bias may underestimate the apparent effect of prophylactic drain insertion. If patients who were deemed high risk for the development of fluid collections were more likely to have drains inserted, and those with a nonelevated risk were not, this would mask an even greater advantage to drain insertion than was detected in the analysis. These fundamental selection issues cannot be overcome without randomization.
There is significant methodological heterogeneity in these studies, which may confound interpretation of the results. For example, the included studies have variable follow-up time with Sidebottom et al17 and Cimen et al24 ceasing to follow up patients after 1 month, well before the end of the peak incidence period for the development of perigraft fluid collections. There were also variable practice patterns for the removal of drains in the postoperative phase. There were also a variable proportion of patients receiving mTOR inhibitors in these studies, a well-known risk factor for the development of fluid collections.14 We also have very little information with respect to follow-up protocol, particularly with respect to imaging.
One study, of apparently high methodological quality, was found. This study by Fahmy et al38 was a prospective randomized controlled trial of 315 live donor recipients from Alexandria, Egypt. The study was only reported in abstract form with only a univariate analysis and without primary outcome data. Therefore, it could not be included in our analysis. Again, there were no definitions of lymphocele, postoperative protocol, or complications. However, the outcomes were in favor of prophylactic drain insertion.
There is also the issue of biological plausibility; it is difficult to explain how drains that are typically only retained for a few days postoperatively prevent the development of fluid collections which have a peak incidence some weeks later. Often there is imaging between the time of drain removal and subsequent collection development, which confirms the absence of peritransplant fluid collection. One could only speculate that the drain incites an inflammatory response or tissue adherence from suction, but this is a matter of conjecture. The lack of a biologically plausible mechanism for prophylactic drainage must increase the suspicion that the data are affected by confounding factors.
It is also interesting to note the meta-analysis suggests no statistically significant differences in terms of complication rates between drain and no drain groups. While it has been suggested that inclusion of drains may decompress a surgical site and reduce the likelihood of wound breakdown and hernia formation, previous research into the effectiveness of drains in kidney transplant surgery13 and other types of surgery39 have suggested that a higher proportion of patients with drains develop surgical site infections. One would assume the inclusion of potent immunosuppressive regimes in transplant surgery would exacerbate this difference; however, it seems, at least in the data we have included, this is not the case. We are unable to posit any plausible mechanism for this difference in complication rates. It may indeed be possible that drains are not a risk factor for the development of surgical site infections in this patient group; however, such a statement would require more evidence than we are able to provide in this article.
Given the significant limitations involved in this meta-analysis, no robust practice recommendations can be made with respect to prophylactic drainage after kidney transplantation. The only way to definitively answer the question is through a prospective randomized study. To be meaningful, this study would have to apply precise definitions of clinically significant peritransplant fluid collections and rational indications for intervention. This would have to occur in the context of a protocol for postoperative imaging and protocol biopsy, which are themselves often the prime drivers behind the diagnosis of collections. Follow-up duration should attempt to go beyond 6 weeks and patient use of mTOR inhibitors should be controlled. Such a study would also provide more clarity on the potential adverse effects of drainage and overall balance of benefit and risk with prophylactic drainage. This would ideally bring us closer to a nuanced approach, where a well-defined group of high-risk patients is identified in whom the benefits of drainage are greater than the risks.
Footnotes
Published online 27 June, 2019.
K.D., A.H., and J.M.L. participated in the performance of the research, data analysis, and writing of the article. S.P.C. participated in the performance of the research. S.L., H.C.P., and C.P. participated in the writing of the article.
The authors declare no conflict of interest.
This project was completely supported with time donations in kind from the contributing authors.
REFERENCES
- 1.Derweesh IH, Ismail HR, Goldfarb DA, et al. Intraoperative placing of drains decreases the incidence of lymphocele and deep vein thrombosis after renal transplantation. BJU Int. 2008;101:1415–1419. [DOI] [PubMed] [Google Scholar]
- 2.Eufrásio P, Parada B, Moreira P, et al. Surgical complications in 2000 renal transplants. Transplant Proc. 2011;43:142–144. [DOI] [PubMed] [Google Scholar]
- 3.Mehrabi A, Fonouni H, Wente M, et al. Wound complications following kidney and liver transplantation. Clin Transplant. 2006;20(Suppl 17):97–110. [DOI] [PubMed] [Google Scholar]
- 4.Tiong HY, Flechner SM, Zhou L, et al. A systematic approach to minimizing wound problems for de novo sirolimus-treated kidney transplant recipients. Transplantation. 2009;87:296–302. [DOI] [PubMed] [Google Scholar]
- 5.Kay R, Fuchs E, Barry JM. Management of postoperative pelvic lymphoceles. Urology. 1980;15:345–347. [DOI] [PubMed] [Google Scholar]
- 6.Khauli RB, Stoff JS, Lovewell T, et al. Post-transplant lymphoceles: a critical look into the risk factors, pathophysiology and management. J Urol. 1993;150:22–26. [DOI] [PubMed] [Google Scholar]
- 7.Malovrh M, Kandus A, Buturović-Ponikvar J, et al. Frequency and clinical influence of lymphoceles after kidney transplantation. Transplant Proc. 1990;22:1423–1424. [PubMed] [Google Scholar]
- 8.Pollak R, Veremis SA, Maddux MS, et al. The natural history of and therapy for perirenal fluid collections following renal transplantation. J Urol. 1988;140:716–720. [DOI] [PubMed] [Google Scholar]
- 9.Silver TM, Campbell D, Wicks JD, et al. Peritransplant fluid collections. Ultrasound evaluation and clinical significance. Radiology. 1981;138:145–151. [DOI] [PubMed] [Google Scholar]
- 10.Ulrich F, Niedzwiecki S, Fikatas P, et al. Symptomatic lymphoceles after kidney transplantation – multivariate analysis of risk factors and outcome after laparoscopic fenestration. Clin Transplant. 2010;24:273–280. [DOI] [PubMed] [Google Scholar]
- 11.Lucewicz A, Wong G, Lam VW, et al. Management of primary symptomatic lymphocele after kidney transplantation: a systematic review. Transplantation. 2011;92:663–673. [DOI] [PubMed] [Google Scholar]
- 12.Goel M, Flechner SM, Zhou L, et al. The influence of various maintenance immunosuppressive drugs on lymphocele formation and treatment after kidney transplantation. J Urol. 2004;171:1788–1792. [DOI] [PubMed] [Google Scholar]
- 13.Fockens MM, Alberts VP, Bemelman FJ, et al. Wound morbidity after kidney transplant. Prog Transplant. 2015;25:45–48. [DOI] [PubMed] [Google Scholar]
- 14.Ranghino A, Segoloni GP, Lasaponara F, et al. Lymphatic disorders after renal transplantation: new insights for an old complication. Clin Kidney J. 2015;8:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowak K, Bönninghoff R, Geiger M, et al. Compression stockings limit the incidence of postoperative lymphocele in kidney transplantation. In Vivo. 2013;27:561–564. [PubMed] [Google Scholar]
- 16.Ebadzadeh MR, Tavakkoli M. Lymphocele after kidney transplantation: where are we standing now? Urol J. 2008;5:144–148. [PubMed] [Google Scholar]
- 17.Sidebottom RC, Parsikia A, Chang PN, et al. No benefit when placing drains after kidney transplant: a complex statistical analysis. Exp Clin Transplant. 2014;12:106–112. [PubMed] [Google Scholar]
- 18.Hetzel GR, Klein B, Brause M, et al. Risk factors for delayed graft function after renal transplantation and their significance for long-term clinical outcome. Transpl Int. 2002;15:10–16. [DOI] [PubMed] [Google Scholar]
- 19.Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968–974. [DOI] [PubMed] [Google Scholar]
- 20.Ramos A, Asensio A, Muñez E, et al. Incisional surgical site infection in kidney transplantation. Urology. 2008;72:119–123. [DOI] [PubMed] [Google Scholar]
- 21.Tobón J, Whitney JD, Jarrett M. Nutritional status and wound severity of overweight and obese patients with venous leg ulcers: a pilot study. J Vasc Nurs. 2008;26:43–52. [DOI] [PubMed] [Google Scholar]
- 22.Zrim S, Furlong T, Grace BS, et al. Body mass index and postoperative complications in kidney transplant recipients. Nephrology (Carlton). 2012;17:582–587. [DOI] [PubMed] [Google Scholar]
- 23.Atray NK, Moore F, Zaman F, et al. Post transplant lymphocele: a single centre experience. Clin Transplant. 2004;18(Suppl 12):46–49. [DOI] [PubMed] [Google Scholar]
- 24.Cimen S, Guler S, Tennankore K, et al. Surgical drains do not decrease complication rates but are associated with a reduced need for imaging after kidney transplant surgery. Ann Transplant. 2016;21:216–221. [DOI] [PubMed] [Google Scholar]
- 25.Petrowsky H, Demartines N, Rousson V, et al. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg. 2004;240:1074–84; discussion 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karthikesalingam A, Walsh SR, Sadat U, et al. Efficacy of closed suction drainage in lower limb arterial surgery: a meta-analysis of published clinical trials. Vasc Endovascular Surg. 2008;42:243–248. [DOI] [PubMed] [Google Scholar]
- 27.Samraj K, Gurusamy KS. Wound drains following thyroid surgery. Cochrane Database Syst Rev. 2007;4:Cd006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomson DR, Sadideen H, Furniss D. Wound drainage after axillary dissection for carcinoma of the breast. Cochrane Database Syst Rev. 2013;10:CD006823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebner F, deGregorio N, Vorwerk E, et al. Should a drain be placed in early breast cancer surgery? Breast Care (Basel). 2014;9:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrosillo N, Drapeau CM, Nicastri E, et al. ; ANIPIO Surgical site infections in Italian hospitals: a prospective multicenter study. BMC Infect Dis. 2008;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fong Y, Brennan MF, Brown K, et al. Drainage is unnecessary after elective liver resection. Am J Surg. 1996;171:158–162. [DOI] [PubMed] [Google Scholar]
- 32.Grim SA, Slover CM, Sankary H, et al. Risk factors for wound healing complications in sirolimus-treated renal transplant recipients. Transplant Proc. 2006;38:3520–3523. [DOI] [PubMed] [Google Scholar]
- 33.Pengel LH, Liu LQ, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? A systematic review of randomized controlled trials. Transpl Int. 2011;24:1216–1230. [DOI] [PubMed] [Google Scholar]
- 34.Webster AC, Lee VW, Chapman JR, et al. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006;81:1234–1248. [DOI] [PubMed] [Google Scholar]
- 35.Flechner SM, Zhou L, Derweesh I, et al. The impact of sirolimus, mycophenolate mofetil, cyclosporine, azathioprine, and steroids on wound healing in 513 kidney-transplant recipients. Transplantation. 2003;76:1729–1734. [DOI] [PubMed] [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- 37.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fahmy A, Youssif M, Aboyoussif T, et al. A prospective randomized study comparing intraoperative placing of drains versus no drain on incidence of lymphocele formation after renal transplantation. J Urol. 2016;195:e430. [Google Scholar]
- 39.Gurusamy KS, Samraj K. Routine abdominal drainage for uncomplicated open cholecystectomy. Cochrane Database Syst Rev. 2007;2Cd006003. [DOI] [PMC free article] [PubMed] [Google Scholar]


