Abstract
Background.
Ischemia-reperfusion (IR) injury remains a significant problem for all solid organ transplants; thus, an important unmet need in transplantation is the prevention of IR injury. PrC-210 has demonstrated superior prevention of reactive oxygen species damage in several preclinical studies as a free radical scavenger. Here, we describe its profound efficacy in suppressing IR injury in a murine model of kidney IR injury.
Methods.
C57/B6 mice underwent laparotomy with the left renal pedicle occluded for 30 minutes to induce IR injury. Right nephrectomy was performed at the time of surgery. Mice received a single systemic dose of the PrC-210, PrC-211, or PrC-252 aminothiols 20 minutes before IR injury. Twenty-four hours following IR injury, blood and kidney tissue were collected for analysis. Kidney caspase-3 level (a marker of cell death), direct histological analysis of kidneys, and serum blood urea nitrogen (BUN) were measured in animals to assess reactive oxygen species scavenger protective efficacies.
Results.
A single systemic PrC-210 dose 20 minutes before IR injury resulted in significant reductions in (1) IR-induced kidney caspase level (P < 0.0001); caspase was reduced to levels not significantly different than control caspase levels seen in unperturbed kidneys, (2) IR-induced renal tubular injury scores (P < 0.0001); brush border loss and tubular dilation were markedly reduced, and (3) serum BUN compared with control IR injury kidneys (P < 0.0001). The ranked protective efficacies of PrC-210 > PrC-211 >> PrC-252 paralleled previous radioprotection studies of the molecules.
Conclusions.
A single PrC-210 dose, minutes before the IR insult, profoundly reduced caspase, renal tubular injury, and serum BUN in mice exposed to standard kidney IR injury. These findings support further development of the PrC-210 molecule to suppress or prevent IR injury in organ transplant and other IR injury settings.
Ischemia-reperfusion (IR) injury remains a significant problem in organ procurement and transplantation due to the severe oxidative injury that occurs in the organ.1 IR injury in kidney transplantation manifests as primary nonfunction or delayed graft function. Approximately one-third of all kidney transplants will develop delayed graft function; this failure rate increases to as high as 50% in kidneys donated after circulatory death.1-4 Delayed graft function is not only a well-established risk factor for inferior graft survival, but it also leads to increased resource utilization and expense in the immediate posttransplant setting as one awaits the return of renal function.5-8 As a result, an important, unmet need in solid organ transplantation is the prevention of IR injury.
The complete mechanisms underlying IR injury are complex and incompletely understood, but oxidative stress, necrosis, cell apoptosis, ATP depletion, and calcium dyshomeostasis all contribute to the mechanisms of renal IR injury.9-11 The generation of reactive oxygen species (ROS) in the reperfusion phase leads to DNA mutation and initiation of apoptotic and necrotic death cascades, ultimately leading to cell death.12 Suppressing IR injury would improve outcomes in any clinical setting in which IR injury is encountered, including transplantation, vascular surgery, urologic surgery, and neurosurgery.
PrC-210 is the prototype of a new family of immediate-acting, small molecule aminothiol ROS scavengers, which can be administered orally, intravenously, or topically, and it has no measurable nausea, emesis, nor hypotension side effects in animal models.13-15 Unlike traditional antioxidants that act indirectly over hours-days by chemical modification of nuclear factor erythroid 2-related factor 2 to activate expression of protective genes, PrC-210 and its analogs directly scavenge ROS to confer 100% protection in seconds to minutes.16-19 To evaluate the ability of PrC-210 to prevent ROS-induced cell death in kidney transplant, we measured the ability of a single systemically administered PrC-210 dose to reduce (1) kidney caspase activation, (2) kidney tubule necrosis, and (3) serum blood urea nitrogen (BUN) elevation using the established mouse renal IR injury model.20 As part of this screening study to determine PrC-210 protective efficacy, 2 structural analogs of PrC-210, PrC-211, and PrC-252, which have known chemical differences in charge and half-life, were compared with PrC-210 for their ability to confer kidney protection.
MATERIALS AND METHODS
Animals
Ten-week-old C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in the University of Wisconsin Laboratory Animal Facility. All procedures were performed in accordance with the Animal Care and Use Policies at the University of Wisconsin. Animal health including animal deaths, room temperature, 12-hour light/dark cycles, and cage cleaning among other sanitation duties were performed daily by animal care staff. Food and water were available ad libitum. This research was prospectively approved by the School of Medicine and Public Health Institutional Animal Care and Use Committee at the University of Wisconsin (protocol number B00000588). Mice were randomized into baseline control, no drug plus IR injury, PrC-210 plus IR injury, PrC-211 plus IR injury, and PrC-252 plus IR injury. All groups contained 4 to 7 animals.
Materials
Synthesis of the PrC-210 HCl aminothiol is described separately.19,21 PrC-210 HCl crystals are stored under a nitrogen atmosphere at −20°C, and even with routine thawing, use, and restorage, crystalline PrC-210 is completely stable for >4 years by mass spectrometry analysis. PrC-211 and PrC-252 were synthesized as described.21 Chemical structures of each molecule are shown in Figure 1. The administered intraperitoneal dose for each of the 3 molecules was 0.24× the maximum tolerated dose (MTD). Intraperitoneal MTDs were previously determined for PrC-210 (504 µg/gm body weight [bw]), PrC-211 (500 µg/gm bw), and PrC-252 (287 µg/gm bw).14 Other chemical reagents were obtained from Sigma Aldrich (St. Louis, MO).
FIGURE 1.
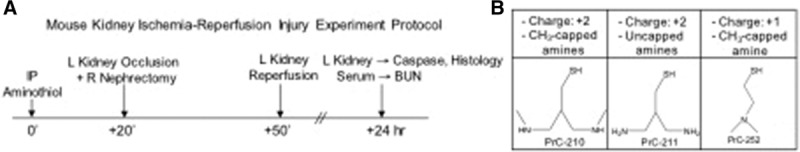
Mouse kidney ischemia-reperfusion injury experiment protocol and chemical structure of aminothiols used. A, Experimental design: At 0 min, mice received either intraperitoneal vehicle or aminothiol; 20 min later all groups underwent 30 min of left (L) kidney occlusion and right (R) nephrectomy, followed by 24 h of reperfusion. Serum and L kidney were retrieved at 24 h. B, Structures and design elements of the 3 aminothiols tested here. BUN, blood urea nitrogen.
Experimental and Surgical Procedure
The schedule in which drug was administered by intraperitoneal injection, left kidneys were occluded to administer IR insult, and right kidneys were removed as controls, is shown diagrammatically in Figure 1A. Surgical manipulation was performed as described previously.20 Briefly, 20 minutes after vehicle or aminothiol intraperitoneal injection, mice were anesthetized with 2% isoflurane. The abdominal region was sterilized with topical alcohol. A midline incision was made to expose renal pedicles bilaterally. The left renal hilum was occluded using a nontraumatic microvascular clamp for 30 minutes to achieve complete cessation of renal blood flow, and a right nephrectomy was performed. After 30 minutes, the clamp was removed to restore blood flow to the left kidney. Successful ischemia or reperfusion was judged by observing the change in kidney color from bright red to dark blue or from dark blue to bright red, respectively. The abdominal wound was sutured after confirming reperfusion to the kidney. At the time of retrieval 24 hours later, blood samples were collected by cardiac puncture, and left nephrectomy was performed. Blood was centrifuged (3000 RPM for 10 min at 4°C) immediately to separate the serum, which was stored at −80°C until assayed. Kidneys were divided in half and immediately frozen in liquid nitrogen and stored at −80°C until used for caspase assay or were placed in formalin for histology. Four to seven animals were included in each treatment group.
Renal Assessments
Caspase-3 and 7 activity in kidney homogenate supernates was determined using the Apo-ONE fluorescent substrate (Promega, Madison, WI). Briefly, thawed kidneys were mixed with an 8-fold excess of lysis buffer containing 50 mM Na HEPES, pH 7.4, 100 mM NaCl, 1 mM EDTA, 10 mM DTT, 10% glycerol, and homogenized at 4°C for 30 seconds with a stainless steel blade homogenizer (5000 RPM). The kidney homogenate was then centrifuged at 4°C at 16 000g in an Eppendorf 5418 microfuge for 20 minutes. The resultant supernates were immediately assayed for caspase activity or frozen and stored at –70°C. Supernate protein was measured by the Bradford method using bovine serum albumin standards. The caspase assay was performed as follows: 38 μg of supernate protein diluted to a total volume of 50 µL with the above lysis buffer, was mixed with 50 μL of the undiluted Apo-ONE substrate in the well of a black, opaque, 96 well plates to initiate the 60-minute reaction. Plates were shaken at 200 RPM at 37°C for 60 minutes. The DEVD caspase substrate peptide cleavage was measured using a BMG Clariostar fluorescent plate reader at an excitation wavelength of 499 nm and emission wavelength of 521 nm. A caspase standard was included for each experiment.
Serum BUN was measured on an IdexxVetTest 8008 bioanalyzer (Idexx Laboratories, West Sacramento, CA) using compatible assay chips. Serum BUN was measured in serial dilutions using no dilution, 1:1, and 1:2 with normal saline.
PrC-210 Protective Lead Time Assay
To establish the lead-time needed for PrC-210 to protect naked plasmid DNA against ROS, exposure to a nearly instantaneous pulse of ·OH DNA insult was required. To accomplish this, pGEM DNA (750 ng) was incubated with PrC-210 (20 mM) added at various times (−5 h to +1 min) before addition of 5 mM H2O2 and immediate irradiation of the tubes with UV light to catalyze essentially instantaneous conversion of H2O2 to ·OH.22 Immediately following the 60-second ·OH pulse that is achieved during the 60-second UV irradiation, triplicate samples of the irradiated plasmid DNA (200 ng) were electrophoresed on a 1% agarose gel in Tris-acetate buffer for 90 minutes at 60 volts. Gels were stained with ethidium bromide, digitally imaged, and supercoiled versus nicked/·OH-damaged DNA band intensities were quantified using Image J software.
Kidney Histology
Kidneys were fixed in 10% formalin and embedded in paraffin; sections were then mounted and stained with hematoxylin-eosin. Images from slides were taken using a Nikon Diaphot microscope with a Nikon D3100 metered camera. IR-induced renal tubular injury scores were determined on blinded microscopic images (6–10 independent images per treatment group). Automated quantification of 10× objective kidney histology images was performed using a custom macro written for ImageJ software (https://imagej.nih.gov/ij/index.html). Briefly, each 10× objective image of kidney sections was processed through an ImageJ macro that was designed to quantify tubular thickness including brush border, the presence of healthy tubules, injured tubules, or necrotic tubules with loss of nuclei. The operator was blinded to the identity of the images. The ratio of nuclei to injured or necrotic tubules (lacking nuclei) was used to calculate a tubular injury score. Scores were then averaged and plotted using GraphPad Prism.
Statistics
Data are expressed as means ± SEMs. One-way Student t tests were used to determine statistical difference and P values using GraphPad Prism 7.03 software. P < 0.05 were considered significant.
RESULTS
PrC-210 Reduced Kidney Cell Death and Improved Renal Function
To test its ability to prevent IR injury, PrC-210 was administered intraperitoneally 20 minutes before initiating kidney ischemia (Figure 1A). Right nephrectomy was performed immediately after the left kidney was clamped. Reperfusion occurred 50 minutes following aminothiol administration. Animals were retrieved 24 hours following IR injury (Figure 1A). Kidney caspase activity was measured as a surrogate marker of cell death. Animals who received PrC-210 demonstrated a significant decrease in caspase activity compared with nontreated animals (Figure 2A; P < 0.0001). PrC-210 reduced cell death to a level not significantly different from that found in kidneys that did not experience IR injury (Figure 2A; P = 0.085). Serum BUN was also measured to assess kidney function in the remaining left kidney after IR injury. Treatment with PrC-210 resulted in a significant decrease in serum BUN compared with nontreated animals (Figure 2B; P < 0.0001).
FIGURE 2.
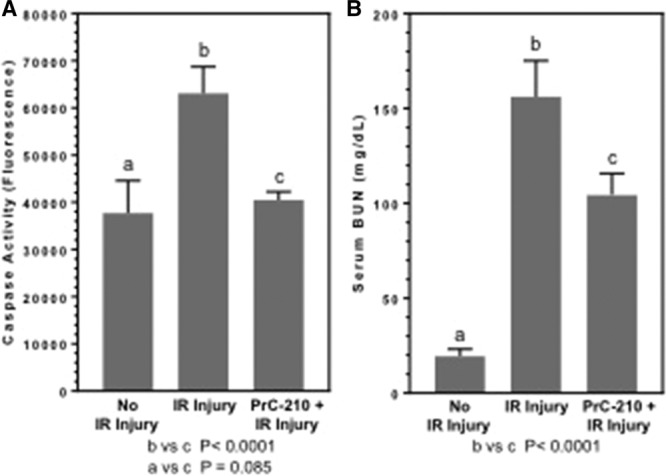
Effects of a single PrC-210 aminothiol dose on kidney caspase and kidney function 24 h after kidney ischemia-reperfusion (IR) injury. Mice received a single intraperitoneal PrC-210 injection 20 min before L kidney occlusion. The PrC-210 dose (121 µg/gm body weight [bw]) was 0.24× maximum tolerated dose (MTD); the PrC-210 MTD (504 µg/gm bw) was previously determined on wild-type mice.13 Kidney supernate caspase activity was measured as described in Materials and Methods in a 60-min reaction. Blood urea nitrogen (BUN) levels were determined on serum retrieved 24 h post-IR injury. P values are indicated.
In an indication of a PrC-210 drug-conferred effect, mice that received decreasing intraperitoneal doses of PrC-210 showed a dose-dependent decrease in PrC-210–conferred protection against kidney IR injury (Figure 3A). A significant reduction in serum BUN at 24 hours was seen in the highest dose, 0.24× MTD group of mice, but lower doses of PrC-210 did not reduce serum BUN (Figure 3B).
FIGURE 3.
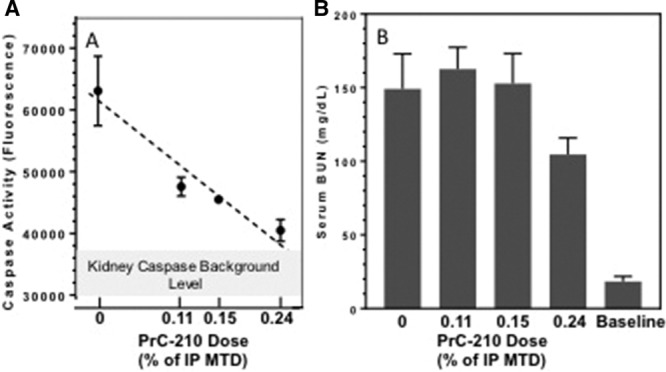
PrC-210 exhibits dose-dependent effect on kidney caspase level and serum blood urea nitrogen (BUN). A, Kidney caspase and (B) kidney function 24 h after kidney ischemia-reperfusion injury. PrC-210 doses are indicated as fractions of the intraperitoneal (IP) maximum tolerated dose (MTD; 504 µg/gm body weight), which was previously determined on wild-type mice.13
PrC-210 Reduces Renal Damage Following IR Injury
Kidneys were retrieved for histologic analysis 24 hours after the 30-minute ischemic injury (Figure 1A). Images were taken from the corticomedullary junction (Figure 4A). The kidneys from mice that underwent sham surgery only showed normal histology as expected, but significant renal changes in mice that underwent IR injury were seen, including loss of tubule brush border, tubular dilation, and tubule luminal congestion (Figure 4B and C). In contrast, renal histology in PrC-210 + IR injury mice showed near normal brush borders within tubules and little dilation of tubules or luminal congestion (Figure 4D). Tubular injury scores were calculated using a custom macro in ImageJ as described in Methods. The PrC-210 + IR injury group showed a significantly improved tubular injury score when compared to the untreated IR injury group (P < 0.0001) (Figure 4E).
FIGURE 4.
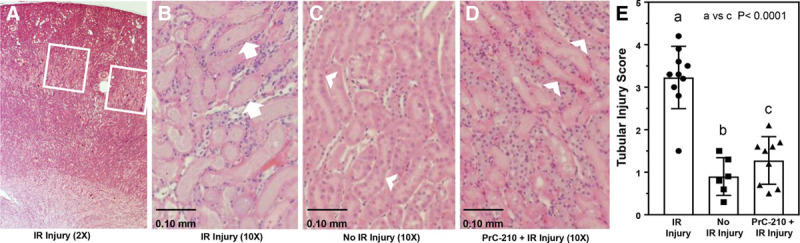
Kidney morphology and scoring of tubular injury 24 h after reperfusion. A, Low power (2× objective) image of an ischemia-reperfusion (IR) kidney; insert boxes are examples of areas from which 10× objective images were taken and scored for IR injury. B–D, Representative (10× objective) hematoxylin-eosin-stained kidney sections. E, Results are expressed as the mean ± SEM; the P value is indicated. B, Kidney 24 h following IR insult. Arrows indicate necrotic tubule with loss of nuclei. C, Kidney with no IR insult. Arrowhead indicates healthy tubule. D, Kidney 24 h following single PrC-210 dose and IR insult. Arrowhead indicates preservation of tubule architecture without necrosis.
PrC-210 Toxicity and 14C PrC-210 Biodistribution
In the first PrC-210 plus IR injury experiments performed here, an intraperitoneal 0.5× MTD dose of PrC-210 (252 µg PrC-210/gm bw) was administered to mice 20 minutes before left kidney occlusion and right nephrectomy. One hundred percent (N = 5) of mice died within the following 24-hour observation period. The toxicity phenotype observed in the mice that died (sleepy, unresponsive, then death) closely resembled that seen in mice previously given increasing, and then lethal doses of an intraperitoneal, oral, or subcutaneous PrC-210 bolus to determine the respective MTDs.13 In our earlier preclinical studies, 0.5× MTD PrC-210 dose did not result in any deaths due to discernible toxicity when given prior to ROS formation (whole-body irradiation, mechanically induced myocardial infarction). Therefore, we reasoned that with diminished glomerular filtration rate due to right nephrectomy and severe left kidney injury, we had shifted the systemic PrC-210 dose-survival curve. An abbreviated intraperitoneal PrC-210 dose-survival curve was then created (Table 1), and a dose of 0.24× MTD (121 µg/gm bw) was adopted for PrC-210. Below, we adopted the same 0.24× MTD fractional doses PrC-211 and PrC-252 to enable their comparison.
TABLE 1.
Survival of WT vs IR-injured mice (R nephrectomy, L kidney hilum occlusion) 24 h following intraperitoneal aminothiol treatments
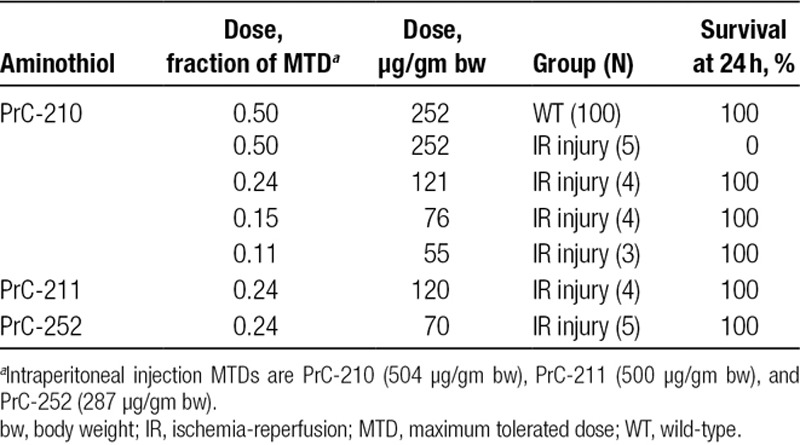
In a previous biodistribution study of systemically administered 14C PrC-210 performed at Covance Laboratory,15 a single bolus of 14C-labeled PrC-210, mixed with cold carrier PrC-210, was administered by intravenous injection to a 300 gm rat. Excreta and animal corpse analysis established that >74% of the systemically administered dose was eliminated in the animal’s urine. This provides a logical explanation for the shift in the PrC-210 dose-toxicity curve seen in the IR injury model presented here.
PrC-210 Provides Greater Renal Protection Compared to PrC-211 and PrC-252
PrC-210, PrC-211, and PrC-252 are 3 unique aminothiol structures designed and synthesized in our lab. Each structure has previously shown systemic- and topically-conferred protection against ionizing radiation,14 where the radiation toxicity primarily results from x-ray–induced ROS formation. To compare the efficacies of these 3 aminothiols in suppressing IR injury, the same fractional doses (0.24× MTD) of PrC-210, PrC-211, or PrC-252 were administered intraperitoneally to animals 20 minutes before renal ischemia (Figure 1). PrC-210 significantly decreased IR injury-induced caspase activity (Figure 5A; P < 0.0001). PrC-211 significantly reduced IR-induced caspase (P < 0.0001), but less effectively than PrC-210 (56% of the PrC-210 effect). PrC-252 treatment conferred no reduction in kidney caspase activity. Though PrC-210 significantly reduced serum BUN levels (P < 0.0001) in IR-injured mice, PrC-211 and PrC-252 had no significant effect on BUN (Figure 5B).
FIGURE 5.
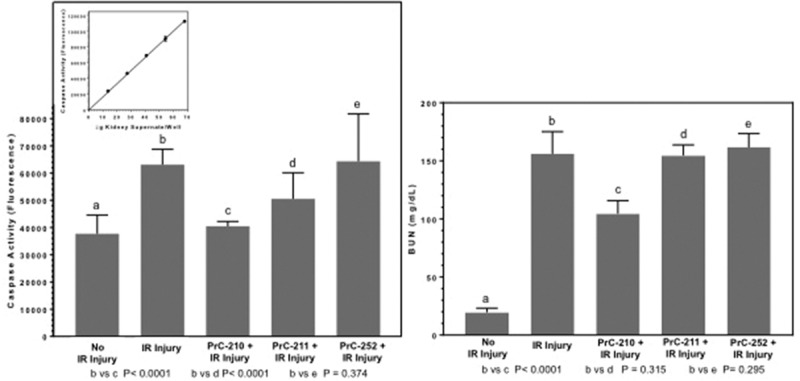
The effects of a single intraperitoneal (IP) dose of 3 different aminothiols on kidney caspase and blood urea nitrogen (BUN). A, Kidney caspase and (B) kidney function 24 h after kidney ischemia-reperfusion (IR) injury. Mice received single IP doses consisting of the 0.24× maximum tolerated dose (MTD) for each aminothiol 20 min before L kidney occlusion. IP MTDs were previously determined on wild-type mice for PrC-210 (504 µg/gm body weight [bw]), PrC-211 (500 µg/gm bw), and PrC-252 (287 µg/gm bw). Inset, Linearity of kidney supernate caspase assay conditions over 60 min was first established.
PrC-210 Protection of Naked DNA
The gel-based assay of ·OH-induced plasmid DNA breaks (Figure 6), in which a 60-second pulse of ·OH was generated, demonstrated that addition of PrC-210 at all time points before the ·OH insult, including as little as 30 seconds before, conferred complete protection against the ·OH pulse that otherwise induced >95% damage to the naked plasmid DNA.22 PrC-210 addition 1 minute after the ROS insult had little or no protective effect.
FIGURE 6.
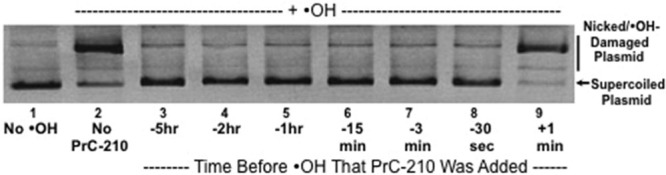
Agarose gel separation of supercoiled and nicked/·OH-damaged forms of pGEM plasmid DNA after exposure of the plasmid DNA to a 60-s pulse of an ·OH generator (H2O2 + UV light).22 Supercoiled DNA was incubated with buffer (lane 1) or 20 mM PrC-210 (lanes 2–9) for the indicated times and then exposed for 1 min to the ·OH generator. Aliquots of each reaction were then immediately electrophoresed, stained with ethidium bromide, and digitally imaged. Three replicate reactions and gels were done.
DISCUSSION
IR injury remains a significant problem for all solid organ transplants, and more broadly, it contributes considerable morbidity to numerous surgical subspecialties, including cardiac and vascular surgery. We undertook this study to determine if PrC-210 would be effective in preventing the ROS damage induced during IR that accompanies all organ transplants. Our data demonstrate that (1) a single intraperitoneal injection of PrC-210 confers a dose-dependent reduction of IR-induced kidney cell death, when measured either by caspase activation or by direct histologic analysis of kidney tubules, to a level not different than that seen in control kidneys; (2) PrC-210 preserves renal function; (3) PrC-210 ROS scavenging efficacy is both immediate (s) and long-lasting (h), which would allow it to be flushed through an organ immediately before transplant and as a systemic drug before and after surgery; and (4) it does not cause nausea, emesis, or hypotension that preclude clinical use of the amifostine aminothiol.15
Numerous reports have studied the use of antioxidants in preclinical models to show suppression of IR injury.23-26 These reports are important for providing proof of concept for this strategy; however, prolonged time to effect and severe side effects have precluded their clinical use for IR injury. Although amifostine is an effective ROS scavenger, it is excluded from clinical use due to its 91% incidence of nausea and emesis and 61% incidence of hypotension.27,28 Other strategies have targeted postsurgical inflammatory mediators or growth factors to decrease IR injury in kidney transplantation. Although these strategies may have some effect, they do not address the primary IR insult, which is the bolus of ROS that is generated within seconds to minutes following reestablishment of blood flow seen in transplantation and other surgical subspecialties.29
To our knowledge, PrC-210 is currently the best available ROS scavenger. In a recent head-to-head comparison of PrC-210 against 13 other commonly studied antioxidants and ROS scavengers, PrC-210 clearly demonstrated its superiority as a ROS scavenger.17 N-acetylcysteine actually increased ROS-induced damage, and other molecules, including glutathione and vitamin E, were without effect or showed only a small effect over hours to days due to indirect mechanisms of action. One exception was WR-1065, the active form of amifostine, which though effective, is clinically precluded due to severe side effects.17
A single systemic dose of PrC-210, when administered 20 minutes before kidney ischemia and 50 minutes before the bolus of IR-associated ROS in the reperfused kidney, essentially eliminated the IR-associated caspase activation. Histologic analysis of kidneys corroborated the PrC-210 conferred protection; the retention of tubule brush border and absence of tubular dilation in PrC-210-treated mice were apparent. The most probable explanation for this PrC-210 effect is loading the kidney prior to occlusion, the maintenance of active PrC-210 thiol within the kidney while occluded, and the utilization of the millimolar PrC-210 thiol level in renal tubules to scavenge labile ROS as it is formed in the reperfused kidney. With a half-life of 3.5 hours at pH 7.2, additional explanations for the protective effect of a single PrC-210 dose could include its reaction with reactive molecules formed in kidney during occlusion or with the ROS produced by inflammatory cells in the kidney after reperfusion. Future studies in our lab will focus on dissecting out these individual PrC-210 contributions.17
There are several limitations to this study. DNA damage that occurred due to IR injury was quantified via histology; however, the damage to proteins and lipids were not quantified. Though PrC-210 reduced IR injury-induced kidney caspase activity to near background, the serum BUN in those animals, though significantly reduced, remained elevated. Gill et al30 in their review enumerate several parameters that affect blood urea that are unrelated to acute tubular necrosis and renal failure. Additionally, studies were not carried past 24 hours to determine if renal function returns to baseline and at what time point this occurs. Future studies will address long-term function in animals and apply the model presented here to a rodent kidney transplant model.
PrC-210, PrC-211, and PrC-252 demonstrated different levels of efficacy in preventing IR-induced renal injury. All 3 molecules share design elements of (1) at least one (+) charged amine group that serves to concentrate the molecule around DNA because it ionically hovers over the (−) charged DNA backbone; and (2) an ROS scavenging, thiol catcher’s mitt that is displaced by 3 bond lengths away from the DNA backbone to more effectively scavenge ROS from the DNA milieu (Figure 7).14 The ranked efficacy of PrC-210 > PrC-211 >> PrC-252 is the same as that observed in previous tests of these molecules to confer radioprotection when administered either systemically (intraperitoneal, oral) or topically to rodents.13-15 As shown in Figure 1B, PrC-210 has 2 amines versus one in PrC-252, hence 2 (+) charges per molecule for attraction to DNA. The capped amines seen in PrC-210 are less easily metabolized by cellular acetylases compared to the bare amines seen in PrC-211. This results in a longer half-life of PrC-210 compared to PrC-211, which likely explains why PrC-210 demonstrated increased efficacy compared to PrC-211.
FIGURE 7.
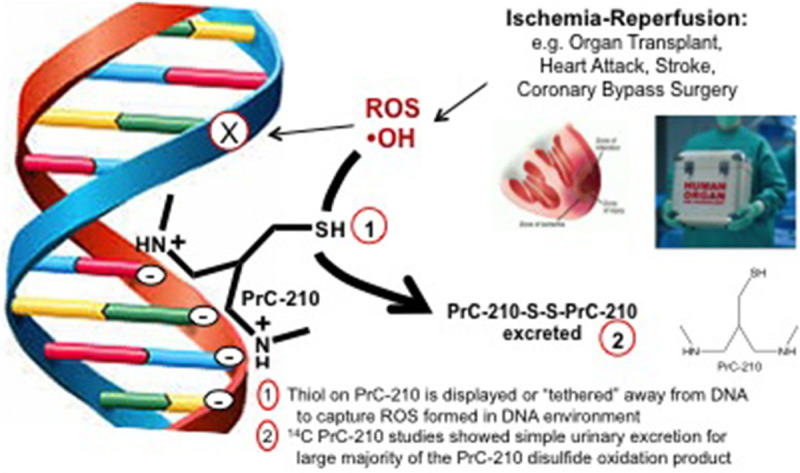
Schematic representation of PrC-210 mechanism of action via direct scavenging of reactive oxygen species (ROS) from the milieu surrounding cellular DNA.
An important advantage of PrC-210 here is its direct mechanism of action, actually immediate action, as a ROS scavenger so that it confers protection within seconds (Figure 6). Importantly, at time points ranging from 5 hours to 30 seconds before ·OH exposure, PrC-210 was equally protective of naked DNA. DNA protection was not seen when PrC-210 was added 1 minute after ·OH exposure; therefore, PrC-210 confers protection by directly scavenging short-lived (milliseconds) ROS before DNA damage occurs. These findings indicate that PrC-210 has both quick onset and a lasting effect when administered before the generation of ROS. This immediate onset of action is a key characteristic that supports PrC-210 as a superior free radical scavenger compared to the other antioxidants previously studied.
Finally, the ability of PrC-210 to confer 100% protection to DNA against ROS, regardless of when administered before ROS insult, combined with its small molecular weight (MW = 148), would allow PrC-210 to be used in a variety of transplant and surgical settings to reduce IR injury. PrC-210 could be (1) added to preservation solutions and flushed through an organ, (2) injected directly into the organ before transplantation, or (3) given intravenously to a patient posttransplant. Importantly, the nature of PrC-210 as a direct-acting, highly effective ROS scavenger would allow it to be used in any environment in which blood flow is stopped and restarted, such as coronary bypass surgeries, neurological procedures following stroke, and during aorta aneurysm repairs (Figure 7).
IR injury remains a profound problem with significant implications for kidney graft function, the ability to use marginal donor organs, and healthcare resource utilization. Therefore, it is imperative that improved strategies to prevent IR injury are developed. Here, we have shown PrC-210 to be a highly effective ROS scavenger that significantly reduces IR injury-associated renal cell death.
Footnotes
Published online 27 June, 2019.
N.M.B. and W.E.F. participated in research design, writing of the article, performance of the research, and data analysis. R.R.R. participated in research design, writing of the article, and data analysis.
The authors declare no conflicts of interest.
The authors thank the following groups for grant support: UW Transplant Research Training grant (T32 AI125231); KL2 grant (KL2TR002374); and grant UL1TR002373 to University of Wisconsin Institute for Clinical and Translational Research from National Institutes of Health/National Center for Advancing Translational Sciences, American Society of Transplant Surgeons (133 AAA1552), American College of Surgeons (133 AAB2176), and grant support to W.E.F. (number R03CA176799).
REFERENCES
- 1.Chatauret N, Badet L, Barrou B, et al. Ischemia-reperfusion: from cell biology to acute kidney injury. Prog Urol. 2014;24(Suppl 1):S4–12. [DOI] [PubMed] [Google Scholar]
- 2.Moers C, Kornmann NS, Leuvenink HG, et al. The influence of deceased donor age and old-for-old allocation on kidney transplant outcome. Transplantation. 2009;88:542–552. [DOI] [PubMed] [Google Scholar]
- 3.Shoskes DA, Cecka JM. Effect of delayed graft function on short- and long-term kidney graft survival. Clin Transpl. 1997297–303. [PubMed] [Google Scholar]
- 4.Orandi BJ, James NT, Hall EC, et al. Center-level variation in the development of delayed graft function after deceased donor kidney transplantation. Transplantation. 2015;99:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irish WD, Ilsley JN, Schnitzler MA, et al. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10:2279–2286. [DOI] [PubMed] [Google Scholar]
- 6.Grosso G, Corona D, Mistretta A, et al. Delayed graft function and long-term outcome in kidney transplantation. Transplant Proc. 2012;44:1879–1883. [DOI] [PubMed] [Google Scholar]
- 7.Moreira P, Sá H, Figueiredo A, et al. Delayed renal graft function: risk factors and impact on the outcome of transplantation. Transplant Proc. 2011;43:100–105. [DOI] [PubMed] [Google Scholar]
- 8.Axelrod DA, Schnitzler MA, Xiao H, et al. The changing financial landscape of renal transplant practice: a national cohort analysis. Am J Transplant. 2017;17:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weight SC, Bell PR, Nicholson ML. Renal ischaemia–reperfusion injury. Br J Surg. 1996;83:162–170. [PubMed] [Google Scholar]
- 10.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloner RA, Przyklenk K, Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation. 1989;80:1115–1127. [DOI] [PubMed] [Google Scholar]
- 12.Gu Y, Huang F, Wang Y, et al. Connexin32 plays a crucial role in ROS-mediated endoplasmic reticulum stress apoptosis signaling pathway in ischemia reperfusion-induced acute kidney injury. J Transl Med. 2018;16:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peebles DD, Soref CM, Copp RR, et al. ROS-scavenger and radioprotective efficacy of the new PrC-210 aminothiol. Radiat Res. 2012;178:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copp RR, Peebles DD, Soref CM, et al. Radioprotective efficacy and toxicity of a new family of aminothiol analogs. Int J Radiat Biol. 2013;89:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soref CM, Hacker TA, Fahl WE. A new orally active, aminothiol radioprotector-free of nausea and hypotension side effects at its highest radioprotective doses. Int J Radiat Oncol Biol Phys. 2012;82:e701–e707. [DOI] [PubMed] [Google Scholar]
- 16.Techapiesancharoenkij N, Fiala JL, Navasumrit P, et al. Sulforaphane, a cancer chemopreventive agent, induces pathways associated with membrane biosynthesis in response to tissue damage by aflatoxin B1. Toxicol Appl Pharmacol. 2015;282:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jermusek F, Jr, Benedict C, Dreischmeier E, et al. Significant suppression of CT radiation-induced DNA damage in normal human cells by the PrC-210 radioprotector. Radiat Res. 2018;190:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brand M, Sommer M, Jermusek F, et al. Reduction of X-ray-induced DNA damage in normal human cells treated with the PrC-210 radioprotector. Biol Open. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copp RR, Peebles DD, Fahl WE. Synthesis and growth regulatory activity of a prototype member of a new family of aminothiol radioprotectors. Bioorg Med Chem Lett. 2011;21:7426–7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karim AS, Reese SR, Wilson NA, et al. Nox2 is a mediator of ischemia reperfusion injury. Am J Transplant. 2015;15:2888–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahl WE, Peebles DD, Copp RR; Inventor. Amino thiol compounds and compositions for use in conjunction with cancer therapy. US Patent 7,314,959. August 9, 2004.
- 22.Floyd RA, Watson JJ, Wong PK. Sensitive assay of hydroxyl free radical formation utilizing high pressure liquid chromatography with electrochemical detection of phenol and salicylate hydroxylation products. J Biochem Biophys Methods. 1984;10:221–235. [DOI] [PubMed] [Google Scholar]
- 23.Han P, Qin Z, Tang J, et al. RTA-408 protects kidney from ischemia-reperfusion injury in mice via activating Nrf2 and downstream GSH biosynthesis gene. Oxid Med Cell Longev. 2017;2017:7612182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou S, Sun Y, Zhuang Y, et al. Effects of kallistatin on oxidative stress and inflammation on renal ischemia-reperfusion injury in mice. Curr Vasc Pharmacol. 2015;13:265–273. [DOI] [PubMed] [Google Scholar]
- 25.Doi K, Suzuki Y, Nakao A, et al. Radical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidney. Kidney Int. 2004;65:1714–1723. [DOI] [PubMed] [Google Scholar]
- 26.van den Akker EK, Manintveld OC, Hesselink DA, et al. Protection against renal ischemia-reperfusion injury by ischemic postconditioning. Transplantation. 2013;95:1299–1305. [DOI] [PubMed] [Google Scholar]
- 27.Chok MK, Conti M, Almolki A, et al. Renoprotective potency of amifostine in rat renal ischaemia-reperfusion. Nephrol Dial Transplant. 2010;25:3845–3851. [DOI] [PubMed] [Google Scholar]
- 28.Rose PG. Amifostine cytoprotection with chemotherapy for advanced ovarian carcinoma. Semin Oncol. 1996;23(4 Suppl 8):83–89. [PubMed] [Google Scholar]
- 29.Feitoza CQ, Câmara NO, Pinheiro HS, et al. Cyclooxygenase 1 and/or 2 blockade ameliorates the renal tissue damage triggered by ischemia and reperfusion injury. Int Immunopharmacol. 2005;5:79–84. [DOI] [PubMed] [Google Scholar]
- 30.Gill N, Nally JV, Jr, Fatica RA. Renal failure secondary to acute tubular necrosis: epidemiology, diagnosis, and management. Chest. 2005;128:2847–2863. [DOI] [PubMed] [Google Scholar]


