Abstract
Background.
Normothermic machine perfusion (NMP) is emerging as a novel preservation strategy in liver transplantation, but the optimal methods for assessing liver grafts during this period have not been determined. The aim of this study was to investigate whether implantable oxygen biosensors can be used to monitor tissue oxygen tension in liver grafts undergoing NMP.
Methods.
Implantable phosphorescence-based oxygen sensors were tested in 3 different experimental groups: (1) in vivo during laparotomy, (2) during NMP of liver grafts with an acellular perfusate (NMP-acellular), and (3) during NMP with perfusate containing red blood cells (NMP-RBC). During in vivo experiments, intrahepatic oxygen tension was measured before and after occlusion of the portal vein (PV). In NMP experiments, intrahepatic oxygen tension was measured as a function of different PV pressure settings (3 vs 5 vs 8 mm Hg) and inflow oxygen concentration (95% O2 vs 6% O2).
Results.
In vivo, intrahepatic oxygen tension decreased significantly within 2 minutes of clamping the PV (P < 0.05). In NMP experiments, intrahepatic oxygen tension correlated directly with PV pressure when high inflow oxygen concentration (95%) was used. Intrahepatic oxygen tension was significantly higher in the NMP-RBC group compared with the NMP-acellular group for all conditions tested (P < 0.05).
Conclusions.
Implantable oxygen biosensors have potential utility as a tool for real-time monitoring of intrahepatic oxygen tension during NMP of liver grafts. Further investigation is required to determine how intrahepatic oxygen tension during NMP correlates with posttransplant graft function.
INTRODUCTION
Normothermic machine perfusion (NMP) has emerged as a novel organ preservation strategy in liver transplantation. During NMP, liver grafts are housed in an ex vivo environment at physiologic temperature and perfused with an oxygenated solution, promoting the restoration of cellular metabolism before transplantation. Several preclinical studies have demonstrated the benefits of NMP in reducing graft injury and improving function in comparison to cold storage.1-4 This has led to clinical trials establishing the safety and feasibility of NMP.5-7 Most recently, a randomized clinical trial in Europe comparing NMP to cold storage demonstrated a significant reduction in peak AST posttransplant and significant reduction in early allograft dysfunction.8
While NMP is rapidly progressing in the clinic, there is no consensus yet on several key parameters, including perfusate composition, pressure/flow settings, and inflow oxygen concentration. Systematic investigation of how these variables affect tissue oxygenation is required to optimize NMP for organ preservation. NMP has also been utilized as a platform for the assessment of marginal liver grafts before making clinical decisions about suitability for transplant.9 At present, however, there is no consensus on parameters that distinguish a viable from nonviable graft. As such, there is a critical need to develop point-of-care tests that provide real-time data to inform organ assessment.
During NMP, adequate delivery of oxygen at the tissue level is critical for maintaining graft viability and promoting aerobic metabolism. At present, there are no established techniques to measure tissue oxygenation during NMP. Phosphorescence-based oxygen biosensors have emerged as a novel technology for the measurement of interstitial tissue oxygenation.10-15 Recently, our group demonstrated the efficacy of an implantable oxygen biosensor for the detection of vascular perfusion and ischemia in different animal models.16
The objective of the present study was to test implantable oxygen biosensors for continuous monitoring of intrahepatic oxygen tension in a rodent model of NMP. We sought to demonstrate how these biosensors can be used to systematically test the effects of key variables during NMP. Specifically, the effects of perfusate composition (acellular vs cellular), portal vein (PV) pressure (PVP) setting, and inflow oxygen concentration on intrahepatic oxygen tension were investigated.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 300 ± 15 g (mean ± SEM) were used for the experiments. All animals were housed in specific pathogen-free conditions in the animal care facilities at the Duke University Medical Center in accordance with national and institutional guidelines. All animal care and procedures were performed under protocols approved by the Institutional Animal Care and Use Committee at Duke University.
Study Design
Intrahepatic oxygen tension was measured in three experimental groups: (1) in vivo during laparotomy, (2) during NMP with an acellular perfusate (NMP-acellular), and (3) during NMP with a perfusate containing RBCs (NMP-RBC). Rats were randomly assigned into 3 groups (N = 3 per group).
Oxygen Biosensor
The biosensors were prepared through incorporating an oxygen-sensing metalloporphyrin15 into a poly (ethylene glycol) diacrylate (PEGDA) hydrogel.17 PEGDA was selected for this application because it is biocompatible, resists nonspecific protein adsorption, has favorable biomechanical properties, and has been approved for internal use in humans by the Food and Drug Administration.18 The metalloporphyrin particles were loaded into the PEGDA hydrogel in the range of 1–20 wt%, based on the total weight of the hydrogel support.
This formulation allows direct measurements of local oxygen concentration through changes in the phosphorescence lifetime as well as imaging via absolute fluorescence (IVIS Kinetic Real-Time Bioluminescent Imaging, Caliper LifeSciences, Hopkinton, MA). Biosensors were fabricated to a size of 3 mm long and 0.5 mm in diameter. To obtain oxygen measurements, the biosensor is excited with ~640 nm wavelength light, and emission is collected at ~810 nm wavelength. The collected raw fluorescence values were converted into oxygen tension through a previously published modified Stern–Volmer equation.19
To ensure complete coverage of the sensor with tissue and minimize risk of dislocation and bleeding, the biosensors were implanted tangentially into the tissue through 19 gauge needles. Due to the size and accessibility of the left lateral lobe (LLL), oxygen biosensors were injected superficially into that lobe of the rat liver for both in vivo and NMP experiments. In preliminary tests in vivo, injection into the inferior right lobe caused severe bleeding and hematoma formation, while injection in the LLL induced minimal self-limited bleeding. The sensor was maintained in stable position at the implantation site without migration or dislodgment. Real-time phosphorescence measurements were detected through an optical reader (National Instruments, Austin, TX), which was placed about 1 cm above the tissue. The phosphorescence was measured every 5 seconds (Figure 1A). Results were recorded in LabView software (National Instruments) and analyzed offline.
FIGURE 1.
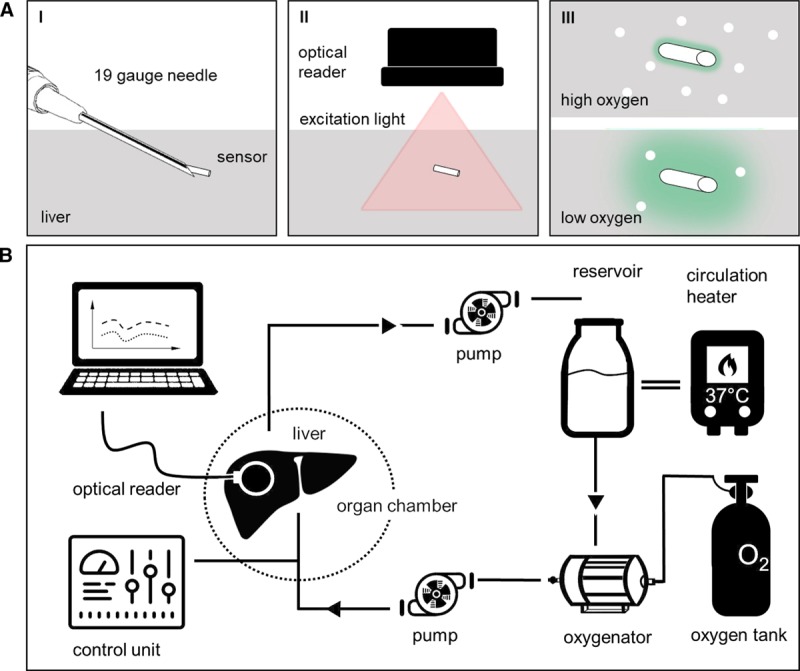
Schematic of (A) oxygen biosensor and (B) normothermic machine perfusion circuit. (A, I) Oxygen biosensor (3 × 0.5 mm) is implanted in liver tissue by injection through a 19 gauge needle. (II) Biosensor is excited by light from the optical reader. (III) Phosphorescence emitted by biosensor is proportional to tissue oxygen tension and detected by optical reader. (B) Schematic of the normothermic machine perfusion (NMP) circuit. NMP was performed using pressure-controlled flow through the portal vein. Acellular and cellular perfusates were tested.
Surgical Procedures
Rats were anesthetized using 2% isoflurane (to effect) in oxygen at 2 L/min. A heating pad was used to maintain body temperature. The abdominal cavity was opened by a midline and transverse incision using monopolar cutting. The hepato-esophageal artery and proper hepatic artery were isolated and divided. A biosensor was implanted in the liver as described above and subsequently in vivo measurements were obtained.
For NMP experiments, a 24 gauge stent (BD Insyte autoguard, Franklin Lakes, NJ) was inserted into the common bile duct and secured. The right suprarenal vein and gastrosplenic and duodeno-pancreatic branches of the PV were separated and divided. The PV was accessed with a perfusion cannula with basket and side port (Harvard Apparatus, Holliston, MA) and the liver was gently flushed with 40 mL cold (4°C) UW solution (Bridge of Life Ltd, Columbia, SC). The infrahepatic vena cava was incised to allow for outflow. The liver was then explanted and weighed, and ex vivo measurements were performed after attachment to an ex vivo perfusion system (Figure 1B).
Machine Perfusion Circuit
Pressure-controlled NMP was performed using a recirculating system (Figure 1B) consisting of a perfusate reservoir, autoclavable organ chamber (Type 834/10), peristaltic pump, oxygenator, and bubble trap (Hugo Sachs Elektronik - Harvard Apparatus GmbH, March-Hugstetten, Germany). The chamber was enclosed in a conditioning system that allowed for precise control of temperature (Optima T100; Grant Instruments, Shepreth, GB).
Measurements of Intrahepatic Oxygen Tension
In Vivo Experiments
After exclusion of severe bleeding or hematoma, heparin (1 U/gram body weight; Fresenius Kabi USA, LLC, Lake Zurich, IL) was injected into the infrahepatic vena cava using a 30 gauge needle. The liver lobe was then positioned on a gauze pad to reduce breathing artifacts (Figure 2A). The optical reader was positioned approximately 1 cm over the liver and fixed. The PV was occluded intermittently using a microvascular clamp placed across the liver hilum. A single cycle of vascular occlusion was limited to 10 minutes and total occlusion time limited to 30 minutes to avoid severe tissue damage.
FIGURE 2.
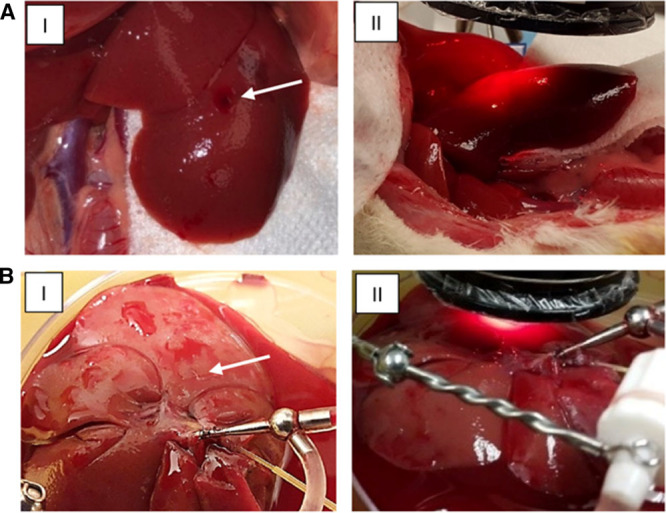
Placement of oxygen biosensor and optical reader for (A) in vivo experiments and (B) NMP experiments. (I) The biosensor is implanted in the left lateral lobe (white arrow). (II) Optical reader is positioned above the biosensor and detects real-time phosphorescence readings from implanted biosensor, which correlates with tissue oxygen tension. NMP, normothermic machine perfusion.
NMP Experiments
Two different perfusate types were tested. In the NMP-acellular group, the perfusate consisted of 100 mL Krebs-Henseleit buffer (Sigma-Aldrich, St. Louis, MO) supplemented with 5 g bovine serum albumin (Hyclone; GE Healthcare Life Sciences, South Logan, UT). In the NMP-RBC group, perfusate consisted of 80 mL Williams’ Medium E (Sigma-Aldrich), supplemented with 5% bovine serum albumin (Hyclone; GE Healthcare Life Sciences), and 20 mL type O+ human red blood cells (RBC) (American Red Cross, Durham, NC). Additives included 0.2 U insulin (Humulin; Eli Lilly, Indianapolis, IN), 29.2 mg L-glutamine (L-Glutamine [200 nM]; Life Technologies, Grand Island, NY), 1 mg hydrocortisone (Solu-Cortef; Pfizer, New York, NY), and 500 IU heparin (Fresenius Kabi USA, LLC).
In both groups, livers were perfused at 37°C. Before initiating NMP, livers were allowed to rewarm for 15 minutes in normal saline at room temperature (equilibration phase). Bile was collected in vials throughout machine perfusion to avoid introducing bile into the perfusion solution. Figure 2B illustrates the rat liver during machine perfusion.
Intrahepatic oxygen tension was measured as a function of perfusate composition, PVP, and inflow oxygen concentration. PV flow rate and PVP were measured continuously (Flow pressure transducer P75, HSE amplifier module TAM-D; Hugo Sachs Elektronik - Harvard Apparatus GmbH). The test was conducted at three different PVPs (3, 5, and 8 mm Hg), and the oxygenator was gassed with two different gas mixtures (95% O2/5% CO2 vs 6% O2/8% CO2/nitrogen balanced; Airgas, Durham, NC). Two cycles at each PVP were performed for each liver graft. LabChartPro software (AD Instruments, Colorado Springs, CO) was used to display and record flow rates and PVP every 10 seconds.
Blood Gas Analysis
Before initiation of NMP, perfusate samples were drawn from the circuit for analysis of partial oxygen pressure (pO2) (iSTAT, cartridge CG4+; Abbott Point of Care Inc., Abbott Park, IL).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (Version 7.04; GraphPad Software Inc., La Jolla, CA). All continuous values are presented as mean ± SEM. Differences between continuous values were analyzed by the Student-Newman-Keuls test (unpaired, 2-sided t-test). P values <0.05 were considered significant. Spearman correlation coefficients (r) were calculated for the intrahepatic oxygen tension measured at the selected PVPs and perfusate oxygen concentrations. Values of r ≥ 0.8 are indicative of high correlation; values of r between 0.5 and 0.8 are indicative of moderate correlation; values of r < 0.5 indicate low correlation.
RESULTS
Intrahepatic Oxygen Tension Measured In Vivo
Effect of Portal Vein Clamping
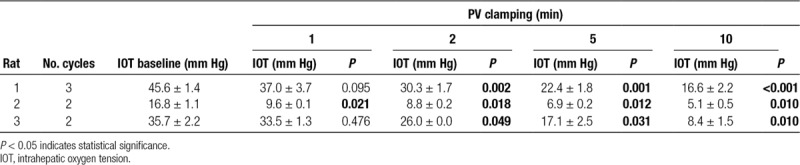
Biosensors were implanted and allowed to equilibrate for 3–5 minutes before obtaining measurements. Blood flow through the PV was intermittently occluded by application of a microvascular clamp across the liver hilum. Oxygen biosensor signals obtained from the LLL are shown in Figure 3. Immediately following PV clamping, the sensors detected a decrease in intrahepatic oxygen tension. In all livers, a significant reduction in tissue oxygenation was observed. Two minutes after clamping, intrahepatic oxygen tension was significantly decreased from baseline (P < 0.05; Table 1). On average, after 10 minutes of PV occlusion, intrahepatic oxygen tension was reduced to 30.1% of baseline (range: 23.4%–36.4%). Restoration of flow through the PV produced a prompt recovery of intrahepatic oxygen tension. Low-frequency artifacts present on tracing were observed due to breathing and pulsatile blood flow.
FIGURE 3.
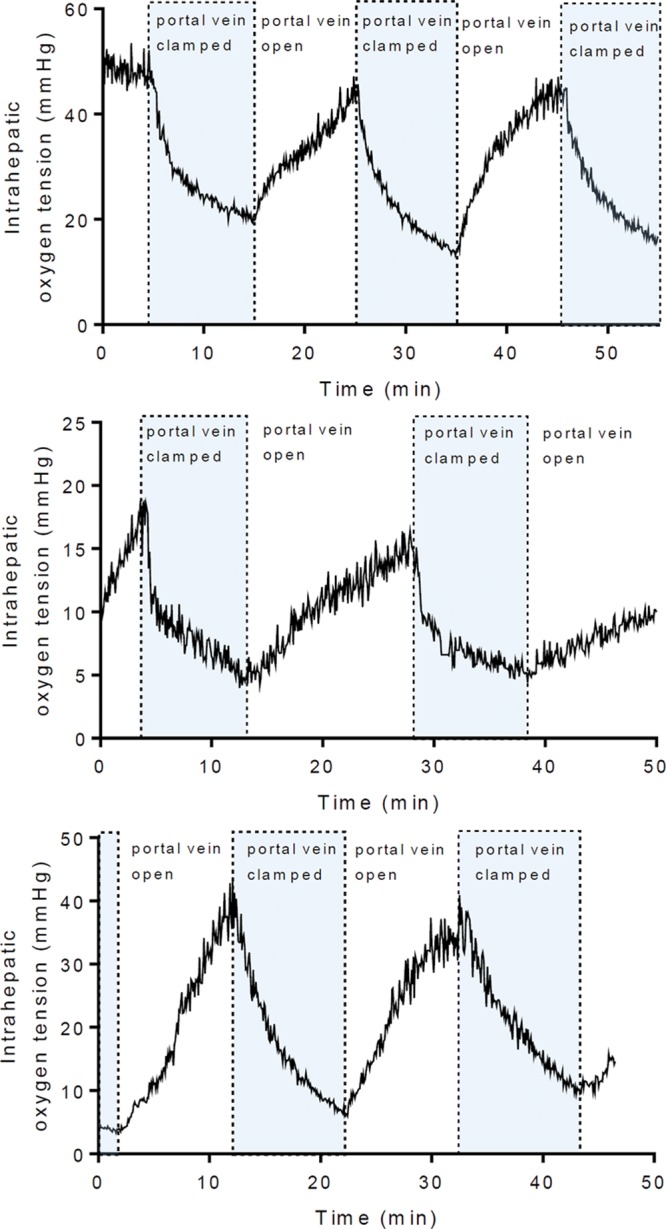
In vivo measurement of intrahepatic oxygen tension with intermittent occlusion of the portal vein. The portal vein was occluded for 10-min intervals by applying a microvascular clamp across the liver hilum. An oxygen biosensor implanted in the left lateral lobe measured oxygen tension continuously (N = 3).
TABLE 1.
In vivo measurement of intrahepatic oxygen tension during inflow occlusion of the portal vein (PV)
Parameters During NMP
Effect of Pressure Setting, Perfusate Composition, and Inflow Oxygen Concentration on Portal Vein Flow Rate
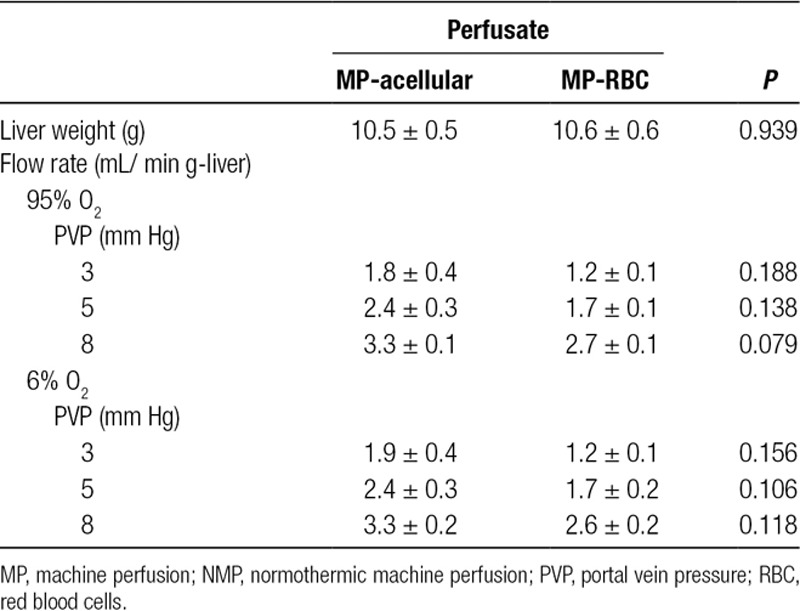
Varying the PV pressure during NMP produced expected changes in PV flow rates for both NMP-acellular and NMP-RBC groups (Figure 4 and Table 2). There were no significant differences in portal flow between NMP-acellular and NMP-RBC groups for any conditions tested (Table 2). In addition, reducing oxygen concentration from 95% to 6% did not impact PV flow rates in either group (Table 2).
FIGURE 4.
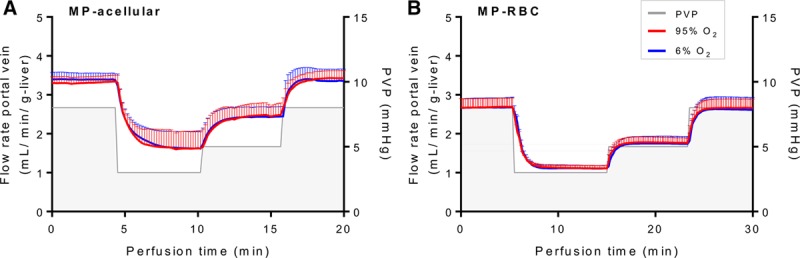
Portal vein flow rates during pressure-controlled NMP using (A) acellular perfusate (NMP-acellular) vs (B) cellular perfusate (NMP-RBC). Portal vein flow rates were measured as a function of portal vein pressure setting (3, 5, or 8 mm Hg) and inflow oxygen concentration (95% O2 vs 6% O2). As expected, portal flow increased with increasing set pressure, but there were no significant differences in portal flow between NMP-acellular and NMP-RBC groups. Portal flow was unchanged by altering inflow oxygen concentration. All data are presented as the mean ± SEM. MP, machine perfusion; NMP, normothermic machine perfusion; PVP, portal vein pressure; RBC, red blood cells; SEM, standard error of the mean.
TABLE 2.
Comparison of portal vein flow during NMP with acellular perfusate (NMP-acellular) vs cellular perfusate (NMP-RBC), while varying portal vein pressure and inflow oxygen concentration
Effect of PV Pressure on Intrahepatic Oxygen Tension During NMP
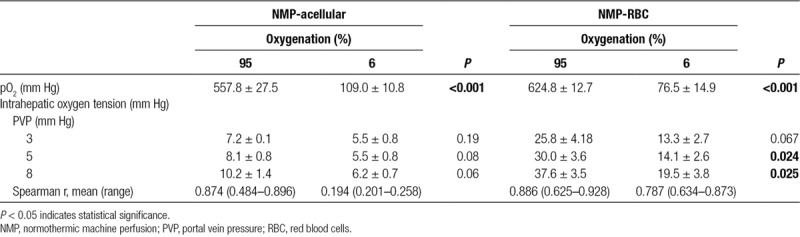
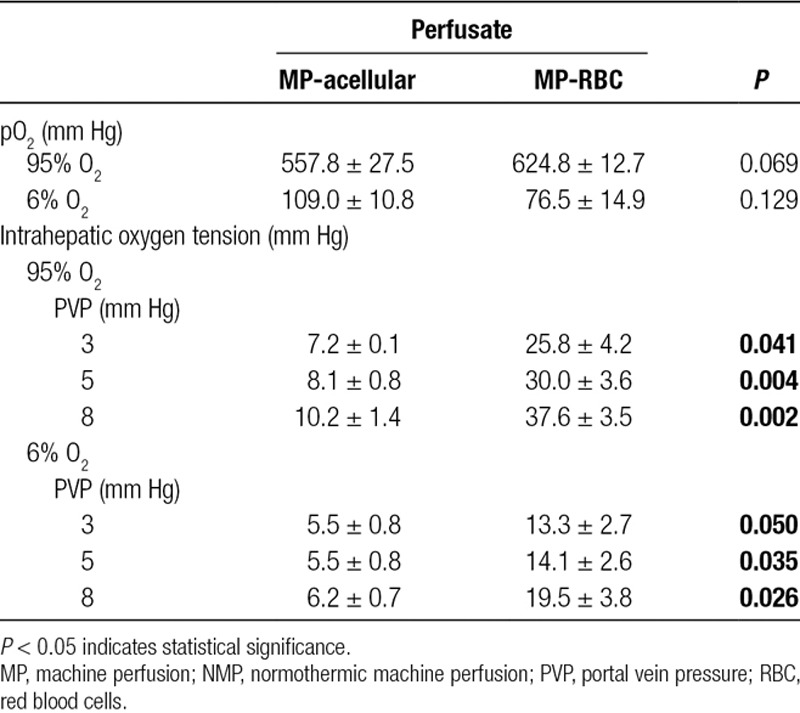
The effect of PVP on intrahepatic oxygen tension was investigated (Figure 5). When the inflow oxygen concentration was high (95%), there was a direct correlation between PVP and intrahepatic oxygen tension for both NMP-acellular and NMP-RBC groups (Spearman r: 0.87 and 0.89, respectively; Table 3). When the inflow oxygen concentration was low (6%), the PVP no longer correlated with intrahepatic oxygen tension for the NMP-acellular group, and the correlation was reduced but still present for the NMP-RBC group. Intrahepatic oxygen tension was significantly reduced in the NMP-RBC group when inflow oxygen concentration was reduced from 95% to 6%, but this was not observed in the NMP-acellular group.
FIGURE 5.
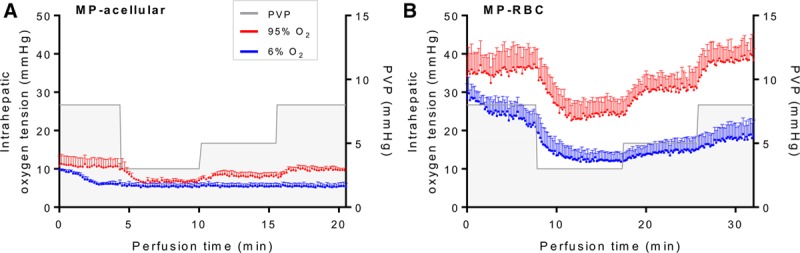
Intrahepatic oxygen tension during NMP using an (A) acellular perfusate (NMP-acellular) vs (B) cellular perfusate (NMP-RBC). Intrahepatic oxygen tension was measured while varying portal vein pressure and inflow oxygen concentration. Intrahepatic oxygen tension was significantly higher for the NMP-RBC group under all conditions tested. All data are presented as the mean ± SEM. MP, machine perfusion; NMP, normothermic machine perfusion; PVP, portal vein pressure; RBC, perfusate containing red blood cell; SEM, standard error of the mean.
TABLE 3.
Comparison of intrahepatic oxygen tension during NMP with high and low inflow oxygen concentration.
Effect of Inflow Oxygen Concentration on Intrahepatic Oxygen Tension During NMP
For the NMP-RBC group, NMP with a high inflow oxygen concentration (95% O2) produced greater intrahepatic oxygen tension than NMP with a low inflow oxygen concentration (6% O2; Figure 5). In the NMP-acellular group, there was a trend toward greater intrahepatic oxygen tension with high inflow oxygen concentration, but the difference did not reach statistical significance (Table 3).
Effect of Perfusate Composition on Intrahepatic Oxygen Tension During NMP
Under all conditions tested, intrahepatic oxygen tension was significantly higher (3–4-fold increase) in livers perfused with a cellular perfusate (containing RBCs) in comparison to livers perfused with an acellular perfusate (Figure 5 and Table 4).
TABLE 4.
Comparison of intrahepatic oxygen tension during NMP with acellular perfusate (NMP-acellular) vs cellular perfusate (NMP-RBC)
DISCUSSION
While NMP has emerged as a promising technology in liver transplantation, there is still no consensus on the optimal perfusate composition, perfusion settings, or how to determine viability of marginal liver grafts. As such, there is an ongoing need to develop point-of-care metrics that provide real-time data on tissue oxygenation, the ultimate end-point of organ perfusion.
In this proof of concept study, we demonstrate the feasibility of utilizing biocompatible oxygen sensors implanted in the graft to continuously monitor oxygen tension during NMP. We also highlight the potential use of these sensors to optimize variables associated with NMP in a systematic fashion. We demonstrated significantly higher intrahepatic oxygen tension when grafts are perfused with a cellular perfusate containing RBCs in comparison to an acellular perfusate. The effect of hemoglobin provided by RBCs in increasing oxygen content and intrahepatic oxygen tension can be readily appreciated when considering the equation for arterial oxygen content (CaO2):
In this study, the partial pressure of oxygen (PO2) was similar for the NMP-RBC and NMP-acellular groups (Table 4), so the observed increase in intrahepatic oxygen tension in the NMP-RBC groups is derived primarily from the additional oxygen transported by hemoglobin.
An important next step in this work is to determine the intrahepatic oxygen tension required for optimal graft preservation during NMP. Both hypoxia and hyperoxia can be injurious, with hypoxia causing ischemic injury and hyperoxia mediating injury through generation of free radicals. Establishing an optimal intrahepatic oxygen tension will require systematic measurement in both standard criteria and marginal grafts during NMP, followed by a robust transplant model to measure graft outcomes.
The potential clinical utility of implantable oxygen sensors extends beyond NMP into the early posttransplant period. At present, graft hypoperfusion remains undetected until organ damage becomes clinically apparent. Real-time monitoring of vascular flow and tissue oxygenation facilitates early detection of malperfusion, but currently available techniques are inconvenient for routine clinical use.20-30 The small size and simplicity of implantable oxygen sensors make them attractive for clinical use, but the lack of a “gold standard” technique for measuring tissue oxygen tension makes validation somewhat challenging. Clinical implementation of this technology will likely require correlation with direct measurement of oxygen tension using electrode-based techniques.
There are some important limitations of our study. First, there appears to be some variability in baseline oxygen tension from graft to graft. Our study used only a single sensor implanted per graft, and variability in sensor position relative to liver vasculature may contribute to this observation. It is likely that a panel of sensors placed in different lobes will be necessary to provide a more comprehensive representation of graft oxygen tension in the future. Fortunately, placement of multiple probes is likely to be feasible in human organs given their size. Second, in this proof of concept study, we performed NMP with only portal perfusion, rather than dual perfusion including the hepatic artery. The contribution of the arterial system to tissue oxygenation is thus not appreciated and requires testing in subsequent experiments.
In conclusion, intrahepatic tissue oxygenation can be continuously monitored during NMP using implantable oxygen biosensors. Our results suggest that these biosensors may be useful tools for optimizing specific parameters of NMP, such as perfusate composition, inflow pressure/flow settings, and oxygen requirement. Future studies will focus on determining how intrahepatic oxygen tension impacts the quality of graft preservation by NMP.
Footnotes
Published online 21 June, 2019.
Uwe Scheuermann and Mohamed M. Ibrahim have contributed equally and share first authorship.
U.S. and M.M.I. participated in research design, writing of the article, performance of the research, and data analysis. J.Y. and M.G.H. participated in the writing of the article and critical review. W.P. and B.K. participated in research design, writing of the article, and critical review. A.S.B. participated in research design, data analysis, writing of the article, and critical review.
The authors declare no conflicts of interest.
Astellas-American Society of Transplant Surgeons Faculty Development Grant 2017 (ASB).
REFERENCES
- 1.Brockmann J, Reddy S, Coussios C, et al. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250:1–6. [DOI] [PubMed] [Google Scholar]
- 2.Boehnert MU, Yeung JC, Bazerbachi F, et al. Normothermic acellular ex vivo liver perfusion reduces liver and bile duct injury of pig livers retrieved after cardiac death. Am J Transplant. 2013;13:1441–1449. [DOI] [PubMed] [Google Scholar]
- 3.Goldaracena N, Echeverri J, Spetzler VN, et al. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transpl. 2017;23:707–708. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Nassar A, Farias K, et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transpl. 2014;20:987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bral M, Gala-Lopez B, Bigam D, et al. Preliminary single-center canadian experience of human normothermic ex vivo liver perfusion: results of a clinical trial. Am J Transplant. 2017;17:1071–1080. [DOI] [PubMed] [Google Scholar]
- 6.Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16:1779–1787. [DOI] [PubMed] [Google Scholar]
- 7.Selzner M, Goldaracena N, Echeverri J, et al. Normothermic ex vivo liver perfusion using steen solution as perfusate for human liver transplantation: first North American results. Liver Transpl. 2016;22:1501–1508. [DOI] [PubMed] [Google Scholar]
- 8.Nasralla D, Coussios CC, Mergental H, et al. ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50–56. [DOI] [PubMed] [Google Scholar]
- 9.Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant. 2016;16:3235–3245. [DOI] [PubMed] [Google Scholar]
- 10.Bodmer SI, Balestra GM, Harms FA, et al. Microvascular and mitochondrial PO(2) simultaneously measured by oxygen-dependent delayed luminescence. J Biophotonics. 2012;5:140–151. [DOI] [PubMed] [Google Scholar]
- 11.Mik EG. Special article: measuring mitochondrial oxygen tension: from basic principles to application in humans. Anesth Analg. 2013;117:834–846. [DOI] [PubMed] [Google Scholar]
- 12.Balestra GM, Aalders MC, Specht PA, et al. Oxygenation measurement by multi-wavelength oxygen-dependent phosphorescence and delayed fluorescence: catchment depth and application in intact heart. J Biophotonics. 2015;8:615–628. [DOI] [PubMed] [Google Scholar]
- 13.Montero-Baker MF, Au-Yeung KY, Wisniewski NA, et al. The first-in-man “si se puede” study for the use of micro-oxygen sensors (moxys) to determine dynamic relative oxygen indices in the feet of patients with limb-threatening ischemia during endovascular therapy. J Vasc Surg. 2015;61:1501–1509.e1. [DOI] [PubMed] [Google Scholar]
- 14.De Santis V, Singer M. Tissue oxygen tension monitoring of organ perfusion: rationale, methodologies, and literature review. Br J Anaesth. 2015;115:357–365. [DOI] [PubMed] [Google Scholar]
- 15.Vanderkooi JM, Wilson DF. A new method for measuring oxygen concentration in biological systems. Adv Exp Med Biol. 1986;200:189–193. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim MM, Schweller RM, Mohammed MM, et al. Implantable real time oxygen biosensors for detection of vascular perfusion and ischemia. Plast Reconstr Surg Glob Open. 2017;5(4 Suppl):110–111. [Google Scholar]
- 17.Peters EB, Christoforou N, Leong KW, et al. Poly(ethylene glycol) hydrogel scaffolds containing cell-adhesive and protease-sensitive peptides support microvessel formation by endothelial progenitor cells. Cell Mol Bioeng. 2016;9:38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Xu B, Puperi DS, et al. Integrating valve-inspired design features into poly(ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomater. 2015;14:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanderkooi JM, Maniara G, Green TJ, et al. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem. 1987;262:5476–5482. [PubMed] [Google Scholar]
- 20.Akl TJ, Wilson MA, Ericson MN, et al. Wireless monitoring of liver hemodynamics in vivo. PLoS One. 2014;9:e102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks AJ, Eastwood J, Beckingham IJ, et al. Liver tissue partial pressure of oxygen and carbon dioxide during partial hepatectomy. Br J Anaesth. 2004;92:735–737. [DOI] [PubMed] [Google Scholar]
- 22.Hickey M, Samuels N, Randive N, et al. Preliminary assessment of abdominal organ perfusion utilizing a fiber optic photoplethysmographic sensor. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:1020–1023. [DOI] [PubMed] [Google Scholar]
- 23.Hickey M, Samuels N, Randive N, et al. A new fibre optic pulse oximeter probe for monitoring splanchnic organ arterial blood oxygen saturation. Comput Methods Programs Biomed. 2012;108:883–888. [DOI] [PubMed] [Google Scholar]
- 24.Hickey M, Samuels N, Randive N, et al. Investigation of photoplethysmographic signals and blood oxygen saturation values obtained from human splanchnic organs using a fiber optic sensor. J Clin Monit Comput. 2011;25:245–255. [DOI] [PubMed] [Google Scholar]
- 25.Leary TS, Klinck JR, Hayman G, et al. Measurement of liver tissue oxygenation after orthotopic liver transplantation using a multiparameter sensor. A pilot study. Anaesthesia. 2002;57:1128–1133. [DOI] [PubMed] [Google Scholar]
- 26.Mudaliar AV, Ellis BE, Ricketts PL, et al. Noninvasive blood perfusion measurements of an isolated rat liver and an anesthetized rat kidney. J Biomech Eng. 2008;130:061013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifalian AM, Mallett S, Piasecki C, et al. Non-invasive measurement of hepatic oxygenation by an oxygen electrode in human orthotopic liver transplantation. Med Eng Phys. 2000;22:371–377. [DOI] [PubMed] [Google Scholar]
- 28.Soller BR, Heard SO, Cingo NA, et al. Application of fiberoptic sensors for the study of hepatic dysoxia in swine hemorrhagic shock. Crit Care Med. 2001;29:1438–1444. [DOI] [PubMed] [Google Scholar]
- 29.Uhlmann D, Pietsch UC, Ludwig S, et al. Paratrend sensor as a novel method for continuous monitoring of hepatic microperfusion. Transplant Proc. 2002;34:3339–3341. [DOI] [PubMed] [Google Scholar]
- 30.Uhlmann D, Pietsch UC, Ludwig S, et al. Assessment of hepatic ischemia-reperfusion injury by simultaneous measurement of tissue pO2, pCO2, and pH. Microvasc Res. 2004;67:38–47. [DOI] [PubMed] [Google Scholar]


