Abstract
Purpose
To establish whether first-order statistical features from [18F]fluoride and 2-deoxy-2-[18F] fluoro-d-glucose ([18F]FDG) positron emission tomography/x-ray computed tomography (PET/CT) demonstrate incremental value in skeletal metastasis response assessment compared with maximum standardised uptake value (SUVmax).
Procedures
Sixteen patients starting endocrine treatment for de novo or progressive breast cancer bone metastases were prospectively recruited to undergo [18F]fluoride and [18F]FDG PET/CT scans before and 8 weeks after treatment. Percentage changes in SUV parameters, metabolic tumour volume (MTV), total lesion metabolism (TLM), standard deviation (SD), entropy, uniformity and absolute changes in kurtosis and skewness, from the same ≤ 5 index lesions, were measured. Clinical response to 24 weeks, assessed by two experienced oncologists blinded to PET/CT imaging findings, was used as a reference standard and associations were made between parameters and progression free and overall survival.
Results
[18F]fluoride PET/CT: In four patients (20 lesions) with progressive disease (PD), TLM and kurtosis predicted PD better than SUVmax on a patient basis (4, 4 and 3 out of 4, respectively) and TLM, entropy, uniformity and skewness on a lesion basis (18, 16, 16, 18 and 15 out of 20, respectively). Kurtosis was independently associated with PFS (p = 0.033) and OS (p = 0.008) on Kaplan-Meier analysis. [18F]FDG PET: No parameter provided incremental value over SUVmax in predicting PD or non-PD. TLM was significantly associated with OS (p = 0.041) and skewness with PFS (p = 0.005). Interlesional heterogeneity of response was seen in 11/16 and 8/16 patients on [18F]fluoride and [18F]FDG PET/CT, respectively.
Conclusion
With [18F]fluoride PET/CT, some first-order features, including those that take into account lesion volume but also some heterogeneity parameters, provide incremental value over SUVmax in predicting clinical response and survival in breast cancer patients with bone metastases treated with endocrine therapy. With [18F]FDG PET/CT, no first-order parameters were more accurate than SUVmax although TLM and skewness were associated with OS and PFS, respectively. Intra-patient heterogeneity of response occurs commonly between metastases with both tracers and most parameters.
Key words: Breast cancer, Bone metastases, Heterogeneity, [18F]fluoride PET/CT, [18F]FDG PET/CT
Introduction
Skeletal metastases are common in patients with advanced breast cancer and are associated with significant morbidity [1]. With the introduction of new systemic therapies that improve survival time, early detection and response assessment of skeletal metastases has become more important. Varied tumour response to treatment is undoubtedly an important factor in the clinical outcome, accentuating the need to have reliable measures to monitor patients for early disease progression in order to allow timely discontinuation of ineffective treatment.
Conventionally, bone scintigraphy has been used to assess breast cancer bone metastases but has certain limitations when assessing treatment response [2]. Molecular and functional imaging can improve diagnosis and treatment response assessment of breast cancer bone metastases [3]. Standardised uptake value (SUV) on positron emission tomography/computed tomography (PET/CT) has been used as a standard semi-quantitative method for monitoring treatment response but other non-heterogeneity parameters such as metabolic tumour volume (MTV) and tumour lesion metabolism (TLM) have also been used to measure metabolic activity within the tumour [4, 5]. Several retrospective studies using 2-deoxy-2-[18F] fluoro-d-glucose ([18F]FDG) PET/CT, mainly focusing on osseous response to treatment, have established that a change in SUVmax can predict disease response or progression [6–8] and a feasibility study has also shown that [18F]fluoride PET may be useful in evaluating treatment response in breast cancer [9]. Despite this, there is limited evidence to support the use of either tracer in routine clinical practice. Measuring volumetric parameters or heterogeneity of tracer activity has also been shown to have incremental predictive or prognostic value in a number of cancers [10–21]. First-order statistics measure global properties of a tumour from individual voxel values and can be obtained from the histogram of voxel intensities and are most commonly used [22], but there are no reports on the use of first-order heterogeneity parameters in evaluating treatment response assessment of breast cancer bone metastases using [18F]fluoride or [18F]FDG PET.
The hypothesis of this study was that global first-order features derived from skeletal metastases, some of which describe heterogeneity in bone metastases, may be a better predictor of response to treatment in comparison with SUVmax.
The objective of this study was to extract first-order features on both [18F]FDG (tumour-specific radiotracer that targets glucose metabolism) and [18F]fluoride (bone-specific tracer that targets osteoblast activity and local blood flow) PET/CT images in breast cancer bone metastases, at baseline and 8 weeks after endocrine treatment, and to compare their ability to predict treatment response determined by a clinical reference standard as well as survival with the most commonly described parameter, SUVmax.
Materials and Methods
Participants
Sixteen female breast cancer patients (mean age 51.6, range 40–79 years) starting endocrine treatment for de novo (n = 5) or progressive bone metastases (n = 11) from an ongoing prospective single-centre exploratory study were included. The endocrine treatments used were letrozole (n = 12), tamoxifen (n = 2), everolimus/exemestane (n = 1) and anastrazole (n = 1). Apart from two patients who had small volume lung and liver metastases, all other patients had bone-only disease. [18F]fluoride and [18F]FDG PET/CT scans were acquired before and 8 weeks after starting treatment. The study was approved by a Research Ethics Committee and the Administration of Radioactive Substances Advisory Committee and all patients signed an informed consent form at the time of recruitment.
[18F]FDG PET/CT and [18F]fluoride PET/CT Image Acquisition
After injection of [18F]FDG (mean 348 ± 18 MBq), PET/CT scans commenced after an uptake time of 60 min. On a separate day following injection of [18F]fluoride (mean 228 ± 15 MBq), scans were performed after an uptake time of 60 min. Imaging comprised a static PET/CT scan using a GE Discovery 710 PET/CT scanner (GE Healthcare, Chicago, USA). Each scan covered the base of the skull to mid-thigh, with an axial field-of-view of 15.7 cm and an 11-slice overlap between bed positions. A low-dose CT scan (140 kV, 10 mA, 0.5 s rotation time and 40 mm collimation) was performed at the start of imaging to provide attenuation correction and an anatomical reference. PET scan duration was set to 3 min per bed position.
PET image reconstruction included standard scanner-based corrections for radiotracer decay, scatter, randoms and dead-time. Emission sinograms were reconstructed with a time-of-flight ordered subset expectation maximisation (OSEM) algorithm (2 iterations, 24 subsets), with a 256 × 256 matrix and a 4-mm full-width at half-maximum (FWHM) Gaussian post-reconstruction smoothing filter on the scanner front end, available from the manufacturer.
Parameter Analysis
Up to five of the most active (SUV ≥ 10) [23] and largest (≥ 1 cm diameter) lesions were first identified for analysis on [18F]fluoride scans in each subject. Regions of interest (ROIs) were delineated around the same metastasis on the static [18F]fluoride and [18F]FDG PET/CT scans by an oncologist and radiologist working in consensus (Figs. 1 and 2). Image heterogeneity analysis was performed using in-house quantitative analysis software implemented in MATLAB (Mathworks, Natick, MA, USA). First-order statistics derived from regional geometry and the histogram distribution of voxel intensities (standard deviation (SD), entropy, uniformity, kurtosis and skewness) on both [18F]FDG and [18F]fluoride PET scans were calculated as well as non-heterogeneity parameters such as SUVmax, SUVmean, SUVpeak, TLM and MTV. All parameters on both PET scans were calculated for the same lesions at baseline and 8 weeks, and changes in the values of these parameters from baseline were used for statistical analyses. The tumour volumes were generally small ((mean volume = 7.1 cm3 (SD = 8.3) on [18F]fluoride PET/CT and (mean volume = 5.7 cm3 (SD = 5.9) on [18F]FDG PET/CT)); therefore, in order to avoid bias from small volumes, second and high-order texture features were not calculated [24, 25]. We also analysed changes in SUVmax between lesions in each individual patient to assess the degree of interlesional heterogeneity of response with both tracers. Interlesional heterogeneity was defined when a metastasis showed a change in parameter that was opposite to the clinical reference standard.
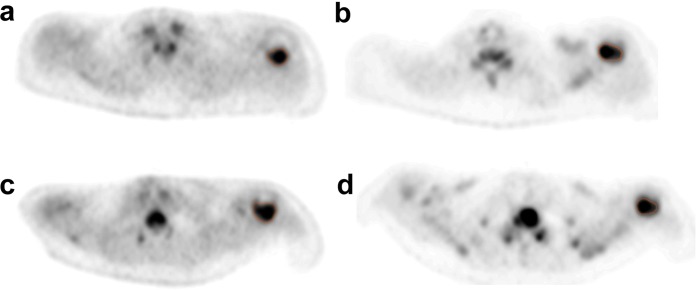
Fig. 1.
Figure demonstrating regions of interest in the left humerus in a patient with clinical progressive disease. a, c [18F]FDG and b, d [18F]fluoride PET transaxial slices a, b before and c, d 8 weeks after treatment. [18F]FDG SUVmax 14.6 at baseline and 15.4 at 8 weeks. [18F]fluoride SUVmax 37.7 at baseline, 56.5 at 8 weeks and 82.4 at 12 weeks (not shown).
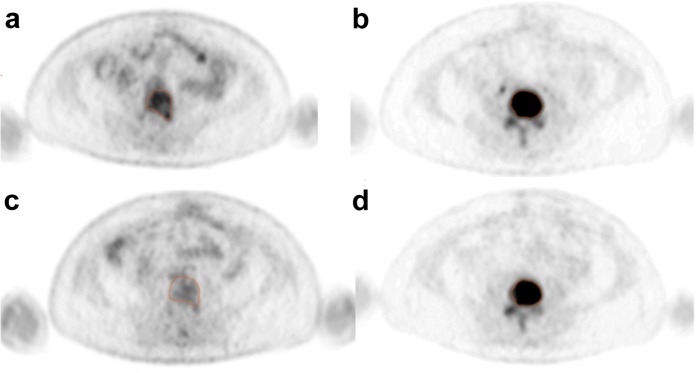
Fig. 2.
Figure demonstrating regions of interest in L4 in a patient with non-progressive disease (partial response). a, c [18F]FDG and b, d [18F]fluoride PET transaxial slices a, b before and c, d 8 weeks after treatment. [18F]FDG SUVmax 8.4 at baseline and 4.4 at 8 weeks. [18F]fluoride SUVmax 72.1 at baseline and 46.5 at 8 weeks.
Two experienced oncologists working in consensus, blinded to PET/CT findings, determined clinical response based on standard imaging including bone scans and contrast-enhanced CT, clinical assessment, including pain scores (using brief pain inventory questionnaire), as well as alkaline phosphatase and carcinoma antigen 15-3 serology up to 24 weeks after the start of treatment or until progression, whichever came first and was used as a reference standard (Table 1). The changes in clinical parameters were used in patient assessment and none of the parameters were used in isolation. Any discrepancy was reviewed by a third clinician and only one went to a third reader. Assessment decisions were made on bone-only disease, given no soft tissue disease in the majority of this group, so soft tissue response (Response Evaluation Criteria In Solid Tumours (RECIST)) was not relevant in our studied population. Patients were grouped into progressive disease (PD) and non-progressive disease (non-PD = partial response (PR) and stable disease (SD)). PR and SD patients were assessed together as clinical management is rarely different in these two groups.
Table 1.
Different parameters used in each patient for categorisation into PD or non-PD
| Patient | Disease status | ALP (alkaline phosphatase) | Ca-153 | Pain score | Clinical assessment | Bone scan | CT scan |
|---|---|---|---|---|---|---|---|
| 1 | PD | Increased | Stable | Worse | New bone pain | Not done | Increase in number of lesions by 24 weeks |
| 2 | PD | Increased | Increased | Stable | Weight loss, vomiting | Increase in number of lesions by 24 weeks | Increase in number of lesions by 24 weeks |
| 3 | PD | Stable | Increased | Worse | New bone pain | Increase in number of lesions by 24 weeks | Increase in number of lesions by 24 weeks |
| 4 | PD | Increased | Stable | Worse | Worsening bone pain | Increase in number of lesions by 24 weeks | Not done |
| 5 | Non-PD | Increased | Increased | Better | Better | No change | No change |
| 6 | Non-PD | Stable | Decreased | Better | Stable symptoms | Not done | No change |
| 7 | Non-PD | Stable | Stable | Better | Asymptomatic | No change | No change |
| 8 | Non-PD | Stable | Stable | Better | Better | No change | Disappearance of some lesions |
| 9 | Non-PD | Better | Better | Better | Stable symptoms | Not done | Disappearance of dome lesions |
| 10 | Non-PD | Better | Stable | Better | Stable symptoms | Not done | No change |
| 11 | Non-PD | Stable | Stable | Better | Asymptomatic | No change | No change |
| 12 | Non-PD | Stable | Stable | Better | Stable symptoms | No change | |
| 13 | Non-PD | Stable | Better | Stable | Asymptomatic | No change | Disappearance of some lesions |
| 14 | Non-PD | Better | Better | Better | Stable symptoms | No change | Disappearance of some lesions |
| 15 | Non-PD | Stable | Better | Better | Stable symptoms | Disappearance of some lesions | Disappearance of some lesions |
| 16 | Non-PD | Better | Stable | Better | Stable symptoms | No change | No change |
Statistical Analysis
Statistical analysis was performed using SPSS for windows version 24 (IBM SPSS Statistics 24). After testing for normality, parametric or nonparametric tests were applied to each set of data. Data that were normally distributed were expressed as a mean and standard deviation and evaluated using the paired t test. Data that were not normally distributed were expressed as median and range and evaluated using Wilcoxon signed rank test or Mann-Whitney U test. For all statistical tests, a P value of ≤ 0.05 was considered statistically significant.
On both [18F]fluoride and [18F]FDG PET/CT scans, optimum threshold values were established with receiver operating characteristic analysis, maximising the sum of sensitivity and specificity by measuring associated areas under the ROC curves as there are no established criteria for these tracers with endocrine treatment, although ± 25 % has been suggested for [18F]FDG post-chemotherapy [26] (Tables 2 and 3). On this basis, we also used a 25 % cut-off for the SUV parameters on [18F]FDG (adapted from the EORTC criteria) and [18F]fluoride PET/CT scans, acknowledging that these criteria were described for [18F]FDG PET/CT. The values of percentage (%) changes in SUVmax, SUVmean, SUVpeak, TLG, MTV, entropy, uniformity and absolute changes in skewness and kurtosis, on [18F]fluoride and [18F]FDG PET/CT scans after 8 weeks of treatment, were calculated for all patients.
Table 2.
Changes in parameter values for PD and non-PD after 8 weeks of treatment on [18F]fluoride PET/CT with associated optimum and 25 % cut-off values and AUC
| PD (20 lesions/4 patients) | Non-PD (52 lesions, 12 patients) | P value for PD vs non-PD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median change (%) | Optimal cut-off (ROC curve analysis) | Number of lesions identified (↑ or ↓) | Number of patients correctly identified (↑ or ↓) | Median change (%) | Number of lesions identified (↑ or ↓) | Number of patients identified (↑ or ↓) | |||||||
| Value | AUC | Opt cut-off | 25 % cut-off | Opt cut-off | 25 % cut-off | Opt cut-off | 25 % cut-off | Opt cut-off | 25 % cut-off | ||||
| SUVmax (%) | − 3.0 | − 15.1 | 0.67 | 15 ↑ | 7 ↑ | 3 ↑ | 2 ↑ | − 20.1 | 29 ↓ | 47 ↓ | 5 ↓ | 9 ↓ | 0.021 |
| SUVmean (%) | 15.1 | − 2.6 | 0.75 | 14 ↑ | 6 ↑ | 3 ↑ | 2 ↑ | − 16.0 | 38 ↓ | 46 ↓ | 8 ↓ | 9 ↓ | 0.001 |
| SUVpeak (%) | 3.0 | − 3.9 | 0.72 | 13↑ | 8 ↑ | 3 ↑ | 2 ↑ | − 17.6 | 31 ↓ | 46 ↓ | 7 ↓ | 9 ↓ | 0.003 |
| MTV (%) | − 2.5 | 19.0 | 0.51 | 14 ↓ | 0 | 1.1 | 44 ↓ | 10 ↓ | 0.806 | ||||
| TLM (%) | 9.2 | − 19.0 | 0.67 | 18 ↑ | 4 ↑ | − 11.2 | 26 ↑ | 8 ↑ | 0.023 | ||||
| SD (%) | 4.9 | 9.2 | 0.67 | 10 ↑ | 2 ↑ | − 25.4 | 43↓ | 8 ↓ | 0.022 | ||||
| Ent (%) | 1.3 | 0.6 | 0.67 | 16 ↑ | 3 ↑ | − 0.5 | 37 ↓ | 5 ↓ | 0.020 | ||||
| Unif (%) | − 6.6 | 1.8 | 0.69 | 16 ↓ | 3 ↓ | 2.9 | 31 ↑ | 7 ↑ | 0.012 | ||||
| Kurt (abs) | − 0.1 | − 0.005 | 0.66 | 14 ↓ | 4 ↓ | 0.04 | 31 ↑ | 8 ↑ | 0.034 | ||||
| Skew (abs) | − 0.05 | 0.1 | 0.5 | 18 ↓ | 3 ↓ | 0.01 | 37 ↓ | 11 ↓ | 0.277 | ||||
MTV metabolic tumour volume, TLM tumour lesion metabolism, SD standard deviation, Ent entropy, Unif uniformity, Kurt kurtosis, Skew skewness, abs absolute change, % percentage change, ↑ ≥ the cut-off value, ↓ < the cut-off value
Table 3.
Changes in parameter values for PD and non-PD after 8 weeks of treatment on [18F]FDG PET/CT with associated optimum and 25 % cut-off values and AUC
| PD (20 lesions/4 patients) | Non-PD (52 lesions/12 patients) | P value for PD vs non-PD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median change | Optimal cut-off (ROC curve analysis) | Number of lesions identified (↑ or ↓) | Number of patients identified (↑ or ↓) | Median change | Number of lesions identified (↑ or ↓) | Number of patients identified (↑ or ↓) | |||||||
| Value | AUC | Opt cut-off | 25 % cut-off | Opt cut-off | 25 % cut-off | Opt cut-off | 25 % cut-off | Opt cut-off | 25 % cut-off | ||||
| SUVmax (%) | − 2.2 | − 20.6 | 0.76 | 18 ↑ | 2 ↑ | 4 ↑ | 0 ↑ | − 27.2 | 33 ↓ | 52 ↓ | 7 ↓ | 12 ↓ | 0.001 |
| SUVmean (%) | 0.4 | − 21.7 | 0.77 | 18 ↑ | 1 ↑ | 4 ↑ | 0 ↑ | − 24.2 | 28 ↓ | 52 ↓ | 4 ↓ | 12 ↓ | 0.001 |
| SUVpeak (%) | 0.6 | − 15.0 | 0.76 | 17 ↑ | 2 ↑ | 3 ↑ | 0 ↑ | − 24.7 | 33 ↓ | 52 ↓ | 6 ↓ | 12 ↓ | 0.001 |
| MTV (%) | 10.7 | − 14.1 | 0.70 | 17 ↑ | 4 ↑ | − 19.7 | 30 ↓ | 6 ↓ | 0.006 | ||||
| TLM (%) | 4.2 | − 24.1 | 0.76 | 18 ↑ | 4 ↑ | − 40.2 | 33 ↓ | 6 ↓ | 0.001 | ||||
| SD (%) | 4.1 | − 23.4 | 0.72 | 16 ↑ | 4 ↑ | − 33.5 | 33 ↓ | 7 ↓ | 0.004 | ||||
| Ent (%) | − 0.7 | − 2.2 | 0.55 | 15 ↑ | 4 ↑ | − 1.1 | 33 ↑ | 7 ↑ | 0.458 | ||||
| Unif (%) | 2.8 | 5.8 | 0.59 | 15 ↓ | 3 ↓ | 4.4 | 27 ↓ | 6 ↓ | 0.235 | ||||
| Kurt (abs) | 0.1 | 0.2 | 0.51 | 16 ↓ | 4 ↓ | 0.09 | 29 ↓ | 7 ↓ | 0.826 | ||||
| Skew (abs) | 0.05 | − 0.13 | 0.55 | 14 ↑ | 0 | 4 ↑ | 0 | − 0.03 | 29 ↑ | 6 ↑ | 0.517 | ||
MTV metabolic tumour volume, TLM tumour lesion metabolism, SD standard deviation, Ent entropy, Unif uniformity, Kurt kurtosis, Skew skewness, abs absolute change, % percentage change, ↑ ≥ the cut-off value, ↓ < the cut-off value
Kaplan-Meier analysis was performed using the median value for each parameter to dichotomise the results with differences in the curves tested with the log-rank test. Progression free survival (PFS) was defined as the time between the date of the start of endocrine treatment and the date of disease progression and overall survival (OS) was calculated from the start of endocrine treatment to the date of death or until censoring on the date of the last follow-up.
Results
There was a total of 16 patients (72 lesions). By the clinical reference standard, 4 patients (20 lesions) had PD at or before 24 weeks and 12 patients (52 lesions) non-PD at 24 weeks. Patients were followed up from between 12 and 49 (median 31.5) months. Four patients died during the follow-up period and all 16 patients progressed at between 2 and 32 (median 11.3) months.
Using the cut-offs that maximised sensitivity and specificity from ROC analysis (Tables 2 and 3), on [18F]fluoride PET/CT, in all 16 patients, MTV, TLM, kurtosis and skewness performed better than SUVmax on a patient basis (10, 12, 12, 11 and 8 out of 16, respectively) and SD, entropy, uniformity, kurtosis and skewness on a lesion basis (53, 53, 47, 45, 55 and 44 out of 72, respectively). In the four patients with PD, TLM and kurtosis predicted PD better than SUVmax on a patient basis (4, 4 and 3 out of 4, respectively) and TLM, entropy, uniformity and skewness on a lesion basis (18, 16, 16, 18 and 15 out of 20, respectively) (Table 2). In the 12 patients with non-PD, [18F]fluoride MTV, TLM, SD, uniformity, kurtosis and skewness predicted non-PD better than SUVmax on a patient basis (10, 8, 8, 7, 8, 11 and 5 out of 12, respectively) and MTV, SD, entropy, uniformity and skewness on a lesion basis (44, 43, 37, 31, 37 and 30 out of 52, respectively (Table 2). On [18F]FDG PET/CT, no parameter provided incremental value over SUVmax overall or in predicting PD or non-PD (Table 3).
In PD and non-PD, on both [18F]fluoride PET/CT and [18F]FDG PET/CT scans, median % change in first-order features was not statistically significantly different from the median % change in the SUV parameters. On a per lesion basis, on [18F]fluoride PET/CT, median % changes in SUVmax, SUVmean, SUVpeak, TLM, SD and entropy were significantly higher in PD than non-PD (− 3 vs − 20.1 % (p = 0.021), 15.1 vs − 16 % (p = 0.001), 3 vs − 17.6 % (p = 0.003), 9.2 vs − 11.2 % (p = 0.023), 4.9 vs − 25.4 % (p = 0.022), 1.3 vs − 0.5 % (p = 0.020), respectively) and median % changes in uniformity and kurtosis were significantly lower in PD than non-PD (− 6.6 vs 2.9 % (p = 0.012), − 0.1 vs 0.04 % (p = 0.034), respectively) (Table 2). On [18F]FDG PET/CT, the median % changes in SUVmax, SUVmean, SUVpeak, MTV, TLM and SD were significantly higher in patients with PD than non-PD (− 2.2 vs − 27.2 % (p = 0.001), 0.4 vs − 24.2 % (p = 0.001), 0.6 vs − 24.7 % (p = 0.001), 10.7 vs − 19.7 % (p = 0.006), 4.2 vs − 40.2 % (p = 0.001), 4.1 vs − 33.5 % (p = 0.004), respectively) (Table 3). Other changes were not statistically significant.
Interlesional heterogeneity of response was seen in 11/16 and 8/16 patients on [18F]fluoride and [18F]FDG PET/CT, respectively. For [18F]fluoride, there was a statistically significant difference between the 15 lesions that showed an increase in SUVmax, in patients with PD and the 22 lesions that showed an increase in SUVmax in patients with non-PD for entropy (p = 0.028), uniformity (p = 0.008) and kurtosis (0.033). No non-PD patient showed new lesions at 8 weeks with either [18F]FDG or [18F]fluoride PET/CT.
Kaplan-Meier analysis showed that on [18F]fluoride PET/CT, at 8 weeks, change in kurtosis had a statistically significant association with PFS (p = 0.033) and OS (p = 0.008) (Fig. 3a, b). On [18F]FDG PET/CT, change in TLM was significantly associated with OS (p = 0.041) and skewness with PFS (p = 0.005).
Fig. 3.
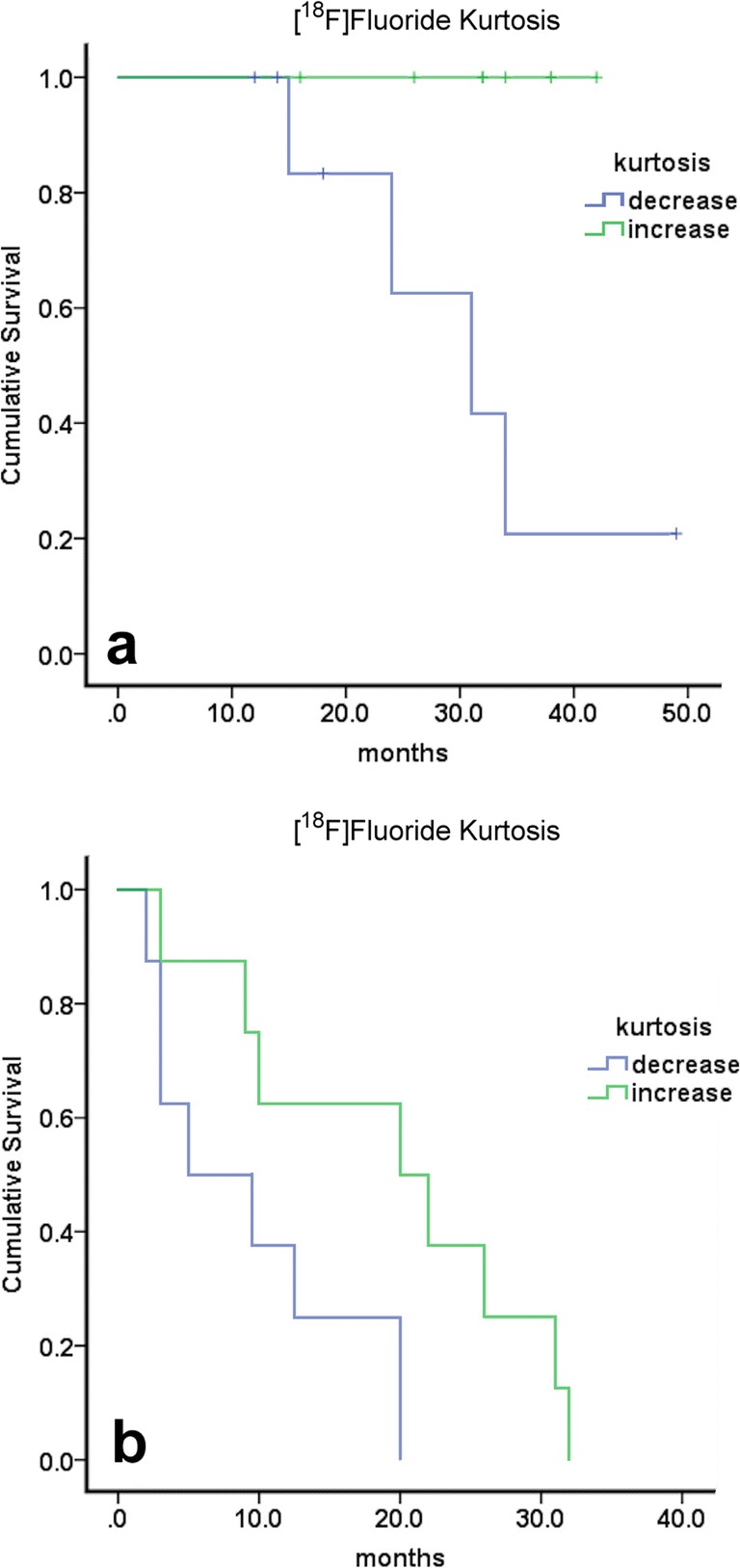
Kaplan-Meier graphs showing worse a OS and b PFS in patients with negative changes in kurtosis less than the median (p = 0.008 and 0.033, respectively) for [18F]fluoride PET/CT scans.
Discussion
Breast cancer is commonly associated with skeletal metastases and early evaluation of response or progression to treatment is vital to the optimisation of patients’ clinical management. To our knowledge, this is the first report that has evaluated several first-order statistical features, including some heterogeneity parameters, for early treatment response assessment of breast cancer bone metastases compared to standard SUV measures using [18F]fluoride and [18F]FDG PET/CT.
For [18F]fluoride PET/CT, several first-order global parameters showed superiority over SUVmax, either on a patient-based or lesion-based analysis in predicting PD, including volume-based parameters (MTV, TLM) and heterogeneity parameters (entropy, uniformity, kurtosis and skewness). Additionally, kurtosis was associated with both PFS and OS. Whilst recognition of PD is of most clinical importance, as these patients will need an early transition to second-line therapy, the majority of first-order parameters were also better than SUVmax at predicting non-PD. The observed changes in patients with PD were as expected, i.e., an increase in activity, volume and/or heterogeneity. In particular, a decrease in kurtosis was also associated with PFS and OS. This relates to an increase in spread (less peakedness) of the voxel intensity histogram (Fig. 4a, b) or greater “heterogeneity” in voxel values within lesions.
Fig. 4.
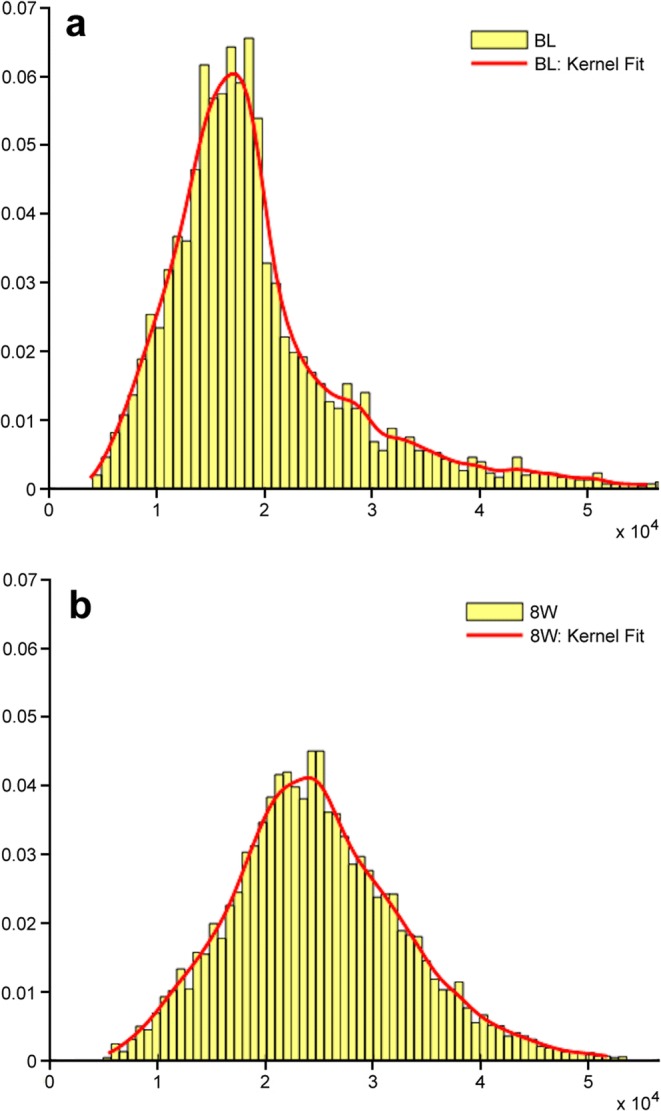
Voxel intensity histograms at a baseline (BL) and b 8 weeks (8W) with kernel fits of a metastasis in a patient with PD on [18F]fluoride PET/CT. As well as an increase in intensity of voxels (SUVmax rose from 32.7 to 38.0 and SUVmean from 12.8–15.3), the histogram becomes less peaked (kurtosis decreased from 5.6 to 3.1).
Whilst changes in SUV and volume parameters, as well as kinetic analysis, have been reported for monitoring therapy with [18F]fluoride PET in bone metastases [9, 27–29], to our knowledge, there are no data describing superiority of heterogeneity parameters in this situation. However, first-order heterogeneity parameters have shown predictive and prognostic ability in other cancers with [18F]FDG PET/CT and increased heterogeneity is usually associated with more aggressive tumours and poor treatment response [11, 13, 21, 30].
For [18F]FDG PET/CT, no parameter performed better than SUVmax in predicting response, although an increase in TLM and skewness (shift of histogram to the right with more high intensity voxels) was associated with poor OS and PFS, respectively, whereas SUVmax was not prognostic. In accordance with our findings, changes in SUVmax have previously been shown to be valuable in assessing treatment response in breast cancer skeletal metastases [7, 8].
Heterogeneity of response between metastases is a recognised phenomenon [31] and occurred in 11/16 of our patients with [18F]fluoride and 8/16 with [18F]FDG. The more frequent occurrence with [18F]fluoride may be partly explained by the flare phenomenon whereby a temporary increase in osteoblastic activity can occur in healing metastases in non-PD patients [32–34]. Despite this and the fact that uptake of the two tracers is dependent on differing underlying biological processes (tumour cell glucose metabolism with [18F]FDG and osteoblastic mineralisation of bone with [18F]fluoride), both tracers offered predictive and prognostic information at a level that would be of clinical utility on a per patient basis. We also observed that entropy, uniformity and kurtosis were significantly different in the 15/20 lesions with a concordant increase in SUVmax in PD patients compared to a discordant increase in the 22/52 lesions in patients with non-PD, the latter that could be attributed to the flare phenomenon. This would require further prospective validation but the potential to differentiate an increase in uptake due to true progression from the flare phenomenon at 8 weeks would be of great clinical utility when using [18F]fluoride PET to measure early response, overcoming one of the limitations of bone-specific imaging.
Limitations of this study include a relatively small number of patients, although we were able to include a large number of individual metastases in the analysis (n = 72). In addition, there was a smaller number of patients with PD (n = 4) compared to non-PD which may have introduced an element of statistical bias. Whilst all patients had similar treatment, i.e., endocrine therapy, treatment regimens were not exactly the same and there was probably heterogeneity in response. Nevertheless, the main objective of this exploratory study was to measure response rather than treatment-specific effects. Though our study was prospective, these findings deserve further evaluation in larger cohorts as well as in the assessment of different types of therapy and bone metastases from other cancers. Whilst there is no gold standard for determining treatment response in bone metastases, our clinical reference standard was made as robust and clinically relevant as possible by including clinical findings, conventional imaging, biochemistry and tumour markers up to 24 weeks assessed by two oncologists in consensus and we were also able to include a survival analysis as an objective assessment of the measured parameters. Whilst criteria for response assessment have previously been reported for [18F]FDG SUVmax or SUVpeak [3, 35], these criteria have not been applied to other first-order parameters and so we used optimal cut-offs in this exploratory study in addition to 25 % cut-offs for [18F]FDG and [18F]fluoride conventional SUV parameters. We acknowledge that repeatability of first-order texture features is variable and that the optimal cut-offs for some parameters that have been reported as showing lower repeatability, such as uniformity and skewness [36], may be within the limits of repeatability measurements. Several novel parameters still performed better, even when an optimal SUVmax cut-off was used for comparison.
Conclusions
Our exploratory data demonstrate that certain first-order statistical features from [18F]fluoride and [18F]FDG PET, related to volume and heterogeneity, may provide incremental value over SUVmax in the prediction of treatment response and survival in breast cancer bone metastases treated with endocrine therapy, a finding that deserves confirmation in further prospective evaluation in future studies. In addition, with [18F]fluoride some heterogeneity parameters can potentially differentiate an increase in SUVmax due to flare from that due to progression of disease. These findings may be of potential clinical utility as the prediction of early PD helps oncologists decide whether an earlier switch to more effective therapies is required, whereas non-PD patients would generally continue the same treatment if there were no significant toxicities. We also observed that intra-patient heterogeneity of response occurs commonly between metastases with both tracers and most parameters.
Funding Information
The authors acknowledge financial support from the King’s College London/University College London Comprehensive Cancer Imaging Centres funded by Cancer Research UK and Engineering and Physical Sciences Research Council in association with the Medical Research Council and the Department of Health (C1519/A16463), Breast Cancer Now (2012NovPR013), the Wellcome Trust EPSRC Centre for Medical Engineering at King’s College London (WT203148/Z/16/Z), the Royal College of Radiologists, Alliance Medical Ltd. and the support of the National Institute of Health Research Clinical Research Network (NIHR CRN).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Domchek SM, Younger J, Finkelstein DM, Seiden MV. Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer. 2000;89:363–368. doi: 10.1002/1097-0142(20000715)89:2<363::AID-CNCR22>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Coombes RC, Dady P, Parsons C, McCready VR, Ford HT, Gazet JC, Powles TJ. Assessment of response of bone metastases to systemic treatment in patients with breast cancer. Cancer. 1983;52:610–614. doi: 10.1002/1097-0142(19830815)52:4<610::AID-CNCR2820520406>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Lecouvet FE, Talbot JN, Messiou C, Bourguet P, Liu Y, de Souza NM, EORTC Imaging Group Monitoring the response of bone metastases to treatment with magnetic resonance imaging and nuclear medicine techniques: a review and position statement by the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer. 2014;50:2519–2531. doi: 10.1016/j.ejca.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, Casilla C, Fazzari M, Srivastava N, Yeung HW, Humm JL, Guillem J, Downey R, Karpeh M, Cohen AE, Ginsberg R. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2:159–171. doi: 10.1016/S1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 5.Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, Yu J. Prognostic significance of metabolic parameters measured by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer. 2011;73:332–337. doi: 10.1016/j.lungcan.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 6.De Giorgi U, Mego M, Rohren EM, et al. 18F-FDG PET/CT findings and circulating tumor cell counts in the monitoring of systemic therapies for bone metastases from breast cancer. J Nucl Med. 2010;51:1213–1218. doi: 10.2967/jnumed.110.076455. [DOI] [PubMed] [Google Scholar]
- 7.Specht J, Tam S, Kurland B, et al. Serial 2-[18F] fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) to monitor treatment of bone-dominant metastatic breast cancer predicts time to progression (TTP) Breast Cancer Res Treat. 2007;105:87–94. doi: 10.1007/s10549-006-9435-1. [DOI] [PubMed] [Google Scholar]
- 8.Stafford SE, Gralow JR, Schubert EK, Rinn KJ, Dunnwald LK, Livingston RB, Mankoff DA. Use of serial FDG PET to measure the response of bone-dominant breast cancer to therapy. Acad Radiol. 2002;9:913–921. doi: 10.1016/S1076-6332(03)80461-0. [DOI] [PubMed] [Google Scholar]
- 9.Doot RK, Muzi M, Peterson LM, Schubert EK, Gralow JR, Specht JM, Mankoff DA. Kinetic analysis of 18F-fluoride PET images of breast cancer bone metastases. J Nucl Med. 2010;51:521–527. doi: 10.2967/jnumed.109.070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orlhac F, Soussan M, Maisonobe JA, Garcia CA, Vanderlinden B, Buvat I. Tumor texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med. 2014;55:414–422. doi: 10.2967/jnumed.113.129858. [DOI] [PubMed] [Google Scholar]
- 11.Vaidya M, Creach KM, Frye J, Dehdashti F, Bradley JD, el Naqa I. Combined PET/CT image characteristics for radiotherapy tumor response in lung cancer. Radiother Oncol. 2012;102:239–245. doi: 10.1016/j.radonc.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Cook GJ, Yip C, Siddique M, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med. 2013;54:19–26. doi: 10.2967/jnumed.112.107375. [DOI] [PubMed] [Google Scholar]
- 13.El Naqa I, Grigsby P, Apte A, et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recogn. 2009;42:1162–1171. doi: 10.1016/j.patcog.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang F, Thomas MA, Dehdashti F, Grigsby PW. Temporal analysis of intratumoral metabolic heterogeneity characterized by textural features in cervical cancer. Eur J Nucl Med Mol Imaging. 2013;40:716–727. doi: 10.1007/s00259-012-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tixier F, Le Rest CC, Hatt M, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52:369–378. doi: 10.2967/jnumed.110.082404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan S, Kligerman S, Chen W, Lu M, Kim G, Feigenberg S, D'Souza WD, Suntharalingam M, Lu W. Spatial-temporal [(1)(8)F]FDG-PET features for predicting pathologic response of esophageal cancer to neoadjuvant chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:1375–1382. doi: 10.1016/j.ijrobp.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong X, Xing L, Wu P, Fu Z, Wan H, Li D, Yin Y, Sun X, Yu J. Three-dimensional positron emission tomography image texture analysis of esophageal squamous cell carcinoma: relationship between tumor 18F-fluorodeoxyglucose uptake heterogeneity, maximum standardized uptake value, and tumor stage. Nucl Med Commun. 2013;34:40–46. doi: 10.1097/MNM.0b013e32835ae50c. [DOI] [PubMed] [Google Scholar]
- 18.Eary JF, O’Sullivan F, O’Sullivan J, et al. Spatial heterogeneity in sarcoma 18F-FDG uptake as a predictor of patient outcome. J Nucl Med. 2008;49:1973–1979. doi: 10.2967/jnumed.108.053397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Caldwell C, Mah K, Poon I, Balogh J, MacKenzie R, Khaouam N, Tirona R. Automated radiation targeting in head-and-neck cancer using region-based texture analysis of PET and CT images. Int J Radiat Oncol Biol Phys. 2009;75:618–625. doi: 10.1016/j.ijrobp.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 20.Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology. 2011;261:165–171. doi: 10.1148/radiol.11110264. [DOI] [PubMed] [Google Scholar]
- 21.Cook GJR, O’Brien ME, Siddique M, Chicklore S, Loi HY, Sharma B, Punwani R, Bassett P, Goh V, Chua S. Non small cell lung cancer treated with erlotinib: heterogeneity of 18F-FDG uptake at PET-association with treatment response and prognosis. Radiology. 2015;276:883–893. doi: 10.1148/radiol.2015141309. [DOI] [PubMed] [Google Scholar]
- 22.Haralick RM, Shanmugan Dinstein KI. Textural features for image classification. IEEE Trans Syst Man Cybern. 1973;3:610–621. doi: 10.1109/TSMC.1973.4309314. [DOI] [Google Scholar]
- 23.Rohren EM, Etchebehere EC, Araujo JC, Hobbs BP, Swanston NM, Everding M, Moody T, Macapinlac HA. Determination of skeletal tumor burden on 18F-fluoride PET/CT. J Nucl Med. 2015;56:1507–1512. doi: 10.2967/jnumed.115.156026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatt M, Majdoub M, Vallieres M, Tixier F, le Rest CC, Groheux D, Hindie E, Martineau A, Pradier O, Hustinx R, Perdrisot R, Guillevin R, el Naqa I, Visvikis D. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med. 2015;56:38–44. doi: 10.2967/jnumed.114.144055. [DOI] [PubMed] [Google Scholar]
- 25.Brooks FJ, Grigsby PW. The effect of small tumor volumes on studies of intratumoral heterogeneity of tracer uptake. J Nucl Med. 2014;55:37–42. doi: 10.2967/jnumed.112.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/S0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 27.Cook GJR, Parker C, Chua S, et al. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223Ra-chloride (Alpharadin) EJMMI Res. 2011;1:4. doi: 10.1186/2191-219X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu EY, Duan F, Muzi M, Deng X, Chin BB, Alumkal JJ, Taplin ME, Taub JM, Herman B, Higano CS, Doot RK, Hartfeil D, Febbo PG, Mankoff DA. Castration-resistant prostate cancer bone metastasis response measured by 18F-fluoride PET after treatment with dasatinib and correlation with progression-free survival: results from American College of Radiology Imaging Network 6687. J Nucl Med. 2015;56:354–360. doi: 10.2967/jnumed.114.146936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etchebehere EC, Araujo JC, Fox PS, Swanston NM, Macapinlac HA, Rohren EM. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56:1177–1184. doi: 10.2967/jnumed.115.158626. [DOI] [PubMed] [Google Scholar]
- 30.Hyun SH, Kim HS, Choi SH, Choi DW, Lee JK, Lee KH, Park JO, Lee KH, Kim BT, Choi JY. Intratumoral heterogeneity of 18F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2016;43:1461–1468. doi: 10.1007/s00259-016-3316-6. [DOI] [PubMed] [Google Scholar]
- 31.Huyge V, Garcia C, Alexiou J, Ameye L, Vanderlinden B, Lemort M, Bergmann P, Awada A, Body JJ, Flamen P. Heterogeneity of metabolic response to systemic therapy in metastatic breast cancer patients. Clin Oncol. 2010;22:818–827. doi: 10.1016/j.clon.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 32.Wade AA, Scott JA, Kuter I, Fischman AJ. Flare response in 18F-fluoride ion PET bone scanning. Am J Roentgenol. 2006;186:1783–1786. doi: 10.2214/AJR.05.0225. [DOI] [PubMed] [Google Scholar]
- 33.Cook GJ, Taylor BP, Glendenning J, et al. Heterogeneity of treatment response in skeletal metastases from breast cancer in 18F-fluoride and 18F-FDG PET. Nucl Med Commun. 2015;36:515–516. doi: 10.1097/MNM.0000000000000303. [DOI] [Google Scholar]
- 34.Cook GJ, Azad GK, Goh V. Imaging bone metastases in breast cancer: staging and response assessment. J Nucl Med. 2016;57(Suppl 1):27s–33s. doi: 10.2967/jnumed.115.157867. [DOI] [PubMed] [Google Scholar]
- 35.JH O, Lodge MA, Wahl RL. Practical PERCIST: a simplified guide to PET respons e criteria in solid tumors 1.0. Radiology. 2016;280:576–584. doi: 10.1148/radiol.2016142043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desseroit MC, Tixier F, Weber WA, Siegel BA, Cheze le Rest C, Visvikis D, Hatt M. Reliability of PET/CT shape and heterogeneity features in functional and morphologic components of non-small cell lung cancer tumors: a repeatability analysis in a prospective multicenter cohort. J Nucl Med. 2017;58:406–411. doi: 10.2967/jnumed.116.180919. [DOI] [PMC free article] [PubMed] [Google Scholar]