Abstract
Cardiac remodeling characterized by cardiac fibrosis is a pathologic process occurring after acute myocardial infarction. Fibrosis can be ameliorated by interferon-gamma (IFN-γ), which is a soluble cytokine showing various effects such as anti-fibrosis, apoptosis, anti-proliferation, immunomodulation, and anti-viral activities. However, the role of IFN-γ in cardiac myofibroblasts is not well established. Therefore, we investigated the anti-fibrotic effects of IFN-γ in human cardiac myofibroblasts (hCMs) in vitro and whether indoleamine 2,3-dioxygenase (IDO), induced by IFN-γ and resulting in cell cycle arrest, plays an important role in regulating the biological activity of hCMs. After IFN-γ treatment, cell signaling pathways and DNA contents were analyzed to assess the biological activity of IFN-γ in hCMs. In addition, an IDO inhibitor (1-methyl tryptophan; 1-MT) was used to assess whether IDO plays a key role in regulating hCMs. IFN-γ significantly inhibited hCM proliferation, and IFN-γ-induced IDO expression caused cell cycle arrest in G0/G1 through tryptophan depletion. Moreover, IFN-γ treatment gradually suppressed the expression of α-smooth muscle actin. When IDO activity was inhibited by 1-MT, marked apoptosis was observed in hCMs through the induction of interferon regulatory factor, Fas, and Fas ligand. Our results suggest that IFN-γ plays key roles in anti-proliferative and anti-fibrotic activities in hCMs and further induces apoptosis via IDO inhibition. In conclusion, co-treatment with IFN-γ and 1-MT can ameliorate fibrosis in cardiac myofibroblasts through apoptosis.
Keywords: Myofibroblast; Interferon-gamma; Indoleamine 2,3-dioxygenase; Apoptosis
Introduction
Cardiac remodeling is a pathologic process leading to structural and functional derangement of damaged cardiac tissue after acute myocardial infarction [1]. The severity of cardiac remodeling is mainly determined by the extent of tissue infarction and the degree of cardiac repair. Cardiac fibrosis is a representative response to various pathophysiological cardiac conditions. It is characterized by the excessive production and accumulation of extracellular matrix (ECM) components (consisting of collagen, fibronectin, glycosaminoglycan, and elastin) in the injured cardiac tissue [2]. This cellular change causes increased stiffness with decreased compliance of the heart, resulting in both systolic and diastolic dysfunction. Cardiac repair requires a balanced healing process from the early inflammatory phase to the reparative and proliferative phases [1, 3]. Appropriate induction and proliferation of cardiac myofibroblasts (CMs) help to prevent cardiac rupture with scar formation, though ongoing pathological activation of CMs causes fibrotic changes to the non-infarcted surrounding tissues followed by a deterioration of cardiac function [4–11]. One potential therapeutic approach is to inhibit the progression of cardiac fibrosis. CMs are widely recognized as a key factor mediating cardiac fibrosis because activated CMs can produce excessive ECMs and express the highly contractile protein α-smooth muscle actin that remodels the surrounding ECM [12].
Interferons (IFNs) are a group of cytokines that elicit pleiotropic biological effects, including immunomodulatory, cell differentiative, anti-angiogenic, anti-proliferative, anti-fibrogenic effects [13–15]. IFN-γ is a sole type II IFNs and mediate their effects by binding to cell surface receptors, consisting of IFN-γR1 and IFN-γR2 subunits [16, 17] and thus activating members of the non-receptor tyrosine kinases Janus-activated kinase (JAK)1 and JAK2. Activation of JAKs phosphorylates the signal transducers and activators of transcription (STAT) family of transcription factors. The JAK/STAT signal activated by IFN-γ induce expression the transcription factor IFN response factor 1 (IRF-1) and then regulate the various target genes, including apoptosis-related caspases, Fas/CD95, Fas ligand, and Bcl-2 [18, 19]. The anti-fibrotic effects of interferon-gamma (IFN-γ) are well established in hepatic fibrosis models. It has been reported that IFN-γ has anti-fibrotic effects on hepatic stellate cells, which play important roles in ECM production in the liver by suppressing ECM production regulated by mothers against decapentaplegic homolog 3 (Smad3), Smad7, Y-box-binding protein 1, or p300/CREB-binding protein [20–24]; inhibition of fibroblast–myofibroblast differentiation [25, 26]; and growth retardation of myofibroblasts [27–30]. In addition, IFN-γ modulates tryptophan metabolism by inducting and activating indoleamine 2,3 dioxygenase (IDO), which inhibits ECM production in human dermal fibroblasts [31]. IDO is a heme-containing enzyme that, in humans, is encoded by the IDO1 gene or the variant IDO2 gene [32, 33] and plays roles in inhibiting ECM production, suppressing inflammatory responses, and promoting immune tolerance [31, 34]. However, no consensus has been reached regarding the role of IFN-γ or whether it is harmful or protective with respect to cardiac remodeling [35, 36]. Moreover, no obvious treatment option is available to reduce cardiac fibrosis [36]. Therefore, we investigated whether IFN-γ shows anti-fibrotic effects in human cardiac myofibroblasts (hCMs) in vitro and whether IDO inhibition, which is induced by IFN-γ, would decrease the viability of hCMs.
Materials and methods
Cell culture
hCMs, isolated from normal adult ventricles, were purchased from Lonza (Walkersville, MD, USA) and maintained in fibroblast growth medium (FGM)-3 with 10% fetal bovine serum, growth factors (recombinant human fibroblast growth factor and insulin), and gentamicin/amphotericin-B. All cell culture media and supplements were purchased from Lonza. IFN-γ (100 ng/ml; R&D Systems, Minneapolis, MN, USA) or 1-MT (0.5 mM; 1-MT (Sigma, San Diego, CA, USA) were used in the experiments. In this study, P4 hCMs were used.
Methylthiazolyldiphenyl-tetrazolium bromide (MTT) assays
hCMs (3500 cells/cm2) were plated in 96-well plates. After 24 h, cells were treated with IFN-γ or 1-MT for the indicated time points and then MTT (Sigma), dissolved in phosphate-buffered saline (PBS), was added to each well at a final concentration of 5 mg/ml. The hCMs were incubated at 37 °C for 2 h. Formazan (formed in the plates during the assay) was dissolved in 100 μl DMSO, and a microplate reader (BioTek Instruments, Winooski, VT, USA) was used to read the optical density of each well at 570 nm.
Cell cycle analysis
The Cycle TEST Plus DNA Reagent Kit (BD Biosciences, San Jose, CA, USA) was used to analyze the cellular DNA contents, as per the manufacturer’s instructions. hCMs were trypsinized and then trypsinization was neutralized by adding FGM-3. Next, hCMs were centrifuged at 1800 rpm for 5 min. The cells were washed twice using the wash buffer provided in the kit. Thereafter, the cells were sequentially treated with solutions A, B, and C in a dark room, in compliance with the manufacturer’s instructions. Cellular DNA contents were analyzed by flow cytometry (BD FACSAria III, BD Biosciences).
Immunoblotting
hCMs were treated with IFN-γ alone or IFN-γ + 1-MT for increasing amounts of time. hCMs were lysed with 1X Laemmli sample buffer (62.5 mM Tris–HCl [pH 6.8], 10% glycerol, 1% sodium dodecyl sulfate [SDS], and 5% β-mercaptoethanol) and boiled for 5 min. Proteins were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins were transferred to an Immobilon-P PVDF membrane (Millipore, Billerica, MA, USA). Then, the membrane was blocked for 30 min in Tris-buffered saline containing 5% non-fat and 0.05% Tween-20, followed by incubation with a primary antibody against IDO1, IDO2, IRF-1, Fas, Fas ligand (FasL), glyceraldehyde 3-phosphate dehydrogenase (Santa Cruz Biotechnology, Santa Cruz, CA, USA), cellular (ED-A) fibronectin (Sigma), or α-smooth muscle actin (SMA) (Abcam, Cambridge, MA, USA). Species-specific horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) were used to detect bound primary antibodies. Visualization of protein bands was performed with the West Pico chemiluminescent substrate (Thermo Scientific, Rockford, IL, USA) and the BioSpectrum imaging system (UVP, Upland, CA, USA).
Measurement of IDO activity
IDO activities of hCMs were determined by detecting the conversion of tryptophan to kynurenine [37, 38]. Cells were treated with IFN-γ ± 1-MT for 48 or 72 h. Briefly, the cell pellets were washed twice in cold PBS. The cells were re-suspended at 1.5 × 107 cells/ml in PBS and frozen at − 80 °C until being analyzed. Cells were rapidly thawed at 37 °C and refrozen in liquid nitrogen, and this process was repeated three times. Cell lysates (250 μl) were mixed with an equivalent volume of 2 × IDO reaction buffer (100 mM potassium phosphate buffer [pH 6.5], 20 μM methylene blue, 40 mM ascorbate, 200 U/ml catalase, and 800 μM l-tryptophan) and incubated at 37 °C for 30 min. The reaction was ceased by adding 30% trichloroacetic acid (Sigma) and then incubated at 50 °C for 30 min. One hundred microliters of each supernatant were transferred to a microfuge tube after pelleting the proteins at 3000×g for 10 min. An equal volume of Ehrlich’s reagent (2% p-dimethylaminobenzaldehyde in glacial acetic acid) was added, and the resulting mixture was incubated at room temperature for 10 min. Kynurenine products were detected at 490 nm using a microplate reader (BioTek Instruments).
Annexin-V and 7-aminoactinomycin (7-AAD) staining
Apoptosis was measured with the PE-Annexin-V Apoptosis Detection Kit I (BD Biosciences) according to the manufacturer’s instructions. hCMs (3500 cells/cm2) were seeded in 60-mm dishes. After 24 h, cells were treated with IFN-γ or 1-MT for 72 h. hCMs were harvested, washed twice in cold PBS, and re-suspended in 1 × binding buffer. Then, hCMs were stained with PE-annexin-V and 7-AAD at room temperature for 15 min in the dark. Cells were rapidly analyzed without washing by flow cytometer within 1 h. To calculate the dead cell population, the percentages of early (Q4; PE-annexin-V/7-AAD, ±) and late apoptotic cells (Q2; PE-annexin-V/7-AAD, +/+) were analyzed.
Statistics
Data are expressed as the mean ± standard error (SE). Differences between groups were analyzed by one-way analysis of variance with Tukey’s test against the control. Statistical analysis was performed using SPSS software, version 22.0 (IBM Corporation, Armonk, NY, USA). Statistical significance was defined as P < 0.05.
Results
Growth retardation of hCMs by IFN-γ
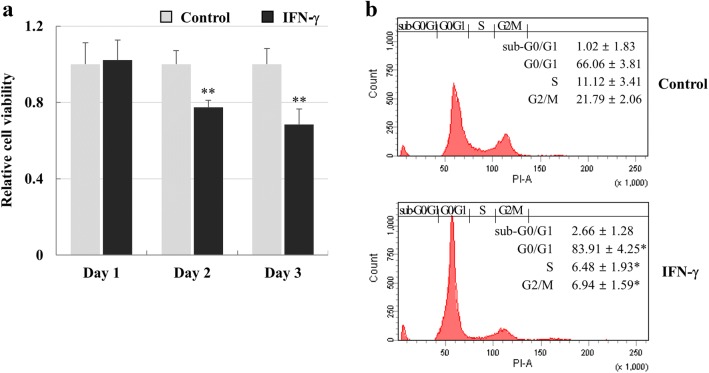
hCMs were treated with 100 ng/ml IFN-γ for 3 days to investigate the growth-inhibitory effect. Cell viability significantly decreased by approximately 22.5% at 2 days and 31.5% at 3 days (Fig. 1a). After treatment with IFN-γ for 3 days, cells in S and G2/M phase decreased in number, but the percentage of the G0/G1 population increased from 66.1 to 83.9% versus the control group. However, the sub-G0/G1 population was not increased by IFN-γ (Fig. 1b). These results suggest that cell cycle arrest in the G0/G1 phase by IFN-γ induced hCM growth retardation.
Fig. 1.
Growth suppression of hCMs by IFN-γ. a Cell viability of hCMs treated with IFN-γ. hCMs were treated with 100 ng/ml IFN-γ. Cell viability was evaluated by performing MTT assays. Data are expressed as the mean ± SE of triplicate experiments. **P < 0.01 b Cell cycle analysis of hCMs treated with IFN-γ for 72 h. Cellular DNA contents were analyzed by flow cytometry. The data shown are from one of three independent experiments. *P < 0.05
IDO induction by IFN-γ in hCMs
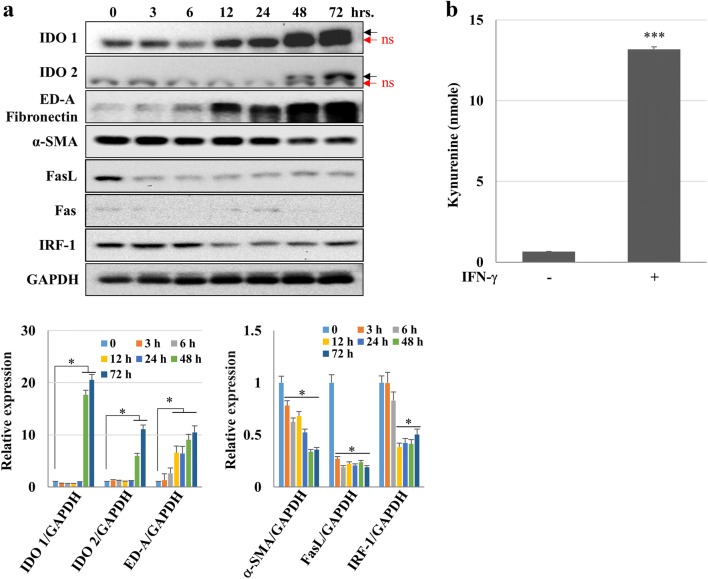
We investigated whether IFN-γ could induce IDO expression and tryptophan depletion in hCMs. The IDO1 and IDO2 expression levels were elevated at 48- and 72-h post-treatments with IFN-γ. ED-A fibronectin increased in a time-dependent manner, but α-SMA expression gradually decreased. The expression levels of apoptosis-related genes such as IRF-1 and FasL were also diminished, although IRF-1 tended to recover somewhat after 24 h (Fig. 2a). To analyze the IDO enzyme activity after IFN-γ treatment, kynurenine production from hCMs treated with IFN-γ for 72 h was calculated. An average of 13 nmoles of kynurenine was produced by IFN-γ treatment for 72 h (Fig. 2b). These data suggest that IFN-γ induce IDO expression, resulting in tryptophan depletion.
Fig. 2.
Signaling pathways activated by IFN-γ in hCMs. a hCMs were treated with IFN-γ (100 ng/ml) for the indicated time points, and the expression of proteins related to apoptosis (i.e., IRF-1, Fas, and FasL) or cell cycle arrest (i.e., IDO1 and IDO2) was detected by immunoblotting. In addition, STAT1 phosphorylation and α-SMA expression were also evaluated. *P < 0.05 Black arrows: IDO bands; red arrows: non-specific (ns) bands. b Kynurenine production in hCMs treated with IFN-γ for 72 h. The data shown are expressed as the mean ± SE of triplicate experiments. ***P < 0.001. (Color figure online)
Cell death after co-treatment with IFN-γ and 1-MT in hCMs via IRF-1, Fas, and FasL expression
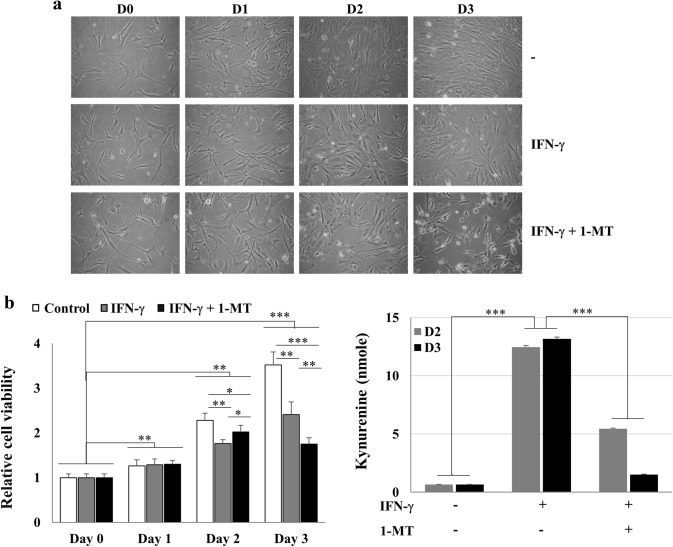
Next, we investigated whether inhibiting IDO activity by 1-MT could restore cell growth. Cell death was observed under a microscope after hCMs had been treated with IFN-γ + 1-MT for 3 days (Fig. 3a). Interestingly, growth retardation by IFN-γ was partially reversed by 1-MT on day 2, but cell viability was further reduced by 1-MT treatment on day 3 (Fig. 3b). Moreover, IFN-γ-derived kynurenine production was significantly reduced by 1-MT (Fig. 3c). These results suggest that 1-MT inhibited tryptophan depletion, thereby partially reversing the growth-inhibitory activity of IFN-γ, but eventually induced cell death regardless of tryptophan depletion.
Fig. 3.
Release from IFN-γ-induced cell cycle arrest by inhibiting IDO activity. a, b Growth-inhibitory effects of IFN-γ (100 ng/ml) and/or 1-MT (0.5 mM) in hCMs. hCMs were treated with IFN-γ or IFN-γ + 1-MT for the indicated time points, and cell viability was examined by light microscopy (a) or MTT assays (b). *P < 0.05, **P < 0.01, and ***P < 0.001 c Kynurenine production in hCMs treated with IFN-γ or IFN-γ + 1-MT for 48 or 72 h. The data shown are expressed as the mean ± SE of triplicate experiments. ***P < 0.001
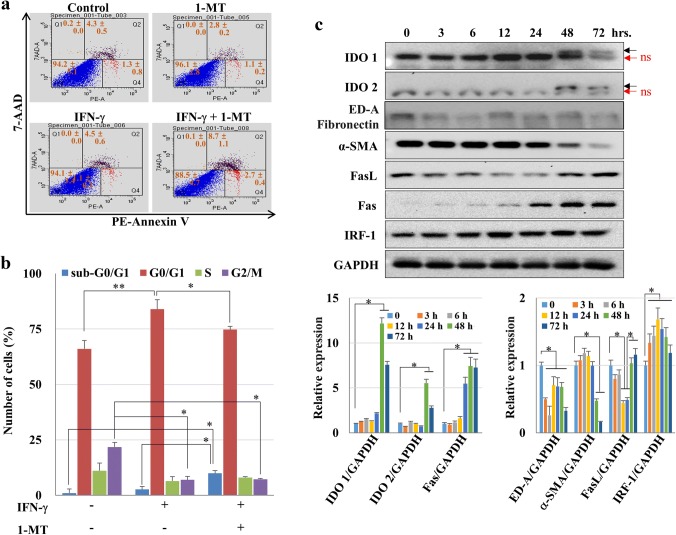
To confirm that cell death occurred in hCMs treated with IFN-γ and 1-MT, PE-annexin-V/7-AAD staining was performed and DNA contents were analyzed by flow cytometry. The percentage of early (PE-annexin-V-PE/7-AAD, ±) and late apoptotic cells (PE-annexin-V/7-AAD, +/+) increased after co-treatment with IFN-γ and 1-MT to 11.5%, compared with 5.8% in the control group (Fig. 4a). The sub-G0 population also increased from 1 to 10% (Fig. 4b). In addition, α-SMA, ED-A fibronectin, IDO1, and IDO2 expression decreased markedly in hCMs co-treated with IFN-γ and 1-MT compared to that of the IFN-γ-treated group (Fig. 2a). On the other hand, IRF-1 expression increased until 12 h and then gradually decreased to 72 h. But, the IRF-1 expression level was still higher than baseline. Fas, an apoptosis-related-gene, gradually increased over time (Fig. 4c). In addition, although FasL was reduced by 24 h, the expression level was restored at 48 and 72 h. These results suggest that 1-MT modulated the activity of IDO; induced apoptosis in myofibroblasts via the expression of apoptosis-related genes such as IRF-1 and Fas and/or FasL; and consequently reduced fibrosis.
Fig. 4.
Cell death induced by IFN-γ and 1-MT in hCMs. a Cell death of hCMs induced by IFN-γ and 1-MT. hCMs treated with IFN-γ + 1-MT for 72 h were analyzed by PE-annexin-V and 7-AAD staining, and cell death was quantified by flow cytometry. Necrotic (PE-annexin-V/7-AAD, ±), late apoptotic (PE-annexin-V/7-AAD, +/+), live (PE-annexin-V/7-AAD, –/–), and early-apoptotic cells (PE-Annexin-V/7-AAD, ±) are shown in Q1–Q4, respectively. b Change in the cell cycle phase induced by IFN-γ + 1-MT. Cellular DNA contents were analyzed by flow cytometry. The data shown are expressed as the mean ± SE of triplicate experiments. *P < 0.05 and **P < 0.01 c Signaling pathways activated by IFN-γ and 1-MT in hCMs. In hCMs treated with IFN-γ (100 ng/ml) and 1-MT (0.5 mM) for the indicated time points, the expression of apoptosis-related proteins (i.e., IRF-1, Fas, and FasL) were analyzed by immunoblotting. The y axis of the lower left graph is the logarithmic scale. *P < 0.05 and **P < 0.01. Additionally, p-STAT1, α-SMA, IDO1, and IDO2 were also detected. Black arrows: IDO bands; red arrows: non-specific (ns) bands. (Color figure online)
Discussion
Activated CMs lead to myocardial scarring and fibrosis of damaged or diseased myocardium by regulating cardiac ECM production [39]. Therefore, CM regulation is important for ameliorating cardiac remodeling. We observed that (1) IFN-γ inhibited hCM proliferation through G0/G1 cell cycle arrest and reduced the expression of α-SMA, a marker of fibrosis [40]. (2) G0/G1 cell cycle arrest was induced by tryptophan depletion through increased IDO activity, induced by IFN-γ. (3) Co-treatment with IFN-γ and IDO inhibitor (1-MT) markedly reduced the activity of hCMs expressing α-SMA and induced apoptosis through up-regulating the IRF-1, Fas, and FasL genes.
Conflicting data have been reported regarding whether IFN-γ is harmful or protective for the heart. IFN-γ overexpression caused chronic active myocarditis, eventually resulting in cardiomyopathy in IFN-γ transgenic mice [41]. Marko et al. [42] reported that IFN-γ blockade reduced inflammation and cardiac fibrosis in IFN-γ receptor-knockout mice. Han et al. [43] also demonstrated that IFN-γ knockout reduced the accumulation of α-SMA-positive cells and α-SMA expression in an angiotensin II-induced cardiac fibrosis model. In contrast, Fairweather et al. [44] reported that IFN-γ deficiency led to increased chronic viral myocarditis following cardiac fibrosis, pericarditis, and dilated cardiomyopathy in IFN-γ-deficient mice. Mast cell degranulation and profibrotic cytokines (i.e., transforming growth factor-β1, interleukin-1β, and interleukin-4) were increased in the heart. Moreover, Afanasyeva et al. found that IFN-γ deficiency increased cardiac inflammation and resulted in dilated cardiomyopathy and heart failure in a mouse model of autoimmune myocarditis [45].
Despite these conflicting results, little information has been reported regarding whether IFN-γ can directly regulate the proliferation and activity of CMs. IFN-γ is well known to express IDO, a rate-limiting enzyme of tryptophan catabolism. Consequently, degradation of the essential amino acid tryptophan to kynurenine causes cell starvation and induces anti-proliferative effects for pathogens, tumor cells, immune cells, and mesenchymal stem cells [46–49], as well as the hCMs used in this study. In addition, tryptophan-derived catabolites including kynurenine inhibited activated T cell and natural killer cell proliferation [50].
Interestingly, co-treatment with IFN-γ and 1-MT induced cell death in hCMs rather than the recovery of proliferative activity. The underlying mechanism is not fully understood. IFNs regulate the expression of interferon regulatory factor protein family members, including IRF-1. IRF-1 regulates the expression of target genes including IDO by binding to an interferon-stimulated response element in their promoters. IRF-1 can also modulate the expression of FasL and induce apoptosis in T cells [51]. In our systems, IFN-γ reduced IRF-1 expression, but co-treatment with IFN-γ and 1-MT gradually increased the expression of IRF-1 by 12 h. However, after 12 h, these expression levels decreased but remained at levels comparable to those of the control group at 72 h. In addition, Fas expression markedly increased in hCMs co-treated with IFN-γ and 1-MT, but FasL expression decreased by 24 h before returning to an increased level by 48 and 72 h. These results suggest that the role of IRF-1 in the expression of IDO and Fas may be different in hCMs. In other words, IFN-γ can induce IRF-1-independent IDO expression, thereby promoting G0/G1 cell cycle arrest through tryptophan depletion in hCMs. However, the IDO inhibitor 1-MT may increase IRF-1-dependent Fas expression and induce apoptosis of hCMs..
Because this in vitro study was performed at the cellular level, in vivo animal models are needed to confirm these results. However, to our knowledge, this is the first study to demonstrate the regulation of activated CMs by IFN-γ and 1-MT. In the future, it should be confirmed whether co-treatment with IFN-γ and 1-MT can control cardiac fibrosis in animal and human models.
In summary, we report that IFN-γ-induced IDO expression decreased cell growth and induced G0/G1 cell cycle arrest in hCMs through tryptophan depletion. Moreover, inhibition of IDO expression with the IDO inhibitor 1-MT increased apoptosis in hCMs through the induction of Fas, FasL, and IRF-1.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [Grant Number NRF- 2017R1D1A1A02019212], the Bio & Medical Technology Development Program of the NRF funded by the Korean government, the Ministry of Science, ICT & Future Planning [Grant Number NRF-2016M3A9B4919711], and Hanmi Pharmaceutical Co., Ltd., Seoul, Republic of Korea. The sponsors did not play any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun-Won Lee and Ji Eun Oh contributed equally to this work.
Contributor Information
Jun-Won Lee, Email: ljwcardio@yonsei.ac.kr.
Ji Eun Oh, Email: skdia7604@naver.com.
Ki-Jong Rhee, Email: kjrhee@yonsei.ac.kr.
Byung-Su Yoo, Phone: +82-33-741-0917, Email: yubs@yonsei.ac.kr.
Young Woo Eom, Phone: +82-33-741-0260, Email: yweom@yonsei.ac.kr.
Sang Wook Park, Email: shinseizi@gmail.com.
Ji Hyun Lee, Email: 89family@hanmail.net.
Jung-Woo Son, Email: soneycar@gmail.com.
Young Jin Youn, Email: younyj@yonsei.ac.kr.
Min-Soo Ahn, Email: heartsaver@yonsei.ac.kr.
Sung-Gyun Ahn, Email: sgahn@yonsei.ac.kr.
Jang-Young Kim, Email: kimjy@yonsei.ac.kr.
Seung-Hwan Lee, Email: carshlee@yonsei.ac.kr.
Junghan Yoon, Email: jyoon@yonsei.ac.kr.
References
- 1.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Z, Guan J. Antifibrotic therapies to control cardiac fibrosis. Biomater Res. 2016;20:13. doi: 10.1186/s40824-016-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobaczewski M, De Haan JJ, Frangogiannis NG. The extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium. J Cardiovasc Transl Res. 2012;5:837–847. doi: 10.1007/s12265-012-9406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González A, Ravassa S, Beaumont J, López B, Díez J. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol. 2011;58:1833–1843. doi: 10.1016/j.jacc.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 6.Van Den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 7.Yarbrough WM, Mukherjee R, Stroud RE, Rivers WT, Oelsen JM, Dixon JA, Eckhouse SR, Ikonomidis JS, Zile MR, Spinale FG. Progressive induction of left ventricular pressure overload in a large animal model elicits myocardial remodeling and a unique matrix signature. J Thorac Cardiovasc Surg. 2012;143:215–223. doi: 10.1016/j.jtcvs.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janicki JS, Brower GL, Gardner JD, Chancey AL, Stewart JA. The dynamic interaction between matrix metalloproteinase activity and adverse myocardial remodeling. Heart Fail Rev. 2004;9:33–42. doi: 10.1023/B:HREV.0000011392.03037.7e. [DOI] [PubMed] [Google Scholar]
- 10.Spinale FG, Janicki JS, Zile MR. Membrane-associated matrix proteolysis and heart failure. Circ Res. 2013;112:195–208. doi: 10.1161/CIRCRESAHA.112.266882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuruda T, Costello-Boerrigter LC, Burnett JC. Matrix metalloproteinases: pathways of induction by bioactive molecules. Heart Fail Rev. 2004;9(1):53–61. doi: 10.1023/B:HREV.0000011394.34355.bb. [DOI] [PubMed] [Google Scholar]
- 12.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res. 2015;116:1269–1276. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 13.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 14.Oh JE, Shim KY, Lee JI, Choi SI, Baik SK, Eom YW. 1-Methyl-l-tryptophan promotes the apoptosis of hepatic stellate cells arrested by interferon-γ by increasing the expression of IFN-γRβ, IRF-1 and FAS. Int J Mol Med. 2017;40:576–582. doi: 10.3892/ijmm.2017.3043. [DOI] [PubMed] [Google Scholar]
- 15.Borden EC, Lindner D, Dreicer R, Hussein M, Peereboom D. Second-generation interferons for cancer: clinical targets. Semin Cancer Biol. 2000;10:125–144. doi: 10.1006/scbi.2000.0315. [DOI] [PubMed] [Google Scholar]
- 16.Ealick SE, Cook WJ, Vijay-Kumar S, Carson M, Nagabhushan TL, Trotta PP, Bugg CE. Three-dimensional structure of recombinant human interferon-gamma. Science. 1991;252:698–702. doi: 10.1126/science.1902591. [DOI] [PubMed] [Google Scholar]
- 17.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 18.Dou L, Liang HF, Geller DA, Chen YF, Chen XP. The regulation role of interferon regulatory factor-1 gene and clinical relevance. Hum Immunol. 2014;75:1110–1114. doi: 10.1016/j.humimm.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Tomita Y, Bilim V, Hara N, Kasahara T, Takahashi K. Role of IRF-1 and caspase-7 in IFN-gamma enhancement of Fas-mediated apoptosis in ACHN renal cell carcinoma cells. Int J Cancer. 2003;104:400–408. doi: 10.1002/ijc.10956. [DOI] [PubMed] [Google Scholar]
- 20.Ulloa L, Doody J, Massagué J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh AK, Yuan W, Mori Y, Chen SJ, Varga J. Antagonistic regulation of type I collagen gene expression by interferon-gamma and transforming growth factor-beta. Integration at the level of p300/CBP transcriptional coactivators. J Biol Chem. 2001;276:11041–11048. doi: 10.1074/jbc.M004709200. [DOI] [PubMed] [Google Scholar]
- 22.Weng H, Mertens PR, Gressner AM, Dooley S. IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor Smads. J Hepatol. 2007;46:295–303. doi: 10.1016/j.jhep.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Higashi K, Inagaki Y, Fujimori K, Nakao A, Kaneko H, Nakatsuka I. Interferon-gamma interferes with transforming growth factor-beta signaling through direct interaction of YB-1 with Smad3. J Biol Chem. 2003;278:43470–43479. doi: 10.1074/jbc.M302339200. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki Y, Kushida M, Higashi K, Itoh J, Higashiyama R, Hong YY, Kawada N, Namikawa K, Kiyama H, Bou-Gharios G, Watanabe T. Cell type-specific intervention of transforming growth factor beta/Smad signaling suppresses collagen gene expression and hepatic fibrosis in mice. Gastroenterology. 2005;129:259–268. doi: 10.1053/j.gastro.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 25.Gu L, Zhu YJ, Guo ZJ, Xu XX, Xu WB. Effect of IFN-gamma and dexamethasone on TGF-beta1-induced human fetal lung fibroblast-myofibroblast differentiation. Acta Pharmacol Sin. 2004;25:1479–1488. [PubMed] [Google Scholar]
- 26.Hasegawa T, Nakao A, Sumiyoshi K, Tsuboi R, Ogawa H. IFN-gamma fails to antagonize fibrotic effect of TGF-beta on keloid-derived dermal fibroblasts. J Dermatol Sci. 2003;32:19–24. doi: 10.1016/S0923-1811(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 27.Poosti F, Bansal R, Yazdani S, Prakash J, Post E, Klok P, van den Born J, de Borst MH, van Goor H, Poelstra K, Hillebrands JL. Selective delivery of IFN-gamma to renal interstitial myofibroblasts: a novel strategy for the treatment of renal fibrosis. FASEB J. 2014;29:1029–1042. doi: 10.1096/fj.14-258459. [DOI] [PubMed] [Google Scholar]
- 28.Yao Y, Zhang J, Tan DQ, Chen XY, Ye DF, Peng JP, Li JT, Zheng YQ, Fang L, Li YK, Fan MX. Interferon-gamma improves renal interstitial fibrosis and decreases intrarenal vascular resistance of hydronephrosis in an animal model. Urology. 2011;77:761. doi: 10.1016/j.urology.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Warner SJ, Friedman GB, Libby P. Immune interferon inhibits proliferation and induces 2′-5′-oligoadenylate synthetase gene expression in human vascular smooth muscle cells. J Clin Invest. 1989;83:1174–1182. doi: 10.1172/JCI113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansson GK, Hellstrand M, Rymo L, Rubbia L, Gabbiani G. Interferon gamma inhibits both proliferation and expression of differentiation-specific alpha-smooth muscle actin in arterial smooth muscle cells. J Exp Med. 1989;170:1595–1608. doi: 10.1084/jem.170.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varga J, Yufit T, Brown RR. Inhibition of collagenase and stromelysin gene expression by interferon-gamma in human dermal fibroblasts is mediated in part via induction of tryptophan degradation. J Clin Invest. 1995;96:475–481. doi: 10.1172/JCI118058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai W, Gupta SL. Molecular cloning, sequencing and expression of human interferon-gamma-inducible indoleamine 2,3-dioxygenase cDNA. Biochem Biophys Res Commun. 1990;168:1–8. doi: 10.1016/0006-291X(90)91666-G. [DOI] [PubMed] [Google Scholar]
- 33.Prendergast GC, Metz R, Muller AJ, Merlo LM, Mandik-Nayak L. IDO2 in immunomodulation and autoimmune disease. Front Immunol. 2014;5:585. doi: 10.3389/fimmu.2014.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 35.Van Linthout S, Miteva K, Tschöpe C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102:258–269. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- 36.Levick SP, Goldspink PH. Could interferon-gamma be a therapeutic target for treating heart failure? Heart Fail Rev. 2014;19:227–236. doi: 10.1007/s10741-013-9393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng GS, Taylor MW. Interferon gamma-resistant mutants are defective in the induction of indoleamine 2,3-dioxygenase. Proc Natl Acad Sci USA. 1989;86:7144–7148. doi: 10.1073/pnas.86.18.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster NL, Wee J, Uren SJ, Boyle W, Sandrin MS. Production and purification of human indoleamine 2,3-dioxygenase (HuIDO) protein in a baculovirus expression system and production and characterization of egg yolk antibody against the purified HuIDO. Immunol Cell Biol. 2005;83:542–548. doi: 10.1111/j.1440-1711.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- 39.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 40.Rao B, Malathi N, Narashiman S, Rajan ST. Evaluation of myofibroblasts by expression of alpha smooth muscle actin: a marker in fibrosis, dysplasia and carcinoma. J Clin Diagn Res. 2014;8:ZC14. doi: 10.7860/JCDR/2014/7820.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reifenberg K, Lehr HA, Torzewski M, Steige G, Wiese E, Küpper I, Becker C, Ott S, Nusser P, Yamamura KI, Rechtsteiner G. Interferon-gamma induces chronic active myocarditis and cardiomyopathy in transgenic mice. Am J Pathol. 2007;171:463–472. doi: 10.2353/ajpath.2007.060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markó L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Müller D. Interferon-gamma signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension. 2012;60:1430–1436. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- 43.Han YL, Li YL, Jia LX, Cheng JZ, Qi YF, Zhang HJ, Du J. Reciprocal interaction between macrophages and T cells stimulates IFN-gamma and MCP-1 production in Ang II-induced cardiac inflammation and fibrosis. PLoS ONE. 2012;7:e35506. doi: 10.1371/journal.pone.0035506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJ, Njoku DB, Rose NR. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883–1894. doi: 10.1016/S0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afanasyeva M, Georgakopoulos D, Belardi DF, Bedja D, Fairweather D, Wang Y, Kaya Z, Gabrielson KL, Rodriguez ER, Caturegli P, Kass DA. Impaired up-regulation of CD25 on CD4 + T cells in IFN-gamma knockout mice is associated with progression of myocarditis to heart failure. Proc Natl Acad Sci USA. 2005;102:180–185. doi: 10.1073/pnas.0408241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. doi: 10.1096/fasebj.5.11.1907934. [DOI] [PubMed] [Google Scholar]
- 47.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Croitoru-Lamoury J, Lamoury FM, Caristo M, Suzuki K, Walker D, Takikawa O, Taylor R, Brew BJ. Interferon-gamma regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO) PLoS ONE. 2011;6:e14698. doi: 10.1371/journal.pone.0014698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow WA, Fang JJ, Yee JK. The IFN regulatory factor family participates in regulation of Fas ligand gene expression in T cells. J Immunol. 2000;164:3512–3518. doi: 10.4049/jimmunol.164.7.3512. [DOI] [PubMed] [Google Scholar]