Supplemental Digital Content is available in the text
Keywords: Living donor liver transplantation, Deceased donor liver transplantation, Hepatocellular carcinoma, Meta-analysis
Abstract
Background:
Although a number of technical problems and donor safety issues associated with living donor liver transplantation (LDLT) have been resolved, some initial clinical studies showed an increased risk of hepatocellular carcinoma (HCC) recurrence in LDLT. This meta-analysis was conducted to assess differences in tumor recurrence between LDLT and deceased donor liver transplantation (DDLT).
Methods:
After systematic retrievals of studies about LDLT and DDLT for HCC, articles were selected with a rationale of emphasizing inter-group comparability. Results from multivariate analyses were combined and discussed together with univariate analyses. In subgroup analysis, the impact of organ allocation policy was taken into consideration.
Results:
Seven articles were included in the meta-analysis. Overall, a salient result that emerged from the seven studies was a significant increased risk of HCC recurrence in the LDLT group than in the DDLT group (P = 0.01). The most significant increase in hazard ratio was found in studies where organs tended to be allocated to non-tumor patients.
Conclusions:
An increased risk for HCC recurrence in LDLT as compared with DDLT patients was found. The relatively shorter preoperative observation windows in LDLT may lead to fewer cases of HCC with invasive features being screened out, which may provide a possible explanation for the high rates of HCC recurrence.
Introduction
Early stage hepatocellular carcinoma (HCC) has become one of the major indications for liver transplantation since successful transplantations for HCC were initially reported by Mazzaferro et al.[1] Liver transplantation has also been reported be beneficial to patients with relatively advanced HCC.[2,3] However, the shortage of deceased liver donors has limited the supply and therefore the application of deceased donor liver transplantation (DDLT). As a result, living donor liver transplantation (LDLT), which provides an alternative for patients waiting for DDLT, has markedly increased of late, especially in East Asia. Although a number of technical problems and donor safety issues associated with LDLT have been resolved, one problematic finding was an increased risk of HCC recurrence in LDLT as reported in some initial clinical studies.[4,5] While it has been speculated that differences in HCC staging or features prior to transplantation may contribute to this recurrence of HCC following LDLT, this issue has yet to be resolved and concerns regarding the impact of LDLT as related to HCC recurrence remain.
During LDLT, the main branches of recipient's portal vein and hepatic artery, as well as the hepatic vein and retrohepatic segment of the inferior vena cava, are typically preserved. This procedure may increase the potential of HCC residue and dissemination. Moreover, the relatively small-sized grafts that are usually used with living donors will quickly grow after LDLT with the result that HCC colonization or growth may be accelerated under such conditions. There are data from animal studies which support such a conclusion. For example, Picardo et al[6] reported increases in HCC growth and cytokine growth factor expression within a partial hepatectomy in a rat model. Similar results were reported by Yang et al[7] in a rat orthotopic liver transplantation model. Therefore, the high recurrence of HCC in LDLT may result from these surgical techniques and use of a small graft in LDLT. As a result of these findings from clinical and animal studies, serious concerns remain regarding LDLT in patients with HCC. Such concerns are revealed in clinical reports where patients with tumors close to the main branches of vessels were only offered DDLT for liver transplants as performed in the Toronto General Hospital[8]; and only patients with HCC in United Network for Organ Sharing (UNOS) T2 stage were considered as candidates for LDLT in the Niguarda Ca’ Granda Hospital.[9] Further, the issue of LDLT in patients with HCC raises many ethical and clinical questions. For example, should this risk be explained to the donor and ethics committee? Should special criteria for LDLT be established? Should changes in surgical techniques or graft size be implemented to reduce HCC recurrence risk in LDLT?
Currently, no randomized clinical trial has been conducted as related to organ driven transplantations. Only findings from retrospective studies have supplied some evidence that can be used to address this issue at present. Though systematic reviews and/or meta-analyses[10–12] exist, variables associated with these reports lack sufficient descriptions. While cohort studies of large samples can provide important information on the relationships between observed factors and events and control for major confounding factors, the complicated nature of liver transplantation limits their utility. Factors including patient selection, donor preservation, surgical technique, post-transplantation treatment, and anti-HCC therapies all contribute to complexities involved with liver transplantation. Moreover, discrepancies in selection criteria, death, and dropout prior to liver transplantation have not received sufficient consideration, and characteristics correlating with HCC staging should also be included in these analyses.
Donor livers are allocated by deferent national regulations, which vary among countries or regions. In most countries/regions, priorities are afforded to patients with relatively small HCC.[13,14] Thus, patients receiving a DDLT comprise a special population of patients with HCC, often in early stages of the disease, with relatively high model for end-stage liver disease (MELD) scores, long waiting periods and favorable prognoses. As recipients in most cases of LDLT are designated by the living donor, the criteria for LDLT in HCC are not the same as those for DDLT. Patients with advanced HCC are more likely to receive LDLT. In clinical practice, characteristics indicating high risks for HCC recurrence are taken into consideration for each patient selected, which may be more precise than that of a stratified HCC staging criterion. Thus discrepancies in HCC features between LDLT and DDLT may remain, even after adjusting for HCC staging. Therefore, detailed characteristics in patient selection should be discussed in any meta-analysis.
We conducted the present meta-analysis after selecting articles based on a rationale emphasizing inter-group comparability. Articles with significant differences in HCC staging and post-transplantation anti-tumor therapies between groups were excluded. Several items were used to grade articles as supplements to the Newcastle-Ottawa scale (NOS), including adjuvant therapy, MELD score, non-tumor factors, patient selection, recurrence rate of HCC, waiting period, patient survival, and methods used to determine HCC staging and screen for tumor recurrence. Results from multivariate analyses were combined and discussed together with univariate analyses. Taken together, we found that a higher incidence of HCC recurrence was observed in LDLT as compared with that of DDLT after adjusting for HCC staging.
Methods
Literature review
“Cochrane Library,” “PubMed,” and “Embase” databases were reviewed and included the period from databases build up until October 1, 2017. Search strategies included the keywords “Liver Transplantation,” “Hepatocellular Carcinoma,” “Living Donor,” “Recurrence Rate,” and their synonymous terms [Supplementary Table S1]. After removal of duplicate articles, titles and abstracts were independently reviewed and assessed by two authors (HZ, YS). Further reviews of full texts were conducted in the same manner to establish whether details of articles met inclusion criteria. Bibliographies from all reviews and reports were examined to identify additional studies for potential inclusion in our analysis.
Evidence quality assessment
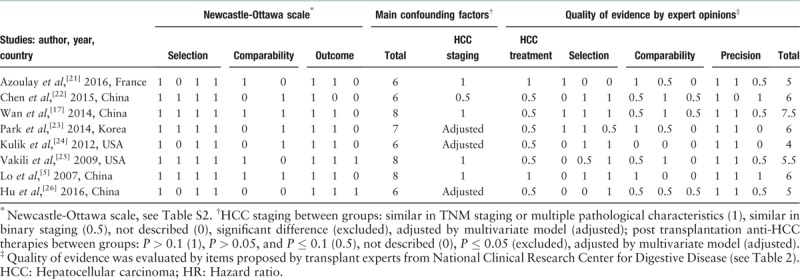
After identifying articles for inclusion in the review, a risk of bias was assessed with use of the NOS [Supplementary Table S2].[15] Articles with less than four stars were regarded as low quality and excluded. A further bias assessment was conducted based on items suggested by transplant experts from the National Clinical Research Center for Digestive Disease, which may have serious impact on the result of analyses. Three items in each category were generated to assess biases in “selection,” “comparability,” and “precision” [Table 1]. According to these inclusion criteria, studies involving only comparisons of unadjusted cumulated tumor recurrences between groups with non-comparable baseline data (HCC staging or post-transplantation anti-HCC therapies) of HCC were excluded. As multivariate analysis and propensity score matching were employed in some studies, baseline discrepancy and statistical matching were both taken into consideration. In univariate analyses, comparisons for HCC recurrence required that they should be conducted between LDLT and DDLT groups with similar HCC staging. In the analysis for HCC recurrence, type of donor (LDLT vs. DDLT) and clinical/pathology information on staging of HCC were required for inclusion in the multivariate models.
Table 1.
Specific criteria for bias assessment made by experts.
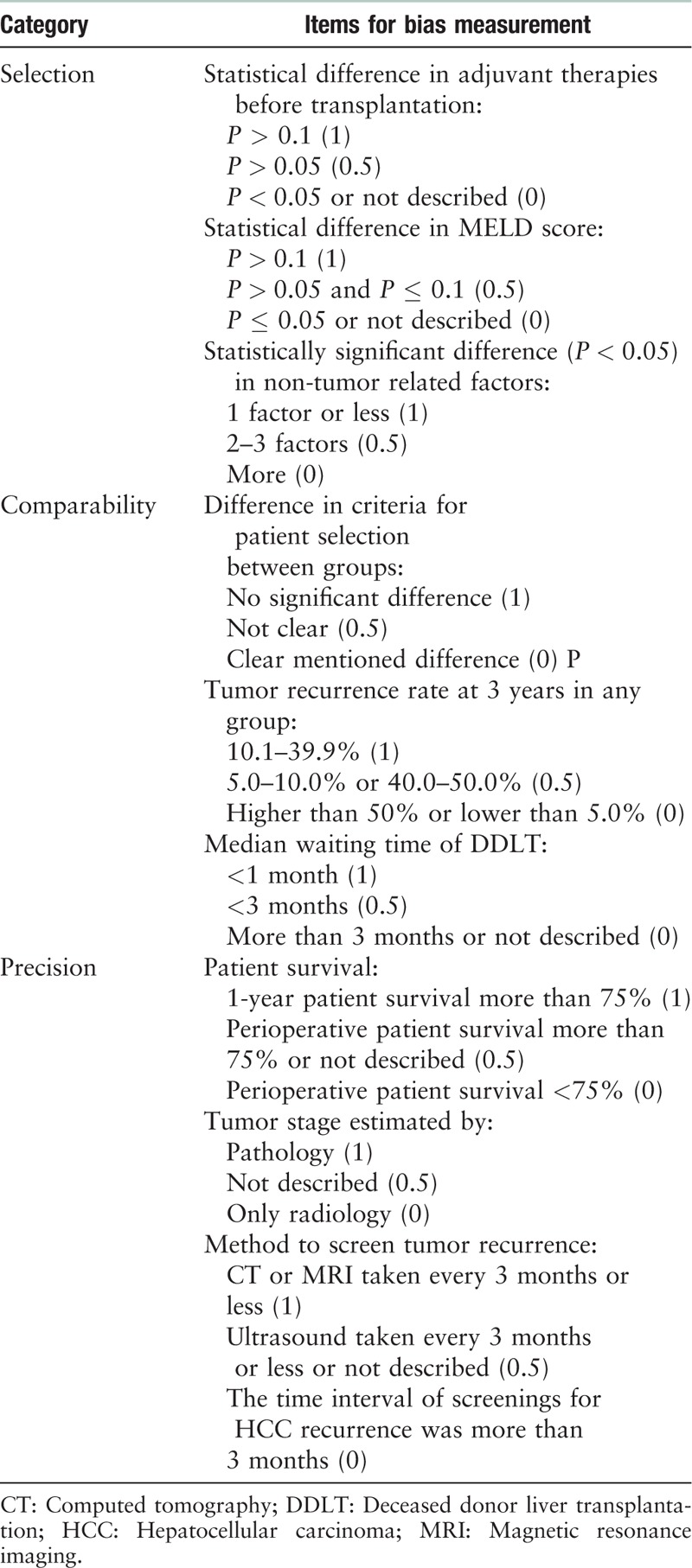
Inclusion and exclusion criteria
Inclusion criteria: (1) reports written in English; (2) cohort studies (prospective and retrospective) with HCC recurrence information in LDLT and DDLT groups.
Exclusion criteria: (1) duplicated reports; (2) case reports and studies with samples less than five in LDLT or DDLT group; (3) reviews without origin data; (4) studies including tumors other than HCC (eg, cholangiocarcinoma) and required data of HCC were not showed alone; (5) HCC staging in LDLT or DDLT group were missing and HCC staging was not adjusted in analysis either; (6) data overlapping with any included report; (7) reports received less than four stars according to NOS; (8) unadjusted statistical difference (P ≤ 0.05) existed in HCC staging at the time of transplantation or in anti-HCC therapies after transplantation between LDLT and DDLT groups; (9) unadjusted discrepancy in patient selection criteria of HCC characteristics between LDLT and DDLT groups.
Outcome measurement
The hazard ratio (HR) that based on accumulated HCC recurrence rates after liver transplantation served as the only outcome measure in the current analysis. Adjusted HRs in multivariate analyses were extracted for the meta-analysis. Unadjusted HRs in univariate analyses were estimated as based on the number of HCC recurrences and P value for accumulated recurrence rates between groups. When only HCC recurrences were defined as the event used in the estimation of recurrence/relapse-free survival (RFS), the P value calculated in analyses of RFS comparison can also be used. However, HCC recurrence and patient death were set as a combined endpoint in the calculation of disease-free survival (DFS). Results of comparisons for DFS were screened and eliminated from the analysis. O-E and variance were calculated following methods described previously[16] and were combined using Review Manager 5.3 software (Cochrance Collaboration, UK).
O-E and variance were calculated by HR and its 95% confidence interval using the following equations.[16]
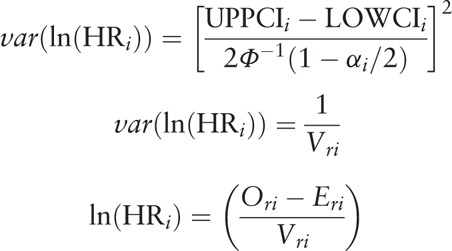 |
O-E and variance were calculated as based upon the number of events and P values using the following equations.[16]
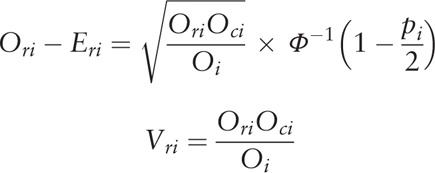 |
where Ori denotes observed number of events in the research group; Oci denotes observed number of events in the control group; Eri denotes log rank expected number of events in the treated group; Eci denotes log rank expected number of events in the control group; and 1/Vri denotes Mantel-Haenszel variance of the log HR.
Results
A summary of the processes involved with retrieval and screening of articles is presented in Figure 1. After removing duplicated articles, a total of 641 articles were screened by title and abstract and 22 full texts of these articles were reviewed. Seven articles with partially overlapping data and two articles with indiscriminate liver cancers (HCC and cholangiocarcinoma) were excluded. In the report of Wan et al,[17] one case of intra-hepatic cholangiocarcinoma (ICC) was included in the DDLT group and one case of combined HCC and cholangiocarcinoma (cHCC-CC) was included in the LDLT group. This report was not excluded, as the proportions of the ICC or cHCC-CC were very low.
Figure 1.
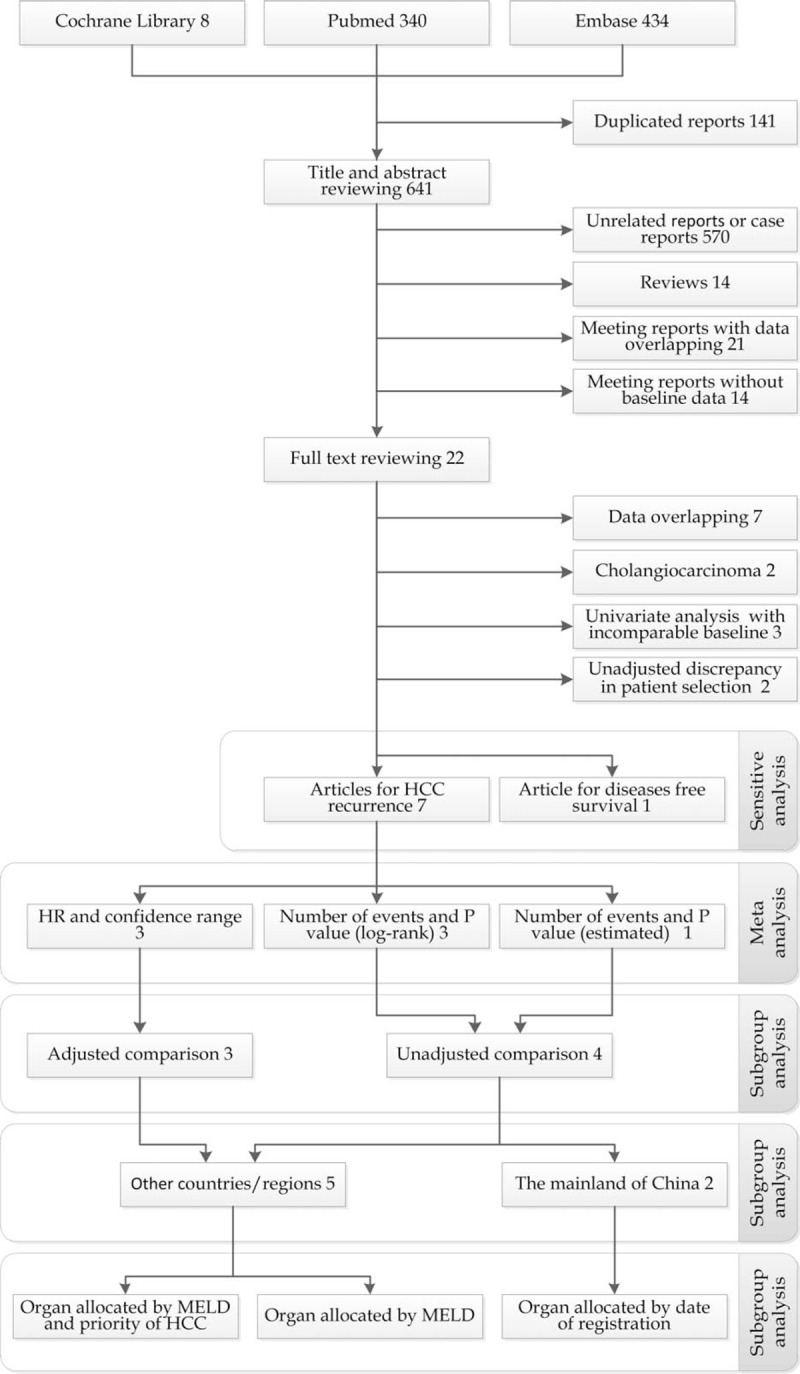
Flow diagram of the meta-analysis (527 cases of living donor liver transplantation and 781 cases of deceased donor liver transplantation from seven articles were included in meta-analysis). HCC: Hepatocellular carcinoma; HR: Hazard ratio; MELD: Model for end-stage liver disease.
Discrepancies in baseline data of HCC staging were present in three articles. For these articles, only univariate analyses were conducted. One report by Sandhu et al[8] noted that “patients with tumors abutting the inferior vena cava, hepatic veins, or porta hepatis were offered only DDLT to prevent the compromising of oncological margins during the course of LDLT.” Although the data of this report was subjected to multivariate analysis, the analysis did not adjust for the factor “tumor abutting vessel.” In another report, Di Sandro et al[9] indicated that “patients with stage II HCC were proposed for LDLT” and only univariate analysis was conducted. After discussing the data of these reports within our group, these five articles were excluded [Table 2].[8,9,18–20]
Table 2.
Articles excluded for baseline or patient selection.
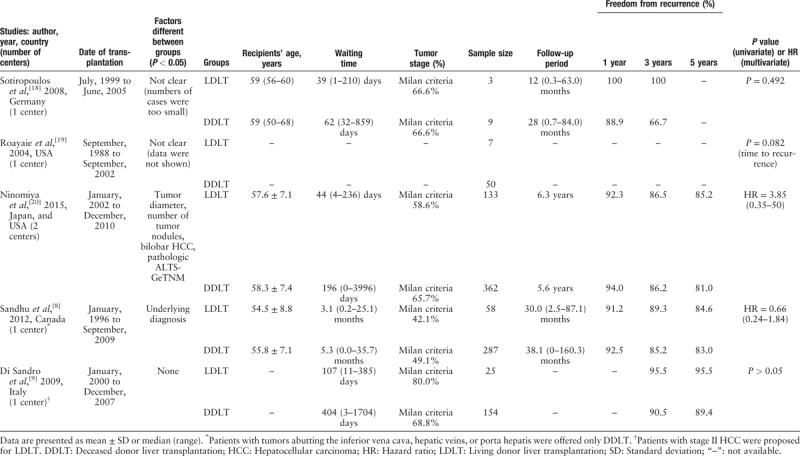
Finally, eight articles remained for our analysis. One article from the mainland of China including 6471 cases of DDLT and 389 cases of LDLT only reported DFS between the groups. The impact of this large sample study was subjected to sensitive analysis, in which the HR for DFS was combined with HR for HCC recurrence after replacing articles with data overlapping with this study. The quality of evidence was assessed by the NOS and items suggested by experts [Table 3].[5,17,21–26]
Table 3.
Quality of evidence.
Characteristics of the eight studies used in our analysis are presented in Table 4. Potential confounding factors in baselines, which showed little variation, are listed for each study. HCC within the Milan criteria was 40% to 75% in each study. Characteristics correlating with HCC staging and invasion were similar in five studies.[5,17,21,22,25] Significant differences in microvascular invasion, number, or diameter of HCC were adjusted with use of the Cox regression model in three other studies,[23,24,26] including the study for DFS.[26] Waiting periods, which were much shorter in the LDLT group, were reported in four of the articles. Differences in factors, such as surgery duration, graft-to-recipient weight ratio (GRWR) and graft size, showed essential distinctions between LDLT and DDLT patients, which were regarded as part of grouping factors. Thus, differences in risk for HCC recurrence between groups could be a direct or indirect result of any of these factors.
Table 4.
Studies included in meta-analysis or sensitive analysis.
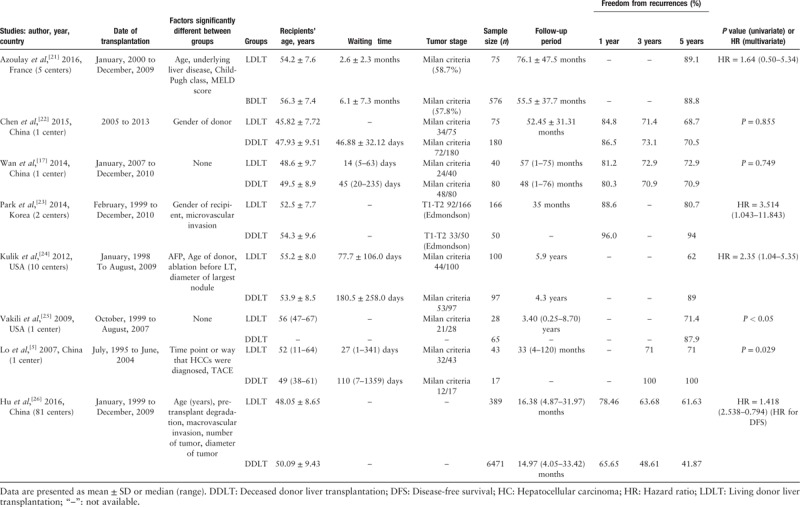
RFSs were relatively lower in the mainland of China (70.5–70.9%) as compared with that of other countries/regions (87.9–100%) and considerable heterogeneity was present between countries/regions. In the DFS study of the mainland of China, the observed high recurrence risk in the DDLT group (P < 0.001) was reversed in the adjusted comparison (P = 0.281). While in the three multivariate studies from other countries/regions, similar results were obtained from the univariate and multivariate analyses. Thus, in the studies from the mainland of China, discrepancies in baseline may reduce HCC recurrence in the LDLT group.
The seven articles included in the meta-analysis [Figure 2], consisted of three articles using multivariate analyses and four using univariate analyses. In one univariate analysis, a P value was only reported as “P < 0.05,” and it was set as 0.05 in the calculation for HR in the current meta-analysis. Overall, a salient result that emerged from the seven studies was a significant increased risk of HCC in the LDLT group (P = 0.01). A high level of heterogeneity was present (I2 = 48%) among the studies, which were grouped by univariate and multivariate analyses [Figure 3]. A high level of heterogeneity was found in univariate studies and a very low level in multivariate studies (I2 = 61%, I2 = 0%). The presence of a high risk in the LDLT groups was only supported by results obtained with multivariate studies.
Figure 2.
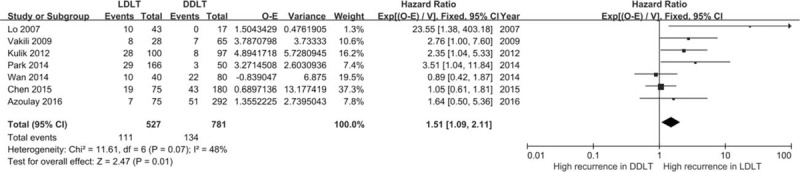
Hazard ratios for hepatocellular carcinoma recurrence from seven included studies. CI: Confidence interval; DDLT: Deceased donor liver transplantation; LDLT: Living donor liver transplantation.
Figure 3.
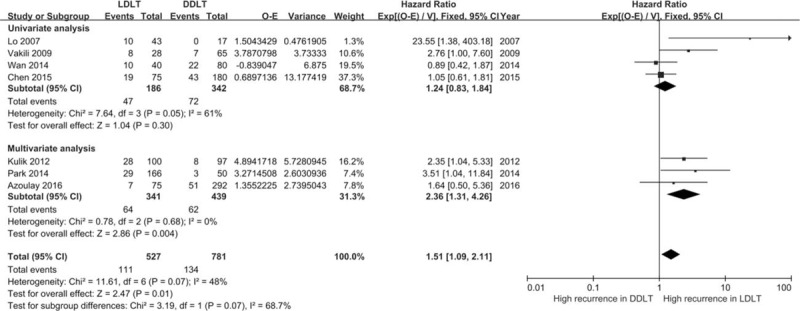
Hazard ratios for hepatocellular carcinoma recurrence grouped by univariate and multivariate analysis. CI: Confidence interval; DDLT: Deceased donor liver transplantation; LDLT: Living donor liver transplantation.
Again, HRs of the two studies from the mainland of China showed apparent differences compared with that of the other studies. Therefore, a subgroup analysis was conducted after studies were grouped in terms of the mainland of China vs. that from the other countries/regions [Supplementary Figure S1]. Heterogeneities disappeared for analyses performed within each subgroup (I2 = 0%, I2 = 0%). The increased risk in LDLT groups was only supported by results from reports of countries/regions other than that of the mainland of China (P = 0.0002). Studies were further grouped by details of policies for organ allocation. The most significant increase in HR was found in studies where organs tended to be allocated to non-tumor patients [Figure 4].
Figure 4.
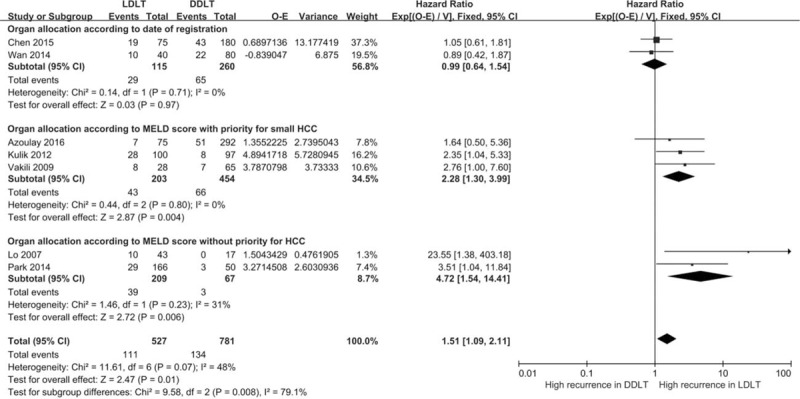
Hazard ratios for hepatocellular carcinoma recurrence grouped by organ policy. CI: Confidence interval; DDLT: Deceased donor liver transplantation; LDLT: Living donor liver transplantation.
A sensitive analysis was performed by removing the study with the maximum weight in the meta-analysis [Supplementary Figure S2]. Another sensitive analysis was performed by introducing the study[26] for DFS, after two studies[17,22] containing overlapping data were removed [Supplementary Figure S3]. In these sensitive analyses, the overall effect or combined HR in each subgroup was not significantly changed.
Discussion
The current meta-analysis was conducted in an attempt to isolate the specific impact of surgical procedure and graft size of LDLT on HCC recurrence after reducing confounding effects from tumor features. For this analysis, the combined data of articles from different countries/regions were included. As noted above, differences in patient selection criteria and death/dropout prior to liver transplantation may result in discrepancies in HCC staging prior to liver transplantation. Such factors can exert a serious impact on HCC recurrence. After scrutinizing the full text of related studies, five articles were excluded from the meta-analysis for uncontrolled confounding in HCC staging or patient selection [Table 2]. No statistically significant differences were found for HCC recurrence in these excluded studies. In two studies, relatively low HCC recurrences were observed in the LDLT group. Only three cases in the LDLT group were included in one study, and a relatively high proportion of small HCC in the LDLT group (80.0% vs. 68.8% conforming to Milan criteria) was found in another study. Thus all excluded articles would not likely show any effects opposite to the combined result of current meta-analysis.
Tumor size/stage along with macro/microvascular invasion is considered as important prognostic factors. Though no statistically significant differences were found between baseline HCC size/staging and macro/microvascular invasion as determined using univariate analyses, differences in HCC staging or other characteristics for invasion still existed within four of the univariate studies included in our analysis. In two studies, proportions of patients with vascular invasion were quite different between the LDLT and DDLT groups (Chen et al[22]: 40.9% vs. 30.7%; Lo et al[5]: 34.9% vs. 17.6%). Data on vascular invasion were not reported in one article[25] and HCC staging was only approximately matched in another.[17] The macro/microvascular invasion variable was adjusted in all three multivariate analyses, together with tumor size/stage. The prognostic values of these factors were also clearly shown in these models. Thus, among studies included in our analysis, results from multivariate analyses were more reliable than that of univariate analyses. The combined HR of meta-analysis for multivariate models showed a high risk for HCC recurrence in the LDLT group, which was concurrent with the overall results of our meta-analysis. Such findings most likely reflect a high HCC recurrence in LDLT. While we identified two meta-analyses studies on similar topics, only unadjusted results were combined in these studies.[11,12] Potential discrepancies on baselines weakened the reliability of results in these studies. No statistically significant differences in HCC recurrence were found in one of these meta-analysis studies.[12] In the other, only a difference in DFS was found and HCC recurrence was not discussed separately.[11] The results in the current meta-analysis show a consistent high risk of HCC recurrence in LDLT.
According to the experience of liver transplant experts at our institution, preserving intra-hepatic branches of vascular or bile ducts may potentially increase HCC residue and dissemination, especially when the HCC is abutting these structures. In one report, not included in our analysis, it was noted that “patients with tumors abutting the inferior vena cava, hepatic veins, or porta hepatis were offered only LDLT to prevent the compromising of oncological margins during the course of LDLT.”[8] Under conditions where similar baselines were present, 5-year cumulated recurrence rates were nearly identical between the LDLT (15.4%) and DDLT (17%) groups. This finding partially reveals the impact of surgical methods. While in one study for LDLT, a decreased RFS was found in the GRWR <0.8 group as compared with the GRWR >0.8 group (P = 0.17), and this difference in RFS was statistically significant in the subgroup of patients with HCC beyond the Milan criteria (P = 0.047).[27] Thus, an impact of graft size on HCC recurrence may also exist and contribute to the high rates of HCC recurrence in LDLT.
In the UNOS system of the United States, patients receive deceased donated livers on the bases of “urgency” and “utility.” As patients with HCC that would benefit from transplantation should have a compatible prognosis with that of benign disease patients, a relatively strict criteria for transplantation was imposed upon these patients with HCC. In 2002, MELD scores were employed for donor liver allocation in the United States. Initially, a priority score was assigned to patients with HCC meeting the Milan criteria (one lesion smaller than 5 cm; or up to three lesions, each smaller than 3 cm; no extra hepatic manifestations; no evidence of gross vascular invasion). Although this priority score policy was revised, a priority score of 22 was still given to patients with HCC meeting the UNOS T2 criteria. Thus, transplants were more frequently performed in patients with smaller HCCs. Similar strategies for liver transplantation in HCC with UNOS criteria have been employed.[13] However, in Korea[28] and Hong Kong, China,[29] no priority policy exists for HCC and donor livers are allocated based only on severity of the liver disease (MELD score). In the mainland of China, the establishment of the national system was relatively late, and before that most patients have received liver transplantations primarily based upon their date of registration. Different policies for DDLT may result in discrepancies of HCC features between groups and studies. Organ allocation policies were either identical or similar with that of UNOS in three studies, which were from the United States and France. Relatively high rates of HCC recurrence in LDLT were obtained in these studies, which were similar to that found in two studies from Korea and Hong Kong, China. The priority for transplant in patients with HCC was based upon the diameter of HCC, number of lesions and vascular invasion, with all of these characteristics being discussed or adjusted in these studies. Therefore, the potential confounding effects associated with this organ policy were reduced and similar results were found among the studies.
Discrepancies in other characteristics of HCC may still have an impact on the results. For example, a long waiting period may increase patient dropout rates as associated with HCC progression and reduce the number of patients with invasive HCC. Under such conditions, patients with relatively advanced HCC may be more likely to select LDLT. As no priority is allocated to patients with HCC in Korea and Hong Kong, China, waiting periods can be quite long. In one study from Hong Kong, China, it was reported that approximately 80% of HCC candidates expired during the waiting period for a DDLT.[29] In the current meta-analysis, the highest HRs were found in two reports. The risk of patient dropout from waiting lists for DDLT and the need of LDLT were usually determined using multi-features of HCC, while only one to three of these features were adjusted in these studies. In this way, discrepancies in other baseline features of HCC may still increase HCC recurrence in LDLT.
One included report from the United States,[24] reviewing data from January 1998 to August 2009, found that a high recurrence of HCC in LDLT was significant before 2002 (in 2002 a priority for small HCCs was employed) and became non-significant after 2002. The number of recurrences decreased in LDLT patients with T2-stage HCC (P = 0.026) while recurrence numbers increased in DDLT patients with T3-stage HCC (P = 0.29). More donor livers were allocated to patients with HCC in the era when MELD scores were employed. Thus, reduced waiting times in patients with HCC may result in relatively increased HCC recurrence rates in DDLT patients. An approximate trend for decreased HRs with time can also be found in the forest graph of the current meta-analysis [Figure 2]. Improvements in surgical techniques and waiting periods were both proposed as explanations for these results.
No mandatory allocation policy has been implemented in the mainland of China before 2010, though tumor stage and MELD scores were typically used in decisions of organ allocation. The mean and median waiting times of 45 to 47 days for DDLT patients in the mainland of China were considerably shorter than that in other countries/regions. As a result, the impact of waiting time was significantly reduced. However, features of HCCs in LDLT and DDLT were not similar. A large sample study from the mainland of China showed significantly reduced DFS in LDLT patients (HR = 0.650, 0.514–0.823) as determined using univariate analysis but slightly increased DFS (HR = 1.418, 2.538–0.794) by Cox analysis. HCC features tended to reduce the recurrence risk in the LDLT group. Stresses in medical practice and requirements for approval by government agencies have resulted in surgeons within the mainland of China usually selecting DDLT for relatively advanced HCC. Such a tendency may then balance the impact of factors like surgical methods and graft size, and result in nearly equal risks in LDLT and DDLT, after combining HRs in this subgroup.
In conclusion, the results of our analysis indicate that there is an overall increased risk for HCC recurrence in LDLT as compared with that of DDLT. Though biases in patient selection and waiting periods may reduce the reliability of such findings, the result of meta-analysis for adjusted studies support the conclusion that increases of HCC recurrence in LDLT were due to factors other than discrepancies in HCC staging by current systems. The relatively shorter preoperative observation windows in LDLT may lead to fewer cases of HCC with invasive features being screened out, which may provide a possible explanation for the high rates of HCC recurrence. The impact of surgical methods and graft size cannot be confirmed as a contributing factor. Further studies are required to establish the exact roles of adjusting for HCC staging, patient selection, waiting periods, and perioperative treatments.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 71603272); National Science and Technology Major Project (No. 2017ZX10203205-001-005).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Zhang HM, Shi YX, Sun LY, Zhu ZJ. Hepatocellular carcinoma recurrence in living and deceased donor liver transplantation: a systematic review and meta-analysis. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000287
References
- 1.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 3.Bonadio I, Colle I, Geerts A, Smeets P, Berardi G, Praet M, et al. Liver transplantation for hepatocellular carcinoma comparing the Milan, UCSF, and Asan criteria: long-term follow-up of a Western single institutional experience. Clin Transplant 2015; 29:425–433. doi: 10.1111/ctr.12534. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS, Jr, et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant 2007; 7:1601–1608. doi: 10.1111/j.1600-6143.2007.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg 2007; 94:78–86. doi: 10.1002/bjs.5528. [DOI] [PubMed] [Google Scholar]
- 6.Picardo A, Karpoff HM, Ng B, Lee J, Brennan MF, Fong Y. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery 1998; 124:57–64. doi: 10.1016/S0039-6060(98)70075-3. [PubMed] [Google Scholar]
- 7.Yang ZF, Poon RT, Luo Y, Cheung CK, Ho DW, Lo CM, et al. Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophage activities through VEGF receptor 2-dependent pathway. J Immunol 2004; 173:2507–2515. doi: 10.4049/jimmunol.173.4.2507. [DOI] [PubMed] [Google Scholar]
- 8.Sandhu L, Sandroussi C, Guba M, Selzner M, Ghanekar A, Cattral MS, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl 2012; 18:315–322. doi: 10.1002/lt.22477. [DOI] [PubMed] [Google Scholar]
- 9.Di Sandro S, Slim AO, Giacomoni A, Lauterio A, Mangoni I, Aseni P, et al. Living donor liver transplantation for hepatocellular carcinoma: long-term results compared with deceased donor liver transplantation. Transplant Proc 2009; 41:1283–1285. doi: 10.1016/j.transproceed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa K, Takada Y. Living vs. deceased-donor liver transplantation for patients with hepatocellular carcinoma. Transl Gastroenterol Hepatol 2016; 1:35.doi: 10.21037/tgh.2016.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant 2013; 27:140–147. doi: 10.1111/ctr.12031. [DOI] [PubMed] [Google Scholar]
- 12.Liang W, Wu L, Ling X, Schroder PM, Ju W, Wang D, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl 2012; 18:1226–1236. doi: 10.1002/lt.23490. [DOI] [PubMed] [Google Scholar]
- 13.De Carlis L, Di Sandro S, Centonze L, Lauterio A, Buscemi V, De Carlis R, et al. Liver-allocation policies for patients affected by HCC in Europe. Curr Transplant Rep 2016; 3:313–318. doi: 10.1007/s40472-016-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology 2008; 134:1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014; 14:45.doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Wan P, Zhang JJ, Li QG, Xu N, Zhang M, Chen XS, et al. Living-donor or deceased-donor liver transplantation for hepatic carcinoma: a case-matched comparison. World J Gastroenterol 2014; 20:4393–4400. doi: 10.3748/wjg.v20.i15.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotiropoulos GC, Beckebaum S, Lang H, Frilling A, Molmenti EP, Cicinnati VR, et al. Single-center experience on liver transplantation for hepatocellular carcinoma arising in alcoholic cirrhosis: results and ethical issues. Eur Surg Res 2008; 40:7–13. doi: 10.1159/000107615. [DOI] [PubMed] [Google Scholar]
- 19.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl 2004; 10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 20.Ninomiya M, Shirabe K, Facciuto ME, Schwartz ME, Florman SS, Yoshizumi T, et al. Comparative study of living and deceased donor liver transplantation as a treatment for hepatocellular carcinoma. J Am College Surg 2015; 220:297.e3–304.e3. doi: 10.1016/j.jamcollsurg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Azoulay D, Audureau E, Bhangui P, Belghiti J, Boillot O, Andreani P, et al. Living or brain-dead donor liver transplantation for hepatocellular carcinoma: a multicenter, western, intent-to-treat cohort study. Ann Surg 2017; 266:1035–1044. doi: 10.1097/sla.0000000000001986. [DOI] [PubMed] [Google Scholar]
- 22.Chen LP, Li C, Wen TF, Yan LN, Li B, Yang JY. Can living donor liver transplantation offer similar outcomes to deceased donor liver transplantation using expanded selection criteria for hepatocellular carcinoma? Pak J Med Sci 2015; 31:763–769. doi: 10.12669/pjms.314.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park MS, Lee KW, Suh SW, You T, Choi Y, Kim H, et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation 2014; 97:71–77. doi: 10.1097/TP.0b013e3182a68953. [DOI] [PubMed] [Google Scholar]
- 24.Kulik LM, Fisher RA, Rodrigo DR, Brown RS, Jr, Freise CE, Shaked A, et al. Outcomes of living and deceased donor liver transplant recipients with hepatocellular carcinoma: results of the A2ALL cohort. Am J Transplant 2012; 12:2997–3007. doi: 10.1111/j.1600-6143.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vakili K, Pomposelli JJ, Cheah YL, Akoad M, Lewis WD, Khettry U, et al. Living donor liver transplantation for hepatocellular carcinoma: increased recurrence but improved survival. Liver Transpl 2009; 15:1861–1866. doi: 10.1002/lt.21940. [DOI] [PubMed] [Google Scholar]
- 26.Hu Z, Qian Z, Wu J, Zhou J, Zhang M, Zhou L, et al. Clinical outcomes and risk factors of hepatocellular carcinoma treated by liver transplantation: a multi-centre comparison of living donor and deceased donor transplantation. Clin Res Hepatol Gastroenterol 2016; 40:315–326. doi: 10.1016/j.clinre.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Lee EC, Kim SH, Shim JR, Park SJ. Small-for-size grafts increase recurrence of hepatocellular carcinoma in liver transplantation beyond milan criteria. Liver Transpl 2018; 24:35–43. doi: 10.1002/lt.24868. [DOI] [PubMed] [Google Scholar]
- 28.Hong G, Lee KW, Suh S, Yoo T, Kim H, Park MS, et al. The model for end-stage liver disease score-based system predicts short term mortality better than the current Child-Turcotte-Pugh score-based allocation system during waiting for deceased liver transplantation. J Korean Med Sci 2013; 28:1207–1212. doi: 10.3346/jkms.2013.28.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo CM, Fan ST, Liu CL, Chan SC, Wong J. The role and limitation of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl 2004; 10:440–447. doi: 10.1002/lt.20097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


