Abstract
Background:
The exact relationship between gastroesophageal reflux disease (GERD) and esophageal squamous cell cancer (ESCC) is far from clarification. The aim of this study was to investigate the epidemiology of GERD in a region with high prevalence of ESCC in China.
Methods:
A population-based, cross-sectional study was conducted in a high ESCC prevalent area, Anyang, Henan, China. All subjects fulfilled questionnaires and underwent gastroendoscopy with routine esophageal biopsy. The subjects were divided into GERD subtypes (reflux esophagitis [RE] and non-erosive reflux disease [NERD]) and controls. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to examine risk factors for RE and NERD.
Results:
A total of 2844 subjects were finally enrolled. The prevalence of GERD (RE + NERD) was 17.3%. Among them, 271 (9.53%) adults were diagnosed with RE. The prevalence of RE increased with age (7.09% in 45–50 years, 8.00% in 51–60 years, and 9.53% in 61–69 years, χ2 = 62.216, P < 0.001). Sixty-seven (2.36%) subjects were diagnosed with the silent RE. A total of 221 (7.77%) subjects were diagnosed with NERD. Frequent liquid food consumption (OR [95% CI]: 1.502 [1.076–2.095]) was independent risk factor for RE as well as age, male gender, high body mass index (BMI), ever smoking. Age was independent risk factor for NERD. For silent RE, age, male gender, and frequent liquid food consumption were risk factors.
Conclusions:
In the population with high prevalence of ESCC, a high prevalence of GERD and inverted proportion of RE/NERD were presented. Age was an independent risk factor for GERD. The male gender, high BMI, smoking, and frequent liquid food consumption may be risk factors for RE but not for NERD.
Keywords: Gastroesophageal reflux disease, Esophageal squamous cell cancer, Prevalence, Risk factors
Introduction
It is well known that gastroesophageal reflux disease (GERD) is a risk factor for esophageal adenocarcinoma (EAC). Western researches presented that with the ascending incidence of GERD, the prevalence of esophageal squamous cell cancer (ESCC) has been declined.[1] However, the development of not only EAC but also ESCC has been shown in rat duodenal content reflux models.[2] Furthermore, the duodenal reflux has been reported to induce genetically unstable and initiate malignancy.[3] Taken together, the exact relationship between GERD and ESCC is far from fully clarification. To find out the prevalence condition and risk factors of GERD in population with high prevalence of ESCC is an essential item to help us definite this relationship.
In recent 20 years, though the incidence of ESCC has been reported to be slightly declined via reducing some high risk factors such as avoiding drinking shallow well water or eating hot foods,[4,5] the areas in the Tai-hang Mountains still showed highest incidence of ESCC throughout the world.[6] In China, the prevalence of at least weekly heart-burn and/or regurgitation has been reported to be around 5.2% in general population,[7] which was much lower than that in western countries.[8] But the prevalence of GERD in Tai-hang Mountains has not been investigated. So we conducted a population-based survey of the epidemiologic situation of GERD subtypes (reflux esophagitis [RE] and non-erosive disease [NERD]) in Anyang, Henan, China, one typical area with high ESCC prevalence in Tai-hang Mountains.
GERD usually presented the classical symptoms of heartburn and acid regurgitation. But in clinical practice, asymptomatic RE (also termed silent RE) was common.[9] Silent RE might progress to asymptomatic injures in esophagus and may do more harm to patients by delaying seeking medical help.[10] Therefore, it is important to reveal the prevalence of silent RE. In Montreal definition, the symptomatic RE was diagnosed when patients with RE reported mild heartburn and/or regurgitation at least 2 days a week, or moderate/severe heartburn and/or regurgitation at least 1 day a week.[11] In some researches, the rest RE except symptomatic RE was defined as asymptomatic RE, and the percent of asymptomatic esophagitis in total esophagitis was reported to be 33.9% to 71.2%.[8,12] However, it has long been known that many Asian patients do not exactly understand the meaning of heartburn and acid regurgitation.[13] Fass and Dickman[14] have defined silent GERD as the presence of typical esophageal mucosal injury during esophagogastroduodenoscopy (EGD) in individuals who lack typical or atypical manifestations of GERD. This proposed definition excludes subjects with well-recognized atypical manifestations of GERD. In our study, we evaluated the prevalence of silent RE according to the Fass’ strict limited definition, and compared it with the “silent RE” using Montreal definition.
Methods
Ethical approval
The study was approved by the Ethical Committee of Peking University Cancer Hospital and Institute (No. 2011101110). All methods were performed in accordance with the relevant guidelines and regulations. All participants provided written informed consent.
Study population
The study was conducted in Hua County of Anyang, China, which has a total of 1.4 million people with high esophageal cancer (EC) incidence exceeding 125/100,000 per year.[6] In January 2012, the Endoscopic Screening for Esophageal Cancer in China (ESECC) randomized controlled trial (Clinical trial: No. NCT01688908) was initiated in Hua County to evaluate the efficacy and cost-effectiveness of endoscopic screening for EC.[15,16] In this trial, 668 target villages were randomly selected from all of the 846 villages with total population sizes ranging from 500 to 3000 in Hua County. As a part of ESECC, we enrolled the data from July 1, 2013 to March 31, 2014 to analyze the GERD prevalence situation. According to the previous reports of the GERD in China, we set up the expected prevalence as 6%, α = 0.05 and allowable error = 0.15, so the estimated sample size was 2788. Totally, 2918 residents were included in the study.
Interview and questionnaires
Demographic and medical history information was got when they presented to the study center. All the subjects were interviewed with the case-report form questionnaire, Chinese version of Gastroesophageal Reflux Disease Questionnaire (GerdQ), and Rome III functional gastrointestinal disorders questionnaire. Subjects with a score ≥8 of GerdQ questionnaire were screened as positive.[17,18] Rome III questionnaire was used to assess the atypical symptoms. The interviewers asked each participant the questions point to point and wrote down the answers in the electronic questionnaires. After the EGD, those without organic diseases were enrolled.
Endoscopic assessment
All the participants underwent the EGD without sedation, and the esophagus, stomach, and duodenum were inspected. Biopsies were taken in all abnormalities and if no lesions were found, one biopsy was taken at the mid-esophagus and gastric antrum respective for pathologic examination. Warthin-Starry staining was used to diagnose the Helicobacter pylori (H. pylori) infection. The specimens were read by two experienced pathologists. RE was diagnosed and classified as grade A to D according to the Los Angles Classification. The subjects were diagnosed as NERD with GerdQ score ≥8 without esophageal mucosal break. Silent RE has been defined as the presence of esophageal mucosal injury during EGD in individuals who lack typical or atypical manifestations of GERD.[14] The other RE was symptomatic RE. The Montreal symptomatic RE was diagnosed when patients with RE reported mild heartburn and/or regurgitation occurring on at least 2 days a week, or moderate/severe heartburn and/or regurgitation occurring on at least 1 day a week.[11] The subjects with the upper gastrointestinal organic diseases such as cancer and gastric ulcer will be excluded in the subsequent analysis. Those subjects without symptoms, RE, or the upper organic gastrointestinal diseases were enrolled as controls. Figure 1 shows typical lesions of ESCC and RE.
Figure 1.
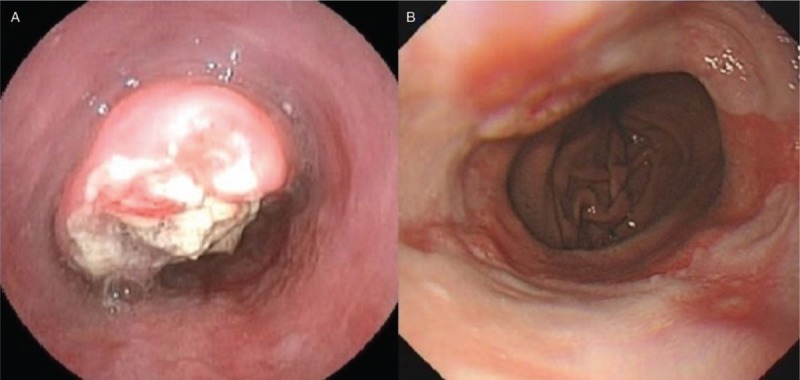
The typical endoscopic images of esophageal squamous cell cancer (A) and reflux esophagitis (B).
Statistical analysis
All the demographic, endoscopic, and questionnaire data were inputted in the database. Statistical analyses were conducted using the IBM SPSS statistical package 19.0 (IBM SPSS Inc., Chicago, IL, USA). Detective data in different age or gender groups were compared by the Chi-square test. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by univariate and multivariate logistic regression to examine factors that are potentially associated with RE (silent RE and symptomatic RE), NERD, respectively. The P < 0.05 was considered statistically significant.
Results
Demographic characteristics of GERD subgroups
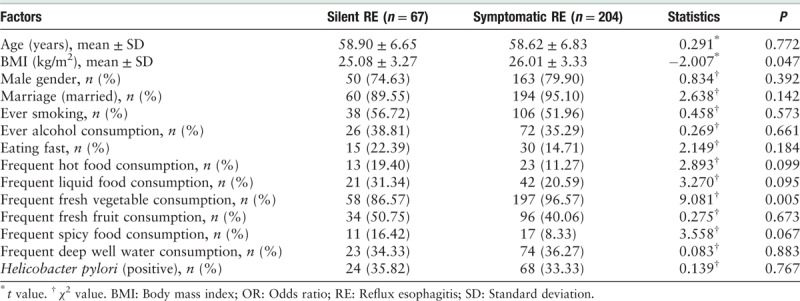
Totally, 2918 residents (average age was 57.36 ± 6.96 years, and male:female = 1454:1464) were screened for eligibility. None of EAC was detected. Seventy-four patients were excluded with the organic gastrointestinal diseases such as ESCC, cardia adenocarcinoma, gastric ulcers, etc. And finally 2844 subjects were enrolled in the subsequent analysis.
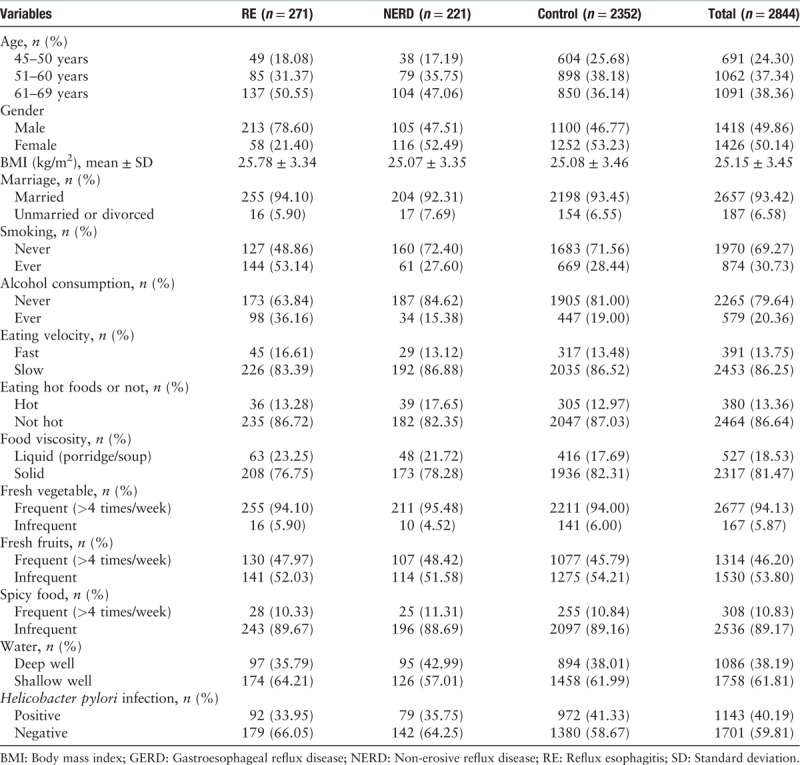
A total of 271 (9.53%) adults were diagnosed with RE. The average age was 58.69 ± 6.78 years and male to female ratio was 213:58 (χ2 = 98.473, P < 0.001). The RE prevalence increased with age (7.09% in 45–50 years, 8.00% in 51–60 years, and 9.53% in 61–69 years, χ2 = 62.216, P < 0.001). A total of 221 (7.77%) were diagnosed with NERD (58.78 ± 6.84 years, and male:female = 105:116, χ2 = 0.045, P = 0.832). Among RE, 67 (24.72%) fulfilled the silent RE diagnoses (58.85 ± 6.70 years, and male:female = 43:24, χ2 = 17.005, P < 0.001). So the prevalence of silent RE was 2.36% (67/2844). The rest 204 patients with RE were symptomatic. Among all the subjects, the H. pylori infection rate was 40.19%. And none of the 2844 subjects had undergone H. pylori eradication. Table 1 shows the demographic characteristics of GERD subgroups.
Table 1.
Demographic characteristics of GERD subgroups.
Risk factors for RE and NERD
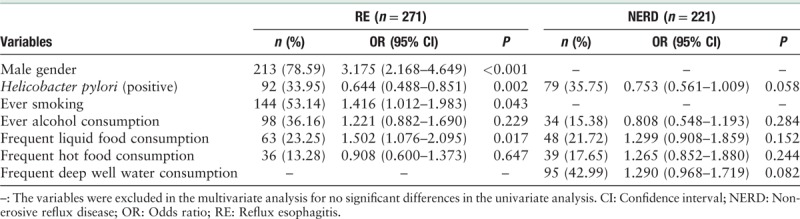
In the univariate analysis, age (OR [95% CI]: 1.035 [1.016–1.054], P < 0.001) and high body mass index (BMI) (OR [95% CI]: 1.059 [1.022–1.097], P = 0.002) were found to be significant risk factors for RE, as well as the male gender, smoking, alcohol consumption, and frequent liquid food consumption were significant risk factors for RE. The subjects with age ≥61 years or BMI ≥28 kg/m2 were more likely to have RE than those younger than 61 years or BMI <28 kg/m2. For NERD, age (OR [95% CI]: 1.037 [1.016–1.058], P < 0.001) was risk factor. H. pylori infection was protective factor for both RE and NERD [Table 2]. The factors with P < 0.1 in univariate analysis were chose for further multivariate analysis. And multivariate analysis showed age (OR [95% CI]: 1.028 [1.008–1.049], P = 0.006) and high BMI (OR [95% CI]: 1.071 [1.030–1.112], P < 0.001) were independent risk factors for RE, also the male gender, smoking, alcohol consumption, and frequent liquid food consumption. Age (OR [95% CI]: 1.036 [1.015–1.057], P = 0.001) was independent risk factors for NERD. H. pylori infection was protective factor for RE [Table 3].
Table 2.
Risk factors for RE and NERD respectively compared with controls (univariate analysis).
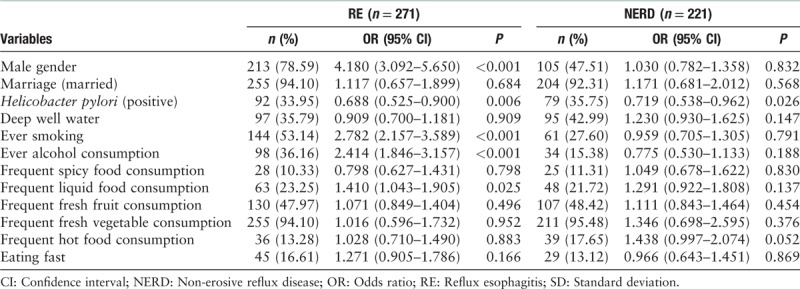
Table 3.
Risk factors for RE and NERD respectively compared with controls (multivariate analysis).
In 204 symptomatic patients with RE, 42 preferred frequent liquid food intake and 162 preferred solid foods. In 67 silent patients with RE, 21 preferred frequent liquid foods and 46 preferred solid foods. No significant differences were found between the group of liquid and solid on the GERD symptoms (χ2 = 3.270, P = 0.095) [Table 4].
Table 4.
Comparisons between silent RE and symptomatic patients with RE.
In 204 symptomatic RE, 169 (82.84%) patients suffered from typical GERD symptoms (163 were heartburn and 9 were regurgitation). Thirty-five (17.16%) patients suffered from atypical symptoms including 13 belching, six non-cardiac chest pain, five nausea, one vomit, eight distension, one abdominal pain, and one choking. A total of 102 (3.59%) patients fulfilled the Montreal silent RE definition.
In 67 silent patients with RE, 32 (47.76%) were RE with Los Angeles A grade and the rest 35 (52.24%) were grade B. In symptomatic RE, 83 (40.69%) were Los Angeles A grade, 103 (50.49%) were grade B and the rest 18 (8.82%) were grade C. There were no significant differences in RE grades between silent RE and symptomatic RE (grade A, χ2 = 1.033, P = 0.309; grade B, χ2 = 0.062, P = 0.804).
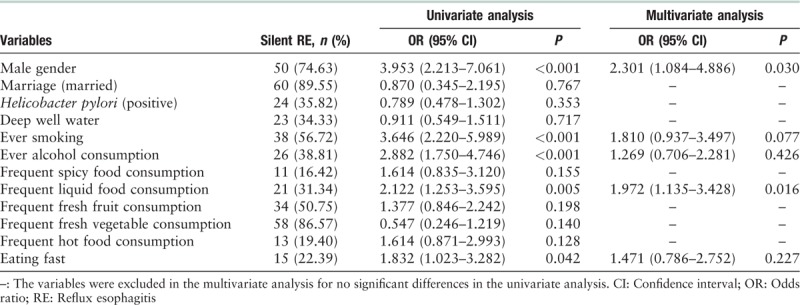
Multivariate analysis showed age (OR [95% CI]: 1.040 [1.002–1.080], P = 0.039) was a risk factor for silent RE, as well as the male gender and frequent liquid food consumption [Table 5].
Table 5.
Risk factors for silent RE compared with control (univariate and multivariate analysis).
Discussion
GERD prevalence presents much higher in ESCC popular population than that in general population of China
In the current study, the prevalence of GERD (RE and NERD) was 17.3%, which was much higher than 2.3% to 5.2% reported in general Chinese population[7,19] and East Asia (2.5–7.8%).[20] Furtherly, we reported a prevalence 9.53% for RE. RE is more common in western countries than in Asia. The prevalence of at least weekly GERD symptoms in the United States is approximately 20%.[8] In Asia, the prevalence of RE in general community were reported to be 6.8% in Japan[21] and 8% in Korea.[22] In China, a mere handful of population-based endoscopy studies were conducted in Chinese general community residents and reported the RE prevalence to vary in 1.99% to 6.40%,[7,12] and it showed a slightly increasing trend. But the data about RE prevalence condition in the ESCC popular area has not been reported yet. The prevalence of 9.53% reported in the current study was higher than that reported in most of the prior studies in China. And the age stratification showed higher prevalence in Anyang than that reported in 2010 (7.09% vs. 6.70% in 40–49 years old, 8.00% vs. 6.72% in 50–59 years old and 9.53% vs. 5.92% in 60–69 years old).[12] RE has been demonstrated to be risk factor for Barrett esophagus and EAC.[23] In one previous study, we have reported that the increasing annual detection rate of type I adenocarcinoma (EAC) of the esophagogastric junction appeared to be positively correlated with that of RE according to the 10 years’ data at a tertiary medical center in Beijing.[24] However, in the current study, none of EAC was detected. One speculation to explain this result is the long time delay from RE to EAC,[25] that means with the increase of RE, the EAC population would come out several years later. If so, we should conduct a longitudinal study to make sure the RE incidence trends over time in this area and to forecast the possible incidence change of EAC in the future. Another important consideration could be deduced that RE may be one of causal factor for the development of ESCC in the current population. It has been speculated that continuous but minimal amounts of reflux can cause ESCC.[2] Therefore, the further study focusing on the reflux contents and extents should be conducted with 24 hour pH-impedance monitoring to clarify this thesis.
We also reported the prevalence of 7.77% for NERD. NERD accounts for the majority of patients with GERD.[26,27] The reports of NERD prevalence in Chinese natural population were rare. In many previous studies, focus on the non-high EAC prevalence outpatients. Our previous study in a tertiary hospital of Beijing also showed a percentage of 71.1% GERD for NERD vs. 28.9% for RE.[28] However, the current study presented a contrary ratio of RE/NERD with more RE. The inverted proportion of RE/NERD is rarely to be reported in China. In the past long time, the view on relationship of RE and NERD are contrary. Some believe NERD is the mild type of RE but others think NERD and RE are two distinct independent diseases. Data from medical literature on the natural history of RE and NERD are scant and mainly retrospective, so the interpretation of them is very difficult[26] and untill now, data from different studies did not help to definitively understand the natural history of GERD.[29] The high prevalence of RE and inverted proportion of RE/NERD reported in the current study may be a new clue to understand the natural history of RE and NERD with future long follow-up study.
The prevalence of GERD (9.53% for RE and 7.77% for NERD) in Anyang was significantly higher than those reported previously in non-high ESCC prevalent area in China and similar to the rate of 20% in western countries.[8] Though the proportion ratio of EAC was reported to be 1.51:1 in high prevalence of EC to that in low prevalence area,[30] the prevalence of EAC is actually low in China. A previous study reported the EAC proportion of 3.9% in EC inpatients in China.[31] In our study with 2844 subjects, none of EAC was detected. While in western countries, especially in North American, the proportions of EAC were reported to be over 50% in EC.[32] We speculate several possible reasons result in the lower incidence of EAC but similarly high prevalence of GERD with western countries. One is due to the genetic heterogeneity of race. Second reason might be, since the duodenogastric reflux has been reported to induce ESCC, the reflux contents differ in the current population from that in western countries. So it presents great value to study the reflux content and extent of these patients with GERD. Another important reason should be the special life habits as discussed as following.
Risk factors for GERD
Multiple factors have been reported to be associated with GERD including age and gender,[33] BMI,[34] alcohol consumption,[35] smoking,[35] etc. In our study, the age, male gender, high BMI, and smoking (former smoker) were also found to be risk factors for RE. For NERD, only age was found independent risk factor. This is partly consistent with previous study in ordinary population.[33] And the different risk factors between RE and NERD suggested that NERD may be not a mild type of RE but an independent disease from RE.
H. pylori infection was found to be protective factor for RE. Similar inverse relationship between RE and H. pylori infection has been found.[36] China is a country with high rates of H. pylori infection of 50% to 69%.[37] The eradication therapy has been widespread used. However, strong evidence was found that eradicating H. pylori infection may increase the prevalence of RE.[38] In our study, the total prevalence of H. pylori was 40.06% in Anyang. And the significant association was found between RE and negative H. pylori infection (OR [95%CI]: 0.644 [0.488–0.851], P = 0.002). This is consistent with a study conducted in Japan.[39] Further high quality and more evidence available studies are needed to define the exact relationship between H. pylori and GERD.
Frequent liquid food consumption was reported to be risk factor for RE. In Anyang, eating hot and/or liquid foods is popular in the residents’ lives. Frequent hot food consumption did not present to be risk factor for GERD. For so long time the liquid food has been suggested to be good for digestive disease patients. Some patients who have reflux symptoms may prefer liquid foods. It seems difficult to explain the causal relationship between liquid food and RE. However, our further analysis showed frequent liquid food consumption was also a risk factor for silent RE (OR [95% CI] 1.972 [1.135–3.428], P = 0.016). These subjects did not experience any symptoms and never underwent EGD before our surveillance. Thus frequent liquid food consumption should be a risk factor for RE. The pathogenic role of liquid food on esophageal mucosa injury was vague. We speculated liquid food increased the gastric volume and then gastric pressure in a short time, and in the following, it was emptied quickly thus resulted in the ineffectively neutralization of gastric acid.
Prevalent situation of silent RE
A Chinese study reported 5.83% (60/1029) Montreal silent RE in 1029 inhabitants of Shanghai.[12] In our current study, the prevalence of Montreal silent RE was 3.59% (102/2844). No consideration of atypical reflux symptoms, the Montreal definition may overestimate the ratio of silent RE.[13] It is well known that many atypical symptoms are GERD related and respond to proton pump inhibitors therapy.[40] We believe the subjects with atypical symptoms should be included in symptomatic RE. So we furtherly calculated the silent RE according to a more strict definition[14] and found the prevalence of silent RE was 2.36% (67/2844). These data were similar to the report of the study conducted by Cho et al. [10] in Korea, who reported a rate of 2.74% of the silent RE in 5301 participants underwent health examination in Korea. The prevalence of 2.36% in natural population is not very low and furthermore, the silent RE present similar grades as that of symptomatic RE. Age and male gender were independent risk factors for silent RE when compared with controls as reported in previous studies.[13] But frequent liquid food consumption was reported to be risk factor for silent RE. The role of liquid food on silent RE was not known yet. Following prospective study should be carried out to observe long-time outcome by avoiding liquid food.
In summary, high prevalence of GERD and inverted proportion of RE/NERD were reported (9.53% for RE and 7.77% for NERD) in the ESCC high prevalence region in China. Compared with many previous studies, a contrary ratio of RE/NERD with more RE in Anyang was presented. And the prevalence of silent RE in term of strict definition was 2.36%.The male gender, high BMI, smoking, and frequent liquid food consumption were found to be risk factors for RE but not for NERD. H. pylori infection was found to be protective factor for RE. The following study should focus on the relationship between GERD and ESCC, and the latent role of gastroesophageal reflux in the pathogenesis of ESCC.
Acknowledgements
The authors thank the following collaborators for their contributions to the field work done for this study, endoscopic examinations, pathologic diagnosis, and database establishment: Chuan-Hai Guo, Li-Xin Zhang, Ying Chen, Rong-Li Cui, He-Jun Zhang, Yu-Jie He, and Cai-Yun Wang from Peking University Cancer Hospital and Institute, Anyang Cancer Hospital, Peking University Third Hospital and School of Public Health, Peking University.
Funding
The work was supported by grants from the Chinese Charity Project of National Ministry of Health (No. 201202014) and the National Natural Science Foundation of China (No. 81400595).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang K, Zhang L, He ZH, Liu ZJ, Zhang L, Hu N, Jin Z, Ke Y, Duan LP. A population-based survey of gastroesophageal reflux disease in a region with high prevalence of esophageal cancer in China. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000275
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Kumagai H, Mukaisho K, Sugihara H, Miwa K, Yamamoto G, Hattori T. Thioproline inhibits development of esophageal adenocarcinoma induced by gastroduodenal reflux in rats. Carcinogenesis 2004; 25:723–727. doi: 10.1093/carcin/bgh067. [DOI] [PubMed] [Google Scholar]
- 3.Ling ZQ, Mukaisho K, Yamamoto H, Chen KH, Asano S, Araki Y, et al. Initiation of malignancy by duodenal contents reflux and the role of ezrin in developing esophageal squamous cell carcinoma. Cancer Sci 2010; 101:624–630. doi: 10.1111/j.1349-7006.2009.01470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He YT, Hou J, Chen ZF, Qiao CY, Song GH, Meng FS, et al. Trends in incidence of esophageal and gastric cardia cancer in high-risk areas in China. Eur J Cancer Prev 2008; 17:71–76. doi: 10.1097/CEJ.0b013e3282b6fd97. [DOI] [PubMed] [Google Scholar]
- 5.Song GH, Ma Q, Ma SR, Chen C, Wei WW. Analysis of the incidence and age characteristics of upper gastrointestinal cancer among 2003-2012 in the high incidence area of esophageal cancer, Cixian county, in Heibei province. Chin J Prev Med 2017; 51:398–402. doi: 10.3760/cma.j.issn.0253-9624.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 2018; 154:360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Ma X, Zhao Y, Wang R, Yan X, Yan H, et al. A population-based survey of the epidemiology of symptom-defined gastroesophageal reflux disease: the Systematic Investigation of Gastrointestinal Diseases in China. BMC Gastroenterol 2010; 10:94.doi: 10.1186/1471-230X-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richter JE, Rubenstein JH. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology 2018; 154:267–276. doi: 10.1053/j.gastro.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018; 67:1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho JH, Kim HM, Ko GJ, Woo ML, Moon CM, Kim YJ, et al. Old age and male sex are associated with increased risk of asymptomatic erosive esophagitis: analysis of data from local health examinations by the Korean National Health Insurance Corporation. J Gastroenterol Hepatol 2011; 26:1034–1038. doi: 10.1111/j.1440-1746.2011.06686.x. [DOI] [PubMed] [Google Scholar]
- 11.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 12.Zou D, He J, Ma X, Chen J, Gong Y, Man X, et al. Epidemiology of symptom-defined gastroesophageal reflux disease and reflux esophagitis: the systematic investigation of gastrointestinal diseases in China (SILC). Scand J Gastroenterol 2010; 46:133–141. doi: 10.3109/00365521.2010.52188. [DOI] [PubMed] [Google Scholar]
- 13.Goh KL, Ho SH. Silent gastroesophageal disease: clinical implications of an unknown disease. J Gastroenterol Hepatol 2011; 26:1034–1038. doi: 10.1111/j.1440-1746.2011.06738.x. [DOI] [PubMed] [Google Scholar]
- 14.Fass R, Dickman R. Clinical consequence of silent gastroesophageal reflux disease. Curr Gastroenterol Rep 2006; 8:195–201. doi: 10.1007/s11894-006-0075-8. [DOI] [PubMed] [Google Scholar]
- 15.He Z, Liu Z, Liu M, Guo C, Xu R, Li F, et al. Efficacy of endoscopic screening for esophageal cancer in China (ESECC): design and preliminary results of a population-based randomized controlled trial. Gut 2019; 68:198–206. doi: 10.1136/gutjnl-2017-315520. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Liu Z, Cai H, Guo C, Li X, Zhang C, et al. A model to identify individuals at high risk for esophageal squamous cell carcinoma and precancerous lesions in regions of high prevalence in China. Clin Gastroenterol Hepatol 2017; 15:1538–1546. doi: 10.1016/j.cgh.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Okimoto E, Ishimura N, Morito Y, Mikami H, Shimura S, Uno G, et al. Prevalence of gastroesophageal reflux disease in children, adults, and elderly in the same community. J Gastroenterol Hepatol 2015; 30:1140–1146. doi: 10.1111/jgh.12899. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Matsuzaki J, Okada S, Hirata K, Fukuhara S, Hibi T. Validation of the GerdQ questionnaire for the management of gastro-oesophageal reflux disease in Japan. United European Gastroenterol J 2013; 1:175–183. doi: 10.1177/2050640613485238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong LS, Chen MH, Chen HX, Xu AG, He LJ, Hu PJ. A population-based epidemiologic study on gastroesophageal reflux disease. Chin J Dig 2006; 26:239–242. doi: 10.3760/j.issn:0254-1432.2006.04.007. [Google Scholar]
- 20.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014; 63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minatsuki C, Yamamichi N, Shimamoto T, Kakimoto H, Takahashi Y, Fujishiro M, et al. Background factors of reflux esophagitis and non-erosive reflux disease: a cross-sectional study of 10,837 subjects in Japan. PLoS One 2013; 8:e69891.doi: 10.1371/journal.pone.0069891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JM, Yang JI, Kang SJ, Han YM, Lee J, Lee C, et al. Association between anxiety and depression and gastroesophageal reflux disease: results from a large cross-sectional study. J Neurogastroenterol Motil 2018; 24:593–602. doi: 10.5056/jnm18069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med 2014; 371:836–845. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Yang CQ, Duan LP, Yang XS, Xia ZW, Cui RL, et al. Changing pattern of adenocarcinoma of the esophagogastric junction in recent 10 years: experience at a large tertiary medical center in China. Tumori 2012; 98:567–573. doi: 10.1700/1190.13196. [DOI] [PubMed] [Google Scholar]
- 25.Malfertheiner P, Nocon M, Vieth M, Stolte M, Jaspersen D, Koelz HR, et al. Evolution of gastro-oesophageal reflux disease over 5 years under routine medical care - the Pro GERD study. Aliment Pharmacol Ther 2012; 35:154–164. doi: 10.1111/j.1365-2036.2011.04901.x. [DOI] [PubMed] [Google Scholar]
- 26.Savarino E, Marabotto E, Bodini G, Pellegatta G, Coppo C, Giambruno E, et al. Epidemiology and natural history of gastro-esophageal reflux disease. Minerva Gastroenterol Dietol 2017; 17: doi: 10.23736/S1121-421X.17.02383-2. [DOI] [PubMed] [Google Scholar]
- 27.Fock KM, Talley N, Goh KL, Sugano K, Katelaris P, Holtmann G, et al. Asia-Pacific consensus on the management of gastro-oesophageal reflux disease: an update focusing on refractory reflux disease and Barrett's oesophagus. Gut 2016; 65:1402–1415. doi: 10.1136/gutjnl-2016-311715. [DOI] [PubMed] [Google Scholar]
- 28.Wang K, Duan LP, Chen H, Xia ZW, Lin SR. Comparison of esophageal acid exposure characteristics between reflux oesophagitis and non-erosive reflux diseases (in Chinese). Chin J Intern Med 2005; 44:5–8. doi: 10.3760/j.issn:0578-1426.2005.01.004. [PubMed] [Google Scholar]
- 29.Savarino E, de Bortoli N, De Cassan C, Della Coletta M, Bartolo O, Furnari M, et al. The natural history of gastro-esophageal reflux disease: a comprehensive review. Dis Esophagus 2017; 30:1–9. doi: 10.1111/dote.12511. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Wang LD. Esophageal adenocarcinoma and esophageal squamous carcinoma of inpatient in high-and low-incidence areas for esophageal cancer from 1973-2012. Zhengzhou: Zhengzhou University; 2014. [Google Scholar]
- 31.Shao SZ, Zhao JJ, Yu XN, Zhang HX, Shen CF, Wang P, et al. Trends in incidence of esophageal and gastric cardia adenocarcinomas in Chongqing city over the past 30 years (in Chinese). Chongqing Medicine 2014; 10:3870–3872. doi: 10.3969/j.issn.1671-8348.2014.29.008. [Google Scholar]
- 32.Rubenstein JH, Shaheen NJ. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology 2015; 149:302–317. doi: 10.1053/j.gastro.2015.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dore MP, Pes GM, Bassotti G, Farina MA, Marras G, Graham DY. Risk factors for erosive and non-erosive gastroesophageal reflux disease and Barrett's esophagus in Northern Sardinia. Scand J Gastroenterol 2016; 51:1281–1287. doi: 10.1080/00365521.2016.1200137. [DOI] [PubMed] [Google Scholar]
- 34.Khan A, Kim A, Sanossian C, Francois F. Impact of obesity treatment on gastroesophageal reflux disease. World J Gastroenterol 2016; 22:1627–1638. doi: 10.3748/wjg.v22.i4.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ness-Jensen E, Lagergren J. Tobacco smoking, alcohol consumption and gastro-oesophageal reflux disease. Best Pract Res Clin Gastroenterol 2017; 31:501–508. doi: 10.1016/j.bpg.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor A, O’Morain CA, Ford AC. Population screening and treatment of Helicobacter pylori infection. Nat Rev Gastroenterol Hepatol 2017; 14:230–240. doi: 10.1038/nrgastro.2016.195. [DOI] [PubMed] [Google Scholar]
- 37.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017; 153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Cui RL, Zhou LY. Helicobacter pylori infection: an overview in 2013, focus on therapy. Chin Med J 2014; 127:568–573. doi: 10.3760/cma.j.issn.0366-6999.20132708. [PubMed] [Google Scholar]
- 39.Sugimoto M, Uotani T, Ichikawa H, Andoh A, Furuta T. Gastroesophageal reflux disease in time covering eradication for all patients infected with Helicobacter pylori in Japan. Digestion 2016; 93:24–31. doi: 10.1159/000441741. [DOI] [PubMed] [Google Scholar]
- 40.Zerbib F. The prevalence of oesophagitis in “silent” gastro-oesophageal reflux disease: Higher than expected? Dig Liver Dis 2015; 47:12–13. doi: 10.1016/j.dld.2014.10.006. [DOI] [PubMed] [Google Scholar]


