Abstract
MiR-101 plays an important role in tumorigenesis. The aim of this study was to estimate diagnostic potential of serum miR-101 in bladder cancer.
Serum level of miR-101 in 122 bladder cancer patients and 110 healthy volunteers was detected using quantitative real-time polymerase chain reaction method. The association between miR-101 expression and clinicopathological characteristic was analyzed via χ2 test. Then receiver operating characteristic (ROC) curve was plotted to evaluate diagnostic value of serum miR-101 in bladder cancer.
MiR-101 expression was statistically down-regulated in bladder cancer patients compared to healthy controls. MiR-101 expression was significantly associated with TNM stage (P = .019), pathological grade (P = .006) and lymph node metastasis (P = .010). ROC analysis suggested that miR-101 had high value in discriminating between bladder cancer patients and healthy individuals with an AUC value of 0.884. The cut-off value for serum miR-101 in bladder cancer diagnosis was 1.645, with a sensitivity of 82.0% and a specificity of 80.9%.
MiR-101 is decreased in bladder cancer patients, and shows negative association with aggressive clinical characteristics. MiR-101 may serve as a bio-marker in diagnosing bladder cancer.
Keywords: bladder cancer, diagnosis, MiR-101, serum
1. Introduction
Bladder cancer is one of the most common malignancies in urinary system, posing great threat to human healthy in the world.[1] In the United States alone, bladder cancer was estimated to see 74,000 new cases and 16,000 deaths in 2015.[2] The cancer is characterized by high recurrent rate. Reportedly, more than half of the patents would undergo recurrence within 5 years after operation.[3,4] Early detection and monitoring are pivotal for clinical outcomes of bladder cancer patients. At present, cystoscopy and cytology are commonly used in early screening of bladder cancer.[5] However, these methods are invasive and costly, and frequently cause infections.[6] Thus, it is in urgent need to explore novel and effective biomarkers for non-invasive diagnosis of bladder cancer.
MicroRNAs (MiRNAs) are a class of small, endogenous, non-coding RNA that regulate gene expression by affecting mRNA translation and stability or by modulating promoter activity of their target genes.[7,8] MiRNAs were originally identified in Caenorhabditis elegans.[9] They are involved in diverse biological processes, including cell growth, apoptosis, and differentiation.[10,11] Cumulated evidences have suggested that the dysregulation of miRNAs may play oncogenic or suppressive roles in cancer development.[12,13] Given their functional roles in tumorigenesis, miRNAs are considered as promising biomarkers for early diagnosis, prognosis evaluation and therapeutic response prediction in oncology.[11]
MiR-101, a common member of miRNAs family, is reported to be a suppressor gene in various human malignancies, such as gastric cancer, prostate cancer, osteosarcoma, and bladder cancer.[14–17] The study carried out by Zhang et al reported that down-regulated miR-101 in bladder transitional cell carcinoma (BTCC) showed obvious association with aggressive clinical characteristics, which might be a promising prognostic biomarker for the disease.[17] However, whether serum miR-101 could serve as a diagnostic biomarker for bladder cancer is still unclear.
In the present study, we aimed to investigate serum level of miR-101 in bladder cancer. Furthermore, we analyzed the association of miR-101 expression with clinicopathological characteristics of the patients with bladder cancer. Additionally, diagnostic value of serum miR-101 in bladder cancer was also investigated in the current study.
2. Materials and methods
2.1. Patients and specimens
A total of 122 patients with bladder cancer and 110 healthy control were recruited in this study from Zhongnan Hospital. The patients were diagnosed with bladder cancer by 2 independent experienced pathologists based on the 1973 diagnosis criteria. TNM staging was performed for the patients according to the American Joint Committee on Cancer staging system (7th edition, 2010). No patients had received preoperative treatment. Detailed clinicopathologic characteristics of the patients with bladder cancer were obtained from their medical records, and summarized in Table 1. In addition, none of the healthy individuals had bladder diseases or malignancy history. The bladder cancer patients and healthy individuals were matched in gender and age. Our study was approved by the Ethics Committee of Zhongnan Hospital. All participants signed written informed consents prior to sampling.
Table 1.
The clinicopathological characteristics of 122 patients and the association with miR-101 expression.
The 5 ml whole blood was collected from every participant on the morning after fasting for 8 to 10 hours. Serum was separated from whole blood through centrifugation, and then stored at −80°C until RNA extraction.
2.2. RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from serum specimens using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. First-strand cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) following the manufacturer's protocol. Relative expression level of miR-101 mRNA was estimated by qRT-PCR method which was performed using SYBR Green PCR Master Mix (Applied Biosystems) in the 7900 Real-Time PCR System (Applied Biosystems). PCR primers used to amplify miR-101 were as follows: 5’-CGGGTACCGGTAGTCCTTCACTTCATGGGGAG-3’ (forward) and 5’-CGGAATTCAAAAAACCCAGCCACCTGTTTCAC-3’ (reverse).[18]U6 was used as reference gene and its primers were as follows: 5’-CTCGCTTCGGCAGCACA-3’ (forward) and reverse 5’-AACGCTTCACGAATTTGCGT-3’ (reverse). Ct values of the samples were recorded, and relative levels of miR-101 were calculated through the 2−△△Ct method. Every sample was tested three times.
2.3. Statistical analysis
All statistical analyses were conducted using SPSS 21.0 software (SPSS, Inc., Chicago, IL) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). Data for gene expression were presented as mean ± standard deviation (SD). Comparisons on miR-101 expression between tumor and normal control groups were performed with 2-tailed paired Student's t test. The relationship between miR-101 expression and clinicopathologic characteristics was analyzed by χ2 test. Receiver-operating characteristic (ROC) curve was constituted to assess diagnostic value of serum miR-101 in bladder cancer, and the results were estimated through calculating the area under the ROC curve (AUC), sensitivity and specificity according to standard formulas. P values less than .05 were considered as statically significant. ∗ P < .05, ∗∗ P < .01 indicated significant difference.
3. Results
3.1. The expression of miR-101 was decreased in bladder cancer
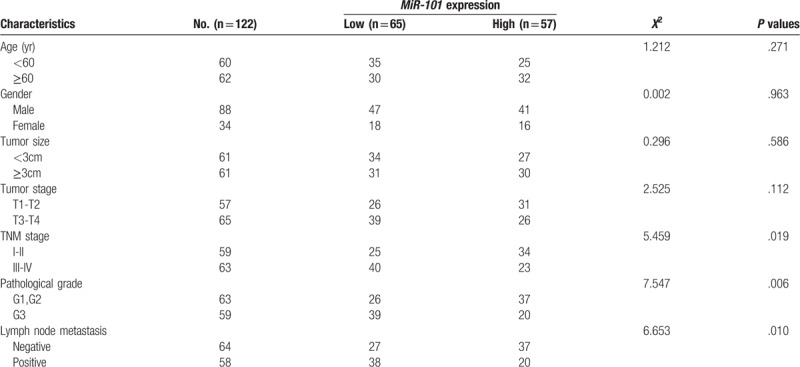
A total of 88 male and 34 female bladder cancer patients were collected in our study, with an average age of 55.62 ± 15.48 years. There were 77 men and 33 women in the control group, and their average age was 53.12 ± 12.22 years. The distributions of gender and age were similar between bladder cancer patients and healthy controls (P > .05 for both). QRT-PCR analysis was used to detect relative expression levels of miR-101 in bladder cancer patients and healthy individuals. As shown in Figure 1, expression level of miR-101 was decreased in bladder cancer patients compared with the normal controls (P < .01).
Figure 1.
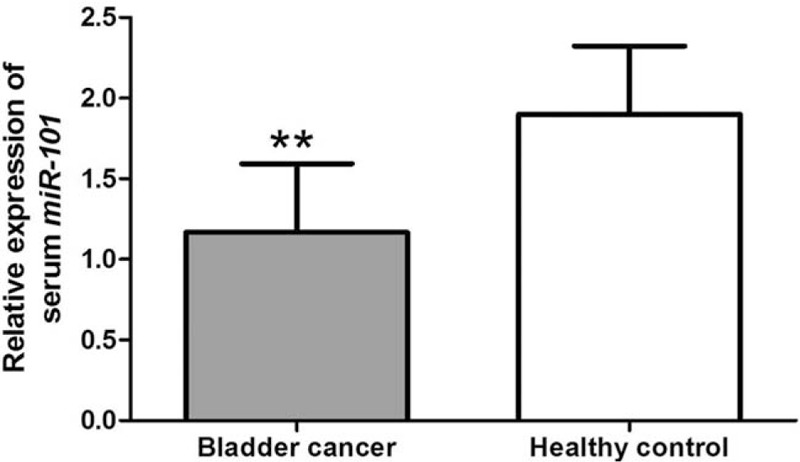
The expression of miR-101 in bladder cancer cases and healthy controls. The result showed that serum miR-101 level was downregulated in bladder cancer patients, compared to healthy individuals (∗∗ P < .01).
3.2. Relationship between miR-101 and clinicopathological parameters
To investigate the relationship between miR-101 expression and clinicopathlogic characteristics of bladder cancer patients, we divided the patients into high- (> mean value) and low-(≤ mean value) expression groups based on their mean expression level of miR-101. Statistical analysis results were summarized in Table 1. The expression of miR-101 was found to be significantly associated with TNM stage (P = .019), pathological grade (P = .006) and lymph node metastasis (P = .010), but not with age, gender, tumor size or tumor stage (all P > .05).
3.3. Diagnostic value of miR-101 in bladder cancer
To investigate diagnostic performance of serum miR-101 in bladder cancer, ROC curve analysis was performed. As shown in Figure 2, miR-101 possessed relatively high accuracy in differentiating bladder cancer patients from healthy individuals with an AUC of 0.884 (95%CI: 0.842–0.927). The optimal cut-off point was 1.645, with a sensitivity of 82.0% and a specificity of 80.9%.
Figure 2.
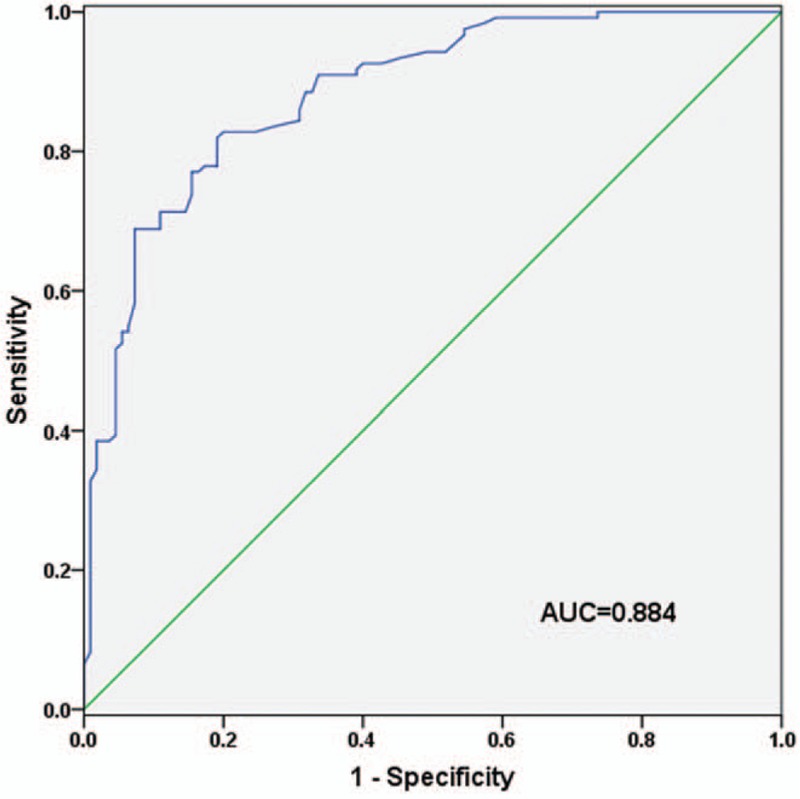
ROC curve was established to evaluate diagnostic value of serum miR-101 in bladder cancer. The AUC value for the curve was 0.884, suggesting that serum miR-101 could discriminate between bladder cancer patients and healthy individuals. The cut-off value of serum miR-101 for bladder cancer diagnosis was 1.645, with the corresponding sensitivity of 82.0% and the specificity of 80.9%.
4. Discussion
Bladder cancer is one of the most common genitourinary malignancies with high morbidity and mortality. Despite great advances in surgery, radiotherapy and chemotherapy, overall survival of bladder cancer patients has not been significantly improved.[19] Early diagnosis is key for clinical outcomes of bladder cancer patients. Currently, standard measures for the diagnosis of bladder cancer contain conventional cystoscopy and biopsy, but cystoscopy is invasive, uncomfortable and costly while urine cytology always shows low sensitivity.[20] Therefore, novel reliable biomarkers with high sensitivity and specificity are urgently needed for non-invasive diagnosis of bladder cancer.
Recent years, more and more researches explored molecular biomarkers for the prediction of tumor development and progression. For instances, Zhang et al reported that DCAMKL1 (doublecortin and CaM kinase-like 1) was up-regulated in bladder cancer tissues and cell lines, moreover, its elevated expression showed positive association with malignant status of the disease. DCAMKL1 might be a potential prognostic biomarker for bladder cancer.[21] A meta-analysis including 6 studies indicated that urine UCA1 exhibited high diagnostic accuracy for bladder cancer.[22] The study carried out by Zhang et al reported that Golgi phosphoprotein 3 (GOLPH3) was involved in the progression of bladder cancer via modulating AKT/mTOR signaling, and that it was a novel prognostic biomarker for the malignancy.[23] Molecular biomarkers may provide new insights in the etiology of bladder cancer, thus contributing to improvements in the disease management.
MiRNAs expression profiles show obvious association with tumor development, progression and therapy response, revealing their potential as biomarkers for early screening and monitoring in oncology.[24,25]MiR-101 is widely accepted as a tumor suppressor in several malignancies. Growing evidences have suggested that miR-101 hold the capacity to serve as a biomarker for cancers. Xie et al suggested that serum miR-101 level could be employed as a non-invasive biomarker to distinguish hBV-associated hepatocellular carcinoma (HBV-HCC) from hBV-associated liver cirrhosis (HBV-LC).[26] The expression of miR-101 also exhibited significant differences between cervical cancer patients and non-cancer patients, which might be a potential biomarker for early diagnosis of the disease.[27] Luo et al reported that the down-regulation of miR-101 predicted malignant clinical characteristics in non-small cell lung cancer, suggesting its potential as a biomarker for prognosis and therapeutic response in this disease.[28] However, predictive function of miR-101 in bladder cancer remains poorly known.
In the present study, we investigated serum level of miR-101 in bladder cancer patients using qRT-PCR analysis. The result proved that the expression of miR-101 was down-regulated in bladder cancer patients compared to the healthy individuals. Then we further analyzed the association of miR-101 expression with clinicopathological characteristics of the bladder cancer patients. The down-regulation of miR-101 was found to be significantly associated with advanced TNM stage, high pathological grade and positive lymph node metastasis, indicating miR-101 as a tumor suppressor gene was participated in the development and progression of bladder cancer. In addition, diagnostic value of serum miR-101 in bladder cancer was also investigated using ROC curve analysis. Analysis results suggested that miR-101 might be employed as a biomarker for bladder cancer detection with high sensitivity and specificity. Serum miR-101 might be a potential biomarker for non-invasive detection of bladder cancer. In previous studies, miR-101 was also reported to be associated with the prognosis of multiple cancers. In the study of He, low serum level of miR-101 was obviously correlated with poor prognosis of colorectal cancer.[29] However, Lv et al reported high expression of miR-101 was an independent prognostic factor for HCC.[30] Therefore, miR-101 may be closely associated with bladder cancer prognosis. In next step, we will explore prognostic value of miR-101 in bladder cancer and underlying mechanism of miR-101 functioning in bladder cancer progression.
However, there were still several limitations in the current study. Firstly, the sample size was relatively small. Secondly, the mechanisms underlying anti-tumor action of miR-101 in bladder cancer was not investigated in the current study. In bladder cancer, there were several targets of miR-101 confirmed in previous studies, including c-Met,[31] VEGF-C (vascular endothelial growth factor C),[18] and c-FOS.[32] Relevant researches might provide ideas for our further investigations.
In conclusion, the expression of serum miR-101 is lower in bladder cancer patients than in healthy individuals. Serum miR-101 may serve as a noninvasive biomarker for the diagnosis of bladder cancer. Further studies are needed to enhance our findings on miR-101 value in the detection of bladder cancer.
Author contributions
Conceptualization: Xiaoyan Chen.
Data curation: Xiaoyan Chen.
Formal analysis: Xiaoyan Chen.
Funding acquisition: Xiaoyan Chen.
Investigation: Xiaoyan Chen.
Methodology: Xiaoyan Chen.
Project administration: Xiaoyan Chen.
Resources: Xiaoyan Chen.
Software: Xiaoyan Chen.
Supervision: Xiaoyan Chen.
Validation: Xiaoyan Chen.
Visualization: Xiaoyan Chen.
Writing – original draft: Xiaoyan Chen.
Writing – review & editing: Xiaoyan Chen.
Footnotes
Abbreviations: AUC = The area under the ROC curve, BTCC = Bladder transitional cell carcinoma, GOLPH3 = Golgi phosphoprotein 3, HBV-HCC = hBV-associated hepatocellular carcinoma, HBV-LC = hBV-associated liver cirrhosis, MiRNAs = MicroRNAs, qRT-PCR = Quantitative real-time polymerase chain reaction, ROC = Receiver-operating characteristic, SD = Standard deviation, VEGF-C = Vascular endothelial growth factor C.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [3].Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol 2017;71:447–61. [DOI] [PubMed] [Google Scholar]
- [4].Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466–5. discussion 475-467. [DOI] [PubMed] [Google Scholar]
- [5].Duan W, Du L, Jiang X, et al. Identification of a serum circulating lncRNA panel for the diagnosis and recurrence prediction of bladder cancer. Oncotarget 2016;7:78850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu C, Shi B, Hao C, et al. Urine gamma-synuclein as a biomarker for the diagnosis of bladder cancer. Oncotarget 2016;7:43432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol 2013;14:475–88. [DOI] [PubMed] [Google Scholar]
- [8].Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- [9].Lee RC, Feinbaum RL, Ambros V, et al. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75:843–54. [DOI] [PubMed] [Google Scholar]
- [10].Zhang LY, Liu M, Li X, et al. miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3). J Biol Chem 2013;288:4035–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Armand-Labit V, Pradines A. Circulating cell-free microRNAs as clinical cancer biomarkers. Biomol Concepts 2017. [DOI] [PubMed] [Google Scholar]
- [12].Shin VY, Chu KM. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J Gastroenterol 2014;20:10432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fazi F, Blandino G. MicroRNAs: non coding pleiotropic factors in development, cancer prevention and treatment. Microrna 2013;2:81. [DOI] [PubMed] [Google Scholar]
- [14].Wu X, Zhou J, Wu Z, et al. MiR-101-3p Suppresses HOX Transcript Antisense RNA (HOTAIR)-induced proliferation and invasion through directly targeting srf in gastric carcinoma cells. Oncol Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang S, Yang Z, Ma Y, et al. miR-101 Enhances Cisplatin-Induced DNA damage through decreasing nicotinamide adenine dinucleotide phosphate levels by directly repressing Tp53-Induced glycolysis and apoptosis regulator expression in prostate cancer cells. DNA Cell Biol 2017;36:303–10. [DOI] [PubMed] [Google Scholar]
- [16].Jiang R, Zhang C, Liu G, et al. MicroRNA-101 inhibits proliferation, migration and invasion in osteosarcoma cells by targeting ROCK1. Am J Cancer Res 2017;7:88–97. [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang H, Qi F, Cao Y, et al. Down-regulated microRNA-101 in bladder transitional cell carcinoma is associated with poor prognosis. Med Sci Monit 2014;20:812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lei Y, Li B, Tong S, et al. miR-101 suppresses vascular endothelial growth factor C that inhibits migration and invasion and enhances cisplatin chemosensitivity of bladder cancer cells. PloS One 2015;10:e0117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhan Y, Lin J, Liu Y, et al. Up-regulation of long non-coding RNA PANDAR is associated with poor prognosis and promotes tumorigenesis in bladder cancer. J Exp Clin Cancer Res 2016;35:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eissa S, Shabayek MI, Ismail MF, et al. Diagnostic evaluation of apoptosis inhibitory gene and tissue inhibitor matrix metalloproteinase-2 in patients with bladder cancer. IUBMB life 2010;62:394–9. [DOI] [PubMed] [Google Scholar]
- [21].Zhang S, Zhang G, Guo H. DCAMKL1 is associated with the malignant status and poor outcome in bladder cancer. Tumour Biol 2017;39:1010428317703822. [DOI] [PubMed] [Google Scholar]
- [22].Cui X, Jing X, Long C, et al. Accuracy of the urine UCA1 for diagnosis of bladder cancer: a meta-analysis. Oncotarget 2017;8:35222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Q, Zhuang J, Deng Y, et al. GOLPH3 is a potential therapeutic target and a prognostic indicator of poor survival in bladder cancer treated by cystectomy. Oncotarget 2015;6:32177–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med 2012;4:143–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rong D, Tang W, Li Z, et al. Novel insights into circular RNAs in clinical application of carcinomas. Onco Targets Ther 2017;10:2183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xie Y, Yao Q, Butt AM, et al. Expression profiling of serum microRNA-101 in HBV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Cancer Biol Ther 2014;15:1248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lin C, Huang F, Zhang YJ, et al. Roles of MiR-101 and its target gene Cox-2 in early diagnosis of cervical cancer in Uygur women. Asian Pac J Cancer Prev 2014;15:45–8. [DOI] [PubMed] [Google Scholar]
- [28].Luo L, Zhang T, Liu H, et al. MiR-101 and Mcl-1 in non-small-cell lung cancer: expression profile and clinical significance. Med Oncol 2012;29:1681–6. [DOI] [PubMed] [Google Scholar]
- [29].He D, Yue Z, Li G, et al. Low Serum Levels of miR-101 Are Associated with Poor Prognosis of Colorectal Cancer Patients After Curative Resection. Med Sci Monit 2018;24:7475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lv X, Li J, Yang B. Clinical effects of miR-101 on prognosis of hepatocellular carcinoma and carcinogenic mechanism of anti-miR-101. Oncol Rep 2016;36:2184–92. [DOI] [PubMed] [Google Scholar]
- [31].Hu Z, Lin Y, Chen H, et al. MicroRNA-101 suppresses motility of bladder cancer cells by targeting c-Met. Biochem Biophys Res Commun 2013;435:82–7. [DOI] [PubMed] [Google Scholar]
- [32].Long Y, Wu Z, Yang X, et al. MicroRNA-101 inhibits the proliferation and invasion of bladder cancer cells via targeting c-FOS. Mol Med Rep 2016;14:2651–6. [DOI] [PubMed] [Google Scholar]


