Abstract
Neutrophil to lymphocyte ratio (NLR) is a simple, noninvasive, inexpensive inflammatory marker that can useful in the assessment of inflammatory activity, especially in pediatric ages. The aim of our study was to establish correlations between the presence of Helicobacter pylori (HP) proved histologically and NLR in children.
A prospective, case–control study was performed on 137 pediatric patients aged between 1 and 18 years, admitted in a Pediatric Tertiary Hospital from Romania, between April 2016 and January 2018. According to the histologic examination, the children were divided into 2 groups: group 1: 50 children with HP infection, and group 2: 87 children without any pathologic findings.
The mean age for the study group was 12.86 ± 3.796 years, whereas for control group, it was 12.10 ± 3.879 years (P = .3001). HP infection was significantly more frequent among children from rural area (P = .0089). Epigastric pain and loss of appetite were significantly associated with HP infection (P = .0350 /P = .0281). We noticed that the leukocyte and neutrophil counts were significantly higher in group 1 (P = .0076/P = .0306). We did not find any significant statistical differences between the 2 groups in terms of lymphocytes, erythrocyte sedimentation rate, and NLR or other assessed laboratory parameters. Regarding the IgA antibodies anti-HP and rapid urease test, they were both significantly associated with histologically confirmed HP infection (P < .0001).
Even though, we did not identify significant differences in term of NLR between HP-induced gastritis children and healthy controls, the mean NLR values were higher HP-positive patients.
Keywords: children, gastritis, Helicobacter pylori, neutrophil/lymphocyte ratio
1. Introduction
Helicobacter pylori (HP), a gram-negative, microaerophilic bacterium, colonizes the human gastric mucosa being able to lead to a long-term persistent infection at this level.[1] Approximately 50% of the world's population are infected with this bacterium and the infection usually occurs during early childhood. Moreover, if left untreated, it may persist for life independent of the innate and adaptive immune responses.[2–5] HP infection may be the leading cause for different conditions: chronic gastritis, peptic ulcers, gastric cancer, lymphomas of the gastric-associated lymphoid tissue, and gastric adenocarcinoma.[6] Nevertheless, HP is not the only leading cause of these disorders.[7,8] The persistence of this infection results in chronic inflammation increasing the risk of subsequent gastric carcinogenesis. Therefore, it is well-documented that approximately 80% of gastric cancers and 5.5% of all malignant conditions worldwide are due to HP induced injury.[9,10]
The HP-related chronic gastritis is defined histologically by lymphoid follicle hyperplasia, intestinal metaplasia, and varying degrees of neutrophil infiltrations within the lamina propria.[11,12] Nevertheless, according to the Sydney Gastritis Classification system, acute gastritis is characterized by an increase in neutrophil predominant inflammatory cells, while in chronic gastritis lymphocytes and plasmacytes predominate at the level of gastric mucosa.[13] After the colonization of the gastric mucosa, HP attracts neutrophils and lymphocytes at this level resulting in the release of different chemotactic proteins in the stomach.[14] Mononuclear cells and macrophages are also present within the gastric mucosa and along with the previously mentioned cells and several signal cytokines will lead to a subclinical systemic low-grade inflammation.[14] A study performed on Japanese underlined an association between increased serum anti-HP levels and interleukin-6 (IL-6), which is secreted by monocytes, lymphocytes, endothelial cells, and mesangial cells.[15] Therefore, due to both the association with IL-6 and the ability to promote chronic inflammation, HP was recognized to cause a systemic inflammatory reaction.[14] Moreover, different gene polymorphisms of IL-6, tumor necrosis factor alpha (TNF-α), and angiotensin-converting enzyme were also associated with HP infection.[16]
C-reactive protein (CRP) is another important inflammatory marker, whose synthesis was regulated by IL-6 and which has also been associated with the presence of HP.[17,18] In addition to IL-6, HP also promotes the secretion of other proinflammatory cytokines, such as TNF-α, IL-1, and IL-8.[19] Therefore, the secretion of these cytokines clearly define a systemic inflammation, being also described in obesity, a condition that is well-known to cause a low grade of systemic inflammation.[20,21] As a response to this systemic inflammation, the leukocytes count will increase based on a relative increase in neutrophil count and a decrease of lymphocyte one. Thus, neutrophil to lymphocyte ratio (NLR) is a simple, noninvasive, inexpensive inflammatory marker that can be obtained from a total complete blood cellular (CBC) count and it was associated with multiple diseases, such as acute coronary syndromes, sepsis, and malignant disorders.[14,22–25] Moreover, NLR was also proved to be related to the mortality rates and the prognosis of the disease.[14] Invasive methods for the detection of HP infection are the most reliable, but upper digestive endoscopy it is even more difficult to be performed in children and a good communication with children and their parents is essential for a good outcome.[26] Therefore, a noninvasive inflammatory marker, such as NLR would be really useful, especially in pediatric ages.
The aim of our study was to establish correlations between the presence of HP proved histologically and NLR in children.
2. Materials and methods
2.1. Study sample
A prospective, case–control study was performed on 187 pediatric patients aged between 1 and 18 years, admitted in a Pediatric Tertiary Hospital from Romania, between April 2016 and January 2018. Parents of only 153 children agreed to their children participating in our study and among them, only 137 children remained after a selection according to sex and age, to comply with the pairing method. According to the histologic examination, the children were divided into 2 groups: group 1 comprised 50 children with HP infection, and group 2 included 87 children without any pathologic findings. The inclusion criteria consisted in gastrointestinal complaints, weight above 12 kg (due to the video endoscope characteristics); while the exclusion ones were: weight under 12 kg, clinical and paraclinical signs of infectious conditions, other histologic types of gastritis, and the refusal to sign the informed consent. All patients underwent clinical examination, laboratory tests (CBC count, CRP, erythrocyte sedimentation rate [ESR], iron, transaminases, IgA antibodies anti-HP), upper digestive endoscopy with at least 2 biopsies (antrum and corpus), rapid urease test, and histologic examination with Giemsa staining for HP detection. The NLR was obtained by dividing neutrophil count to lymphocyte count. All upper digestive endoscopies were performed by a single trained person, as well as the assessment of the histologic examinations. The laboratory parameters were assessed using a Cobas Integra 400 plus automated analyser.
2.2. Ethics
All mothers signed the informed consent for their children. Our study was approved by the Ethics Committee of the University of Medicine and Pharmacy of Târgu Mures (No 27/March 17, 2016), and it was accepted according to the principles of the Helsinki declaration.
2.3. Statistical analysis
The characteristics of children were presented as mean ± standard deviation and median. Continuous variables were defined as mean ± standard deviation, and categorical variables were given as percentages. D’Agostino and Pearson normality test was used to determine variables distribution. Both Student t test and Mann–Whitney test were used for mean and median comparison and unpaired data. The significance threshold was settled at a P-value of <.05. All the statistical analyses were conducted using GraphPad Prism.
3. Results
Among the 137 patients included in our study, the mean age for the study group was 12.86 ± 3.796 years, whereas for control group, it was 12.10 ± 3.879 years (P = .3001) (Fig. 1). Regarding gender distribution, we did not find a significant difference regarding the distribution of girls and boys in the 2 groups (P = .9005). Therefore, we can say that the groups were similar regarding age and gender distribution. HP infection was significantly more frequent among children from rural area than those from the urban one (P = .0089). The most frequent complaints encountered in our study were: diffuse abdominal pain, epigastric pain, nausea, loss of appetite, heart burn, and vomiting. Among these symptoms, only epigastric pain and loss of appetite were significantly associated with HP infection (P = .0350 and P = .0281).
Figure 1.
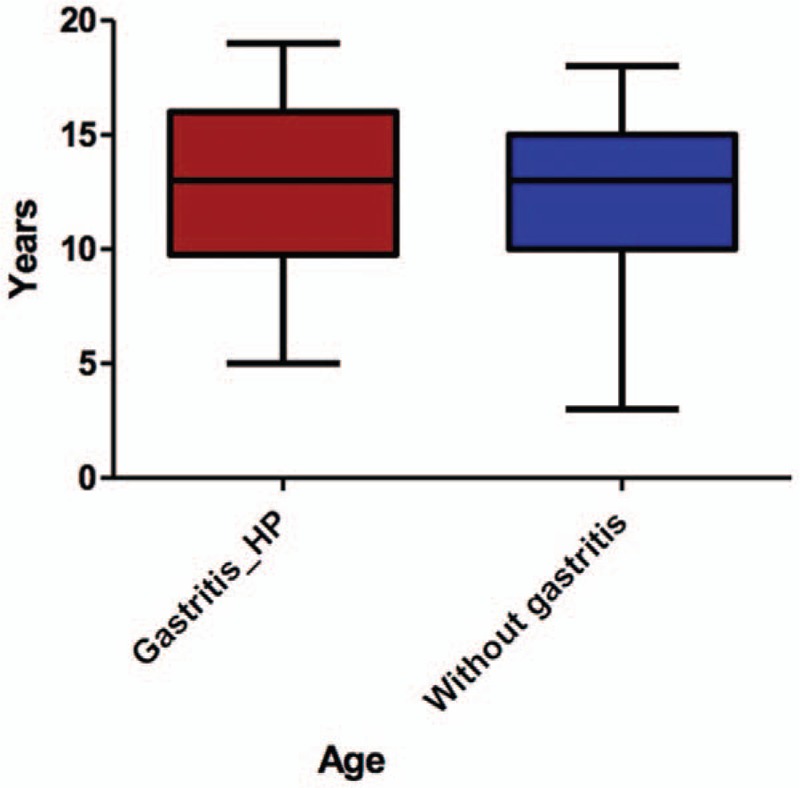
Age distribution.
All the laboratory data assessed in our study are represented in Table 1. After comparing these data between the 2 groups, we noticed that the leukocyte (Fig. 2) and neutrophil (Fig. 3) counts were significantly higher in children with HP gastritis (P = .0076, and P = .0306) as compared to control group. Nevertheless, we did not encounter significant statistical differences between the 2 groups in terms of lymphocytes, ESR, and NLR (P = .4733/P = .1593/.2147), or other assessed laboratory parameters. Regarding the IgA antibodies anti-HP and rapid urease test, they were both significantly associated with histologically confirmed HP infection (P < .0001).
Table 1.
The analysis of the laboratory parameters.
Figure 2.
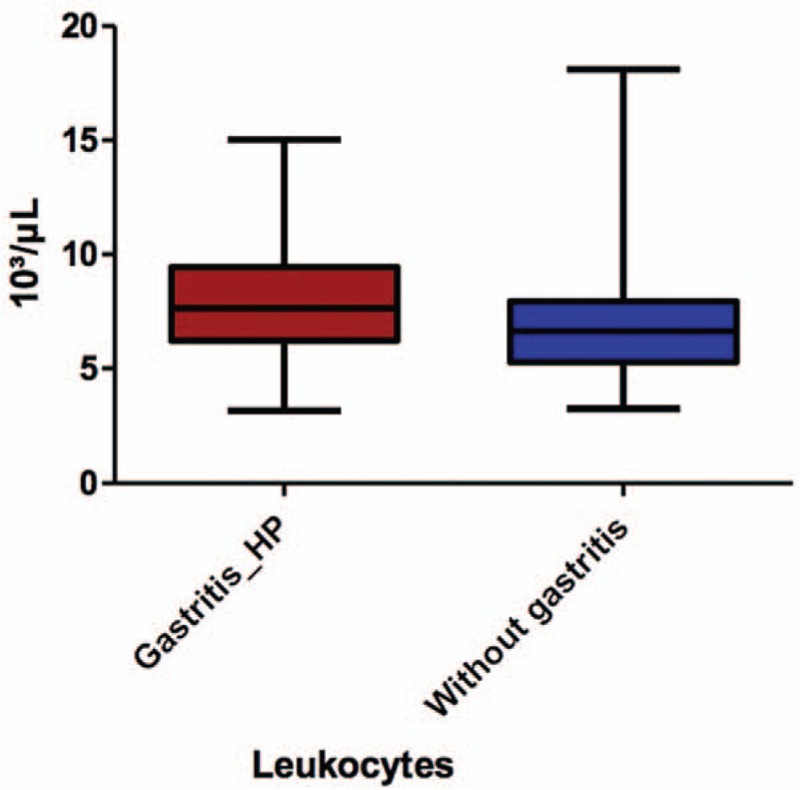
The comparison of leukocytes count between the 2 groups.
Figure 3.

The comparison of neutrophil count between the 2 groups.
4. Discussion
Despite the fact that theoretically HP infection is localized within the gastric mucosa, due to its chemotactic abilities, it leads to a systemic inflammatory reaction. HP is the most common pathogen worldwide, and its frequency is more common in areas with a low socioeconomic status.[27] Similarly, in our study, we found a significantly higher incidence in children from rural areas as compared to those from the urban ones. Histopathologic examination is the most reliable diagnostic tool for this infection, but unfortunately, it requires upper digestive endoscopy, an invasive method that is particularly hard to be performed in children. Therefore, the identification of a noninvasive method to detect this inflammation and along with other noninvasive diagnostic tools to establish the diagnosis of HP induced gastritis would be of real help especially in pediatrics area. Moreover, due to this HP-related systemic inflammation, studies have proved that this bacteria is associated with other conditions such stroke, cardiovascular diseases, glaucoma, anemia, rosacea, eczema, chronic hives, Alzheimer disease, idiopathic thrombocytopenic purpura, diabetes, and thyroid disease.[28] Also, other studies showed that increased HP antibodies were significantly associated with coronary artery disease,[29] arterial stiffness, and high systolic blood pressure in diabetic subjects.[30] Recent studies focused on assessing the markers of systemic inflammatory status that are associated with HP infection to delineate between the individuals that require treatment and those who are asymptomatic carriers. Thus, it was proved that acute phase reactants are significantly increased in HP-positive patients.[31] Moreover, another study showed that leukocytes and NLR represent reliable indicators of systemic inflammation.[32] Its value was also proved in case of obesity,[33] which is known to have a multifactorial determinism[34–37] and to be associated with a systemic inflammatory response. A recent study performed on 50 adults with HP gastritis and 50 with gastritis without HP infection, underlined a significant increase in leukocytes, neutrophils, and lymphocytes count in HP-positive patients in comparison to the negative ones.[38] The same authors stated showed also a positive association between both the neutrophils count, NLR and the severity of the symptoms.[38] These findings were supported also by the study of Atayan and Hacisalihoglu.[39] Similarly to the previously mentioned findings, in our study leukocyte and neutrophil counts were significantly higher in children with HP-induced gastritis, but we failed to prove the same association for lymphocytes and NLR. Nevertheless, we found higher mean NLR values in HP positive patients, but without statistical significance. A more recent study, also involving adults, found no difference in terms of NLR, neutrophils or leukocytes in HP positive patients, but in exchange, it proved a significant increase of lymphocytes to be associated with the severity of this infection.[14] In our, study the number of lymphocytes did not differ significantly between the 2 groups. This contradictory result may be explained by the fact that we enrolled children in our study and this infection becomes more severe as the individual ages, being well-known that if left untreated it may persist, leading to chronic inflammation and increasing the risk of carcinogenesis.[2–4,6] Thus, in individuals at risk for developing HP-related gastric cancer, screening programs would be major importance to prevent further complications.[40,41] A more complex study, performed on 3 groups of patients, HP-positive gastritis, HP-negative gastritis, and patients with no pathologic findings and the histopathologic examination, proved that both mean neutrophil count and NLR were higher in HP-positive patients.[42] In addition, a more recent study underlined that the combination between NLR and platelet to lymphocyte ratio might an even better predictor of HP infection and its related gastrointestinal complications than NLR alone.[43]
The limitations of this study consist in the small number of cases, the inclusion of children from a single area of Romania, and the fact that we did not assess the laboratory parameters after the eradication therapy. On the contrary, the strengths of this study comprise the fact that all the upper digestive endoscopies were performed by a single trained person as well as the histopathologic interpretations. Moreover, to the best of our knowledge, this is the 1st study that assessed the role of NLR in children's HP gastritis.
5. Conclusion
Even though, we did not identify significant differences in term of NLR between HP-induced gastritis children and healthy controls, the mean NLR values were higher HP-positive patients. On the contrary, we identified significant increase in leukocytes and neutrophils counts in children with HP gastritis. Therefore, the assessment of peripheral blood cell counts and NLR might represent useful tools in children with HP-induced gastritis not only at the time of initial diagnosis, but also for the follow-up of proper eradication. Moreover, in children, it is of special interest because a proper eradication of HP infection in small ages might prevent the future development of gastric cancers.
Author contributions
Dr Melit Lorena Elena, Dr Mărginean Cristina Oana, and Dr Mărginean Maria Oana conceptualized and designed the study, drafted the initial manuscript, and revised the manuscript.
Dr Mărginean Maria Oana and Mărginean Cristian Oana designed the data collection instruments, collected data, carried out the initial analyses, and revised the manuscript.
Dr Simona Mocanu performed all the histopathologic examination of the gastric samples.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conceptualization: Lorena Elena Melit, Cristina Oana Mărginean.
Investigation: Lorena Elena Melit, Maria Oana Mărginean, Simona Mocan, Cristina Oana Mărginean.
Methodology: Cristina Oana Mărginean.
Supervision: Lorena Elena Melit.
Validation: Cristina Oana Mărginean.
Writing – original draft: Lorena Elena Melit, Maria Oana Mărginean, Cristina Oana Mărginean.
Writing – review & editing: Lorena Elena Melit, Maria Oana Mărginean, Cristina Oana Mărginean.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, CBC = complete blood cellular, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, GGT = gamma glutamil transferase, HP = Helicobacter pylori, NLR = neutrophil/lymphocyte ratio, TNF-α = tumor necrosis factor alpha.
This research was partially supported by the Internal Scientific Research Grants of the University of Medicine, Pharmacy, Sciences and Technology Târgu Mures, Romania (“The role of genomic and inflammatory markers in determinism of child's gastritis” no. 615/11/17.01.2019).
The authors have no conflicts of interest to disclose.
References
- [1].Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002;347:1175–86. [DOI] [PubMed] [Google Scholar]
- [2].Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer 2010;10:403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McColl KEL. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010;362:1597–604. [DOI] [PubMed] [Google Scholar]
- [4].Salama NR, Hartung ML, Müller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol 2013;11:385–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Melit LE, Mărginean CO, Mărginean CD, et al. The relationship between toll-like receptors and Helicobacter pylori-related gastropathies: still a controversial topic. J Immunol Res 2019, doi:10.1155/2019/8197048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sijun H, Yong X. Helicobacter pylori vaccine: mucosal adjuvant & delivery systems. Indian J Med Res 2009;130:115–24. [PubMed] [Google Scholar]
- [7].Meliţ LE, Mărginean CO, Mocanu S, et al. A rare case of iron-pill induced gastritis in a female teenager: a case report and a review of the literature. Medicine (Baltimore) 2017;96:e7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mărginean CO, Meliţ LE, Moldovan H, et al. Lead poisoning in a 16-year-old girl: a case report and a review of the literature (CARE compliant). Medicine (Baltimore) 2016;95:e4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Amieva M, Peek RM. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology 2016;150:64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- [11].Bodger K, Wyatt JI, Heatley RV. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut 1997;40:739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mărginean CO, Cotoi OS, Pitea AM, et al. Assessment of the relationship between Helicobacter pylori infection, endoscopic appearance and histological changes of the gastric mucosa in children with gastritis (a single center experience). Rom J Morphol Embryol 2013;54:709–15. [PubMed] [Google Scholar]
- [13].Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- [14].Guclu M, Faruq Agan A. Association of severity of Helicobacter pylori infection with peripheral blood neutrophil to lymphocyte ratio and mean platelet volume. Euroasian J Hepatogastroenterol 2017;7:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nakagawa H, Tamura T, Mitsuda Y, et al. Significant association between serum interleukin-6 and Helicobacter pylori antibody levels among H. pylori-positive Japanese adults. Mediators Inflamm 2013;2013:142358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mărginean MO, Mărginean CO, Meliţ LE, et al. The impact of host's genetic susceptibility on Helicobacter pylori infection in children. Medicine (Baltimore) 2017;96:e7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jafarzadeh A, Hassanshahi GH, Nemati M. Serum levels of high-sensitivity C-reactive protein (hs-CRP)in Helicobacter pylori-infected peptic ulcer patients and its association with bacterial CagA virulence factor. Dig Dis Sci 2009;54:2612–6. [DOI] [PubMed] [Google Scholar]
- [18].Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990;265:621–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mehmet N, Refik M, Harputluoglu M, et al. Serum and gastric fluid levels of cytokines and nitrates in gastric diseases infected with Helicobacter pylori. New Microbiol 2004;27:139–48. [PubMed] [Google Scholar]
- [20].Mărginean CO, Bănescu C, Duicu C, et al. Angiotensin-converting enzyme gene insertion/deletion polymorphism in nutritional disorders in children. Eur J Nutr 2015;54:1245–54. [DOI] [PubMed] [Google Scholar]
- [21].Mărginean C, Bănescu C, Duicu C, et al. The role of IL-6 572 C/G, 190 C/T, and 174 G/C gene polymorphisms in children's obesity. Eur J Pediatr 2014;173:1285–96. [DOI] [PubMed] [Google Scholar]
- [22].Tamhane UU, Aneja S, Montgomery D, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol 2008;102:653–7. [DOI] [PubMed] [Google Scholar]
- [23].Kemal Y, Yucel I, Ekiz K, et al. Elevated serum neutrophil to lymphocyte and platelet to lymphocyte ratios could be useful in lung cancer diagnosis. Asian Pac J Cancer Prev 2014;15:2651–4. [DOI] [PubMed] [Google Scholar]
- [24].Feng J-F, Huang Y, Chen Q-X. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 2014;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Melit LE, Mărginean CO, Georgescu A, et al. Complications of sepsis in infant. A case report. J Crit Care Med (Targu Mures) 2016;2:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mărginean CO, Meliţ LE, Chinceşan M, et al. Communication skills in pediatrics - the relationship between pediatrician and child. Medicine (Baltimore) 2017;96:e8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cave DR. Transmission and epidemiology of Helicobacter pylori. Am J Med 1996;100:12S–7S. [DOI] [PubMed] [Google Scholar]
- [28].Szlachcic A. The link between Helicobacter pylori infection and rosacea. J Eur Acad Dermatol Venereol 2002;16:328–33. [DOI] [PubMed] [Google Scholar]
- [29].Jha HC, Prasad J, Mittal A. High immunoglobulin A seropositivity for combined Chlamydia pneumoniae, Helicobacter pylori infection, and high-sensitivity C-reactive protein in coronary artery disease patients in India can serve as atherosclerotic marker. Heart Vessels 2008;23:390–6. [DOI] [PubMed] [Google Scholar]
- [30].Ohnishi M, Fukui M, Ishikawa T, et al. Helicobacter pylori infection and arterial stiffness in patients with type 2 diabetes mellitus. Metab Clin Exp 2008;57:1760–4. [DOI] [PubMed] [Google Scholar]
- [31].Jackson L, Britton J, Lewis SA, et al. A population-based epidemiologic study of Helicobacter pylori infection and its association with systemic inflammation. Helicobacter 2009;14:108–13. [DOI] [PubMed] [Google Scholar]
- [32].Papa A, Emdin M, Passino C, et al. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta 2008;395:27–31. [DOI] [PubMed] [Google Scholar]
- [33].Furuncuoğlu Y, Tulgar S, Dogan AN, et al. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci 2016;20:1300–6. [PubMed] [Google Scholar]
- [34].Mărginean C, Mărginean CO, Bănescu C, et al. Impact of demographic, genetic, and bioimpedance factors on gestational weight gain and birth weight in a Romanian population: A cross-sectional study in mothers and their newborns: the Monebo study (STROBE-compliant article). Medicine (Baltimore) 2016;95:e4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mărginean C, Mărginean CO, Iancu M, et al. The role of TGF-β1 869 T>C and PPAR γ2 34 C>G polymorphisms, fat mass, and anthropometric characteristics in predicting childhood obesity at birth: A cross-sectional study according the parental characteristics and newborn's risk for child obesity (the newborns obesity's risk) NOR study. Medicine (Baltimore) 2016;95:e4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mărginean C, Mărginean CO, Iancu M, et al. The FTO rs9939609 and LEPR rs1137101 mothers-newborns gene polymorphisms and maternal fat mass index effects on anthropometric characteristics in newborns: a cross-sectional study on mothers-newborns gene polymorphisms-The FTO-LEPR Study (STROBE-compliant article). Medicine (Baltimore) 2016;95:e5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mărginean CO, Mărginean C, Voidăzan S, et al. Correlations between leptin gene polymorphisms 223 A/G, G/A, 492 G/C, 976 C/A, and anthropometrical and biochemical parameters in children with obesity: a prospective case-control study in a Romanian population-the Nutrichild study. Medicine (Baltimore) 2016;95:e3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Farah R, Khamisy-Farah R. Association of neutrophil to lymphocyte ratio with presence and severity of gastritis due to Helicobacter pylori infection. J Clin Lab Anal 2014;28:219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Atayan Y, Hacisalihoglu P. The correlation between tissue Helicobacter pylori severity and the increase in serum neutrophil/lymphocyte ratio in patients with active chronic gastritis. Biomed Res 2018;28: Available at: http://www.alliedacademies.org/abstract/the-correlation-between-tissue-helicobacter-pylori-severity-and-the-increase-in-serum-neutrophillymphocyte-ratio-in-patients-with--7577.html. Accessed December 9, 2018. [Google Scholar]
- [40].Groselj U, Tansek MZ, Smon A, et al. Newborn screening in southeastern Europe. Mol Genet Metab 2014;113:42–5. [DOI] [PubMed] [Google Scholar]
- [41].Zerjav Tansek M, Groselj U, Angelkova N, et al. Phenylketonuria screening and management in southeastern Europe - survey results from 11 countries. Orphanet J Rare Dis 2015;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zeren S, Bayhan Z, Kocak E, et al. Diagnostic value of platelet/lymphocyte ratio and neutrophil/lymphocyte ratio in investigations for Helicobacter pylori gastritis. Int J Clin Exp Med 2016;9:5102–6. [Google Scholar]
- [43].Kaplan M, Ates I, Yuksel M, et al. The role of the PLR-NLR combination in the prediction of the presence of Helicobacter pylori and its associated complications. Saudi J Gastroenterol 2018;24:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]


