Abstract
Background
Several observational studies have suggested a protective effect of oral bisphosphonates (BP) on the risk of breast cancer, but no such association has been seen in randomized control trials. The role of oral BP in breast cancer prevention remains unclear.
Aim
To investigate the association between different levels of BP exposure and breast cancer incidence in a cohort of osteoporotic post-menopausal women.
Subjects and methods
This historical prospective study was conducted using the computerized databases of Maccabi Healthcare Services (MHS) in Israel. Included in the study were osteopenic and osteoporotic women aged 55–75 years who started BP therapy between 1998 and 2012. The subjects were enrolled in MHS for at least 3 years before therapy initiation, and had a minimum follow-up of 5 years in MHS. Women with a previous cancer, and women treated with selective estrogen receptor modulators (SERMs) were excluded. BP exposure was expressed in quintiles of proportion of days covered (PDC) with BP during follow-up period and cancer incidence was ascertained by the Israel National Tumor Registry. Person-years of follow-up began on January 1st, 1998 and ended at the date of cancer diagnosis, death, or December 31st, 2012, whichever occurred first.
Results
A total of 11,717 patients (mean age = 66.87 ± 4.38) were eligible for the analysis. During a total of 130,252 person-years of follow-up, (mean 7.2 years) 173 incident cases of breast cancer were diagnosed. Compared to women with a PDC with BP of 20% or lower, the adjusted hazard ratio for breast cancer were HR = 0.81 (95%CI: 0.48–1.39), HR = 0.82 (95%CI: 0.50–1.33), HR = 0.72 (95%CI:0.45–1.15) and HR = 1.14 (95%CI:0.76–1.70) among women with 20–40%, 40–60%, 60%–80%, and 80% or higher, PDC, respectively.
Conclusion
In this study, we did not find a significant association between oral BP therapy for osteoporosis and the risk of breast cancer in postmenopausal women. The discrepancy between our results and the reports of such an association in observational studies might originate from an indication bias.
Keywords: Bisphosphonates, Breast cancer, Osteoporosis
1. Introduction
Bisphosphonates (BP) may affect cell function and survival and reduce tumor cell viability via inhibition of the mevalonate pathway [1], [2], [3], [4], [5]. Furthermore, it is now apparent that nitrogen-containing bisphosphonates have immunomodulatory properties as they can activate gamma delta lymphocytes. These non-conventional T cells exhibit characteristics of natural killer cells and cytotoxic T cells and are thought to play an essential role in tumor surveillance [6], [7], [8], [9]. BP comprise a well-established treatment for bone metastases and hypercalcemia of malignancy but, their potential role in reducing the risk of breast cancer has also become the focus of investigation in recent years.
Several population-based studies examined whether long-term use of oral bisphosphonates in women with postmenopausal osteoporosis may be associated with a reduced risk of breast cancer [10], [11], [12]. Newcomb and colleagues have suggested that increasing duration of use of BP was linked to a greater reduction in breast cancer risk [10]. This finding came from a case-control analysis of more than 6000 women in Wisconsin, half of whom were diagnosed with invasive breast cancer. The use of BP was associated with a 30% reduction in the risk for breast cancer, which accorded the findings in two other studies. One study by Cheblowsky et al. included more than 150,000 postmenopausal women participating in the Women's Health Initiative study and showed that BP use reduced the risk for invasive breast cancer by 32%, and an increased incidence of in situ breast cancer (DICS) [11].
The other study by Rennert et al. demonstrated a 28% reduction in the risk of postmenopausal breast cancer among BP users in a cohort of 4039 postmenopausal women in Israel [12].
Although a large cohort study from Denmark confirmed the finding of a reduced risk for breast cancer in patients treated with BP for osteoporosis, this report suggested that most of the effect might be attributed to low cumulative exposure to estrogen and not to a direct antitumor effect of BP [13]. Liu et al. summarized these results in a meta-analysis which showed an overall 32% risk reduction for breast cancer (pooled RR 0.68 95% CI 0.59, 0.80) in BP users compared to nonusers. They further concluded that a significant protective effect of BP was observed in patients who used BP for more than one year before the diagnosis of breast cancer [14]. More recently, Cardwell et al. reported a lower risk of breast (HR = 0.71, 95% CI 0.62, 0.81) and colon cancer (HR = 0.74 95% CI 0.60, 0.91) in a cohort of 41,826 BP users compared to a control cohort in the United Kingdom. Importantly, these investigators also noted that the potential confounding effect of low bone density per se on this finding could not be defined [15] (Table 1).
Table 1.
Observational studies that found a significant association between BP exposure and decreased risk of breast cancer.
 |
Low BMD has been associated with a lower incidence of breast cancer [16], [17] and the associations reported in observational studies might result from confounding. The aim of this study was to further investigate the association between BP exposure and breast cancer incidence. In order to try and overcome the possibility of confounding by indication, we restricted the study population to a cohort of osteopenic and osteoporotic post-menopausal women.
2. Settings, study design and study population
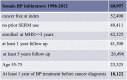
A historical prospective study was conducted using the computerized databases of Maccabi Healthcare Services (MHS). Person-years of follow-up began on January 1st, 1998 and stopped at the date of cancer diagnosis, death, or December 31st, 2011, whichever occurred first. Members of the cohort who left MHS were included in the follow-up to the last day of membership. Women aged 55–75 years who started BP therapy between 1998 and 2012 were included. Women enrolled in MHS 3 years before index and with at least five years of follow-up were included. Previous cancer was an exclusion criterion as was the previous use of SERM. Subjects diagnosed with breast cancer within one year from index were also excluded (Table 2).
Table 2.
Attrition table.
 |
Cancer incidence was ascertained by the Israel National Cancer Registry (INCR). The completeness of the National Cancer Registry's database for solid tumors is about 95% [18]. The use of BP was determined from the MHS pharmacy records. It was measured by calculating the PDC (Proportion of Days covered=monthly packs × 100%/months of follow-up). BP exposure was expressed in quintiles of the proportion of days covered with BP during follow-up period (PDC) [19], [20]. We included prescriptions for the commonly prescribed oral BP for the treatment of osteoporosis in Israel: Alendronate, and Risedronate. Detailed prescription information enables us to evaluate dose–response relationship between duration of use and risk of breast cancer. Because of the very low copayment, it is unlikely that medications were purchased in private non-MHS pharmacies. The use of hormone replacement therapy was also recorded, including type and duration use by MHS pharmacy records.
2.1. Study variables
Socio-demographic characteristics: the personal characteristics that were considered as potential determinants of cancer included age at index date, sex, marital status (categorized into: married, single, divorced or widowed), place of residency at index date (categorized into: northern, southern or central Israel) and religion ancestry (classified into Jews and non-Jews). This was based on self-reported data obtained by MHS for marketing purposes. Socioeconomic level was determined according to the poverty index of the member's enumeration area. Socioeconomic status (SES) was defined by the 2008 national census [21] according to the poverty index of the member's enumeration area, ranging between 1 (lowest) and 20 (highest). Enrollees that are regularly paid an income support benefit by the National Insurance Institute, which is aimed to assist individuals and families who are not able to ensure themselves a basic minimum income for subsistence, were categorized in the lowest SES quintile.
Health status: body mass index (BMI), the smoking status, the diagnosis of diabetes and hypertension, the general physician number of visits, the number of gynecologist consultations, the number of mammograms and bone mineral density exams performed were identified based on outpatient diagnoses, BMD values were electronically available. BMI was divided into quintiles: underweight BMI < 20 kg/m2, normal 20–25, overweight 25–30, obese (class I) > 30 and obese (class II-III) > 35 [22].
Smoking status was divided into three categories: never smoked, past smoker, or current smoker. BMD was measured by DXA at the lumbar spine, femoral neck and total hip using GE Lunar Prodigy systems). The BMD results were expressed as T-scores; a standardized score, comparing BMD to average values for young healthy women. The results were divided into four categories according to T-scores reflecting the severity of the osteoporosis according to the WHO definition [23], [24]. Normal reference until T-score −1.0, osteopenia from −1.0 to −2.5, osteoporosis from −2.5 and severe osteoporosis below −3.0. BMD was measures at the lumbar spine, femoral neck ant proximal femur and whichever was lower was retained. GP visits were divided into quartiles according to the frequency of the visits ranging from less than seven visits, between 7–9, between 10 and 15 and more than 15 visits annually. We reported the percentage of subjects who visited an obstetric gynecologist and the percentage of subjects who had a mammogram performed before the index date. Subjects were defined as hypertensive or diabetic according to the database based on diabetes and hypertension registries of the Maccabi health services [25], [26], [27].
2.2. Data management and quality control
Good quality data was essential for this investigation. Although MHS electronic data systems are used for current clinical practice, we examined the quality of the obtained data using a combination of logical checks. To ensure the validity of the automated linkages among different data sources, a sample of the data was manually checked.
We used data from the osteoporosis registry of Maccabi Healthcare Services (MHS), the second largest sick fund in Israel, ensuring 25% of the population with a nationwide and representative distribution. MHS databases are derived from electronic medical records of a stable population of over 2 million ensured members.
2.3. Sample size calculation and statistical analysis
Sample size calculations were performed using Winpepi statistical program version 11.4. We took into account that the incidence of breast cancer could be slightly lower than expected in an osteopenic–osteoporotic population. We used Cox's proportional hazards model to build the multivariable survival model. The multivariate model was based on a stepwise forward selection of the data (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY). To examine the effect of minimal exposure period on study outcomes, we performed sensitivity analyses of our survival models using several minimal exposure periods such as one year, three years, and five years. The proportional hazard assumption was tested based on Schoenfeld residuals regressed on follow-up time, and it was met by all covariates (p > 0.1).
3. Results
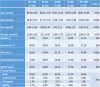
A total of 18,122 eligible MHS members were identified, out of which 11,717 remained for analysis due to missing data on BMI and HRT use mainly. The total follow-up period was of 130,252 person-years, the mean follow-up was 7.2 years and 173 cases of breast cancer were diagnosed. The mean age was 66.87 ± 4.38 in the highest PDC quintile and was similar to the mean age in the other quintiles. The mean BMI was 27.08 ± 4.91 in the highest quintile of exposure and was slightly lower than in the other groups. Smoking, diabetes, and hypertension were slightly more prevalent in the lowest quintile of exposure. The women with the higher exposure performed more mammograms. Ninety percent of the subjects received Alendronate. The baseline characteristics are shown in Table 3 below.
Table 3.
Baseline characteristics of the study population.
 |
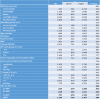
Compared to women with a PDC with BP of 20% or lower, the hazard ratio for breast cancer were HR = 0.95 (95%CI:0.55–1.62), HR = 0.74 (95%CI:0.43–1.25), HR = 0.82 (95%CI:0.50–1.32) and HR = 1.32 (95%CI:0.86–2.02) among women with 20–40%, 40–60%, 60%−80%, and 80% or higher respectively. Compared to women with a PDC with BP of 20% or lower, the adjusted hazard ratio for breast cancer were HR = 0.81 (95%CI: 0.48–1.39), HR = 0.82 (95%CI: 0.50–1.33), HR = 0.72 (95%CI:0.45–1.15) and HR = 1.14 (95%CI:0.76–1.70) among women with 20–40%, 40–60%, 60%−80%, and 80% or higher, PDC, respectively. The hazard ratio was adjusted for age, BMI, SES, smoking status, HRT use, mammograms, physician visits, and T-score (Table 4).
Table 4.
Adjusted hazard ratio for breast cancer according to adherence with bisphosphonates and baseline characteristics (n = 11,717).
 |
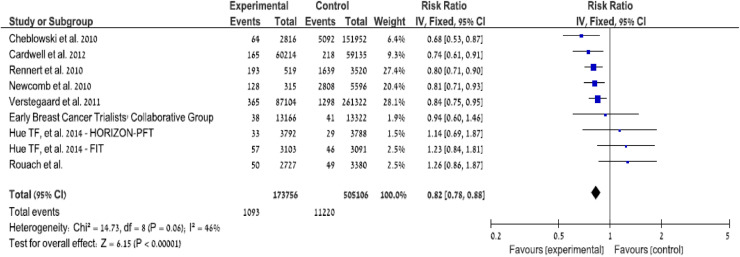
We found a non-significant increase in the risk of breast cancer in the highest quintile of adherence to BP. These highly adherent women seem to have a different "health behavior", with more mammograms performed (p < 0.001), a possible example of the health adherer phenomenon. We were not able to identify special risk factors in this subset of subjects. We looked into the data and found slightly more in situ breast cancers diagnoses. We further divided the cohort into deciles per PDC, and what seemed at first as a U shape relationship was not convincing. We observed very small differences between the deciles with overlapping confidence intervals (Supplement, Fig. 1).
Fig. 1.
Summary of the results of observational and randomized trials on the association between BP and breast cancer incidence.
4. Discussion
In this large cohort of post-menopausal osteoporotic women, we did not find any significant negative association between oral BP adherence and breast cancer incidence. Our study has limitations, yet it raises the possibility that the protective effect of BP treatment on the risk of breast cancer reported by previous studies [10], [11], [12], [13], [14], [15], may have resulted from a confounder effect, i.e., confounding by indication.
Most of the observational studies which reported a significant reduction in the risk of breast cancer used a control population of non-osteoporotic women, raising the possibility that the effect ascribed to BP use might, in fact, reflect a potential low cumulative exposure to estrogen and not a direct antitumor effect of BP.
Indeed, low estrogen exposure (late menarche, oophorectomy, and early menopause) is one of the main risk factors for osteoporosis. On the other hand, high estrogen exposure is one of the main risk factors for breast cancer. Keeping these associations in mind, the comparison between BP treated osteoporotic women with healthy non-osteoporotic non-BP treated women, may not provide the proper opportunity to truly assess the association between BP and breast cancer incidence.
Cheblowsky et al. considered this issue and reported total hip BMD measurements in a sample of 10,693 subjects. Total Hip BMD was significantly lower in BP users. In order to try and overcome this potential confounding by indication they used the 5-year hip fracture score in the multivariate model to adjust for potential BMD difference between bisphosphonate users and nonusers. A strong significant association was established between a non-BMD containing, calculated 5-year hip fracture risk estimate and both BMD as well as breast cancer incidence, which supports the use of the 5-year hip fracture score in the multivariate model [11].
In order to further address the possibility of confounding, Hue et al. [28] examined the results of two randomized clinical trials designed to assess the efficacy of BP for the prevention of osteoporotic fractures (the Fracture Intervention Trial FIT [29] and the Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis HORIZON pivotal trial [30]. Data were collected at clinical centers in the United States (FIT and HORIZON-PFT), in Asia and the Pacific, Europe, North America and South America (HORIZON-PFT). Women, in either study, with recurrent breast cancer or who reported a history of breast cancer were excluded from analyses. In each trial, a blinded review was conducted of each cancer adverse event report to verify incident invasive breast cancer cases. There was no significant difference in the rate of breast cancer in FIT: 1.5% (n = 46) in the placebo group and 1.8% (n = 57) in the alendronate group (hazard ratio [HR], 1.24 [95% CI, 0.84–1.83]). In HORIZON-PFT, there was also no significant difference: 0.8% (n = 29) in the placebo group and 0.9% (n = 33) in the zoledronic acid group (HR, 1.15 [95% CI, 0.70–1.89]). There was also no significant difference when the data from FIT and HORIZON-PFT were pooled (HR, 1.20 [95% CI, 0.89–1.63]). These two randomized clinical trials did not support the findings from observational research: contrary to the results from observational studies, 3 to 4 years of BP treatment did not decrease the risk of invasive postmenopausal breast cancer.
To the best of our knowledge, our study is the only one which expressed the exposure to BP as PDC and not in years of treatment. PDC is considered a more accurate way to assess adherence [18], but this might be a limitation regarding BP which display a unique pharmacology. BP is taken up preferentially by the skeleton and decrease osteoclast-mediated bone resorption. There are differences in the affinities of different BP for bone as well as in their anti-resorptive potency. The capacity of the skeleton to retain BP is large, and there is no indication for saturation of binding sites with the doses used in osteoporosis [31]. Because BP accumulate in the bone, it is very challenging to evaluate the actual effect of different lengths of treatment or different levels of adherence.
Bisphosphonates have profound effects on bone physiology, and could modify the process of metastasis and breast cancer outcome. The Early Breast Cancer Trialists' Collaborative Group (EBCTCG) has concluded that adjuvant bisphosphonates reduce the rate of breast cancer recurrence in the bone and improve breast cancer survival in post-menopausal women. The subjects in the studies included in this meta-analysis were breast cancer patients. The focus of this meta-analysis was not on the incidence of breast cancer, but one should notice that no significant effect was seen on the incidence of local recurrence as first event (RR 1•10, 0•94–1•28; 2p = 0•25) or of contralateral breast cancer as first event (RR 0•96, 0•74–1•25; 2p = 0•79) [32].
The potential role of BP in the prevention of cancer remains unclear.
4.1. Strengths and limitations
The main strength of our study is the restriction of the study population to osteopenic and osteoporotic women, all BP users, with different levels of BP exposure. All the subjects were from the same source population and shared the same period of follow up. This allowed us to exclude the possibility of confounding by indication, a concern which was raised in previous published observational studies.
Moreover, our study has several major strengths: a large population, a relatively long retrospective follow-up, an automated collection of comprehensive data, including medical diagnosis, prescribed therapies, and standardized densitometry measurements. The completeness of the National Cancer Registry database for solid tumors is about 95% [18], and the database of the Maccabi Health Services represents a large proportion of the general population in Israel. The use of such qualitative data sources reduces the potential for selection and information biases.
Nonetheless, several limitations and potential methodological issues should be discussed. (A) Power; the assumed risk of breast cancer was based on the incidence rate of breast cancer in MHS in 2003 among members aged 55–74 in Israel and according to the recently published studies on the subject. The minimal sample size to detect a hazard ratio of at least 0.7 at a significance level of 5% and a power of 80% was 2102 subjects in each group. We took into account that breast cancer incidence may be lower than expected in a cohort of osteopenic–osteoporoic women. We did not find any significant association between low and high adherence, we then divided the cohort into 5 different levels of adherence to look for a possible dose response, but this multiple comparisons require a larger sample and this is a limitation.
(B) Adherence with BPs: the evaluation of adherence to treatment was based on dispensing data, which is the most feasible method of estimating medication use in large populations, but does not ensure that the drug is actually consumed. We might have failed to identify partial adherence, and this may lead to some information bias. (C) Surveillance bias: persistent BP users are frequently under more tight medical surveillance and thus are thus more likely to undergo tests for early detection of breast cancer. This could lead to overestimation of an association between persistence with BPs and cancer. To address this bias, stage and grade of the tumor at diagnosis, as well as physician visits and mammograms performed were included in the model.
(D) Confounders: based on known published findings several other variables may be associated with bisphosphonate use and breast cancer. These include family history of breast cancer, vegetable consumption, physical activity, alcohol consumption, age at firs pregnancy, and breastfeeding. We were not able to investigate these exposures as they are not routinely reported in the personal medical files, and they might exert some confounding effects. (E) Missing data: data on BMI and smoking status are missing in about 50% of the records. Missing data are ubiquitous in epidemiologic studies. Individuals with missing data may differ from those with no missing data regarding the outcome. Data imputation was done for missing values of BMI: missing BMI values were replaced by the median value of all non-missing BMI values in the study population. The analysis was repeated with imputed BMI values. Mean imputation is a simple method which preserves the mean of the observed data but does not fully account for the uncertainty of missing data.
5. Conclusions
Our data did not provide evidence that oral BP therapy for osteoporosis, at any level of adherence, reduces the risk of breast cancer in postmenopausal women. The discrepancy between our findings and the reports of associations in observational studies might result from an indication bias. Low bone mineral density (BMD) is both an indication for BP use and is associated with lower breast cancer incidence and the inability to control for BMD as a potential confounding might explain the different results observed. The use of BPs to prevent aromatase inhibitors bone loss and reduce the risk of fracture in postmenopausal women with ER-positive disease is well established. Whether or not oral BPs should be considered in a broader range of postmenopausal women for cancer prevention needs to be clarified.
Acknowledgments
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2018.10.001.
Appendix. Supplementary materials
References
- 1.Luckman S.P., Hughes D.E., Coxon F.P., Graham R., Russell G., Rogers M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J. Bone Miner. Res. 1998;13(4):581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 2.Russell R.G. Bisphosphonates: from bench to bedside. Ann N.Y. Acad. Sci. 2006;1068:367–401. doi: 10.1196/annals.1346.041. [DOI] [PubMed] [Google Scholar]
- 3.Dunford J.E., Thompson K., Coxon F.P. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 2001;296(2):235–242. [PubMed] [Google Scholar]
- 4.Kavanagh K.L., Guo K., Dunford J.E. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc. Natl. Acad. Sci. U S A. 2006;103(20):7829–7834. doi: 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russel R.G., Rogers M.J., Frith J.C. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J. Bone Miner. Res. 1999;14(suppl 2):53–65. doi: 10.1002/jbmr.5650140212. [DOI] [PubMed] [Google Scholar]
- 6.Caraglia M., Santini D., Marra M. Emerging anti-cancer molecular mechanisms of aminobisphosphonates. Endocr. Relat. Cancer. 2006;13:7–26. doi: 10.1677/erc.1.01094. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman S. Y/6 and other unconventional T lymphocytes: what do they see and what do they do? PNAS. 1996;93:2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson K., Roelofs A.J., Jauhiainen M. Activation of γδ T cells by bisphosphonates. Adv. Exp. Med. Biol. 2010;658:11–20. doi: 10.1007/978-1-4419-1050-9_2. [DOI] [PubMed] [Google Scholar]
- 9.Cezardin P. Bisphosphonattes antitumor activity: an unraveled side of a multifaceted drug class. Bone. 2011;48:71–79. doi: 10.1016/j.bone.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Newcomb P.A., Trentham-Dietz A, Hampton JM. Bisphosphonates for osteoporosis treatment associated with reduced breast cancer risk. Br. J. Cancer. 2010;102(5):799–802. doi: 10.1038/sj.bjc.6605555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chlebowski R.T., Chen Z., Cauley J.A. Oral bisphosphonate use and breast cancer incidence in postmenopausal women. J. Clin. Oncol. 2010;28(22):3582–3590. doi: 10.1200/JCO.2010.28.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rennert G., Pinchev M., Rennert H.S. Use of bisphosphonates and risk of postmenopausal breast cancer. J. Clin. Oncol. 2010;28(22):3577–3581. doi: 10.1200/JCO.2010.28.1113. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard P., Fischer L., Mele M., Mosekilde L., Christiansen P. Use of bisphosphonates and risk of breast cancer. Calcif. Tissue Int. 2011;88(4):255–262. doi: 10.1007/s00223-011-9463-7. [DOI] [PubMed] [Google Scholar]
- 14.Liu J., Huang W., Zhou R., Jia S., Tang W., Luo Y., Zhang J. Bisphosphonates in the treatment of patients with metastatic breast, lung, and prostate cancer: a meta-analysis. Med. (Baltimore) 2015;94(46):e2014. doi: 10.1097/MD.0000000000002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardwell C.R., Abnet C.C., Veal P., Hughes C.M., Cantwell M.M., Murray L.J. Exposure to oral bisphosphonates and risk of cancer. Int. J. Cancer. 2012;131(5):E717–E725. doi: 10.1002/ijc.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cauley J.A., Lucas F.L., Kuller L.H., Vogt M.T., Browner W.S., Cummings S.R. Bone mineral density and risk of breast cancer in older women. The study of osteoporotic fractures. JAMA. 1996;276(17):1404–1408. [PubMed] [Google Scholar]
- 17.Chen Z., Arendell L., Aickin M., Cauley J., Lewis C.E., Chlebowski R. Hip bone density predicts breast cancer risk independently of gail score-results from the women's health initiative. Cancer. 2008;113(5):907–915. doi: 10.1002/cncr.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Y. Fishler, A. Chetrit, M. Barchana, B. Modan, Estimation of Completeness of the Cancer Registry in Israel (in Hebrew). Israel Center for Disease Control, Ministry of Health 2003.
- 19.Peterson A.M., Peterson A.M., Nau D.P., Cramer J.A. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 20.Karve S., Cleves M.A., Helm M., Hudson T.J., West D.S., Martin B.C. Prospective validation of eight different adherence measures for use for use with administrative claims data among patients with schizoprenia. Value Health. 2009;12(6):989–995. doi: 10.1111/j.1524-4733.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 21.Israel Central Bureau of Statistics . Israel Central Bureau of Statistics; 2013. Characterization of Geographical Units by the Socio-Economic Level of the Population 2008.http://www.cbs.gov.il/webpub/pub/text_page_eng.html?publ=100&CYear=2008&CMonth=1 [Google Scholar]
- 22.BMI Classification . World Health Organization; 2006. Global Database on Body Mass Index. Archived from the original on April 18, 2009. Retrieved July 27, 2012. [Google Scholar]
- 23.Prevention and Management of Osteoporosis . World Health Organization; Geneva: 2003. Report of a WHO Scientific Group. WHO Technical Report Series, No. 921. [Google Scholar]
- 24.Kanis J.A. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 25.Chodick G., Heymann A.D., Shalev V., Kookia E. The epidemiology of diabetes in a large Israeli HMO. Eur. J. Epidemiol. 2003;18:1143–1146. doi: 10.1023/b:ejep.0000006635.36802.c8. [DOI] [PubMed] [Google Scholar]
- 26.Heymann A.D.1, Chodick G., Halkin H., Kokia E., Shalev V. Description of a diabetes disease register extracted from a central database. Harefuah. 2007;146(1):15–17. [PubMed] [Google Scholar]
- 27.Shalev V., Chodick G., Goren I., Silber H., Kokia E., Heymann A.D. The use of an automated patient registry to manage and monitor cardiovascular conditions and related outcomes in a large health organization. Int. J. Cardiol. 2011;152:345–349. doi: 10.1016/j.ijcard.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Hue T.F., Cummings S.R., Cauley J.A., Bauer D.C., Ensrud K.E., Barrett-Connor E., Black D.M. Effect of bisphosphonate use on risk of postmenopausal breast cancer: results from the randomized clinical trials of alendronate and zoledronic acid. JAMA Intern. Med. 2014;174(10):1550–1557. doi: 10.1001/jamainternmed.2014.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings S.R., Black D.M., Thompson D.E., Applegate W.B., Barrett-Connor E., Musliner T.A., Palermo L., Prineas R., Rubin S.M., Scott J.C., Vogt T., Wallace R., Yates A.J., LaCroix A.Z. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the fracture intervention trial. JAMA. 1998;280(24):2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 30.Black D.M., Delmas P.D., Eastell R., Reid I.R., Boonen S., Cauley J.A., Cosman F., Lakatos P., Leung P.C., Man Z., Mautalen C., Mesenbrink P., Hu H., Caminis J., Tong K., Rosario-Jansen T., Krasnow J., Hue T.F., Sellmeyer D., Eriksen E.F., Cummings S.R. HORIZON pivotal fracture trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 2007;356(18):1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 31.Papapoulos S. Bisphosphonates: how do they work? Best Pract. Res. Clin. Endocrinol. Metab. 2008;22(5):831–847. doi: 10.1016/j.beem.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361. doi: 10.1016/S0140-6736(15)60908-4. http://www.ctsu.ox.ac.uk/research/meta-trials/ebctcg/ebctcg-page Full list of members available at. [PubMed] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.