Abstract
This study tries to evaluate the associations between circulating C-reactive protein (CRP) and the overall survival of patients with non-small cell lung cancer (NSCLC).
One hundred ninety-two patients with advanced NSCLC who treated with chemotherapy were enrolled in this study. The cut-off value of CRP concentration was 5.0 mg/L. The patients were divided into low, intermediate and high 3 groups respectively according to the baseline level of CRP before the treatment. Kaplan–Meier analysis and Cox proportional-hazard models were used to evaluate the relationship between the CRP and overall survival time of patients.
After adjusting for age, gender, smoking history, pathologic type, CRP was a significant independent impact which predicts the survival prognosis of patients with NSCLC. For all patients, the hazard ratio with high CRP levels for NSCLC-specific survival was 1.83 [95%confidenceinterval (CI) = 0.96, 3.48] compared with low CRP levels. The level of CRP was significantly correlated with survival time (hazard ratio = 1.77; 95% CI = 0.73, 4.26) for the patient with first-line chemotherapy. Patients with high level of circulating CRP also responded poorly to chemotherapy.
A high level of circulating CRP was associated with a poor response and worse survival in patients with NSCLC.
Keywords: C-reactive protein, non-small cell lung cancer, prognosis
1. Introduction
Lung cancer is one of the leading causes of cancer-related mortality worldwide, attribute to first causes among women and second among men.[1] For patients with advanced lung cancer, the five year survival rate was 2%, and the median survival time was about 6 to 12 months.[2] Non-small cell lung cancer (NSCLC) is the most common histological type, accounting for more than 80% of lung cancer.[3] For treatment biomarker of NSCLC, many studies have identified the driving genes of lung cancer, including epidermal growth factor receptor (EGFR) gene mutation, anaplastic lymphoma kinase (ALK) gene rearrangement.[4,5]At the same time, other studies have confirmed the potential markers such as phosphatase and tensin homolog on chromosometen (PTEN), ribonucleotide reductase M1 (RRM1) and excision repair cross-complementing 1 (ERCC1) as prognostic markers in patients with lung cancer,especially for those who received chemotherapy[6,7,8,9] However, these indicators are not widely used clinically. Therefore, it is very important to find a simple and easy marker which is not only easy to be measured, but also can be used to identify specific types of patients. C-reactive protein (CRP) is a representative acute-phase reactant whose levels rapidly increase in response to inflammation and is accepted as one of the most widely used systemic inflammation markers in vivo.[10] But this inflammatory marker may also increase in patients with malignant tumors without concomitant bacterial or viral infections and the reason is not clear. The inflammation may be secondary to tumor necrosis or local tissue damage, thereby releasing inflammatory factors. [11]
Previous study had reported a correlation between high levels of inflammatory markers and poor prognosis in patients with malignant tumors, including colorectal cancer, hepatocellular carcinoma, renal cell carcinoma, ovarian cancer.[12,13,14,15] Until now, the underlying mechanism by which CRP affects the prognosis of patients with cancer is unclear. Elevated CRP may contribute to tumor progression. Furthermore, systemic inflammation may lead to energy consumption and weight loss in patients with lung cancer.[16] Therefore, it is important to determine whether CRP can be used as an independent prognostic factor in patients with NSCLC, especially for patients who receiving chemotherapy. In this study, we compare the different level of CRP before treatment to assess the effect on the survival and prognosis in patients with NSCLC. Meanwhile, we evaluate the relationship between CRP levels and treatment effect
1.1. Patients and methods
1.1.1. Design and clinical data
This retrospective study recruited 192 NSCLC patients who were admitted to the affiliated Tongji Hospital of Huazhong University of Science and Technology during january 2010 to january 2014. Histopathological specimens were obtained by fiberoptic bronchoscopy, computed tomography (CT)-guided transthoracic needle aspiration, or cervical lymph node biopsy.
The inclusion criteria of study were listed below: Eastern Cooperative Oncology Group (ECOG) score: 0 to 2; essentially normal routine blood, liver and kidney function tests before treatment; measurable lesion that can be evaluated by radiology and have not received chemotherapy or radiotherapy; ethnic Chinese; at least 18 years old; the expected survival time is 3 months or more; and informed consent.
The exclusion criteria of study were listed below: severe infection, diarrhea, or any other serious systemic diseases; presence of contraindications to chemotherapy; secondary primary tumors; history of allergies to biological products; pregnant and lactating women.
The study was approved by the medical ethics committee of Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology. All patients enrolled in this study provided written informed consent.
1.2. Treatment
All the patients received platinum-based combination chemotherapy. Appropriate platinum drug (cisplatin, carboplatin) was selected based on the patient's general physical condition, concurrent diseases and tolerance to the chemotherapy. The optimal regimen of first-line or second-line chemotherapy was selected according to the guideline of National Comprehensive Cancer Network (NCCN). The combined chemotherapeutic agents include pemetrexed, paclitaxel, docetaxel, gemcitabine. Thoracic radiation may cause lung inflammation. The patients who received chest radiotherapy were excluded from this study for the influence of local inflammation on CRP.
1.3. CRP test
The value of CRP could be obtained from routine blood tests. CRP was measured at 2 definite time points: the time before and after the chemotherapy respectively. The measurements were performed in the central biochemical laboratory at the Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology. The cut-off value of CRP is 5 mg/L, which is the upper normal value measured by the test. All patients were divided into three groups based on the 5 mg/L and 20 mg/L as the critical cut-off value.
1.4. Follow-up visit
All patients needed to undergo thoracic and abdominal CT scan before the chemotherapy, during every 2 cycles of chemotherapy until the progression of tumors. Radiographic evaluation of the tumor could be repeated in the event of clinical need or suspected disease progression. The evaluation strategies of each patient should be consistent, and the total tumor evaluation parameters were recorded before and after chemotherapy. The follow-up visit was done every 3 months after the end of treatment. The median follow-up period was 46 months.
1.5. Study endpoints
The primary endpoint of the study was overall survival (OS). OS is defined as the time from the start of the treatment until death caused by any reason. The date of death is confirmed by hospitalization records and the local police station computer network query system. The secondary endpoint of the study was the short-term efficacy of chemotherapy. The efficacy of chemotherapy response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 standard.
1.6. Data analysis
Three groups could be divided according to the serum level of CRP: the low level CRP group (<5 mg/L), the middle level CRP group (5∼20 mg/L) and the high level CRP group (>20 mg/L) respectively. All patients were divided into two subgroups according to the criteria of smoking: never smokers; smokers (regular smoking for more than 6 months). The pathologic types of NSCLC patients were divided into adenocarcinoma and squamous cell carcinoma. The clinical stage was classified to IIIb and IV stage. The baseline characteristics and clinical information of three groups with different levels of CRP were compared by means of Poisson chi square test and 1-way ANOVA test.
Kaplan–Meier method was used to assess the relationship between the survival time of patient and the level of CRP, further to analysis the relationship of different pathologic types (adenocarcinoma vs squamous cell carcinoma) and chemotherapy state (first-line vs second-line chemotherapy) between the survival time and CRP. The difference of survival curves between groups was evaluated by log-rank test. Cox proportional hazards model was used to evaluate the relationship between CRP level and survival time in different age, gender, smoking history, pathological type and clinical stage. All of the above analysis was realized by SPSS19.0 (SPSS Inc, Chicago, IL). All statistical tests were two-sided, and the P value of less than .05 is defined as having a statistically significant difference.
2. Results
2.1. Basic information on patients with different CRP levels
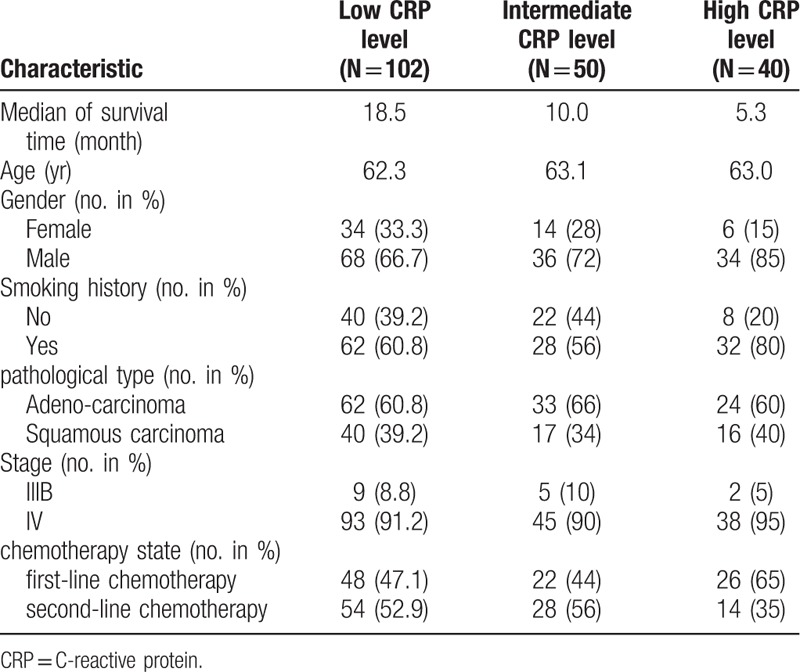
With basis of the CRP value, the average ages of patients in the 3 groups were 62.3, 63.1, 63.0, respectively. The median survival time between groups had significant difference (P < .001). The higher CRP level meaned the shorter survival time. The median survival time of the low level of the CRP group was about 3 times higher than that of the high level group. The median survival time of two groups was 18.5 months and 5.3 months respectively. Of a total of 192 patients, we included only 16 patients with stage III B. The 16 patients were unable to receive thoracic radiotherapy due to different contraindications of concurrent radiotherapy and chemotherapy. Furthermore, the patient with the characteristic of male, smoking, adenocarcinoma, and stage IV was more likely to have higher CRP levels (see Table 1).
Table 1.
Baseline characteristics of patients.
2.2. The correlation between CRP level and survival time
The results of Kaplan–Meier survival analysis and log-rank test were shown in Figure 1, Figure 2. There were significant differences in survival time between the three groups of patients with different CRP levels (P < .001, Fig. 1a). There were also significant differences between patients with adenocarcinoma (N = 119) and squamous cell carcinoma (N = 73) (P < .001, P < .001; Fig. 1b, c respectively). In addition, CRP levels were significantly associated with survival for the patients with chemotherapy for the first time (P < .001, Fig. 2a). However, there was no significant correlation between CRP level and survival time in patients receiving second-line chemotherapy (P = .142, Fig. 2b).
Figure 1.
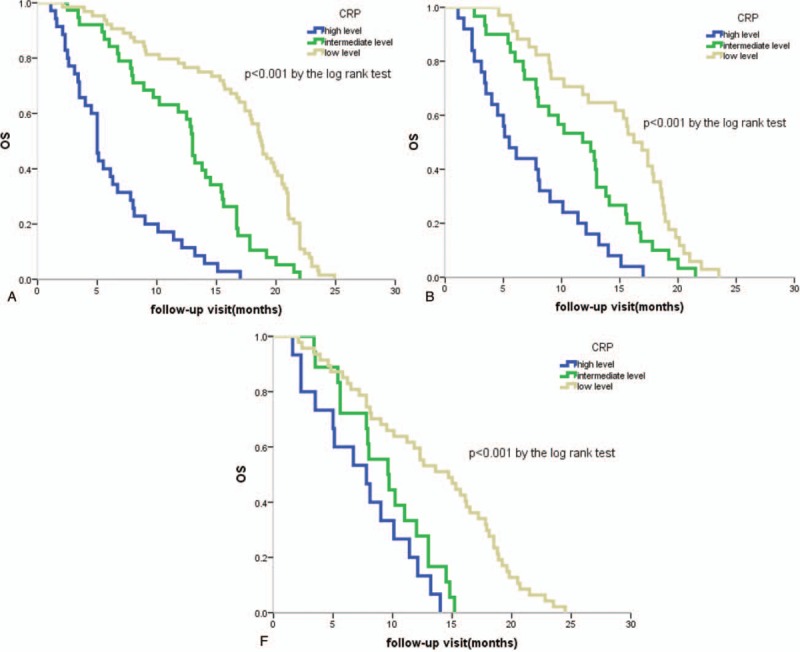
Kaplan–Meier survival estimates by plasma CRP concentration. (A) Kaplan–Meier curves for lung cancer-specific survival are shown for all patients; (B) Survival curves for patients with adenocarcinoma;(C) Survival curves for patients with squamous carcinoma. OS = overall survival; CRP = C-reactive protein.
Figure 2.
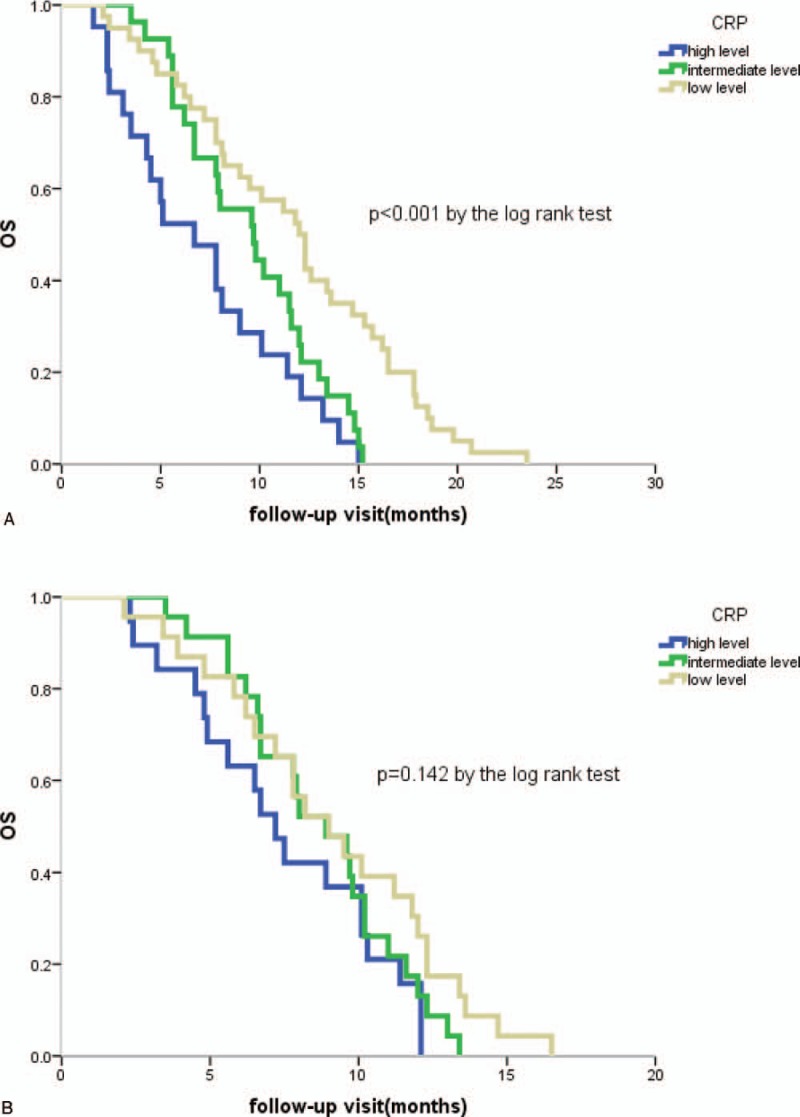
Kaplan–Meier curves for lung cancer-specific survival by chemotherapy status. (A) Survival curves for patients with first-line chemotherapy; (B) Survival curves for patients with second-line chemotherapy. OS = overall survival; CRP = C-reactive protein.
In different subgroups, we used Cox's proportional hazard model to further analyze the relationship between the level of CRP in patients with NSCLC and intervention factors. The higher level of CRP was associated with the worse prognosis and the hazard ratio was 1.83[95% confidence interval (CI) = 0.96,3.48] (see Table 2). Compared with stage IIIB, stage IV was more likely to reflect the patient's survival time (hazard ratio = 1.37; 95% CI = 0.63, 2.95). The results were consistent with the analysis with different pathologic types. For squamous cell carcinoma with high level CRP, the hazard ratio was 2.06[95% CI = 0.44, 7.56]. For adenocarcinoma with high level CRP, the hazard ratio was 1.88[95% CI = 0.91, 3.89]. For the patients with different chemotherapy status (see Table 3), the level of CRP was significantly correlated with survival time (hazard ratio = 1.77; 95% CI = 0.73, 4.26) for the patient with first-line chemotherapy, and there was no significant correlation between the patients with second-line chemotherapy.
Table 2.
Multivariate analysis of survival stratified by pathologic type.
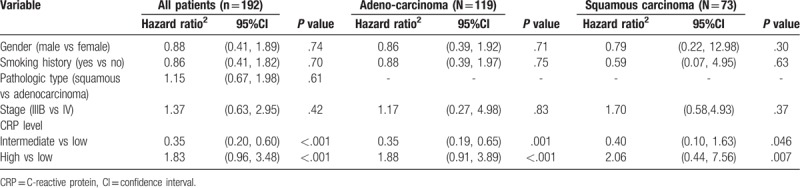
Table 3.
Multivariate analysis stratified by the chemotherapy state.
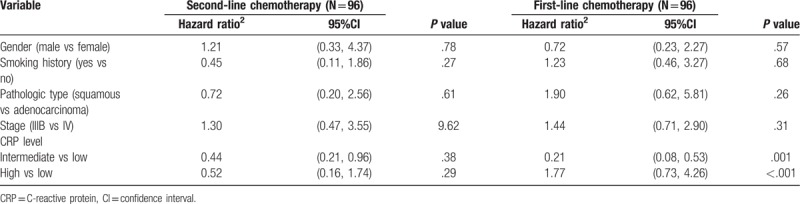
Table 4.
Chemotherapy response for patients.
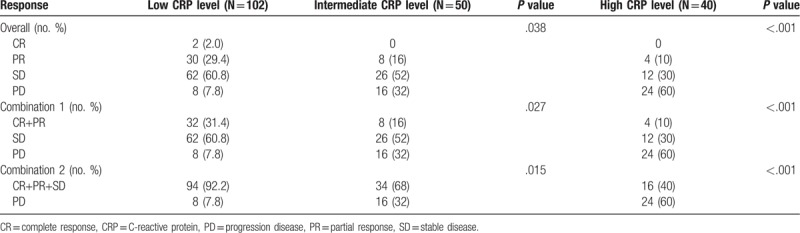
2.3. The correlation between CRP level and chemotherapy efficacy
In addition, we analyzed the relationship between short-term chemotherapy efficacy and CRP level in patients with NSCLC. Compared with the low level CRP group, the proportion of patients with progression disease (PD) was higher in the intermediate and high level CRP group, which was 32% and 60% respectively. The short-term effect of chemotherapy between high and low level of CRP group also had significant differences (P = .015; P < .001). Therefore, the CRP level can be used as a potential indicator to evaluate the efficacy of chemotherapy.
3. Discussion
A promising breakthrough to improve the outcome for NSCLC patients is the application of validated biomarkers into clinical management. Chronic airway inflammation plays an important role in the alternations of bronchial epithelium and lung microenvironment, provoking a milieu conducive to pulmonary carcinogenesis and progression of lung cancer. [17] The relationships between inflammation and lung cancer are complex and not yet fully understood. Previous studies have shown that elevated CRP levels are associated with decreased survival time. [18] The elevation of pretreatment serum CRP was defined as an independent poor prognosis factor for several types of solid tumors. Several studies found that the higher pretreatment serum CRP level was associated with unfavorable pathological characters. Lee reported that the preoperative CRP levels were associated with tumor size and lymphovascular invasion in resected NSCLC.[19] Hefler found that the ovarian cancer patients with elevated preoperative CRP had worse disease stage.[15]Hashimoto found that the patients with higher preoperative CRP level had larger tumor size and portal vein invasion in hepatocellular carcinoma.[20] Consistently, this result was also confirmed by our study. In this study, the results shown that the higher level of CRP indicated a shorter survival time, which was not affected by age, gender, smoking history, clinical stage and pathologic type of lung cancer. In addition, patients with elevated CRP tended to be men with a history of smoking, which was also consistent with previous studies.
So far, the biological mechanisms underlying the effects of serum CRP on survival in patients with NSCLC are not clear. Studies conducted in non-Asian countries all selected “≥10 mg/L” as the cut-off value.[16] While with the development of a high sensitivity technique, a minor elevation of CRP reflecting chronic low-grade inflammation shows clinically significant. In my study, we select “≥5 mg/L” as the cut-off value of CRP and try to confirm the relationship between CRP and NSCLC in Asian population. As an inflammatory marker, CRP may increase the risk of inflammation related cancer. Elevated CRP plays a critical role in the progression of cancer and high levels of CRP are associated with poor prognosis in advanced cancers. Furthermore, CRP value may be much easier in clinical use to predict the prognosis of patients with NSCLC than other parameter.
The underlying molecular mechanism by which circulating CRP is associated with worse outcome of NSCLC is still not clear. One possible explanation is IL-6. Several studies have shown that IL-6 was associated with cancer resistance.[21] Wang founded that the expression of IL-6 was upregulated in human ovarian cancer cells by the use of paclitaxel.[22] Poth also confirmed that platinum induced IL-6 expression in brain cancer, thereby increasing the tumorigenic potential of cancer cells.[23] IL-6 was the only inflammatory cytokine independently associated with CRP concentrations in patients with advanced NSCLC, suggesting that CRP was a useful surrogate marker of IL-6 activity in patients with NSCLC.[24] It has been reported that IL-6 contribute to the cancer growth and progression via IL-6R/JAK1/STAT3 signal pathway.[25,26] STAT proteins are a family of transcription factors which play a key role in various tyrosine kinase signal pathways, including the EGFR pathway.[27] Persistent STAT3 activation is associated with cell cycle progression, tumor invasion, metastasis and angiogenesis. IL-6 could stimulate and induce the production of CRP.[28] However, the correlation between CRP and cancer resistance needs further study.[29,30]
The therapeutic effect of tyrosine kinase inhibitor (TKI) is better than that of traditional chemotherapy for the sensitive mutation of EGFR. But in this study, owing to the limitation of this study started earlier and sensitive EGFR mutation affects the survival of the patient. The patients who received TKI treatment were not included in this study. This is the limitation of this study and deserved further study. More large-scale, high-quality and prospective studies are needed to confirm whether the level of CRP after chemotherapy can be used as indicators of chemotherapy efficacy and independent prognostic indicators in patients with NSCLC.
4. Conclusion
In summary, the results of this study show that the higher CRP level is associated with poor prognosis. CRP can be used as an independent prognostic factor in patients with NSCLC chemotherapy.
Author contributions
Data curation: Xiaoguang Xiao, Shujing Wang.
Formal analysis: Xiaoguang Xiao, Shujing Wang.
Investigation: Xiaoguang Xiao.
Methodology: Guoxian Long.
Project administration: Guoxian Long.
Resources: Xiaoguang Xiao, Shujing Wang.
Software: Shujing Wang.
Supervision: Guoxian Long.
Writing – original draft: Xiaoguang Xiao, Shujing Wang.
Writing – review & editing: Guoxian Long.
Footnotes
Abbreviations: ALK = anaplastic lymphoma kinase, CI = confidence interval, CRP = c reactive protein, CT = computed tomography, ECOG = eastern cooperative oncology group, EGFR = epidermal growth factor receptor, ERCC1 = excision repair cross-complementing1, NCCN = National Comprehensive Cancer Network, NSCLC = non-small cell lung cancer, OS = overall survival, PTEN = phosphatase and tensin homolog on chromosometen, RECIST = response evaluation criteria in solid tumors, RRM1 = ribonucleotide reductase M1, TKI = tyrosine kinase inhibitor.
This work was supported by Hubei Provincial Natural Science Foundation (Grant No. 2016CFB137).
The authors report no conflicts of interest.
References
- [1].Xiao X, Wang S, Xia S, et al. Retrospective study of irinotecan/cisplatin followed by etoposide/cisplatin or the reverse sequence in extensive-stage small cell lung cancer. Onco Targets Ther 2015;21:2209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Brambilla E, Travis WD, Colby TV, et al. The new World Health Organization classification of lung tumours. Eur Respir J 2001;18:1059–68. [DOI] [PubMed] [Google Scholar]
- [4].Bepler G, Kusmartseva I, Sharma S, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol 2006;24:4731–7. [DOI] [PubMed] [Google Scholar]
- [5].Sequist LV, Mart ins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 2008;2442–9. [DOI] [PubMed] [Google Scholar]
- [6].Bepler G, Sharma S, Cantor A, et al. RRM1 and PTEN as prognostic parameters for overall and disease-free survival in patients with non-small-cell lung cancer. J Clin Oncol 2014;22:1878–85. [DOI] [PubMed] [Google Scholar]
- [7].Zheng Z, Chen T, Li X, et al. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med 2007;356:800–8. [DOI] [PubMed] [Google Scholar]
- [8].Pircher A, Ulsperger E, Hack R, et al. Basic clinical parameters predict gefitinib efficacy in non-small celllung cancer. Anticancer Res 2011;31:2949–55. [PubMed] [Google Scholar]
- [9].Zhuo Y, Lin L, Wei S, et al. Pretreatment elevated serum lactate dehydrogenase as a significant prognostic factor in malignant mesothelioma: a meta-analysis. Medicine (Baltimore) 2016;95:e5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vander Meer V, Neven AK, vanden Broek PJ, et al. Diagnostic value of C reactive protein in infections of the lower respiratory tract: systematic review. BMJ 2005;331:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Scott HR, McMillan DC, Forrest LM, et al. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer 2012;87:264–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Crozier JE, McKee RF, McArdle CS, et al. Preoperative but not postoperative systemic inflammatory response correlates with survival in colorectal cancer. Br J Surg 2014;94:1028–32. [DOI] [PubMed] [Google Scholar]
- [13].Vinocha A1, Grover RK1, Deepak R1. Clinical significance of interleukin-6 in diagnosis of lung, oral, esophageal, and gall bladder carcinomas. J Cancer Res Ther 2018;14:758–60. [DOI] [PubMed] [Google Scholar]
- [14].Karakiewicz PI, Hutterer GC, Trinh QD, et al. C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer 2007;110:1241–7. [DOI] [PubMed] [Google Scholar]
- [15].Hefler LA, Concin N, Hofstetter G, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res 2008;14:710–4. [DOI] [PubMed] [Google Scholar]
- [16].Koch A, Fohlin H, Sorenson S. Prognostic significance of C-reactive protein and smoking in patients with advanced non-small cell lung cancer treated with first-line palliative chemotherapy. J Thorac Oncol 2009;4:326–32. [DOI] [PubMed] [Google Scholar]
- [17].Chechlinska M, Kowalewska M, Nowak R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat Rev Cancer 2010;10:2–3. [DOI] [PubMed] [Google Scholar]
- [18].Tomita M, Shimizu T, Ayabe T, et al. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res 2012;32:3535–8. [PubMed] [Google Scholar]
- [19].Lee JG, Cho BC, Bae MK, et al. Preoperative C-reactive protein levels are associated with tumor size and lymphovascular invasion in resected non-small cell lung cancer. Lung Cancer 2009;63:106–10. [DOI] [PubMed] [Google Scholar]
- [20].Hashimoto K, Ikeda Y, Korenaga D, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer 2005;103:1856–64. [DOI] [PubMed] [Google Scholar]
- [21].Li J, Jiao XD, Yuan ZF, et al. C-reactive protein and risk of ovarian cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang TH, Chan YH, Chen CW, et al. Paclitaxel (Taxol) upregulates expression of functional interleukin-6 in human ovarian cancer cells through multiple signaling pathways. Oncogene 2006;25:4857–66. [DOI] [PubMed] [Google Scholar]
- [23].Poth KJ, Guminsk iAD, Thomas GP, et al. Cisplatin treatment induces a transient increse in tumorigenic potential associated with high Interleukin-6 expression in head and neck squamous cell carcinoma. Mol Cancer Ther 2010;9:2430–9. [DOI] [PubMed] [Google Scholar]
- [24].Guthrie GJ, Roxburgh CS, Horgan PG, et al. Does interleukin-6 link explain the link between tumour necrosis, local and systemic inflammatory responses and outcome in patients with colorectal cancer? Cancer Treat Rev 2013;39:89–96. [DOI] [PubMed] [Google Scholar]
- [25].Masago K, Fujita S, Togashi Y. Clinical significance of pretreatment C-reactive protein inpatients with advanced nonsquamous, non-small cell lung cancer who received gefitinib. Oncology 2010;79:355–62. [DOI] [PubMed] [Google Scholar]
- [26].Ondrej F, Milos P, Jindrich F, et al. High serum level of C-reactive protein is associated with worse outcome of patients with advanced-stage NSCLC treated with erlotinib. Tumor Biol 2015;36:9215–22. [DOI] [PubMed] [Google Scholar]
- [27].Kim SM, Kwon OJ, Hong YK, et al. Activation of IL-6R/JAK1/STAT3 signaling induces de novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790 M resistance mutation. Mol Cancer Ther 2012;11:2254–64. [DOI] [PubMed] [Google Scholar]
- [28].Haura EB, Zheng Z, Song L, et al. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res 2005;11:8288–94. [DOI] [PubMed] [Google Scholar]
- [29].Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci 2011;48:155–70. [DOI] [PubMed] [Google Scholar]
- [30].Jin Y, Sun Y, Shi X, et al. Prognostic value of circulating C-reactive protein levels in patients with non-small cell lung cancer: a systematic review with meta-analysis. J Can Res Ther 2014;10:160–6. [DOI] [PubMed] [Google Scholar]


