Abstract
Introduction:
A progressive decline in lean body mass and increase in fat mass occur with aging, and result in progressive weakness and impaired mobility; these features are altogether landmarks of the ageing frailty syndrome. High-fat mass and low muscle mass are both associated with an increased risk of cardiovascular events and are supposed to be risk factors for arterial stiffness. Little data analyzing the relationship between body composition and cardio-ankle vascular index (CAVI) are currently available. The main objective of this study was to verify whether low muscle mass and/or high fat mass could be associated with arterial stiffness measured by CAVI.
Methods:
Data are from the cross-sectional assessment of the “Al passo con la tua salute”, a clinical study aimed to promote physical function among free-living elderly subjects.
After a screening interview and a clinical visit aimed to exclude ineligible persons, 52 volunteers were enrolled in the study. All underwent: clinical examination, physical performance assessment, an interview on lifestyle and dietary habits, and lastly, a blood sample collection after at least 8 hours of fasting.
Results:
CAVI was statistically significantly higher in those participants in the highest tertile of distribution for fat mass compared to all other subjects (P = .03). In those participants in the lowest tertile of distribution of muscle mass, compared to all other, CAVI was also statistically significant higher (P = .01) independently of age, sex, body mass index, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and systolic blood pressure.
Conclusions:
Low muscle mass and high fat mass were landmarks in the frailty model of aging; therefore, it is not inconsistent that both clinical conditions might share with the “aging vessel” a common pathway, probably mediated through signaling network deregulation and/or through alteration of the balance between energy availability and energy demand.
Keywords: body composition, cardio-ankle vascular index, sarcopenia, stiffness
1. Introduction
“Aging vessels” are associated with an increase in intimal media thickness accompanied by both luminal dilatation and by a reduction in compliance or distensibility, with an increase in vessel stress/strain relationship (stiffness).[1] Changes in the carotid stiffness are accelerated by coexistent cardiovascular disease or risk factors, independent from the effects of age.[2]
There is clear evidence of a vicious cycle in the vascular stiffness control and in the genesis/control of atherosclerosis.[3] Atherosclerosis is influenced by mechanical properties of the vessel wall, and in turn, atherosclerosis can influence: structural changes within the matrix, endothelial modulation of vascular smooth muscle tone, and vascular wall structure/function.[3]
Obesity is a known risk factor for arterial stiffness[4]; moreover, increased aortic stiffness has been shown to be more related to abdominal visceral fat, rather than an elevated body mass index (BMI).[5] Arterial stiffness, as measured by brachial-ankle pulse wave velocity (baPWV), was also associated with sarcopenia,[6,7] a reduction of muscle volume and strength, typically described in the aging processes.[8]
The cardio-ankle vascular index (CAVI), a new arteriosclerosis disease index, has been proposed, and it seems to be unbiased by blood pressure (BP).[9,10]
Several studies have addressed the relationship between body composition, namely low muscle mass and high fat mass, and CAVI. Among them, 3 studies considered large cohort of free-living subjects, and another one enrolled hemodialysis patients; all the studies were carried out in an Asiatic catchment area.[6,7,11,12]
The concept of sarcopenic obesity, introduced by Baumgartner,[13] defines a condition in which older adults experience both a low muscle mass and a high fat mass. It was suggested that approximately 15% of those subjects with sarcopenia were also obese[13] and showed a higher risk of disability.[14] The cardiovascular and metabolic implications of sarcopenic obesity have not been extensively addressed, even if it was suggested that it could represent a clinical landmark of the frailty syndrome.[15]
The main objective of this study was to verify to what extent low muscle mass and/or high fat mass could be associated with arterial stiffness measured with CAVI.
2. Materials and methods
Data are from an ancillary study, of the cross-sectional assessment of the “Al passo con la tua salute” (Keep pace with your health project), a clinical study aimed to contrast the effect of: a moderate-intense physical exercise intervention program (aerobic exercise arm), with an education intervention program on light physical activity (healthy aging education arm), extensively described elsewhere.[16]
One hundred and eighty-four free-living subjects (mean age 63.4 ± 5.9), therefore subjects who were living at home, and were not dependent in activity of daily living, belonging to a local social and cultural association (University of the third age) participated in this initiative. A screening interview and a clinical visit excluded 132 ineligible persons. Therefore, 52 (14 men and 38 women) subjects were enrolled in this study.
The eligibility criteria in this study were aimed at identifying: those subjects who were at risk for safety clinical conditions, those who had a low physical activity level, and those who could have lower extremity functional limitations, but who had not yet developed mobility disability.[16]
Briefly, a structured interview was developed for the study, to assess whether clinical conditions, and pharmacological prescriptions, that could interfere with lower limb physical function and gait speed, could be excluded.
Inclusion criteria were: age >65 years and independent community-dwelling status; ability to perform self-care activities of daily living without difficulties or need for help, and able to walk independently for at least 400 m. Exclusion criteria were: impaired cognitive status (<24 on Mini Mental State Examination); patients affected by major or minor neurocognitive disorder, could express deficit in cognitive domain as: complex attention, executive function, and perceptual-motor function, that could interfere with exercise training; neurological disorders (stroke with disabling outcomes, Parkinson disease, multiple sclerosis); psychiatric disorders (depression or anxiety) requiring drug treatment; history of cardiovascular disease (including angina, myocardial infarction, congestive heart failure, but not controlled hypertension); active cancer; kidney or liver diseases; important sensory deficits (any condition that precluded subjects from being tested with performance oriented disability scales or neuropsychological tests); cochleo-vestibular diseases; previous lower limb surgery; acute diseases; and diabetes requiring insulin or oral lowering glycaemic drugs (subjects with impaired glucose tolerance, defined as serum fasting glucose values ≥110 mg/dL but <140 mg/dL were included).
Therefore, all enrolled subjects had an independent community-dwelling status; they could perform self-care activities of daily living without difficulties and/or without help; moreover, they were able to walk independently for at least 400 m without help or assistive devices; and lastly were cognitively able to understand and to follow a standardized exercise protocol.
Volunteers who satisfied the above inclusion/exclusion criteria gave their informed consent, in accordance with procedures approved by the Helsinki Committee.
2.1. Protocol
The study was designed and conducted by the laboratory of Medicine and Sports Cardiology of the University Centre of Sports Medicine of the “G. d’Annunzio” University (Chieti-Pescara, Italy).
All subjects underwent a careful clinical examination, a physical performance assessment, a detailed interview on habits and lifestyle, dietary habits investigation, and lastly a blood sample collection after at least 8 hours of fasting. The research protocol was approved by the ethics review board of the University “Gabriele d’Annunzio” (n° 1070; Pescara, 10/24/2013), and written informed consent was obtained from all participants in the study.
2.2. CAVI
CAVI was measured using a Vasera VS-1000 (Fukuda Denshi, Tokyo, Japan). Cuffs were applied to the 4 extremities, and electrocardiographic electrodes were attached to the upper arm. A microphone was placed on the sternal angle for phonocardiography. The subjects rested in the supine position for 5 minutes. To minimize cuff inflation effects on blood flow dynamics, pulse waves were measured with cuffs inflated to lower than the diastolic BP (DBP, 50 mm/Hg). Then, the extremity BP was measured by oscillometry. Systolic BP (SBP, mm/Hg), DBP (mm/Hg), and pulse pressure (PP) were obtained by measuring the BP at the right brachial artery.[9]
Vasera produces a lower limbs ankle brachial index (ABI), calculated as the relationship between the measurements of the systolic pressure of each ankle and the right arm.
2.3. Anthropometry and body composition
Body weight and height were measured, to the nearest 0.1 kg and 0.1 cm, respectively, in standardized position, using a stadiometer with a balance-beam scale (SECA 220, SECA, Hamburg, Germany), subjects were dressed in light clothing and without shoes. BMI was calculated as weight (kilograms)/height (square meter).
The body composition (fat mass, fat-free mass, muscle mass, and total body water expressed in kilogram) was assessed by means of electrical bioimpedance technique, using leg-to-leg 50 kHz frequency bioelectrical impedance scale (BC-420MA, Tanita, Tokio, Japan). The test was executed in standing position, without socks, after emptying the bladder, and after at least 2 hours of fasting. Participants also abstained from alcohol consumption within 48 hours of the test.
Tertiles of distribution of fat mass (19–26 kg) and muscle mass (39–46 kg) were considered in the analysis.
2.4. Daily physical activity measurement
Daily physical activity was measured during 5 consecutive days, which included also 1 weekend day, using SenseWear Pro3 armbands (BodyMedia, Pittsburg). The armbands sensors integrate the spatial solicitations and provide estimated qualitative and quantitative information about spontaneous daily physical activity.
2.5. Cardiovascular assessment and evaluation for walking training
First step in cardiovascular evaluation was a resting 12-lead electrocardiogram (ECG) that was performed after 10 minutes in supine rest.
The eligibility for aerobic training and fitness level of subjects selected were assessed through a graded maximal exercise test, supervised by a Sports Medicine physician specialist. The exercise test was executed under continuous ECG monitoring, and BP was measured at the end of each step.
2.6. Dietary assessment
A structured questionnaire concerning information on food intake was proposed to subjects involved in the study. The questionnaire is organized into 2 parts: the first part consists of questions focusing on general dietary habits or specific dietary approach; the second part consists of items aimed to assess, and investigates how frequently each specific food was generally consumed.
2.7. Blood sampling
After an 8-hour overnight fasting, venous blood samples were obtained for measurements of blood glucose (mg/dL), total serum cholesterol (mg/dL), high-density lipoprotein cholesterol (HDL-cholesterol) (mg/dL), and triglycerides (mg/dL). Fasting blood glucose, total serum cholesterol, HDL-cholesterol, and triglycerides were assessed through commercial enzymatic kits, whereas low-density lipoprotein cholesterol (LDL-cholesterol) was calculated according to a standardized procedure.[17]
2.8. Other covariates
The diagnosis of major medical conditions was ascertained according to preestablished criteria that combined information from medical history, physical examination, blood tests, and medical records.[18]
2.9. Statistical Analysis
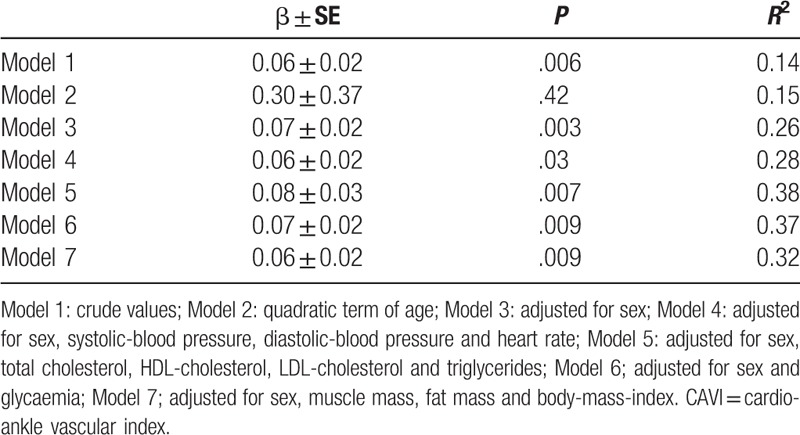
The association between CAVI and age was analyzed with generalized linear models, and the potential effects of different confounders was also accounted: Model 1, crude values; Model 2, quadratic term of age; Model 3, adjusted for sex; Model 4, adjusted for sex, SBP, DBP, and heart rate; Model 5, adjusted for sex, total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides; Model 6, adjusted for sex and glycemia; Model 7, adjusted for sex, muscle mass, fat mass, and BMI.
Continuous variables are reported as mean ± standard deviation (SD) and ordinal categorical variables as frequencies and percentage. Differences between sex were evaluated by generalized linear model for continuous variables and with χ2 test for dichotomous or categorical variables; P values for descriptive analysis were adjusted for age.
To analyze whether statistically significant differences could be found in the CAVI and ABI mean values, between subjects in the lowest tertiles of muscle mass compared to all other persons, and between subjects in the highest tertiles of fat mass compared to all other persons, generalized linear models were used. To minimize the role of potential confounders, in the association between CAVI, ABI, and body composition, the 4 models were adjusted for: age, sex, BMI, LDL-cholesterol, HDL-cholesterol, and SBP.
Lastly to evaluate the contemporaneous and independent effect of low muscle mass and high fat mass on vascular stiffness (by means of CAVI), generalized linear models were used: Model 1, crude values; Model 2, age and sex adjusted; Model 3, full adjusted model (age, sex, BMI, LDL-cholesterol, HDL-cholesterol, and SBP). Estimated regressor coefficients (β ± SE) were reported.
A P value ≤.05 was considered statistically significant. All Analyses were performed using the SAS statistical package version 9.2 (SAS Institute, Inc.,Cary, NC)
3. Results
The study demonstrated a statistically significant direct association between arterial stiffness and age (Fig. 1). The age-associated increase in arterial stiffness was linear; indeed introducing a quadratic term in the model, the fit was not significantly improved (Table 1, Model 2), even when sex was considered. Moreover, several factors potentially mediating the association between CAVI and age were analyzed (Table 1). Only in Model 4, wherein the association between CAVI and age was adjusted for sex, BP, and heart rate, the strength of the association was reduced but was still statistically significant (P = .03).
Figure 1.
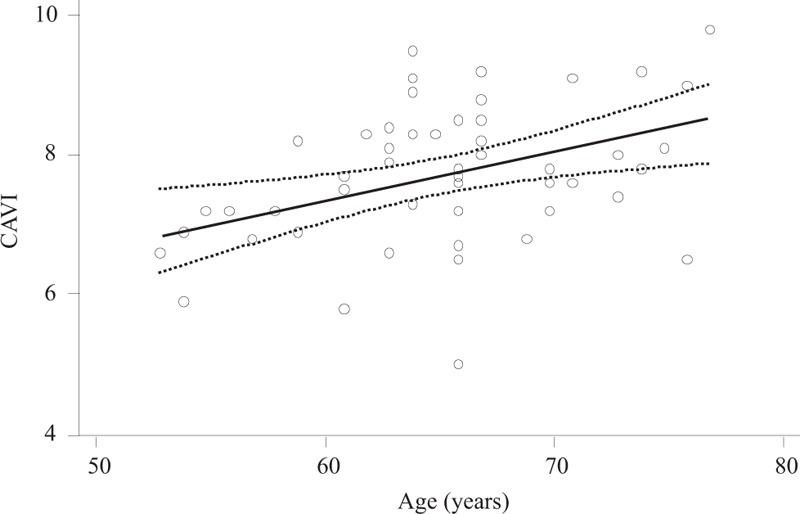
Association between cardio-ankle vascular index and age; dotted lines represent 95% confidence intervals.
Table 1.
Generalized Linear Models assessing the association between arterial stiffness (CAVI) and age, accounting for different potential confounders.
In Table 2 were reported demographics, body composition, main clinical characteristics, and laboratory parameters of the population enrolled in the study, according to sex. Male subjects compared to females, as expected, were taller, heavier, and had a statistically significant different body composition. In other words, independent of BMI, muscle mass (P < .001), fat-free mass (P < .001), and total body water (P < .001) were higher in male participants.
Table 2.
Demographics, body composition, clinical characteristic and laboratory parameters in the population enrolled in the study, according to sex. Data were reported as mean ± standard deviation.
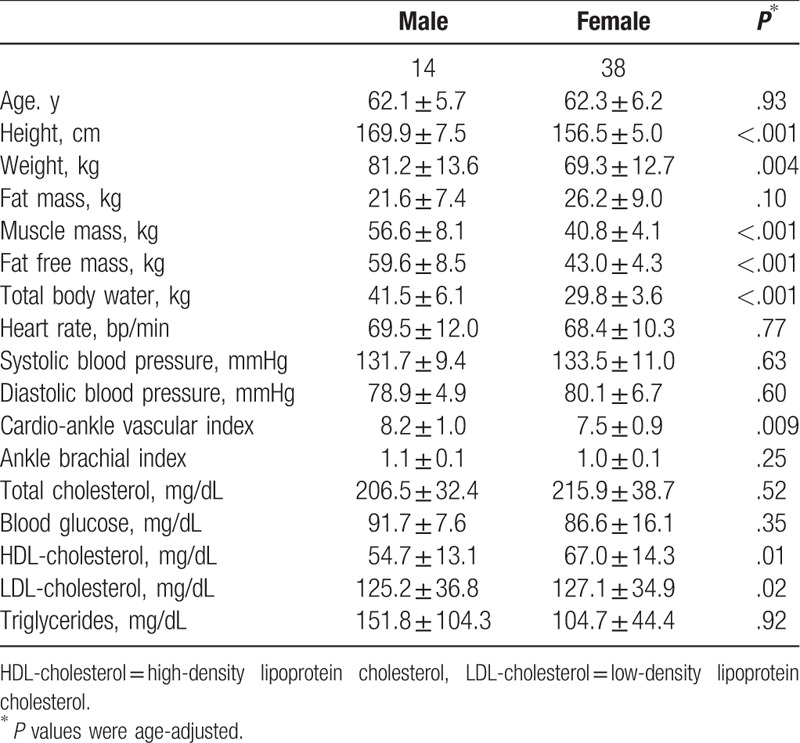
Serum level of HDL-cholesterol was higher in women, whereas LDL-cholesterol was more elevated in male subjects (respectively P value: P = .01; P = .02). Lastly, CAVI but not ABI was statistically significant higher in male (8.2 ± 1.0) compared to female (7.5 ± 0.9) subjects (P = .009), independently from age and sex.
Fat-free mass (β ± SE: 5.33 ± 1.36; P < .001), muscle mass (β ± SE: 5.01 ± 1.29; P < .001), and total body water (β ± SE: 4.63 ± 0.97; P < .001) were statistically significant greater in subjects in the highest tertile of distribution for fat mass compared with those subjects in the intermediate and lowest tertiles; similarly among laboratoristic parameters only fasting blood glucose level was higher in subjects in the highest tertile of distribution for fat mass compared with all other persons (β ± SE:14.59 ± 4.77; P = .003).
CAVI but not ABI was statistically significant higher in the group in the upper tertile of fat mass compared to all other subjects enrolled in the study (Fig. 2A). Adjusting the analysis for age, sex, BMI, LDL-cholesterol, HDL-cholesterol, and for blood-pressure, the strength of the association between CAVI and tertiles of fat mass was unaltered (β ± SE: 1.17 ± 0.30; P = .001), meaning that high fat mass (>26 kg) could raise CAVI of about one unit independently of the potential confounders considered.
Figure 2.
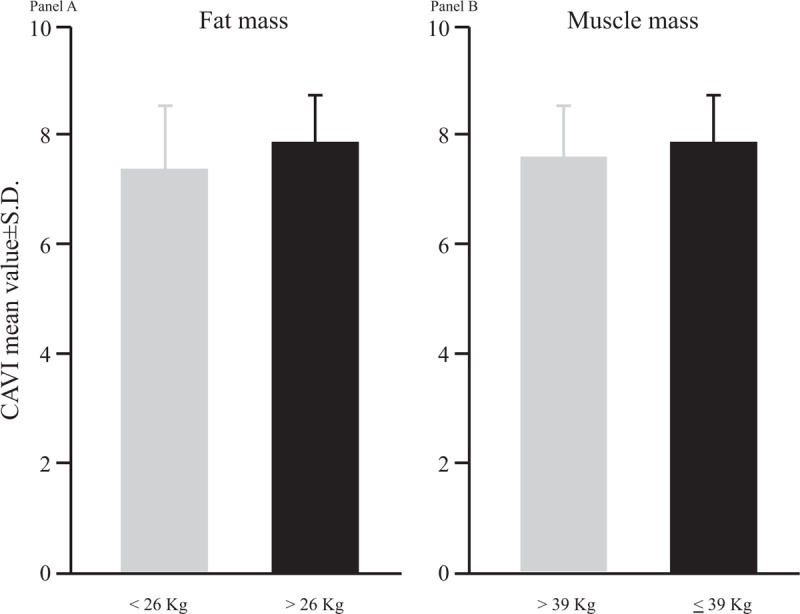
Cardio-ankle vascular index mean values according to tertiles of distribution of fat mass and muscle mass. In panel A, black box represents the highest tertiles of distribution for fat mass, whereas in panel B, black box represents the lowest tertiles of distribution for muscle mass. SD = standard deviation.
Those subjects in the lowest tertiles of distribution of muscle mass compared to all other subjects showed a statistically significant lower fat-free mass (β ± SE: −5.59 ± 1.59; P = .002) and total body water (β ± SE: −4.55 ± 1.18; P = .001).
Again CAVI but not ABI was statistically significantly higher in those subjects with a lower muscle mass compared to the group with intermediate higher muscle mass (Fig. 2B); also in this case, adjusting the analysis for age, sex, BMI, LDL-cholesterol, HDL-cholesterol, and for systolic-blood-pressure, the strength of the association was practically unaffected (β ± SE: 0.67 ± 0.31; P = .03).
In Table 3 were reported the independent and simultaneous effects of low muscle mass, and high fat mass, in vascular stiffness.
Table 3.
Mean differences in arterial stiffness (by means of CAVI) between group considered low tertile of muscle mass (≤39 kg) versus intermediate high tertiles of muscle mass, and high fat mass (≥27 kg) versus intermediate low fat mass.
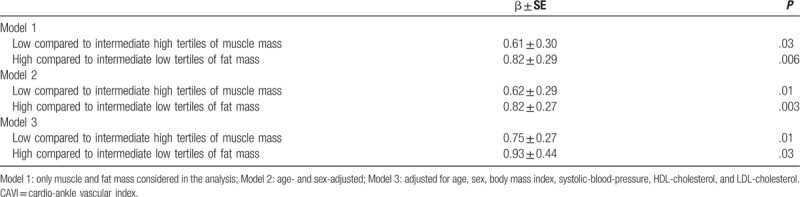
Low muscle mass and high fat mass groups compared to all other persons showed higher values of vascular stiffness, independently from age, sex, BMI, SBP, HDL-cholesterol, and LDL-cholesterol. The interaction term between low muscle mass and high fat mass was also evaluated but did not reach a statistically significant difference in CAVI mean value among the groups considered.
4. Discussion
The findings of this study support the notion that low muscle mass and high fat mass were associated with an increase in the cardio-ankle vascular index, independently from age, sex, BMI, SBP, HDL-cholesterol, and LDL-cholesterol.
Increased vascular stiffness has traditionally been linked to structural alterations in the vascular media, and more recently also associated to an error in the regulation of endothelium, an alteration in the vascular smooth muscle tone, and in other aspects of vascular wall structure/function.[1]
Beyond the typical aging processes, abnormalities of the endothelium were also identified in the early stage of atherosclerosis, supporting the hypothesis that atherosclerosis and vascular stiffness could be two knots, part of a vicious cycle, leading to an increase in cardiovascular risk.[19,20]
The effect of fat mass on arterial stiffness was in depth evaluated. Independently from age, increasing visceral adiposity was accompanied by an increase of arterial wall stiffness; weight loss was associated with a decrease in arterial stiffness.[21,22] The Baltimore Longitudinal Study of Aging demonstrated that leptin could mediate the observed relationship between abdominal adiposity and arterial stiffness, and adiponectin and resistin were also independent correlates of baPWV.[4]
Several factors were hypothesized to be responsible for the age-related processes of sarcopenia; among them arterial stiffness should be also included.[8] In a Japanese population, alteration of glycation of serum proteins was proposed as a mediator of the relationship between sarcopenia and arterial stiffness.[7] Another Japanese study demonstrated that arterial stiffness and sarcopenia could share a common pathway leading to an increase in cardiovascular risk; moreover, it showed that at least in males hormonal derangement could also be a part of this pathway.[6] Lastly in a cohort of hemodialysis patients, therefore exposed to a higher cardiovascular risk, the reduced thigh muscle mass area was independently related to CAVI and ABI. This suggests that sarcopenia in the lower limbs is closely associated with systemic changes of arteriosclerosis in those patients.[11,23]
In this study, of free-living healthy subjects, low muscle mass and high fat mass were independently associated with an increase in CAVI, which could indicate an increase of cardiovascular risk, independently of well-known underlying risk factors.
Low muscle mass and high fat mass were landmarks of the Fried loop model of the frailty of aging.[24] It is not inconsistent therefore that both clinical conditions share with the “aging vessel” a common pathway, probably mediated through signaling network deregulation and/or through alteration of the balance between energy availability and energy demand.
Among the limitations of our study, the following must be considered: the cross-sectional design of the data considered in the analysis, that did not enable us to disentangle the causal-effect relationship between body composition and arterial stiffness; sarcopenia was estimated through tertiles of distribution of muscle mass; and lastly, the relatively small sample size of the study population, that could introduce the “small sample size effect”, namely smaller study could show larger effect than those expected. Altogether such limitations could restrict the external validity of the study.
Finally, it is important to consider that the population enrolled in the project consisted of volunteers; therefore, a “healthy workers effect” bias may be present.
5. Conclusion
In conclusion, low muscle mass and high fat mass were both associated with an increase in the cardiovascular risk profile, estimated by means of CAVI, in a healthy free-living population, even if these data need to be validated in a larger and prospective cohort. A pharmacological and clinical intervention aimed to increase muscle mass and/or to reduce highfat mass could also positively reflect on arterial stiffness.
Author contributions
Conceptualization: Angelo Di Iorio, Andrea Di Blasio, Patrizio Ripari, Roberto Paganelli, Francesco Cipollone.
Data curation: Patrizio Ripari, Roberto Paganelli, Francesco Cipollone.
Formal analysis: Angelo Di Iorio, Giorgio Napolitano, Roberto Paganelli.
Investigation: Andrea Di Blasio, Francesco Cipollone.
Methodology: Angelo Di Iorio, Andrea Di Blasio, Giorgio Napolitano, Francesco Cipollone.
Supervision: Francesco Cipollone.
Validation: Giorgio Napolitano.
Writing – original draft: Angelo Di Iorio, Andrea Di Blasio, Giorgio Napolitano, Patrizio Ripari, Roberto Paganelli, Francesco Cipollone.
Writing – review & editing: Angelo Di Iorio.
Angelo Di Iorio orcid: 0000-0003-2899-146X.
Footnotes
Abbreviations: β±SE = regression beta coefficients±Standard Error, ABI = ankle brachial index, baPWV = brachial-ankle pulse wave velocity, BMI = body mass index, CAVI = cardio-ankle vascular index, DBP = diastolic blood pressure, ECG = Electrocardiogram, HDL-cholesterol = high-density lipoprotein cholesterol, LDL-cholesterol = low-density lipoprotein cholesterol, PP = pulse pressure, SBP = systolic blood pressure.
The authors report no conflicts of interest.
References
- [1].Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 2003;107:346–54. [DOI] [PubMed] [Google Scholar]
- [2].Lee HY, Oh BH. Aging and arterial stiffness. Circ J 2010;74:2257–62. [DOI] [PubMed] [Google Scholar]
- [3].Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 2003;107:139–46. [DOI] [PubMed] [Google Scholar]
- [4].Windham BG, Griswold ME, Farasat SM, et al. Influence of leptin, adiponectin, and resistin on the association between abdominal adiposity and arterial stiffness. Am J Hypertens 2010;23:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sutton-Tyrrell K, Newman A, Simonsick EM, et al. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension 2001;38:429–33. [DOI] [PubMed] [Google Scholar]
- [6].Ochi M, Kohara K, Tabara Y, et al. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis 2010;212:327–32. [DOI] [PubMed] [Google Scholar]
- [7].Sanada K, Miyachi M, Tanimoto M, et al. A cross-sectional study of sarcopenia in Japanese men and women: reference values and association with cardiovascular risk factors. Eur J Appl Physiol 2010;110:57–65. [DOI] [PubMed] [Google Scholar]
- [8].Di Iorio A, Abate M, Di Renzo D, et al. Sarcopenia: age-related skeletal muscle changes from determinants to physical disability. Int J Immunopathol Pharmacol 2006;19:703–19. [DOI] [PubMed] [Google Scholar]
- [9].Saiki A, Sato Y, Watanabe R, et al. The role of a novel arterial stiffness parameter, cardio-ankle vascular index (CAVI), as a surrogate marker for cardiovascular diseases. J Atheroscler Thromb 2016;23:155–68. [DOI] [PubMed] [Google Scholar]
- [10].Bonarjee VVS. Arterial stiffness: a prognostic marker in coronary heart disease. available methods and clinical application. Front Cardiovasc Med 2018;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kato A, Ishida J, Endo Y, et al. Association of abdominal visceral adiposity and thigh sarcopenia with changes of arteriosclerosis in haemodialysis patients. Nephrol Dial Transplant 2010;26:1967–76. [DOI] [PubMed] [Google Scholar]
- [12].Im IJ, Choi HJ, Jeong SM, et al. The association between muscle mass deficits and arterial stiffness in middle-aged men. Nutr Metab Cardiovasc Dis 2017;27:1130–5. [DOI] [PubMed] [Google Scholar]
- [13].Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci 2000;904:437–48. [DOI] [PubMed] [Google Scholar]
- [14].Stenholm S, Harris TB, Rantanen T, et al. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care 2008;11:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Janssen I. Evolution of sarcopenia research. Appl Physiol Nutr Metab 2010;35:707–12. [DOI] [PubMed] [Google Scholar]
- [16].Di Blasio A, Izzicupo P, Di Baldassarre A, et al. Walking training and cortisol to DHEA-S ratio in postmenopause: an intervention study. Women Health 2018;58:387–402. [DOI] [PubMed] [Google Scholar]
- [17].Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- [18].Simonsick EM, Maffeo CE, Rogers SK, et al. Methodology and feasibility of a home-based examination in disabled older women: the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci 1997;52:M264–74. [DOI] [PubMed] [Google Scholar]
- [19].Safar ME, Blacher J, Mourad JJ, et al. Stiffness of carotid artery wall material and blood pressure in humans: application to antihypertensive therapy and stroke prevention. Stroke 2000;31:782–90. [DOI] [PubMed] [Google Scholar]
- [20].De Simone G, Roman MJ, Koren MJ, et al. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension 1999;33:800–5. [DOI] [PubMed] [Google Scholar]
- [21].Barinas-Mitchell E, Kuller LH, Sutton-Tyrrell K, et al. Effect of weight loss and nutritional intervention on arterial stiffness in type 2 diabetes. Diabetes Care 2006;29:2218–22. [DOI] [PubMed] [Google Scholar]
- [22].Wildman RP, Farhat GN, Patel AS, et al. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension 2005;45:187–92. [DOI] [PubMed] [Google Scholar]
- [23].Rodríguez AJ, Karim MN, Srikanth V, et al. Lower muscle tissue is associated with higher pulse wave velocity: a systematic review and meta-analysis of observational study data. Clin Exp Pharmacol Physiol 2017;44:980–92. [DOI] [PubMed] [Google Scholar]
- [24].Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–63. [DOI] [PubMed] [Google Scholar]


