Supplemental Digital Content is available in the text
Keywords: drug-induced liver injury, prednisone, severity reduction
Abstract
There is limited information about the effects of corticosteroids on severe drug-induced liver injury (DILI). This study aimed to investigate the efficacy and safety of prednisone in severe DILI.
Ninety patients with severe DILI were enrolled and studied retrospectively. They were divided into prednisone (n = 66) and control groups (n = 24), undergoing the same treatment regimen except that patients in the prednisone group received a median daily dose of 40 mg prednisone. The primary endpoint was severity reduction (serum total bilirubin [TBIL] <86 μmol/L).
During the study, the cumulative rates of severity reduction at 4-, 8-, and 12 days were comparable between the 2 groups (prednisone versus control: 7.6%, 33.3%, and 60.6% versus 12.5%, 37.5%, and 66.7%, P = .331), and were markedly lower in the high-dose group than in the low-dose group (0%, 28.6%, and 35.7% versus 9.6%, 34.6%, and 67.3%, P = .012) or in the control group (0%, 28.6%, and 35.7% versus 12.5%, 37.5%, and 66.7%, P = .023). The 30-day overall survival rate in the prednisone group was significantly higher than in the control group (100% versus 91.7%, P = .018). Serum bilirubin and transaminase values gradually decreased in both groups, which were not significantly different mostly. Cox-regression models revealed that baseline TBIL (hazard ratio: 0.235; 95% confidence interval: 0.084–0.665; P = .006) was the only predictor for severity reduction. No severe adverse event was noted in both groups.
Prednisone therapy is safe but not beneficial, and even detrimental at a daily dose > 40 mg for the treatment of severe DILI.
1. Introduction
Drug-induced liver injury (DILI) refers to a type of liver injury driven by various prescription or nonprescription drugs, Traditional Chinese Medicine (TCM), healthcare products, and dietary supplements.[1] It is a serious health issue worldwide that accounts for approximately 50% of acute liver failure (ALF) cases in developed countries.[2–4] Recently, DILI cases have been increasingly reported. For example, the annual incidence rates for DILI were 19.1 and 34 cases per 100,000 in Iceland and Spain, respectively.[5,6] In China, reports about DILI cases were also increasing due to its huge population and easy access to the vast number of drugs, particularly the Chinese herbal medicines.[1]
DILI may present either asymptomatically with mildly increased liver enzymes, or with severe hepatic damage requiring liver transplantation, though the majority of patients have a favorable outcome. When the serum bilirubin is elevated above 3 times the standard value, DILI may progress to ALF with increased mortality.[4] Despite its pathogenesis being not fully clarified, accumulating evidences suggest that the immune and inflammatory responses play a very important role in the development and progression of DILI.[7] It is, thus, logical to hypothesize that immunosuppression with corticosteroids may be effective in the treatment of severe DILI or drug-induced ALF. However, previous studies reported that corticosteroids were not beneficial for the treatment of patients with drug-induced ALF, and were even detrimental in patients with model for end stage liver disease (MELD) scores above 40.[8–13] One possible explanation for this phenomenon may be that the liver damage in drug-induced ALF has been too advanced and beyond the point of salvage. Presumably, treatment with corticosteroids may be beneficial when DILI has not progressed to liver failure yet.
Therefore, we conducted this study to investigate the safety and efficacy of corticosteroids in treating patients with severe DILI that is at high risk of progression to ALF.
2. Patients and methods
All data were analyzed anonymously with waived individual patient consent due to the retrospective design of the study. The protocol was approved by the ethics committee of the Second Affiliated Hospital of Kunming Medical University and conformed to the Helsinki Declaration of 1975, as revised in 2008.
2.1. Patients and study design
We conducted a retrospective study of all patients with severe DILI who were treated at the Gastroenterology Department of the Second Affiliated Hospital of Kunming Medical University between January 2015 and June 2018. DILI was diagnosed according to the American College of Gastroenterology Guidelines for the diagnosis and management of drug-induced liver injury, which takes into account the patient's recent history of drug use, the relationship between suspicious drug intake and onset of liver test abnormalities, clinical presentation, biochemical tests, with or without histological examination, after excluding other forms of liver disease.[14] ALF was also diagnosed according to the American criteria that include evidence of jaundice (serum total bilirubin [TBIL] ≥171 μmol/L), coagulopathy with usually an international normalized ratio (INR) ≥1.5, and hepatic encephalopathy (any degree of mental alteration) in a patient without preexisting cirrhosis and with an illness of <26 weeks’ duration.[15,16] Severe DILI was defined as a type of DILI that is at high risk of progression to ALF, and its diagnosis is established when serum TBIL≥86 μmol/L along with normal or abnormal coagulation function, but not sufficient for the diagnosis of ALF.
Of a total of 195 consecutive patients with a diagnosis of DILI, only 90 patients were enrolled in this study. Inclusion criteria were age ≥18 years, and severe DILI. Exclusion criteria were coinfection with hepatitis B or C virus, chronic alcohol consumption, autoimmune liver disease (autoimmune hepatitis, primary biliary cholangitis, or primary sclerosing cholangitis), Wilson disease, biliary obstruction, acetaminophen toxicitiy, pregnancy, chronic heart, pulmonary or renal diseases, cirrhosis, or any known malignancy. The enrollment process of our study population is presented in Figure 1.
Figure 1.
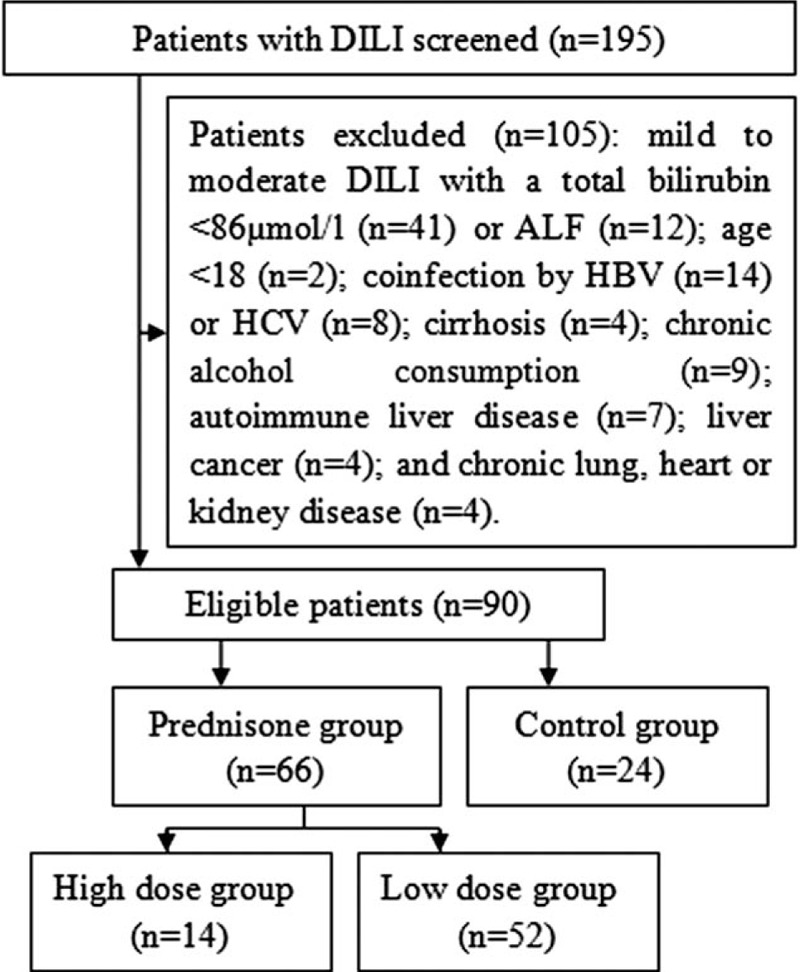
The flow chart of the study.
2.2. Treatment and follow-up protocol
During the hospitalization period, all patients received intravenous hepatic protective and regenerative agents (i.e., reduced glutathione, polyene phosphatidylcholine, glycyrrhizin, and ademetionine). Intravenous antibiotics and blood transfusion (i.e., human serum albumin, red blood cells, platelets, and fresh frozen plasma) were administered when indicated. Most patients underwent artificial liver support by either therapeutic plasma exchange (TPE) or double plasma molecular absorption system (DPMAS) as previously described.[17–19] Other supportive and symptomatic treatments were at the discretion of the responsible physicians. Whether to administer corticosteroids was entirely decided by a patient's responsible physician. However, the specific criteria adopted by each physician to administer corticosteroids were not available. The timing, route of administration, and dosing of corticosteroids were also at the discretion of each responsible physician, and were not uniform. After being discharged, all patients received oral hepatic protective and regenerative agents (i.e., polyene phosphatidylcholine, glycyrrhizin, and silymarin). Patients were followed up on a daily basis during the hospitalization period, and every 2 to 4 weeks when discharged at the outpatient department until the time of death or at least 3 months. Clinical, laboratory, and imaging data were prospective collected on admission, at 1 week, 2 weeks, and 3 months.
2.3. Endpoints
Primary endpoint was serum TBIL<86 μmol/L. Secondary endpoints were clinical improvement, changes of alanine aminotransferase (ALT), aspatate aminotransferase (AST), total bile acids (TBA), alkaline phosphatase (ALP), and γ-glutamyl transpeptidase (γ-GT), side effects, and overall mortality.
2.4. Statistical analysis
Continuous data were presented as median (range) and compared by Mann–Whitney U test. Categorical data were expressed as proportions and analyzed by χ2 test or the Fisher exact test. Survival curves were plotted by the Kaplan–Meier method and analyzed by the log-rank test. Univariate and multivariate analyses were performed to evaluate the predictors of primary endpoint using the Cox-proportional hazards regression model. To avoid the problem of overfitting and collinearity, only predictors with P < .05 in the univariate analysis were evaluated in the multivariate model, and then sequentially removed to establish the final model. Group was included in all multivariate models as it was the central predictor to evaluate. Moreover, TBIL and direct bilirubin (DBIL) were not included together in multivariate analysis because TBIL was derived from summation of DBIL and indirect bilirubin. Statistical analysis was performed using SPSS 17 (Chicago, IL). A P value of <.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of the study population
The baseline characteristics of patients in the 2 groups were summarized in Table 1. Overall, both groups were comparable with regard to age, gender, BMI, suspected cause of liver injury, concomitant diseases, liver function indices, pattern of liver injury, ascites, artificial liver support treatment, and hospital stay. Notably, TCM was the suspected cause of DILI in about a half of patients in the prednisone group and one-third in the control group. The median TBIL was 226.0 μmol/L in the prednisone group and 234.8 μmol/L in the control group (P = .562). The median PT was 13.7 seconds in the prednisone group and 14.0 seconds in the control group (P = .477). Hepatocellular pattern of liver injury accounted for 68.2% in the prednisone group and 58.3% in the control group (P = .543). Ascites was not detectable in 92.4% and 87.5% of patients in the prednisone and control groups, respectively (P = .245). If patients treated by prednisone were stratified into low-dose group receiving prednisone ≤ 40 mg daily and high-dose group receiving prednisone > 40 mg daily, the baseline characteristics of patients were also comparable among the 3 groups (Supplementary Table 1).
Table 1.
Baseline characteristics of the study population.

3.2. Corticosteroid dosage and timing
Overall, there were 41 patients in the prednisone group who received a daily dose of 40 mg oral prednisone, which resulted in a median daily dose of 40 mg (range: 15–70 mg). The prednisone was started at a median period of 21.5 days (range: 7–86 days) from the onset of liver injury, presenting mainly with anorexia, fatigue, nausea, vomiting, sickness of greasy food, and jaundice.
3.3. Effect of prednisone on severity reduction and its predictors
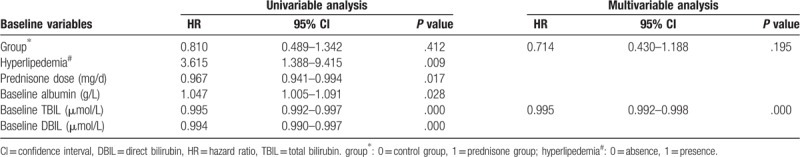
Since the definition of severe DILI by TBIL is set at the threshold of 5 times the upper limit of normal (ULN), we arbitrarily define the primary endpoint of our study as TBIL< 86 μmol/L (i.e., 5ULN). During the study, the cumulative rates of patients who reached the primary endpoint (i.e., severity reduction) at 4 days, 8 days, and 12 days were not different between the 2 group (prednisone group versus control group: 7.6%, 33.3%, and 60.6% versus 12.5%, 37.5%, and 66.7%, P = .331; Fig. 2A). However, when patients treated by prednisone were stratified into low-dose group receiving prednisone ≤ 40 mg daily and high-dose group receiving prednisone > 40 mg daily, the cumulative rates of patients who achieved severity reduction (i.e., primary endpoint) at 4 days, 8 days, and 12 days in the high-dose group were markedly lower than that in the low-dose group (0%, 28.6%, and 35.7% versus 9.6%, 34.6%, and 67.3%, P = .012; Fig. 2B) and the control group (0%, 28.6%, and 35.7% versus 12.5%, 37.5%, and 66.7%, P = .023; Fig. 2B), which were not different between the low-dose group and the control group (9.6%, 34.6%, and 67.3% versus 12.5%, 37.5%, and 66.7%, P = .816; Fig. 2B). In univariate analysis, group, prednisone dose, hyperlipidemia, baseline albumin, TBIL, and DBIL were significantly associated with the development of severity reduction (Table 2). In multivariate analysis, only baseline TBIL (hazard ratio[HR]: 0.995, 95% confidence interval [CI]: 0.992–0.998, P = .000) remained significantly associated with the occurrence of severity reduction (Table 2).
Figure 2.
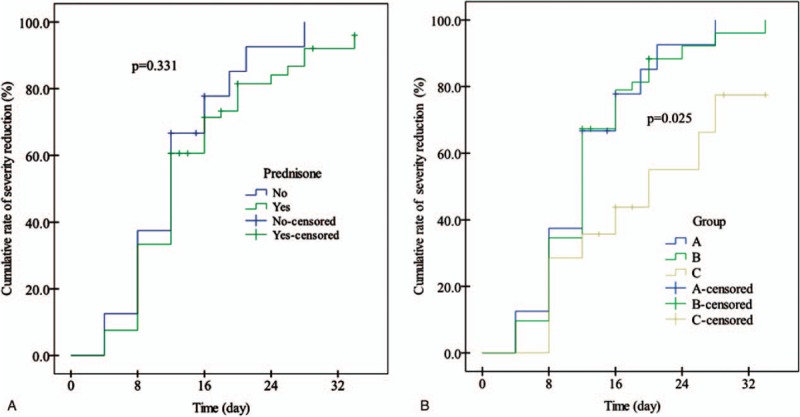
A, Cumulative rates (%) of reduction from severe DILI to moderate DILI in patients treated with and without prednisone (P = .331, by log-rank test). B, Cumulative rates (%) of reduction from severe DILI to moderate DILI among the 3 groups (P = .025), between A and C groups (P = .023), between B and C groups (P = .012), and between A and B groups (P = .816) (by log-rank test).
Table 2.
Univariate and multivariate analyses of the baseline factors predictive of severity reduction.
3.4. Effect of prednisone on clinical improvement and overall mortality
All patients had clinical improvement in fatigue, appetite, nausea, vomiting, greasy food sickness, and jaundice after 4 to 12 days of treatment, except 2 patients in the control group who experienced no clinical improvement and succumbed to ALF eventually on the 17th and 24th day, respectively. Thus, the cumulative rate of 30-day overall survival was markedly higher in the prednisone group than in the control group (100% versus 91.7%, P = .018; Fig. 3A). When the prednisone group was again divided into low-dose and high-dose groups, the cumulative rate of 30-day overall survival was significantly lower in the control group than in the low-dose group (91.7% versus 100%, P = .035; Fig. 3B), but was similar to the high-dose group (91.7% versus 100%, P = .275; Fig. 3B). Due to limited cases of death, we did not evaluate the predictors of mortality.
Figure 3.

A, Cumulative rate (%) of overall survival between patients treated with and without prednisone (P = .018, by log-rank test). B, Cumulative rates (%) of overall survival among the 3 groups (P = .060), between A and C groups (P = .257) and between A and B groups (P = .035) (by log-rank test).
3.5. Effect of prednisone on changes of liver function indices
During the study period, ALT, ALP, TBIL, and prothrombin time (PT) were gradually decreased, which though were not significantly different between the 2 groups (Fig. 4). Similarly, other liver function indices were also improved to comparable degrees at various time points, except ALT at the 12th day, γ-GT at the 4th and 8th days, and PT at the 3rd month in the control group were markedly lower than in the prednisone group (Supplementary Table 2).
Figure 4.
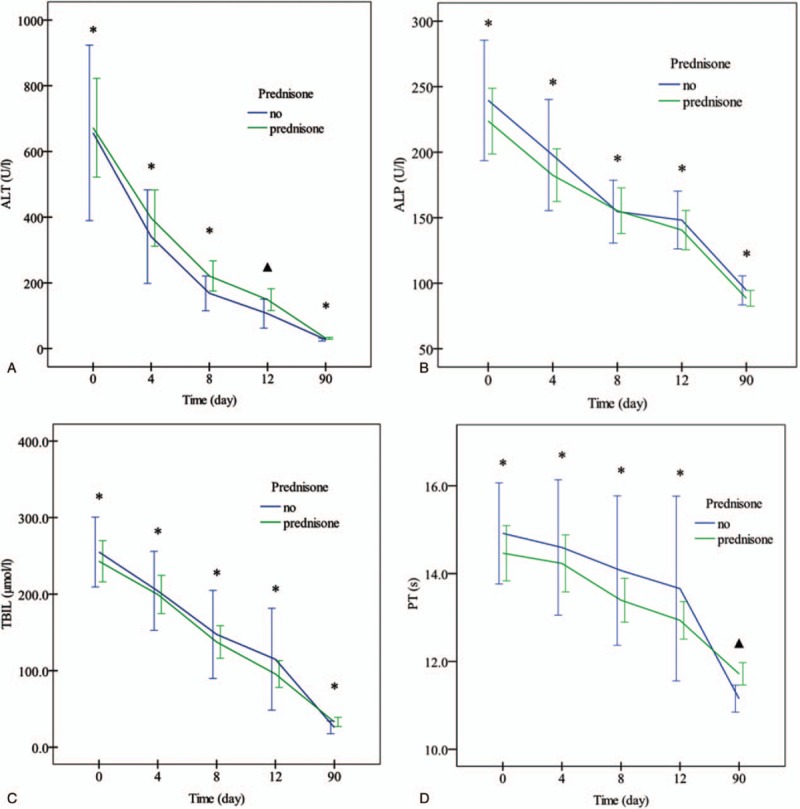
Serial changes of ALT (A), ALP (B), TBIL (C), and PT (D) in patients treated with and without prednisone.
3.6. Adverse event
Overall, prednisone was well tolerated in the prednisone group. Infection occurred in 12 patients in the prednisone group and 3 in the control group (12/66 versus 3/24, P = .522). No other severe adverse events were recorded.
4. Discussion
In the present study, we clearly demonstrated that prednisone treatment was not associated with improved 4-, 8-, and 12-day cumulative rates of achieving severity reduction from severe DILI to moderate or mild DILI compared to the control group (Fig. 2A). Similarly, prednisone treatment failed to improve at various time points other liver function indices, some of which (ALT, γ-GT, and PT) were even worse than the control group at some time points (supplementary Table 2). Despite the prednisone treatment resulted in statistically improved 30-day cumulative rate of overall survival, there were only 2 death cases in the control group and none in the prednisone group, which is too limited to make a definitive conclusion. Moreover, when the prednisone group was divided into 2 subgroups, the subgroup analyses even showed that prednisone when given at high dose (>40 mg/d) was associated with lower rates of achieving severity reduction than the control group or the low-dose group (Fig. 2B).
Patients with severe DILI are at risk of progression to ALF, presenting at the earliest stage with INR>1.5, development of ascites, or hepatic encephalopathy.[20] The therapeutic algorithm for severe DILI is poorly defined except for discontinuation of the culprit agents, supportive and symptomatic treatment, and close monitoring. For the treatment of ALF, the use of corticosteroids remains controversial. One recent study reported that corticosteroids improved the prognosis of ALF patients with the highest response rates observed in those with lower MELD scores and coma grades but with extremely high ALT levels,[21] whereas numerous other studies showed that corticosteroids were not effective in improving the prognosis of patients with ALF.[8–13] The discrepant results may be related to the following factors: the diagnostic criteria for ALF are not uniform in the these studies, which may cause heterogeneity in patient selection, and the condition of some patients may be too severe to allow for rescue with corticosteroid therapy; the causes of ALF are not identical in the these studies, some of which may be unresponsive to corticosteroid therapy; the timing and dose of corticosteroid intervention was different in these studies. Because corticosteroids have limited effect on hepatic regenerative capability over a short period of time, different timing and dose of corticosteroid intervention may lead to varied outcomes.[13]
In the present study, we chose to examine the effects of corticosteroids on patients with severe DILI that had identical cause of liver injury (exclusive of other causes of liver injury), less severe liver disease, and earlier corticosteroid intervention compared to ALF. However, our study still failed to demonstrate the benefit of corticosteroid therapy, which is in agreement with the previous studies[8–13] and contradicts with our previous hypothesis. Moreover, both the univariate and multivariate analyses further illustrated that prednisone treatment was not associated with severity reduction in our study. One possible explanation for lack of benefit with corticosteroid therapy for patients with severe DILI may be that the pathogenesis of severe DILI or ALF is very complicated, involving a complex interaction between chemical properties of the drug, environmental, genetic, immune, and other host factors,[22] which cannot be simplified into immune and inflammatory responses that are responsive to corticosteroid therapy. And other facets of its pathogenesis may possibly be unresponsive to corticosteroids, and DILI cased by different culprit agents may have a different pathogenesis. This is true in autoimmune ALF, in which patients with more chronic presentations have been shown to be more responsive to corticosteroids than patients with acute presentations. Histologically, centrilobular necro-inflammatory feature, a feature of severe and therapy-refractory autoimmune hepatitis, and acute cellular rejection in transplant recipients, were more frequently seen in patients with acute presentations of autoimmune ALF than those with classical autoimmune hepatitis.[8,23,24] Another possible explanation for lack of benefit with corticosteroid therapy in severe DILI may be that corticosteroid dose and duration in the present study is not sufficient to achieve potential benefits or that corticosteroids may achieve the potential benefits when used in combination with other agents. Indeed, a previous study reported that combined therapy using prednisone in high doses (2–20 mg/kg/d) with ursodesoxycholic acid was effective in the treatment of DILI, in which the bilirubin and transaminase levels decreased to below half of the peak values within 2 weeks and normalized in 4 to 8 weeks after receiving combined therapy.[25] Nonetheless, in our study, subgroup analysis revealed that high-dose group (prednisone>40 mg/d) was associated with markedly lower rates of severity reduction when compared to the low-dose group (prednisone≤40 mg/d) or the control group, which suggested that prednisone when given at high dose might be detrimental in patients with severe DILI (Fig. 2B). Because prednisone is a prodrug that is biologically active only after being converted to prednisolone by the liver, a third possible explanation for lack of benefit may be that patients with severe DILI or ALF were unable to convert prednisone into prednisolone due to severely impaired liver function, thus it is not effective in this cohort of patients.
The prognosis of DILI is generally favorable. In the majority, clinical improvement is observed after drug cessation, although liver injury may worsen for up to a few weeks in some patients.[25,26] DILI is rarely associated with mortality.[4,27] In line with these studies,[4,27] there were only 2 death cases (2.2%) in our study. As for the effect of corticosteroid therapy on the prognosis of patients with severe DILI, data are very limited due to its low mortality rate. In our study, the 30-day overall survival rate in the prednisone group was shown to be statistically higher than in the control group. However, the death cases were too limited to draw any meaningful conclusion, and a larger study may give a different result.
It is generally concerned that corticosteroid therapy may be associated with increased risk of infectious and gastrointestinal bleeding complications. In our study, the rates of infection were not significantly different between the 2 groups, which is in agreement with the previous study.[8] No patient had gastrointestinal bleeding. Other side effects such as disturbed control of blood glucose and blood pressure were not common despite daily measurements, which is probably related to the low enrollment rate of patients with hypertension or diabetes mellitus (Table 1).
Our study had several limits: first, it is a retrospective study and the use of prednisone was neither randomized nor standardized. Thus, the dose and duration was not uniform, which might bias or confound our results. However, we performed subgroup analyses which still indicated that prednisone therapy was not beneficial in increasing the rate of severity reduction, and high-dose group (>40 mg/d) might even be associated with worse results than the control group or the low-dose group. Second, the sample size is small, and follow-up duration is relatively short. However, because all survived patients had completely or markedly improved liver function at 3 months, follow-up beyond 3 months will not impact our results. Third, we failed to measure the serum level of prednisolone, the bioactive form of prednisolone, in all patients; therefore, we cannot provide evidence to clarify the third possible explanation for lack of benefit, which warrants future investigations.
In conclusion, our study demonstrates no benefit of prednisone therapy for patients with severe DILI, though its safety is favorable.
Author contributions
Wan Yue-Meng: patient management; acquisition of data; analysis and interpretation of data; drafting manuscript; draft revision; critical revision of the manuscript for important intellectual content.
Jie-Fang Wu: acquisition of data; analysis and interpretation of data.
Yu-Hua Li, Hua-Mei Wu: patient management; acquisition of data; analysis and interpretation of data.
Xi-Nan Wu: study concept and design; statistical analysis.
Ying Xu: study design; administrative, technical, or material support; study coordination and supervision.
Conceptualization: Yue-Meng Wan, Ying Xu.
Data curation: Yue-Meng Wan, Jie-Fang Wu, Yu-Hua Li, Hua-Mei Wu, Xi-Nan Wu.
Formal analysis: Yue-Meng Wan, Yu-Hua Li, Hua-Mei Wu, Xi-Nan Wu, Ying Xu.
Investigation: Yue-Meng Wan, Jie-Fang Wu, Yu-Hua Li, Hua-Mei Wu.
Project administration: Ying Xu.
Writing – original draft: Yue-Meng Wan.
Writing – review & editing: Ying Xu.
Supplementary Material
Footnotes
Abbreviations: γ-GT = γ-glutamyl transpeptidase, ALF = acute liver failure, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspatate aminotransferase, CI = confidence interval, DBIL = direct bilirubin, DILI = drug-induced liver injury, DPMAS = double plasma molecular absorption system, HR = hazard ratio, INR = international normalized ratio, MELD = model for end stage liver disease, PT = prothrombin time, TBA = total bile acids, TBIL = total bilirubin, TCM = Traditional Chinese Medicine, TPE = therapeutic plasma exchange, ULN = upper limit of normal.
Y-MW and J-FW share the first authorship.
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Individual written informed consent for participation in this study was obtained from all patients or their next-of-kins.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Drug-induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the management of drug-induced liver injury (In Chinese). J Clin Hepatol 2015;31:1752–8. [Google Scholar]
- [2].Grant LM, Rockey DC. Drug-induced liver injury. Curr Opin Gastroenterol 2012;28:198–202. [DOI] [PubMed] [Google Scholar]
- [3].Canbay A, Jochum C, Bechmann LP, et al. Acute liver failure in a metropolitan area in Germany: a retrospective study (2002-2008). Z Gastroenterol 2009;47:807–13. [DOI] [PubMed] [Google Scholar]
- [4].Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008;135:1924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bjornsson E, Bergmann OM, Bjornsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013;144:1419–25. [DOI] [PubMed] [Google Scholar]
- [6].Andrade RJ, Lucena MI, Fernández MC, et al. Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005;129:512–21. [DOI] [PubMed] [Google Scholar]
- [7].Sebode M, Schulz L, Lohse AW. Autoimmune(-Like)” drug and herb induced liver injury: new insights into molecular pathogenesis. Int J Mol Sci 2017;18: E1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Karkhanis J, Verna EC, Chang MS, et al. Steroid use in acute liver failure. Hepatology 2014;59:612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ware AJ, Jones RE, Shorey JW, et al. A controlled trial of steroid therapy in massive hepatic necrosis. Am J Gastroenterol 1974;62:130–3. [PubMed] [Google Scholar]
- [10].Randomised trial of steroid therapy in acute liver failure. Report from the European Association for the Study of the Liver (EASL). Gut 1979; 20:620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rakela J, Mosley JW, Edwards VM, et al. A double-blinded, randomized trial of hydrocortisone in acute hepatic failure. The Acute Hepatic Failure Study Group. Dig Dis Sci 1991;36:1223–8. [DOI] [PubMed] [Google Scholar]
- [12].Ichai P, Duclos-Vallée JC, Guettier C, et al. Usefulness of corticosteroids for the treatment of severe and fulminant forms of autoimmune hepatitis. Liver Transpl 2007;13:996–1003. [DOI] [PubMed] [Google Scholar]
- [13].Fujiwara K, Yasui S, Yonemitsu Y, et al. Efficacy of high-dose corticosteroid in the early stage of viral acute liver failure. Hepatol Res 2014;44:491–501. [DOI] [PubMed] [Google Scholar]
- [14].Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950–66. [DOI] [PubMed] [Google Scholar]
- [15].Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology 2005;41:1179–97. [DOI] [PubMed] [Google Scholar]
- [16].Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology 2012;55:965–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wan YM, Li YH, Xu ZY, et al. Therapeutic plasma exchange versus double plasma molecular absorption system in hepatitis B virus-infected acute-on-chronic liver failure treated by entercavir: a prospective study. J Clin Apher 2017;32:453–61. [DOI] [PubMed] [Google Scholar]
- [18].Yue-Meng W, Yang LH, Yang JH, et al. The effect of plasma exchange on entecavir-treated chronic hepatitis B patients with hepatic de-compensation and acute-on-chronic liver failure. Hepatol Int 2016;10:462–9. [DOI] [PubMed] [Google Scholar]
- [19].Wan YM, Li YH, Xu ZY, et al. Tenofovir Versus Entecavir for the treatment of acute-on-chronic liver failure due to reactivation of chronic hepatitis B with genotypes B and C. J Clin Gastroenterol 2019;53:e171–7. [DOI] [PubMed] [Google Scholar]
- [20].Wei G, Bergquist A, Broomé U, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med 2007;262:393–401. [DOI] [PubMed] [Google Scholar]
- [21].Zhao B, Zhang HY, Xie GJ, et al. Evaluation of the efficacy of steroid therapy on acute liver failure. Exp Ther Med 2016;12:3121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kaplowitz N. Drug-induced liver injury. Clin Infect Dis 2004;38suppl 2:S44–8. [DOI] [PubMed] [Google Scholar]
- [23].Stravitz RT, Lefkowitch JH, Fontana RJ, et al. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology 2011;53:517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kessler W, Cummings O, Eckert G, et al. Fulminant hepatic failure as the initial presentation of acute autoimmune hepatitis. Clin Gastroenterol Hepatol 2004;2:625–31. [DOI] [PubMed] [Google Scholar]
- [25].Wree A, Dechêne A, Herzer K, et al. Steroid and ursodesoxycholic acid combination therapy in severe drug-induced liver injury. Digestion 2011;84:54–9. [DOI] [PubMed] [Google Scholar]
- [26].Björnsson E. The natural history of drug-induced liver injury. Semin Liver Dis 2009;29:357–63. [DOI] [PubMed] [Google Scholar]
- [27].Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 2005;42:481–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


