Abstract
Zika virus (ZIKV) is a re-emerged flavivirus transmitted by Aedes spp mosquitoes that has caused outbreaks of fever and rash on islands in the Pacific and in the Americas. These outbreaks have been associated with neurologic complications that include congenital abnormalities and Guillain-Barré syndrome (GBS). The pathogenesis of ZIKV-associated GBS, a potentially life-threatening peripheral nerve disease, remains unclear. Because Schwann cells (SCs) play a central role in peripheral nerve function and can be the target for damage in GBS, we characterized the interactions of ZIKV isolates from Africa, Asia and Brazil with human SCs in comparison with the related mosquito-transmitted flaviviruses yellow fever virus 17D (YFV) and dengue virus type 2 (DENV2). SCs supported sustained replication of ZIKV and YFV, but not DENV. ZIKV infection induced increased SC expression of IL-6, interferon (IFN)β1, IFN-λ, IFIT-1, TNFα and IL-23A mRNAs as well as IFN-λ receptors and negative regulators of IFN signaling. SCs expressed baseline mRNAs for multiple potential flavivirus receptors and levels did not change after ZIKV infection. SCs did not express detectable levels of cell surface Fcγ receptors. This study demonstrates the susceptibility and biological responses of SCs to ZIKV infection of potential importance for the pathogenesis of ZIKV-associated GBS.
Subject terms: Pathogens, Dengue virus, Viral pathogenesis, Peripheral nervous system
Introduction
Zika virus (ZIKV) is a plus-strand enveloped RNA virus that belongs to the genus Flavivirus and is related to other mosquito-borne flaviviruses, including dengue, yellow fever, West Nile, Japanese encephalitis and Spondweni viruses1. Aedes spp. mosquitoes are the main vectors for ZIKV, yellow fever virus (YFV) and all four types of dengue virus (DENV) but ZIKV can also be transmitted through infected semen and from mother to fetus2–4. ZIKV was first isolated from primates in Uganda in 1947, from Aedes africanus mosquitoes in 19485 and from a febrile man working in Uganda in 19636. For the next five decades sporadic cases of benign, self-limiting acute illness characterized by fever, headache, myalgia and rash were identified in Asia and Africa7–9.
The first recognized large outbreak of human ZIKV infection occurred in 2007 on the Pacific island of Yap, in the Federated States of Micronesia10. Subsequent outbreaks beginning in October 2013 and continuing throughout 2014 were recognized across Oceania in four groups of Pacific islands: French Polynesia, Easter Island, the Cook Islands, and New Caledonia with 8,750 reported cases11. In May 2015, locally acquired ZIKV disease was recognized in Brazil12 followed by rapid spread through the Americas and Caribbean islands with accompanying reports of neurological complications and congenital malformations including microcephaly13–15.
The first association of ZIKV infection with Guillain-Barré syndrome (GBS) was a retrospective study of the French Polynesian outbreak where there was a 20-fold increase in GBS incidence and all of the 42 patients hospitalized with GBS had anti-ZIKV antibody16. During the ZIKV outbreak in South America, 66 of the 68 patients with GBS in Colombia had symptoms compatible with ZIKV infection before the onset of GBS17 and in Martinique, a prospective study determined that the incidence rate of GBS during the 2016 ZIKV outbreak was 4.52 times that of prior years18. In 2016, the World Health Organization officially recognized ZIKV as a cause of GBS and microcephaly19.
GBS, a potentially life-threatening peripheral nerve disease, is characterized by a rapid onset of bilateral weakness progressing to paralysis that may be accompanied by sensory symptoms. GBS typically occurs 1–3 weeks after an infectious disease postulated to trigger a pathogen-specific immune response that cross-reacts with peripheral nervous system (PNS) antigens20. The most common subtypes of GBS are acute motor axonal neuropathy (AMAN) and acute inflammatory demyelinating polyneuropathy (AIDP), differentiated by the site of immune-mediated injury21. In AMAN, membranes of the nerve axon are the primary targets of cross-reactive anti-ganglioside antibodies while in AIDP the Schwann cell (SC)-produced myelin sheath is the target for damage but the relative roles and specificities of antibody and cellular immune responses are unclear20.
Several studies have sought to determine the type of GBS associated with ZIKV infection. Although in silico comparison of the ZIKV protein sequence with human proteins has identified shared peptides22, there is no evidence of disease-relevant cross reactivity so the pathogenesis of ZIKV-associated GBS remains unclear. The electrophysiological findings from the French Polynesian GBS cases were reported to be compatible with the AMAN subtype of GBS, but the typical AMAN-associated anti-ganglioside antibodies were rarely detected16. In Colombia, nerve-conduction studies and electromyography were consistent with the AIDP subtype of GBS17 and the electrophysiologic findings from Martinique were also consistent with AIDP further suggesting that SCs were the target for ZIKV-associated damage18.
SCs develop from neural crest cells, are the myelinating glial cells of the PNS and play a central role in peripheral nerve function, maintenance and repair. The SC myelin sheath enables saltatory conduction of action potentials by large diameter axons but SCs also ensheath and maintain axons that are not myelinated. In response to nerve injury SCs can trans-differentiate into a proliferating cell capable of secreting inflammatory mediators that enhance macrophage-mediated myelin removal23 and can initiate and regulate local immune responses24,25. For instance, SCs can be induced to express MHC class I and II molecules, inflammatory cytokines (e.g. TNFα, IL-1β, IL-6, LIF, IL-12, IL-18), chemokines (e.g. CCL2, CCL3, CXCL10) and nitric oxide synthase (iNOS) in response to damage or toll-like receptor (TLR) stimulation26–29. Induction of cytokines, chemokines and cell surface immunoregulatory proteins by infection of SCs could promote development of potentially damaging virus- or host cell-specific immune responses.
Because GBS is associated with ZIKV infection and often occurs when symptoms of ZIKV infection are still present and ZIKV RNA is detectable, it has been postulated that GBS might be directly associated with ZIKV infection of peripheral nerve cells or the immune response to viral antigens expressed by these cells17,30–32. To evaluate the potential for direct ZIKV-induced peripheral nerve damage, we assessed the susceptibility of immortalized primary human Schwann cells (hSCs)33 to infection with strains of ZIKV from Africa, Asia and Brazil. Infection with ZIKV was compared to infection with flaviviruses YFV 17D and DENV2 that are less commonly associated with GBS34,35. All strains of ZIKV and YFV, but not DENV2, replicated well in hSCs and induced innate responses and cytopathic effect.
Results
Human Schwann cells are more susceptible to infection with ZIKV and YFV than DENV
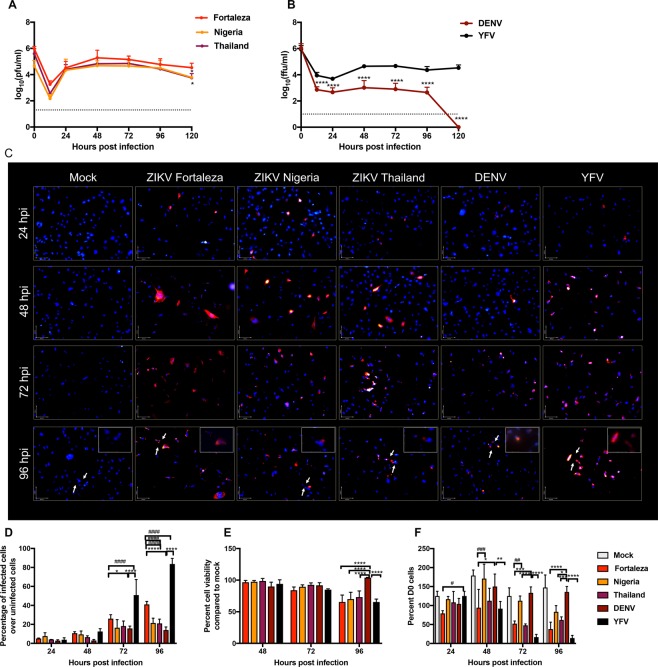
Immortalized hSCs express SC-characteristic proteins (e.g. S100B), transcription factors (e.g. Slug, TWIST), cell surface receptors (e.g. p75NTR), chemokines (e.g. CCL2) and neurotrophic factors (e.g. NGF, BDNF, NT-3) and can myelinate axons in vitro36, but have not been evaluated for susceptibility or response to virus infection. To determine susceptibility of hSCs to flavivirus infection, cells were incubated (MOI = 5) with YFV 17D, DENV2 and three strains of ZIKV: 1968 Nigeria, 2014 Thailand and 2015 Brazil (Fortaleza) chosen to represent the historical shifts of ZIKV as it moved from Africa to Asia and finally the Americas37. Supernatant fluids, collected from 0 to 120 h after infection, were analyzed by plaque assay (ZIKV, Fig. 1A) or focus forming assay (YFV and DENV, Fig. 1B). The three ZIKV strains replicated well with the highest virus production by ZIKV Brazil (Fortaleza) that peaked at 48 h and continued through 120 h (Fig. 1A). YFV 17D also replicated in hSCs, but DENV2 infection resulted in little virus production (Fig. 1B).
Figure 1.
- Replication of ZIKV strains 1968 Nigeria (IBH 30656), 2014 Thailand (SCV0127/14) and 2015 Brazil (Fortaleza), YFV (17D) and DENV2 (NGC) in immortalized human Schwann cells. (A) hSC cells were infected with strains of ZIKV from Africa (Nigeria), Asia (Thailand) and Brazil (Fortaleza) (MOI = 5). Virus production was measured by plaque formation in Vero cells. Each value represents the average +/− standard deviation from three independent experiments. *P < 0.05 (Fortaleza vs Nigeria/Thailand). (B) hSC cells were infected with DENV2 and YFV (MOI = 5). Virus production was measured by focus formation in Vero cells. Each value represents the average +/− standard deviation from three independent experiments ****P < 0.0001 (DENV vs YFV). (C) Immunofluorescence images of mock, ZIKV Fortaleza, ZIKV Nigeria, ZIKV Thailand, DENV, and YFV infected hSCs at 24-96 h after infection (MOI = 5). Cells were stained with pan flavivirus 4G2 antibody followed by anti mouse Alexafluor594 (red). Nuclei were stained with DAPI (blue). The insets show a higher magnification of infected cells (400X). (D) The number of infected cells (red) as a percentage of total cells remaining in the culture (blue). Each value represents the average +/− standard deviation from three different fields. *P < 0.05 ****P < 0.0001 DENV vs Fortaleza/YFV, ####P < 0.0001 Fortaleza vs Nigeria/Thailand/DENV/YFV (E) hSC viability after infection with three strains of ZIKV, YFV and DENV (MOI = 0.1) as determined by MTT assay. Readings taken at 570 nm were plotted as a percentage of the value for mock-infected cells. Each value represents the average +/− standard deviation from four independent infections. ****P < 0.0001 (DENV vs Fortaleza/Nigeria/Thailand/YFV) (F) hSC viability after infection with three strains of ZIKV, YFV and DENV (MOI = 5) as determined by trypan blue exclusion. Each value represents the average +/− standard deviation from three independent experiments of the numbers of viable cells compared to d0 expressed as a percentage. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (DENV vs Fortaleza/Nigeria/Thailand/YFV), #P < 0.05, ##P < 0.01, ###P < 0.001 (Fortaleza vs Nigeria/YFV).
To further evaluate the effects of ZIKV, YFV and DENV infection of hSCs, cells were stained for expression of viral protein and visualized by immunofluorescence microscopy 24–96 h after infection (Fig. 1C). Percentages of cells remaining in the cultures that were infected were quantified (Fig. 1D) and cell viability was determined by MTT metabolism (Fig. 1E) and trypan blue exclusion (Fig. 1F). The percentages of cells expressing detectable viral protein at 48 h when there is little cytopathic effect were: ZIKV Fortaleza 10.7%; ZIKV Nigeria 9.5%; ZIKV Thailand 6.5%; DENV 3.0%; and YFV 12.5%. At 72 and 96 h the numbers of cells in cultures infected with ZIKV Fortaleza, ZIKV Thailand and YFV were greatly diminished (Fig. 1F) and most of the remaining cells were infected (Fig. 1D). Individual ZIKV and YFV-positive cells showed more extensive expression of viral proteins than DENV2-infected cells (Fig. 1C).
To evaluate the response of hSCs to flavivirus infection, cell viability was determined using the MTT mitochondrial function (Fig. 1E) and trypan blue exclusion cell permeability assays (Fig. 1F). Infection at a low MOI of 0.1 for the MTT assay showed significant cell death at 96 h for all three strains of ZIKV and YFV but not DENV2. Infection at a higher MOI of 5 for the trypan blue assay showed evidence of cell death in ZIKV Fortaleza-infected cells at 24 h ZIKV Thailand-infected cells at 48 h, while many ZIKV Nigeria-infected cells were viable 96 h after infection. Cell death was most extensive for YFV-infected cells and few cells remained by 72 h (Fig. 1D,F) and most of the remaining cells were infected (Fig. 1D). Therefore, hSCs were similarly susceptible to evolutionarily distinct ZIKV strains with the Brazilian strain producing the most virus and the African strain the least cytopathic effect. However, hSCs were comparatively resistant to infection with DENV2.
ZIKV Fortaleza and YFV infections of human Schwann cells induced expression of innate responses
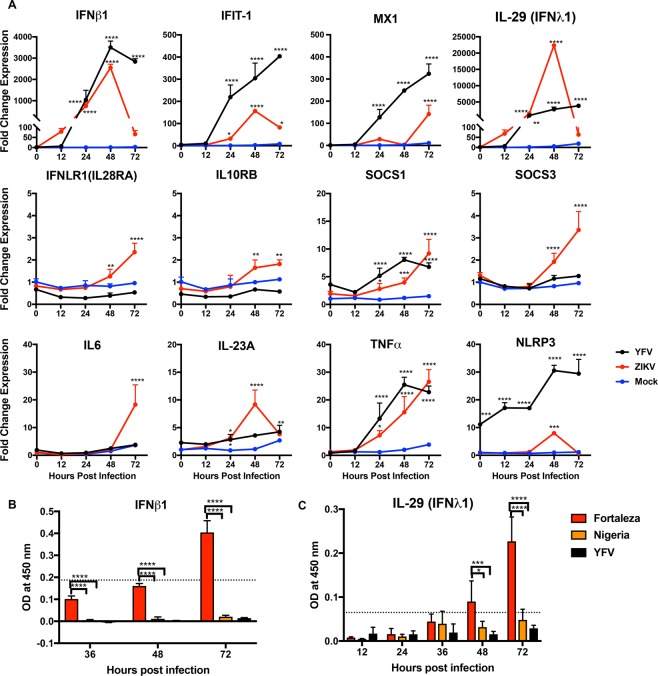
Interferon (IFN) is an important regulator of cell susceptibility to infection. To determine the responses of hSCs to ZIKV Fortaleza and YFV 17D infection, which had similar levels of replication and similar response (Fig. 1), we analyzed expression of mRNAs for IFN and other IFN pathway proteins (Fig. 2). Levels of mRNAs for IFNβ1 and IFN response gene proteins Mx1, a GTPase induced by type I and type III IFNs38, and IFN-induced protein with tetratricopeptide repeats (IFIT)-1 were upregulated after infection with both YFV and ZIKV with the greatest response induced by YFV. However, IFN β1 protein production was detectable only in ZIKV Fortaleza-infected cells (119 ± 18.9 pg/ml at 72 h) (Fig. 2B). IFNλ1 (IL-29) was highly induced in ZIKV-infected hSCs at 48 h after infection and in YFV-infected hSCs at 48 h and 72 h (Fig. 2A). ZIKV infection also increased expression of mRNAs for both IFNLR1 and IL10RB components of the IFNλ heterodimeric receptor complex and the SOCS1 and SOCS3 negative regulators of IFN signaling39. The levels of IFNλ1 (IL-29) protein at 24 and 36 h were similar, but at 48 and 72 h ZIKV Fortaleza-infected cells produced more IFNλ1 (Fig. 2B). At 48 h protein levels for ZIKV Fortaleza-infected cells were 37.8 ± 10.8 pg/ml and at 72 h were 75.2 ± 16.4 pg/ml for ZIKV Fortaleza and 11.05 pg/ml for ZIKV Nigeria.
Figure 2.
Expression of immune response protein mRNAs and proteins induced by ZIKV and YFV infection of human Schwann cells. (A) hSC cells were infected with ZIKV Fortaleza and YFV (17D) (MOI = 5) and mRNA expression was measured by qRT-PCR. ZIKV-infected cells (red) and YFV-infected cells (black) were compared to mock-infected cells (blue). CT values were normalized to Gapdh and fold change was calculated relative to uninfected 0-hour (ΔΔCT) data. Each value represents the average +/− SD from three independent experiments *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (mock vs infected). (B,C) hSC cells were infected with ZIKV (Fortaleza and Nigeria) and YFV (17D) (MOI = 5) and supernatant fluids were collected. Levels of IFNβ1 (B) and IFNλ1/IL-29 (C) protein were measured by EIA and optical density was plotted. Data are presented as the mean ± SD for three independent infections. *P < 0.05, ***P < 0.001, ****P < 0.0001(Fortaleza vs YFV/Nigeria).
To determine whether ZIKV or YFV upregulates hSC expression of other innate response gene mRNAs, levels of mRNAs for representative innate cytokines interleukin (IL)-6, IL-23A and tumor necrosis factor (TNF)-α as well as inflammasome protein NLRP3 were measured (Fig. 2). IL-6 and IL-23A, an innate cytokine related to IL-12 that is associated with induction of autoimmunity40, were induced only by ZIKV. Expression of TNFα mRNA was induced in both ZIKV and YFV-infected hSCs. The NLRP3 inflammasome protein mRNA was induced by YFV, but only transiently by ZIKV. Therefore, ZIKV Fortaleza and YFV infections induced overlapping, but distinct innate immune responses in hSCs.
Expression of potential receptor and immunomodulatory protein mRNAs by hSCs
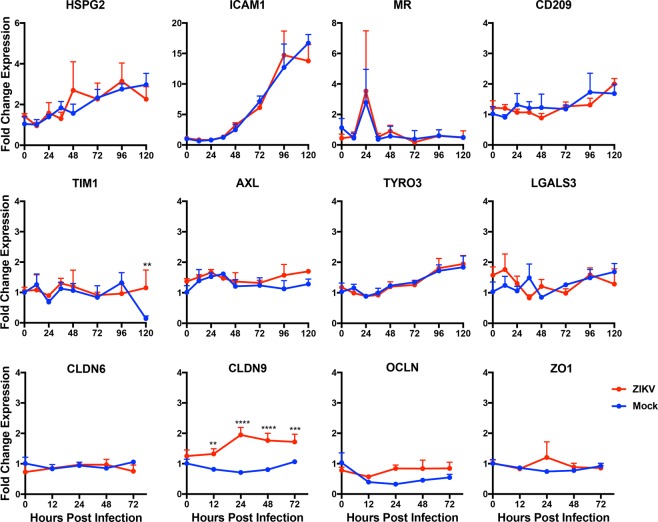
Like other flaviviruses, ZIKV probably can use multiple receptors for host cell attachment, clathrin-mediated endocytosis, pH-dependent membrane fusion and entry41–43. Cell surface molecules of potential importance for ZIKV infection include the C-type lectin receptors CD209 (DC-SIGN), CLEC5a and mannose receptor (MR) and the phosphatidyl serine receptors T-cell immunoglobulin and mucin domain (TIM)-1 and TAM receptors Tyro3 and AXL42–45. To begin to identify factors that may facilitate flavivirus infection of hSCs, we analyzed expression of mRNAs for protein receptor families reported to mediate entry (Fig. 3). Baseline transcripts were present for heparan sulfate proteoglycan (HSPG2), an attachment protein for many flaviviruses42; intercellular adhesion molecule -1 (ICAM-1); MR, a receptor for DENV46 that colocalizes with MHC class II during phagocytosis by SCs47; C-type lectin CD20948,49; and TIM1 and TAM receptors42,50. We also assessed the modulation of receptor expression during culture and infection. HSPG2 and ICAM-1 mRNA expression increased during culture of hSCs, but this was not affected by ZIKV infection. MR mRNA expression showed a transient increase at 24 h in both infected and uninfected cells. There was little change and no effect of infection on expression of CD209, TIM1, AXL or Tyro3. The intercellular cell adhesion molecule (ICAM-1) a cell surface receptor that can be up regulated by IFNγ, IL1β and TNFα in SCs51 showed increased mRNA expression during culture but was not modulated by ZIKV infection.
Figure 3.
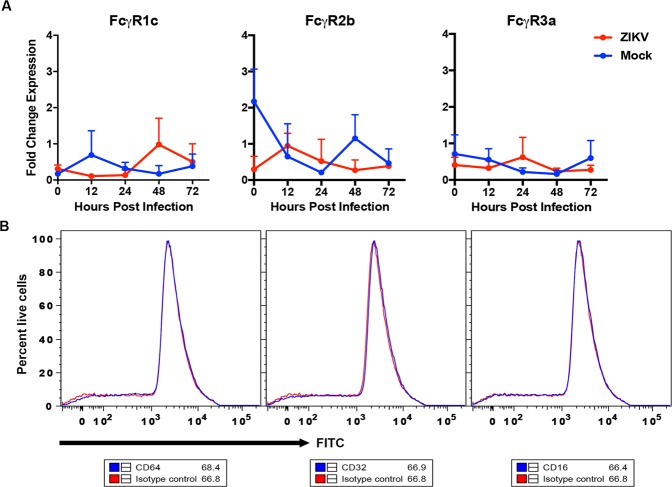
Expression of Fcγ before and after ZIKV infection of human Schwann cells. (A) hSC cells were infected with ZIKV Fortaleza at an MOI of 5 and Fcγ receptor mRNA was measured by qRT-PCR. CT values were normalized to Gapdh, and fold change was calculated relative to uninfected 0-hour (ΔΔCT) data. Each value represents the mean +/− SD from three independent experiments. (B) hSC cells were stained with Fcγ receptor-specific antibodies and analyzed by flow cytometry. The blue line in the histogram corresponds to specific staining with the receptor antibody while the red line corresponds to staining with the isotype control antibody. Data are representative of 3 independent sets of experiments.
Expression and modulation of functionally important SC protein mRNAs
Because SC responses to injury can affect peripheral nerve function we also assessed the effect of ZIKV infection on expression of several response-relevant protein mRNAs (Fig. 3). Galectin3 (LGALS3) promotes proinflammatory cytokine expression through the activation of pattern recognition receptor and boosts the phagocytic activity of SCs in injured sciatic nerve in Wallerian degeneration52, but levels of mRNA were not modulated by ZIKV infection. Several types of junctional specializations are formed in myelinating SCs between membrane lamellae, act as autotypic junctions and are disrupted during infection of retinal epithelial cells53,54. In addition, claudin 1 interacts with the prM protein of DENV to facilitate entry55,56. Therefore, we assessed expression of mRNAs for claudins (CLDN) 6 and 9, occludin (OCLN) and zona occludens (ZO)-1. CLDN9 was up regulated in ZIKV-infected hSCs, but other junctional protein mRNAs were not affected by infection.
Expression of Fcγ receptors (FcγR) by hSCs
Cross-reacting non- or sub-neutralizing antibodies can enhance flavivirus infection of FcγR-bearing cells by providing a route of infection for virions coated with non-neutralizing antibodies distinct from binding to the virus cellular receptor and endocytosis57–61. SCs in peripheral nerves have been reported to express FcγRII and FcγRIII62,63. To determine whether immortalized hSCs express FcγR mRNAs and whether expression is modulated by infection, we assessed the levels of FcγRIc, FcγRIIb or FcγRIIIa mRNAs before and after ZIKV infection (Fig. 4A). Baseline transcripts were detected, but levels were not affected by ZIKV infection. To determine whether FcγR protein was expressed on the cell surface uninfected hSCs were stained with antibodies specific for CD64 (FcγRI), CD32 (FcγRII) and CD16 (FcγRIII) and compared with isotype-specific control antibodies by flow cytometry (Fig. 4B). There was no evidence of FcγR expression on the cell surface.
Figure 4.
Expression of potential flavivirus receptors. hSC cells were infected with ZIKV (Fortaleza) at an MOI of 5 and levels of cellular receptor mRNA were measured by qRT-PCR. Mock-infected cells (blue) were compared to ZIKV-infected cells (red). CT values were normalized to Gapdh, and fold change was calculated relative to uninfected 0-hour (ΔΔCT) data. Each value represents the average +/− SD from three independent experiments **P < 0.01, ***P < 0.001, ****P < 0.0001 (mock vs infected).
Discussion
ZIKV was declared a “Public Health Emergency of International Concern” by the World Health Organization because of the neurological complications of infection: microcephaly and GBS19. Evidence that ZIKV triggers the AIDP type of GBS that targets SCs suggested the possibility that ZIKV induces GBS by damaging SCs either directly through infection or through the immune response to viral rather than cellular antigens17,64. To assess the potential for ZIKV to infect SCs in comparison with other flaviviruses we analyzed the outcome of ZIKV, YFV and DENV interactions with immortalized primary human SCs33. SCs were susceptible to infection with evolutionarily distinct strains of ZIKV and to infection with YFV 17D, but not DENV2. SCs responded to infection with increased expression of mRNAs for IFNβ, IFNλ, IFIT-1, Mx1, NLRP3, IL-23A, IL-6 and TNFα. Baseline transcripts were present for several potential ZIKV receptors and claudin 9 mRNA was increased by infection. hSCs expressed Fcγ receptor mRNAs, but FcγR protein was not detected on the cell surface. Therefore, hSCs are susceptible to ZIKV infection and mount a multifaceted innate response, but are unlikely to be targets for antibody-dependent enhanced infection.
There is substantial variation in susceptibility of human cells to flavivirus infection and ZIKV tropism is generally broader than that of DENV45,65. The current studies show that hSCs are susceptible to infection with ZIKV and YFV 17D, but not DENV2. Previous in vitro studies have shown ZIKV replication in cells from the skin, lung, placenta, muscle, retina, brain, liver, colon, prostate, testes and kidney43,53,65–68. Phylogenetic analysis identifies African and Asian lineages of ZIKV with recent outbreaks due to spread of an Asian strain that had acquired mutations potentially relevant to neurovirulence into the Americas37,69–71. However, there is limited evidence of strain-specific variation in ZIKV replication in vitro or virulence to explain differences in induction of human neurologic disease72. African strains replicate better than Asian strains in 293 T embryonic kidney cells and neural cells and induce more cell death while Asian strains are more likely to establish persistent infection66,73,74. American strains replicate better in placental explants and endothelial cells than African strains45,75. In our studies Brazilian ZIKV Fortaleza replicated better and induced more rapid cell death in hSCs than African ZIKV Nigeria.
The 17D vaccine strain of YFV also infects a wide range of human cell types in vitro76,77. Compared to wild type YFV, YFV 17D infects human cells more efficiently, replicates better and induces more robust activation of innate antiviral responses. Studies of YFV 17D infection of HeLa, 293 T, hepatocyte and dendritic cells have shown induction of IFNβ, IL29/IFNλ, IFIT-1, CCL5 and CXCL10 mRNAs that is dependent on a clathrin-independent, dynamin-dependent mode of entry76. Our studies showed that YFV 17D also replicated well in hSCs with virus production similar to ZIKV (Fig. 1) and induced strong IFNβ, IFNλ and ISG mRNA synthesis (Fig. 2). However, despite efficient in vitro replication in SCs, GBS is a rare complication of YFV 17D immunization potentially because in vivo spread of the virus is restricted35,78.
Flaviviruses can activate pathogen recognition receptors RIG-I, MDA-5, TLR3 and TLR7 to induce expression of IFN and cytokine genes43,79,80. The cellular responses of hSCs to ZIKV Fortaleza and YFV 17D infections showed innate antiviral mRNA responses that were similar. Both viruses induced expression of type I and type III IFNs and IFN-stimulated genes (ISGs) IFIT1 and MX1, as previously described for ZIKV infection of A549 respiratory epithelial cells and glomerular podocytes67,68. The IFNβ and ISG response to YFV was greater than to ZIKV, perhaps reflecting the ability of ZIKV to reduce type I IFN induction and NS5 to degrade STAT2 required for IFN signaling81,82.
Type III IFN exhibits antiviral and anti-proliferative activities produced through a different receptor but the same downstream signaling pathways as IFNα and IFNβ83. Both ZIKV and YFV-infected cells showed significant increases in IL-29 (IFNλ1) mRNA at 48 h although larger amounts of both IFNβ and IFNλ proteins were produced by ZIKV-infected cells than YFV-infected cells perhaps because of better viability (Fig. 1F). Increased expression of the IFNλ heterodimeric receptor complex and negative regulators SOCS1/3 were also observed during ZIKV infection.
The mechanism(s) of ZIKV-induced GBS is not understood. Speculated mechanisms include: Antibody dependent enhancement with DENV sera, an immune mediated mechanism inducing damage through molecular mimicry, cellular mediated inflammation and demyelination induced by complement and macrophage activation, and a direct viral pathogenic effect64. Similar mechanisms have been proposed to explain the increased incidence of GBS associated with hepatitis E virus (HEV) infection84–86. HEV can replicate in neural cells and virus is often detectable at the onset of GBS, but tropism for SCs has not been examined87. Our study of the relationship between ZIKV and GBS, specifically examining hSCs, suggests that GBS could be mediated by pathogen-specific antibodies/T cells or a direct viral effect on SCs rather than cross-reactive antibodies to host proteins/lipids. However, pathologic evaluation of the limited tissue available has not detected ZIKV in peripheral nerves of patients dying with ZIKV-associated GBS88,89. The understanding that ZIKV can replicate within hSCs and induces a cellular response that this study demonstrates facilitates a better understanding of the role of ZIKV and GBS in the most recent epidemic.
Methods
Cell cultures
Human SCs, a cell line derived from 60–80 day fetal sciatic nerves and immortalized with SV40 large T antigen and human telomerase reverse transcriptase have been previously described and were obtained from Ahmet Hoke (Department of Neurology, Johns Hopkins School of Medicine)33,36. hSCs were grown in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO Life Technologies) supplemented with 0.2% glucose, 2mM L-glutamine, 10% heat-inactivated fetal bovine serum (FBS), 2 µM forskolin, penicillin (100 U/ml) and streptomycin (100 µg/ml) at 37 °C in 5% CO2. Vero cells (American Type Culture Collection) were grown in DMEM with 10% FBS, 2mM L-glutamine, penicillin and streptomycin at 37 °C in 5% CO2. C6/36 Aedes albopictus cells (American Type Culture Collection) were grown in DMEM supplemented with 10% FBS and minimal essential amino acids, at 28 °C in 5% CO2. Cell viability was determined by trypan blue exclusion.
Viruses and virus assays
YFV (17D), DENV2 (NGC) and ZIKV strains 2014 Thailand (SCV0127/14) and 2015 Brazil (Fortaleza) were obtained from Anna Durbin (Johns Hopkins Bloomberg School of Public Health). ZIKV strain 1968 Nigeria (IBH 30656) was obtained from Andrew Pekosz (Johns Hopkins Bloomberg School of Public Health). All virus stocks were grown in C6/36 mosquito cells. Stocks and supernatant fluids from infected cells (MOI = 5) were assayed on Vero cells by focus-formation for DENV and YFV and by plaque-formation for ZIKV. Samples serially diluted in DMEM plus 1% FBS were incubated with Vero cell monolayers for an hour. For focus-forming assays, cells were overlaid with 1% methylcellulose in Optimem (Gibco) with 2% FBS, 2 mM glutamine, 50μg/ml gentamicin (Sigma Aldrich) and incubated for 3 days. Cells were fixed with 80% methanol for 10 min, blocked with 5% nonfat milk for 10 min and incubated for 1 h with pan-flavivirus mouse 4G2 monoclonal antibody (purified from hybridoma cells, American Type Culture Collection)90 diluted 1:2000 in 5% milk followed by horse radish peroxidase-conjugated goat anti-mouse IgG (KPL) diluted 1:3000 in 5% milk for 1 h. Foci were developed with KPL TrueBlue Peroxidase Substrate (Sera Care). For plaque assays, cells were overlaid with 0.6% Bacto Agar in Modified Eagle Medium (MEM, Gibco), incubated for 5 days, fixed with 10% formaldehyde in PBS and stained with 0.2% crystal violet in 20% ethanol.
MTT cell viability assay
hSCs were infected with three strains of ZIKV, YFV and DENV (MOI = 0.1). At different times after infection the supernatant fluid was removed and 50μl of MTT reagent (5 mg/ml; thiazolyl blue tetrazolium bromide, Affymetrix USB) in DMEM was added. After 2 h 50μl lysis buffer (20% sodium dodecyl sulfate [Biorad] in 50% N, N′-dimethyl formamide [Applied Biosystems]) was added and incubated for 4 h. Readings at 570 nm were used to calculate the percentage of viable infected cells compared to mock-infected cells.
Immunofluorescence
hSCs grown on poly-L-lysine-coated cover slips were infected with the three strains of ZIKV, DENV2 and YFV at a MOI of 5. At 24–96 h after infection, cells were fixed with 4% formaldehyde, permeabilized with 0.2% triton X 100 in PBS, and blocked with 5% normal goat serum in PBS. The cells were incubated with pan-flavivirus 4G2 primary antibody (1:1000 in PBS) for 1 h followed by anti-mouse IgG Alexafluor594 (Invitrogen). Nuclei were stained with DAPI. Images were captured using a Zeiss Axio Imager M2 microscope and analyzed using Volocity software. Numbers of infected and uninfected cells in three fields were counted to determine percentages of cells infected for each virus.
Quantitative real-time RT-PCR (qRT-PCR)
hSCs infected with ZIKV Fortaleza and YFV (MOI = 5) were collected in RLT buffer at 0, 12, 24, 48, and 72 h after infection in triplicate. RNA was isolated from cell lysates using the RNeasy Plus Mini Kit (Qiagen) and quantified using a Nanodrop-1000 spectrophotometer. cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) on an Applied Biosystems 2720 thermal cycler with an RNA concentration of 500 ng/µl in a 20 µl total reaction volume. qPCR was performed with the Promega GoTaq qPCR Master Mix for Dye-Based Detection using gene-specific oligonucleotide primers for SYBR Green-based measurements or Applied Biosystems TaqMan probes (Table 1). Applied Biosystems 7500 Real Time PCR System was used under the following conditions: initial hold for 2 min at 50 °C and hold for 10 min at 95 °C followed by 40 cycles to denature for 15 s at 95 °C and anneal for 60 s at 60 °C. All gene expression data were normalized against GAPDH levels and fold change compared to uninfected cells was calculated as 2−∆∆ct.
Table 1.
Primers used for the study.
| TaqMan Probes (Applied Biosystems) | |||
|---|---|---|---|
| Gene | RefSeq. | Taqman Assay ID | |
| IFNB1 | NM_002176 | Hs01077958_s1 | |
| IFIT-1 | NM_001270929 | Hs01675197_m1 | |
| NLRP3 | NM_001079821 | Hs00918082_m1 | |
| TNFα | NM_000594 | Hs00174128_m1 | |
| IL-29 | NM_172140 | Hs00601677_g1 | |
| SOCS1 | NM_003745 | Hs00705164_s1 | |
| SOCS3 | NM_003955 | Hs02330328_s1 | |
| MX1 | NM_001144925 | Hs00895608_m1 | |
| IDT Primers | |||
| Gene | RefSeq. | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
| IL-23A | NM_016584 | AGCCGCCCGGGTCTT | TCCTTGAGCTGCTGCCTTTAG |
| IL6 | NM_000600 | CCAGCTATGAACTCCTTCTC | GCTTGTTCCTCACATCTCTC |
| IFNLR1 | NM_173065 | GTAGATGGTTCTGGCACTGAG | GATCTGAAGTATGAGGTGGCA |
| IL10RB | NM_000628 | CTCATCCGACAATGGAAAGGA | AAGTACGCCTTCTCCCCTA |
| FCGR1C | NR_027484 | TGGAGACCTGCAGTAGTGG | CCCAGCTACAGATCACCTC |
| FCGR2B | NM_004001 | GATTAGTGGGATTGGCTGGTT | GTAGTGGCCTTGATCTACTGC |
| FCGR3A | NM_001127596 | TGGGAGATCAGTCCGCAT | TGAGCTAAATCCGCAGGAC |
| HSPG2 | NM_005529 | CATAGAGACCGTCACAGCAAG | AGGGCTCGGAAATAAACCATC |
| ICAM1 | NM_000201.2 | CAATGTGCTATTCAAACTGCCC | CAGCGTAGGGTAAGGTTCTTG |
| MR | NM_006039.4 | GTCATCATTGTGATCCTCCTG | GATGACCGAGTGTTCATTCTG |
| CD209 | NM_021155.3 | TGGACACTGGGGGAGAGTGG | CATGGCCAAGACACCCTGCT |
| TIM1 | NM_001099414 | AACAGATGGGAATGACACCG | GAAGCACCAAGACAGAAATACAG |
| AXL | NM_001699 | TTTATGACTATCTGCGCCAGG | TGTGTTCTCCAAATCTTCCCG |
| TYRO3 | NM_006293.3 | GCCGCCGCAGGTCTGAAG | GTCAGGCTCCTCCATCCCCT |
| LGALS3 | NM_002306.3 | CTGGGAGTATTTGAGGCTCG | TTTCCAGACCCAGATAACGC |
| GAPDH | NM_002046.5 | CCATCACTGCCACCCAGAAGAC | GGCAGGTTTTTCTAGACGGCAG |
| CLDN1 | NM_021101 | GATTCTATTGCCATACCATGCTG | TGTATGAAGTGCTTGGAAGACG |
| CLDN6 | NM_021195 | GAGACCAGGCCATTCACC | AATTTCCCTTATCTCCTTCGCA |
| CLDN9 | NM_020982 | AAAGCGTCCGTAGCATCTG | GTTTCTGTCCTGAGCCTGTT |
Protein quantification by enzyme immunoassay
hSCs were infected with ZIKV Fortaleza, YFV and Nigeria and supernatant fluids were collected at 0, 12, 24, 36, 48 and 72 h after infection. IFN β was measured using the VeriKine Human IFN Beta ELISA kit (PBL Assay Science) and IL-29 (IFNλ1) was measured using the Human IL29 Uncoated ELISA kit (Invitrogen) according to the manufacturer’s instructions. Supernatant fluids from 3 independent infections were tested and data are presented as pg per ml or OD at 450 nm. Assay range was 50 to 4000 pg/ml for IFNβ and 15.6 to 1000 pg/ml for IL-29. The limit of detection is marked as the OD value obtained for lowest concentration of assay range.
Fcγ receptor staining
hSCs were collected in ice cold PBS and live/dead staining (Invitrogen) was done on ice for 30 min in the dark. Cells were stained with FITC-conjugated mouse antihuman CD64 (FcγRI; BD-560970), CD32 (FcγRII; ebio 11-0329), CD16 (FcγRIII; BD-555406) and mouse IgG1 (isotype control; BD-551954) for 1 h on ice and analyzed on a FACSCanto flow cytometer. Histograms were plotted to determine mean fluorescence intensity.
Statistics
Data were compared using two-way ANOVA and are presented as mean +/− standard deviation. P < 0.05 was considered significant. All statistical analyses were performed with GraphPad Prism 5.
Acknowledgements
These studies were supported by a supplement to research grant R01 NS87539 (D.E.G.) from the U.S. National Institutes of Health. We thank Ahmet Hoke (Johns Hopkins School of Medicine) for the immortalized human Schwann cells and Anna Durbin and Andrew Pekosz (Johns Hopkins Bloomberg School of Public Health) for viruses and reagents used in this study.
Author Contributions
Experiments were performed by G.D. and R.A. All authors conceived and designed the experiments and wrote the manuscript.
Competing Interests
G.D. and R.A. declare no competing interests. D.E.G. is a member of the Takeda Zika virus vaccine data monitoring committee.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gaurav Dhiman and Rachy Abraham contributed equally.
References
- 1.Lanciotti RS, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerging infectious diseases. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abushouk AI, Negida A, Ahmed H. An updated review of Zika virus. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2016;84:53–58. doi: 10.1016/j.jcv.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Russell K, et al. Male-to-Female Sexual Transmission of Zika Virus-United States, January-April 2016. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017;64:211–213. doi: 10.1093/cid/ciw692. [DOI] [PubMed] [Google Scholar]
- 4.Noronha L, Zanluca C, Azevedo ML, Luz KG, Santos CN. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz. 2016;111:287–293. doi: 10.1590/0074-02760160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 6.Haddow AD, Woodall JP. Distinguishing between Zika and Spondweni viruses. Bulletin of the World Health Organization. 2016;94:711–711A. doi: 10.2471/BLT.16.181503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson JG, Ksiazek TG. Suhandiman & Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1981;75:389–393. doi: 10.1016/0035-9203(81)90100-0. [DOI] [PubMed] [Google Scholar]
- 8.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. The Journal of hygiene. 1979;83:213–219. doi: 10.1017/S0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrera BB, et al. Continued Transmission of Zika Virus in Humans in West Africa, 1992-2016. The Journal of infectious diseases. 2017;215:1546–1550. doi: 10.1093/infdis/jix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffy MR, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. The New England journal of medicine. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 11.Ioos S, et al. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44:302–307. doi: 10.1016/j.medmal.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Zanluca C, et al. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White MK, Wollebo HS, David Beckham J, Tyler KL, Khalili K. Zika virus: An emergent neuropathological agent. Annals of neurology. 2016;80:479–489. doi: 10.1002/ana.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatherer D, Kohl A. Zika virus: a previously slow pandemic spreads rapidly through the Americas. The Journal of general virology. 2016;97:269–273. doi: 10.1099/jgv.0.000381. [DOI] [PubMed] [Google Scholar]
- 15.Pierson TC, Diamond MS. The emergence of Zika virus and its new clinical syndromes. Nature. 2018;560:573–581. doi: 10.1038/s41586-018-0446-y. [DOI] [PubMed] [Google Scholar]
- 16.Cao-Lormeau VM, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parra B, et al. Guillain-Barre Syndrome Associated with Zika Virus Infection in Colombia. The New England journal of medicine. 2016;375:1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 18.Roze B, et al. Guillain-Barre Syndrome Associated With Zika Virus Infection in Martinique in 2016: A Prospective Study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017;65:1462–1468. doi: 10.1093/cid/cix588. [DOI] [PubMed] [Google Scholar]
- 19.Krauer F, et al. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barre Syndrome: Systematic Review. PLoS medicine. 2017;14:e1002203. doi: 10.1371/journal.pmed.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet. 2016 doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg B, et al. Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10:469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 22.Lucchese G, Kanduc D. Zika virus and autoimmunity: From microcephaly to Guillain-Barre syndrome, and beyond. Autoimmun Rev. 2016;15:801–808. doi: 10.1016/j.autrev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Armati PJ, Mathey EK. An update on Schwann cell biology–immunomodulation, neural regulation and other surprises. J Neurol Sci. 2013;333:68–72. doi: 10.1016/j.jns.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Meyer zu Horste G, Hu W, Hartung HP, Lehmann HC, Kieseier BC. The immunocompetence of Schwann cells. Muscle Nerve. 2008;37:3–13. doi: 10.1002/mus.20893. [DOI] [PubMed] [Google Scholar]
- 25.Ydens E, et al. The neuroinflammatory role of Schwann cells in disease. Neurobiology of disease. 2013;55:95–103. doi: 10.1016/j.nbd.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Lu MO, Zhu J. The role of cytokines in Guillain-Barre syndrome. J Neurol. 2011;258:533–548. doi: 10.1007/s00415-010-5836-5. [DOI] [PubMed] [Google Scholar]
- 27.Lee H, et al. Double-stranded RNA induces iNOS gene expression in Schwann cells, sensory neuronal death, and peripheral nerve demyelination. Glia. 2007;55:712–722. doi: 10.1002/glia.20493. [DOI] [PubMed] [Google Scholar]
- 28.Tzekova N, Heinen A, Kury P. Molecules involved in the crosstalk between immune- and peripheral nerve Schwann cells. Journal of clinical immunology. 2014;34(Suppl 1):S86–104. doi: 10.1007/s10875-014-0015-6. [DOI] [PubMed] [Google Scholar]
- 29.Spierings E, et al. Mycobacterium leprae-specific, HLA class II-restricted killing of human Schwann cells by CD4+ Th1 cells: a novel immunopathogenic mechanism of nerve damage in leprosy. Journal of immunology. 2001;166:5883–5888. doi: 10.4049/jimmunol.166.10.5883. [DOI] [PubMed] [Google Scholar]
- 30.Simon O, et al. Zika virus outbreak in New Caledonia and Guillain-Barre syndrome: a case-control study. Journal of neurovirology. 2018;24:362–368. doi: 10.1007/s13365-018-0621-9. [DOI] [PubMed] [Google Scholar]
- 31.Roze B, et al. Zika virus detection in urine from patients with Guillain-Barre syndrome on Martinique, January 2016. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2016;21:30154. doi: 10.2807/1560-7917.ES.2016.21.9.30154. [DOI] [PubMed] [Google Scholar]
- 32.Dirlikov E, et al. Clinical Features of Guillain-Barre Syndrome With vs Without Zika Virus Infection, Puerto Rico, 2016. JAMA Neurol. 2018;75:1089–1097. doi: 10.1001/jamaneurol.2018.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann HC, et al. Human Schwann cells retain essential phenotype characteristics after immortalization. Stem Cells Dev. 2012;21:423–431. doi: 10.1089/scd.2010.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowman LR, Rocklov J, Kroeger A, Olliaro P, Skewes R. A comparison of Zika and dengue outbreaks using national surveillance data in the Dominican Republic. PLoS neglected tropical diseases. 2018;12:e0006876. doi: 10.1371/journal.pntd.0006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahon AW, et al. Neurologic disease associated with 17D-204 yellow fever vaccination: a report of 15 cases. Vaccine. 2007;25:1727–1734. doi: 10.1016/j.vaccine.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann HC, Hoke A. Use of engineered Schwann cells in peripheral neuropathy: Hopes and hazards. Brain Res. 2016;1638:97–104. doi: 10.1016/j.brainres.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 37.Faria NR, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verhelst J, Parthoens E, Schepens B, Fiers W, Saelens X. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. Journal of virology. 2012;86:13445–13455. doi: 10.1128/JVI.01682-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brand S, et al. SOCS-1 inhibits expression of the antiviral proteins 2′,5′-OAS and MxA induced by the novel interferon-lambdas IL-28A and IL-29. Biochemical and biophysical research communications. 2005;331:543–548. doi: 10.1016/j.bbrc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. The Journal of experimental medicine. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rey FA, Stiasny K, Heinz FX. Flavivirus structural heterogeneity: implications for cell entry. Current opinion in virology. 2017;24:132–139. doi: 10.1016/j.coviro.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perera-Lecoin M, Meertens L, Carnec X, Amara A. Flavivirus entry receptors: an update. Viruses. 2013;6:69–88. doi: 10.3390/v6010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamel R, et al. Biology of Zika Virus Infection in Human Skin Cells. Journal of virology. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowakowski TJ, et al. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell. 2016;18:591–596. doi: 10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Shufeng, DeLalio Leon J., Isakson Brant E., Wang Tony T. AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circulation Research. 2016;119(11):1183–1189. doi: 10.1161/CIRCRESAHA.116.309866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller JL, et al. The mannose receptor mediates dengue virus infection of macrophages. PLoS pathogens. 2008;4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baetas-da-Cruz W, et al. Schwann cells express the macrophage mannose receptor and MHC class II. Do they have a role in antigen presentation? J Peripher Nerv Syst. 2009;14:84–92. doi: 10.1111/j.1529-8027.2009.00217.x. [DOI] [PubMed] [Google Scholar]
- 48.Davis CW, et al. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. Journal of virology. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tassaneetrithep B, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. The Journal of experimental medicine. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meertens L, et al. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lisak RP, Bealmear B. Upregulation of intercellular adhesion molecule-1 (ICAM-1) on rat Schwann cells in vitro: comparison of interferon-gamma, tumor necrosis factor-alpha and interleukin-1. J Peripher Nerv Syst. 1997;2:233–243. [PubMed] [Google Scholar]
- 52.Mietto BS, et al. Lack of galectin-3 speeds Wallerian degeneration by altering TLR and pro-inflammatory cytokine expressions in injured sciatic nerve. Eur J Neurosci. 2013;37:1682–1690. doi: 10.1111/ejn.12161. [DOI] [PubMed] [Google Scholar]
- 53.Salinas, S. et al. Zika Virus Efficiently Replicates in Human Retinal Epithelium and Disturbs Its Permeability. Journal of virology91, 10.1128/JVI.02144-16 (2017). [DOI] [PMC free article] [PubMed]
- 54.Alanne MH, et al. Tight junction proteins in human Schwann cell autotypic junctions. J Histochem Cytochem. 2009;57:523–529. doi: 10.1369/jhc.2009.951681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Che P, Tang H, Li Q. The interaction between claudin-1 and dengue viral prM/M protein for its entry. Virology. 2013;446:303–313. doi: 10.1016/j.virol.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Gao F, et al. Novel binding between pre-membrane protein and claudin-1 is required for efficient dengue virus entry. Biochemical and biophysical research communications. 2010;391:952–957. doi: 10.1016/j.bbrc.2009.11.172. [DOI] [PubMed] [Google Scholar]
- 57.de Alwis R, et al. Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS pathogens. 2014;10:e1004386. doi: 10.1371/journal.ppat.1004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dejnirattisai W, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nature immunology. 2016;17:1102–1108. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wahala WM, Silva AM. The human antibody response to dengue virus infection. Viruses. 2011;3:2374–2395. doi: 10.3390/v3122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Priyamvada L, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:7852–7857. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayala-Nunez NV, et al. How antibodies alter the cell entry pathway of dengue virus particles in macrophages. Sci Rep. 2016;6:28768. doi: 10.1038/srep28768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vedeler CA, Scarpini E, Beretta S, Doronzo R, Matre R. The ontogenesis of Fc gamma receptors and complement receptors CR1 in human peripheral nerve. Acta Neuropathol. 1990;80:35–40. doi: 10.1007/BF00294219. [DOI] [PubMed] [Google Scholar]
- 63.Vedeler CA, Matre R, Kristoffersen EK, Ulvestad E. IgG Fc receptor heterogeneity in human peripheral nerves. Acta Neurol Scand. 1991;84:177–180. doi: 10.1111/j.1600-0404.1991.tb04933.x. [DOI] [PubMed] [Google Scholar]
- 64.Munoz LS, Barreras P, Pardo CA. Zika Virus-Associated Neurological Disease in the Adult: Guillain-Barre Syndrome, Encephalitis, and Myelitis. Semin Reprod Med. 2016;34:273–279. doi: 10.1055/s-0036-1592066. [DOI] [PubMed] [Google Scholar]
- 65.Chan JF, et al. Differential cell line susceptibility to the emerging Zika virus: implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg Microbes Infect. 2016;5:e93. doi: 10.1038/emi.2016.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simonin Y, et al. Zika Virus Strains Potentially Display Different Infectious Profiles in Human Neural Cells. EBioMedicine. 2016;12:161–169. doi: 10.1016/j.ebiom.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frumence E, et al. The South Pacific epidemic strain of Zika virus replicates efficiently in human epithelial A549 cells leading to IFN-beta production and apoptosis induction. Virology. 2016;493:217–226. doi: 10.1016/j.virol.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Alcendor DJ. Zika Virus Infection of the Human Glomerular Cells: Implications for Viral Reservoirs and Renal Pathogenesis. The Journal of infectious diseases. 2017;216:162–171. doi: 10.1093/infdis/jix171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan L, et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science. 2017;358:933–936. doi: 10.1126/science.aam7120. [DOI] [PubMed] [Google Scholar]
- 70.Haddow AD, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS neglected tropical diseases. 2012;6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong Z, Gao Y, Han GZ. Zika Virus: Two or Three Lineages? Trends in microbiology. 2016;24:521–522. doi: 10.1016/j.tim.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Simonin Y, van Riel D, Van de Perre P, Rockx B, Salinas S. Differential virulence between Asian and African lineages of Zika virus. PLoS neglected tropical diseases. 2017;11:e0005821. doi: 10.1371/journal.pntd.0005821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramos da Silva Suzane, Cheng Fan, Huang I‐Chueh, Jung Jae U., Gao Shou‐Jiang. Efficiencies and kinetics of infection in different cell types/lines by African and Asian strains of Zika virus. Journal of Medical Virology. 2018;91(2):179–189. doi: 10.1002/jmv.25306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anfasa, F. et al. Phenotypic Differences between Asian and African Lineage Zika Viruses in Human Neural Progenitor Cells. mSphere2, 10.1128/mSphere.00292-17 (2017). [DOI] [PMC free article] [PubMed]
- 75.Tabata T, et al. Zika Virus Replicates in Proliferating Cells in Explants From First-Trimester Human Placentas, Potential Sites for Dissemination of Infection. The Journal of infectious diseases. 2018;217:1202–1213. doi: 10.1093/infdis/jix552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernandez-Garcia MD, et al. Vaccine and Wild-Type Strains of Yellow Fever Virus Engage Distinct Entry Mechanisms and Differentially Stimulate Antiviral Immune Responses. mBio. 2016;7:e01956–01915. doi: 10.1128/mBio.01956-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liprandi F, Walder R. Replication of virulent and attenuated strains of yellow fever virus in human monocytes and macrophage-like cells (U937) Archives of virology. 1983;76:51–61. doi: 10.1007/BF01315703. [DOI] [PubMed] [Google Scholar]
- 78.Oliveira AC, et al. Occurrence of Autoimmune Diseases Related to the Vaccine against Yellow Fever. Autoimmune Dis. 2014;2014:473170. doi: 10.1155/2014/473170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savidis G, et al. The IFITMs Inhibit Zika Virus Replication. Cell Rep. 2016;15:2323–2330. doi: 10.1016/j.celrep.2016.05.074. [DOI] [PubMed] [Google Scholar]
- 80.Mandl JN, et al. Distinctive TLR7 signaling, type I IFN production, and attenuated innate and adaptive immune responses to yellow fever virus in a primate reservoir host. Journal of immunology. 2011;186:6406–6416. doi: 10.4049/jimmunol.1001191. [DOI] [PubMed] [Google Scholar]
- 81.Grant A, et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe. 2016;19:882–890. doi: 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar Anil, Hou Shangmei, Airo Adriana M, Limonta Daniel, Mancinelli Valeria, Branton William, Power Christopher, Hobman Tom C. Zika virus inhibits type‐I interferon production and downstream signaling. EMBO reports. 2016;17(12):1766–1775. doi: 10.15252/embr.201642627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelm NE, et al. The role of IL-29 in immunity and cancer. Crit Rev Oncol Hematol. 2016;106:91–98. doi: 10.1016/j.critrevonc.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van den Berg B, et al. Guillain-Barre syndrome associated with preceding hepatitis E virus infection. Neurology. 2014;82:491–497. doi: 10.1212/WNL.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 85.Dalton HR, et al. Hepatitis E virus and neurological injury. Nat Rev Neurol. 2016;12:77–85. doi: 10.1038/nrneurol.2015.234. [DOI] [PubMed] [Google Scholar]
- 86.Bazerbachi F, Haffar S, Garg SK, Lake JR. Extra-hepatic manifestations associated with hepatitis E virus infection: a comprehensive review of the literature. Gastroenterol Rep (Oxf) 2016;4:1–15. doi: 10.1093/gastro/gov042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou X, et al. Hepatitis E Virus Infects Neurons and Brains. The Journal of infectious diseases. 2017;215:1197–1206. doi: 10.1093/infdis/jix079. [DOI] [PubMed] [Google Scholar]
- 88.Dirlikov E, et al. Postmortem Findings in Patient with Guillain-Barre Syndrome and Zika Virus Infection. Emerging infectious diseases. 2018;24:114–117. doi: 10.3201/eid2401.171331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ritter JM, Martines RB, Zaki SR. Zika Virus: Pathology From the Pandemic. Arch Pathol Lab Med. 2017;141:49–59. doi: 10.5858/arpa.2016-0397-SA. [DOI] [PubMed] [Google Scholar]
- 90.Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. The American journal of tropical medicine and hygiene. 1982;31:830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]