Abstract
Trigeminal Neuralgia (TN) is a chronic neuropathic pain syndrome characterized by paroxysmal unilateral shock-like pains in the trigeminal territory most frequently attributed to neurovascular compression of the trigeminal nerve at its root entry zone. Recent advances in the study of TN suggest a possible central nervous system (CNS) role in modulation and maintenance of pain. TN and other chronic pain patients commonly experience alterations in cognition and affect, as well as abnormalities in CNS volume and microstructure in regions associated with pain perception, emotional modulation, and memory consolidation. However, the microstructural changes in the hippocampus, an important structure within the limbic system, have not been previously studied in TN patients. Here, we use grey matter analysis to assess whether TN pain is associated with altered hippocampal subfield volume in patients with classic TN. Anatomical magnetic resonance (MR) images of twenty-two right-sided TN patients and matched healthy controls underwent automated segmentation of hippocampal subfields using FreeSurfer v6.0. Right-sided TN patients had significant volumetric reductions in ipsilateral cornu ammois 1 (CA1), CA4, dentate gyrus, molecular layer, and hippocampus-amygdala transition area – resulting in decreased whole ipsilateral hippocampal volume, compared to healthy controls. Overall, we demonstrate selective hippocampal subfield volume reduction in patients with classic TN. These changes occur in subfields implicated as neural circuits for chronic pain processing. Selective subfield volume reduction suggests aberrant processes and circuitry reorganization, which may contribute to development and/or maintenance of TN symptoms.
Keywords: Trigeminal neuralgia, Chronic pain, Facial pain, Hippocampus, Structural MR analysis
Highlights
-
•
Selective Hippocampal subfields alteration in trigeminal neuralgia patients
-
•
Ipsilateral hippocampal volume reduction in TN patients
-
•
Females but not males show bilateral hippocampal volume reduction.
-
•
Pain duration correlates with hippocampal volume reduction.
-
•
Abnormal neurogenesis could explain hippocampal alterations.
1. Introduction
Trigeminal Neuralgia (TN) is the most common chronic neuropathic facial pain disorder, characterized by paroxysmal unilateral electric shock-like pain in the trigeminal nerve subdivisions. TN is closely linked to trigeminal neurovascular compression at its root entry zone, which frequently necessitates surgical interventions to alleviate symptoms (Love and Coakham, 2001; Nurmikko and Eldridge, 2001). As a type of severe neuropathic pain, TN has several unique features that make it an ideal model for the investigation of the effect of pain on brain structure. TN overwhelmingly unilateral, severe and is generally not associated with numbness or sensory deficits reported in different chronic pain syndromes. This distinguishes TN from other syndromes where pain is more diffuse, or axial, and where there is greater inter-individual heterogeneity in the expression of pain.
Grey matter (GM) and white matter (WM) abnormalities within the central nervous system (CNS) occur with chronic pain (Cauda et al., 2014; Henry et al., 2011; Smallwood et al., 2013). Likewise, chronic neuropathic pain including TN results in GM volume changes within areas such as insular cortex (Pan et al., 2015). GM abnormalities may occur as a consequence of chronic pain, as some abnormalities show reversibility with effective pain treatment (Gwilym et al., 2010; Rodriguez-Raecke et al., 2013; Seminowicz et al., 2011). Alternatively, chronic pain can produce a maladaptive stress response, which triggers functional reorganization of pain-related networks, including those involving the hippocampus (Baliki et al., 2010; Baliki et al., 2011; Vachon-Presseau et al., 2013).
We have previously demonstrated that GM and WM changes in CNS structures are important for pain perception, pain modulation, and motor function (Desouza et al., 2013; DeSouza et al., 2014). Furthermore, altered diffusivities in the pontine segment of trigeminal nerve can help to predict and prognosticate responders from non-responders to surgical treatments (Hung et al., 2017). Recently, we reported that TN results in changes to specific affect-related circuits, and reduced GM volumes in multiple regions including the hippocampus (Hayes et al., 2017).
The hippocampus has been long investigated for its widespread anatomical connections, with key roles in processes including cognition, memory and the limbic circuitry of emotion (Fortin et al., 2002; Kim et al., 2015; Nestler et al., 2002). While its role has been studied in conditions such as dementia, stress, and cognitive disorders; its involvement in chronic pain has not been well defined. Yet, patients with chronic pain uniformly report cognitive, memory changes, and pain-associated negative affect (McCarberg and Peppin, 2019). Thus, investigation of the role of the hippocampus in chronic pain is timely.
The hippocampus receives complex integrated sensory and cognitive information, including pain, from different regions of the brain and limbic system and has diverse cortical projections. It comprises different anatomical components, or subfields, with distinct morphologies and connections. As such, these subfields, notably the subiculum, cornu ammonis (CA1 – CA4), and dentate gyrus (DG), demonstrate functional specialization with key involvement in distinct processes such as verbal fluency, memory, spatial navigation, and emotional processing. For example, prior studies have suggested CA3 and DG involvement in memory encoding and early retrieval, whereas CA1 is primarily responsible for recognition and late retrieval (Acsády and Káli, 2007; Hunsaker and Kesner, 2008; Kesner and Hunsaker, 2010; Rolls and Kesner, 2006). Additionally, previous studies have delineated hippocampal circuit involvement in pain processing (Liu and Chen, 2009) and neurogenesis deregulation in chronic pain animal models (Apkarian et al., 2016), however the potential level of functional specialization of the hippocampus with respect to pain has not been investigated. Assessing the impact of hippocampal subfields may further elucidate the relationship between chronic neuropathic pain and CNS changes, for which TN, a severe form of unilateral neuropathic pain, is a particularly apt model for study. Thus, in this study, we hypothesize there will be selective subfield hippocampus GM alterations associated with TN. Specifically, we anticipate reduction in subfields associated with neurogenesis, such as DG and CA4 regions, with similar effects on CA1 subregion which is primarily involved in encoding and processing of neuronal input. To study this, we aim to use MRI and grey matter analysis to segment the hippocampus and analyze individual subfield volume changes in TN patients. We further investigate the effect of sex as well as pain duration on hippocampal changes.
2. Methods
2.1. Ethics
The University Health Network (UHN) Research Ethics Board approved this retrospective study of classical TN patients. Patient data was analyzed retrospectively and there was no active participation by patients. Individual patient consent was not required for this retrospective study. In addition, the UHN Research Ethics Board approved recruitment of healthy control subjects and the procedure to obtain written informed consent. Each healthy control participant provided written informed consent. All MRI scans where anonymized prior to analysis and stored in secure databases.
2.2. Participants
Twenty-two TN subjects seen at the Toronto Western Hospital between May 2008 and February 2011 were selected for the study using the criteria for classic TN as outlined by The International Classification of Headache Disorders (Headache Classification Committee of the International Headache S, 2013). Briefly, the inclusion criteria were: 1) unilateral (right-sided) pain involving one or more branches of trigeminal nerve; 2) stereotypical pain attacks involving intense, sharp, superficial, or stabbing paroxysmal facial pain precipitated from trigger areas or by trigger factors, and not associated with clinical evidence of neurological or sensory deficits or another disorder; 3) no previous surgical procedures for TN. Demographic and clinical details for patients were obtained via retrospective chart reviews (Table 1). Patients were age- and sex-matched to a cohort of twenty-two healthy pain-free control participants.
Table 1.
Demographic summary of the 21 right-sided TN patients included in this study. Pain distribution delineates the affected peripheral branches of the trigeminal nerve (V1: ophthalmic branch, V2: maxillary branch, V3: mandibular branch).
| Patient | Sex | Age (yrs) | TN Side | Pain distribution | Pain duration (yrs) | Age at pain onset (yrs) | Medication |
|---|---|---|---|---|---|---|---|
| P1 | M | 46 | R | V2, V3 | 8 | 38 | Ibuprofen |
| P2 | F | 52 | R | V2, V3 | 10 | 42 | ACV, SNRI |
| P3 | M | 43 | R | V2, V3 | 2 | 41 | CBZ, BNZ |
| P4 | F | 61 | R | V1, V2, V3 | 4 | 57 | CBZ, GBP |
| P5 | F | 40 | R | V1, V2, V3 | 9 | 31 | GBP, BNZ, Baclofen, Opioid |
| P6 | F | 33 | R | V2, V3 | 4 | 29 | GBP, PGB |
| P7 | F | 38 | R | V3 | 6 | 32 | CBZ |
| P8 | M | 52 | R | V1 | 5 | 47 | CBZ, ACV, Opioid |
| P9 | F | 59 | R | V2, V3 | 4 | 55 | CBZ, PGB |
| P10 | F | 38 | R | V3 | 5 | 33 | CBZ, PGB |
| P11 | F | 67 | R | V3 | 1 | 67 | GBP, Opioid |
| P12 | F | 63 | R | V2 | 7 | 56 | CBZ |
| P13 | M | 61 | R | V1, V2 | 2 | 59 | PGB |
| P14 | F | 45 | R | V2, V3 | 2 | 43 | None |
| P15 | F | 47 | R | V1, V2, V3 | 3 | 44 | CBZ, GBP |
| P16 | F | 76 | R | V1, V2 | 3 | 73 | CBZ |
| P17 | M | 38 | R | V3 | 1 | 37 | CBZ, PGB |
| P18 | M | 23 | R | V3 | 3 | 20 | CBZ |
| P19 | F | 52 | R | V1, V2 | 3 | 49 | CBZ |
| P20 | F | 52 | R | V2, V3 | 2 | 50 | CBZ |
| P21 | M | 24 | R | V3 | 2 | 22 | CBZ, TCA |
| P22 | M | 38 | R | V2 | 2 | 36 | CBZ |
Abbreviations: ACV: Anticonvulsant; CBZ: carbamazepine; PGB: pregabalin; GBP: Gabapentin; TCA: tricyclic antidepressant; BNZ: Benzodiazepine.
2.3. Imaging
All imaging was conducted with a 3 T GE Signa HDx MRI system fitted with an eight-channel phased array head coil. Scans of T1-weighted 3D FSPGR axial brain images were obtained from the top of the cranium to below the foramen magnum (0.9 × 0.9 × 0.9 mm3 voxels derived from a 256 × 256 matrix and field of view of 24 cm, echo time = 5 ms, repetition time = 12 ms, inversion time = 300 ms).
2.4. Automated volumetric hippocampal segmentation
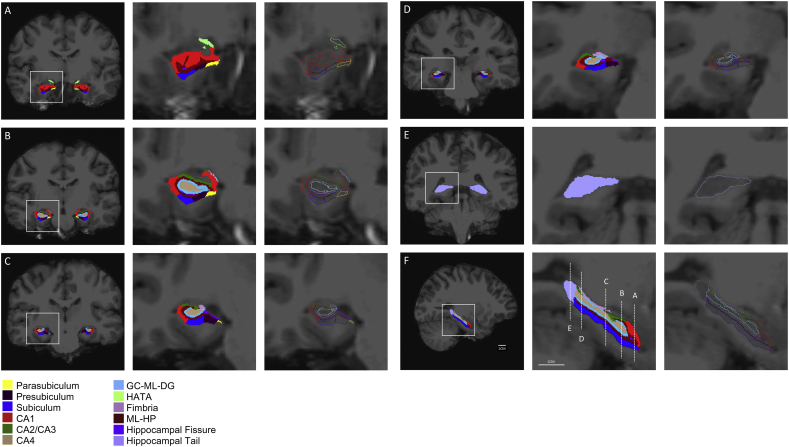
Volumetric segmentation was performed with the FreeSurfer 6.0 image analysis suite as previously described by the software developers (http://surfer.nmr.mgh.harvard.edu/) (Fischl, 2012; Iglesias et al., 2015). In addition, we used the Hippocampal Subfields segmentation protocol built into FreeSurfer 6.0 which automatically segments 12 subfields of the hippocampal formation in each hemisphere (Fig. 1). The segmentation procedure is based on previously established ex-vivo histological segmentation with ultra-high-resolution MRI data. The reliability and validity of the FreeSurfer 6.0 hippocampal segmentation protocol has been previously demonstrated (Iglesias et al., 2015).
Fig. 1.
Representative images of FreeSurfer 6.0 automated hippocampal subfield segmentation in a control subject. Right two panels are 5× magnified of the left panel. A-E are coronal views. F is a sagittal view.
2.5. Intracranial volume correction
The estimated total intracranial volume (eTIV) is calculated by Freesurfer upon registration of images to the MNI305 Talairach space, as previously described (Buckner et al., 2004). The volume of the hippocampal formation scales with head size, or total intracranial volume. Hippocampal subfield volumes are corrected for natural inter-subject variability in TIV. Based on previous work suggesting the advantages of the residual method compared with the proportion method of assessment of inter-subject variability, the former was used in the present project. (Sanfilipo et al., 2004). We adjusted whole hippocampal volume and its subfields using the residual method with regression analysis using the following formula as previously described (Buckner et al., 2004):
Here, VOIadj is the adjusted volume of interest, VOI is the output volume of interest from FreeSurfer automated hippocampal segmentation, b is the slope of the VOI linear regression on eTIV, and the eTIVm is the sample mean of the eTIV. Only the slope of the healthy controls' VOIs were calculated, and subsequently used to calculate the VOIadj for both R-TN subjects and controls. All reported volumes are adjusted. Additionally, subfield volume difference between R-TNs and controls are calculated as the VOIadj of R-TN - ipsilateral VOIadj of matched control for each subfield.
2.6. Manual volumetric hippocampal segmentation
Methods for manual hippocampal segmentation have been previously described in detail (Kulaga-Yoskovitz et al., 2015). Briefly, T1-weighted scans were processed as described above. The hippocampal subfields in five randomly selected R-TN subjects and their respective controls were manually segmented by a neuroanatomist (MV) using 3D Display software, part of the MINC tools (www.bic.mni.mcgill.ca/ServicesSoftwareVisualization/). Segmented areas include 1) Subiculum, 2) cornu ammois 1 (CA1), CA2, and CA3 (CA1-CA2-CA3); and 3) CA4 and dentate gyrus (CA4-DG). The volume of the whole hippocampus was calculated as the sum of the 3 segments. Anatomical landmarks used to guide segmentation are previously described (Kulaga-Yoskovitz et al., 2015).
2.7. Statistical analysis
All statistical analyses were performed using GraphPad Prism version 7.0c for Mac, GraphPad Software, La Jolla, California, USA (www.graphpad.com), including: 1) student's t-test to compare mean age of R-TN subjects versus controls; 2) linear regression of left and right VOI over eTIV of healthy controls for ICV correction; 3) linear regression of left and right subfield volume difference over pain duration; 4) one-way ANOVA with matching, Geisser-Greenhouse's correction, and Bonferroni's multiple comparison tests to analyze VOIadj of R-TN subjects versus controls; and 5) two-way ANOVA with Tukey's multiple comparison to analyze differences in VOIadj of R-TN subjects versus controls separated by sex (males and females). First-stage analysis compared R-TN group (right or left VOIadj) to their respective control group, and this was followed with Bonferroni's multiple comparison test correction. All the reported p-values are corrected for multiple comparisons and statistical analyses were determined significant if p < .05.
3. Results
3.1. Subject demographics
The R-TN group had a mean age ± SD of 47.6 ± 13.5 years and was comprised of 8 males and 14 females who had a mean age of 40.6 ± 13.0 years and 51.6 ± 12.5 years respectively, that was not statistically different (p = .0635). Male and female subjects had a mean right-sided TN pain duration for 3.1 ± 2.3 years and 4.5 ± 2.7 years respectively (p = .2350), and an average pain onset at 37.5 ± 12.6 years and 47.2 ± 13.5 years respectively (p = .1131). All patients with the exception of one had pharmacological therapy at the time of imaging, the most common being Carbamazepine (CBZ). Patients were sex and age matched to a healthy control group with a mean age of 46.0 ± 11.6 years (14F: 49.0 ± 9.9; 8 M: 40.8 ± 11.6). The age is not statistically different between control group and the R-TN group (p = .6952). Patient demographic information is shown in Table 1.
3.2. Automated hippocampal subfield segmentation in R-TN subjects and matched controls
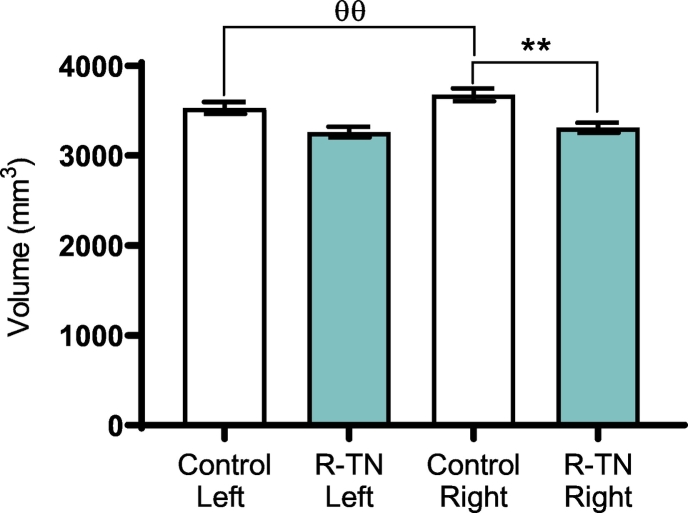
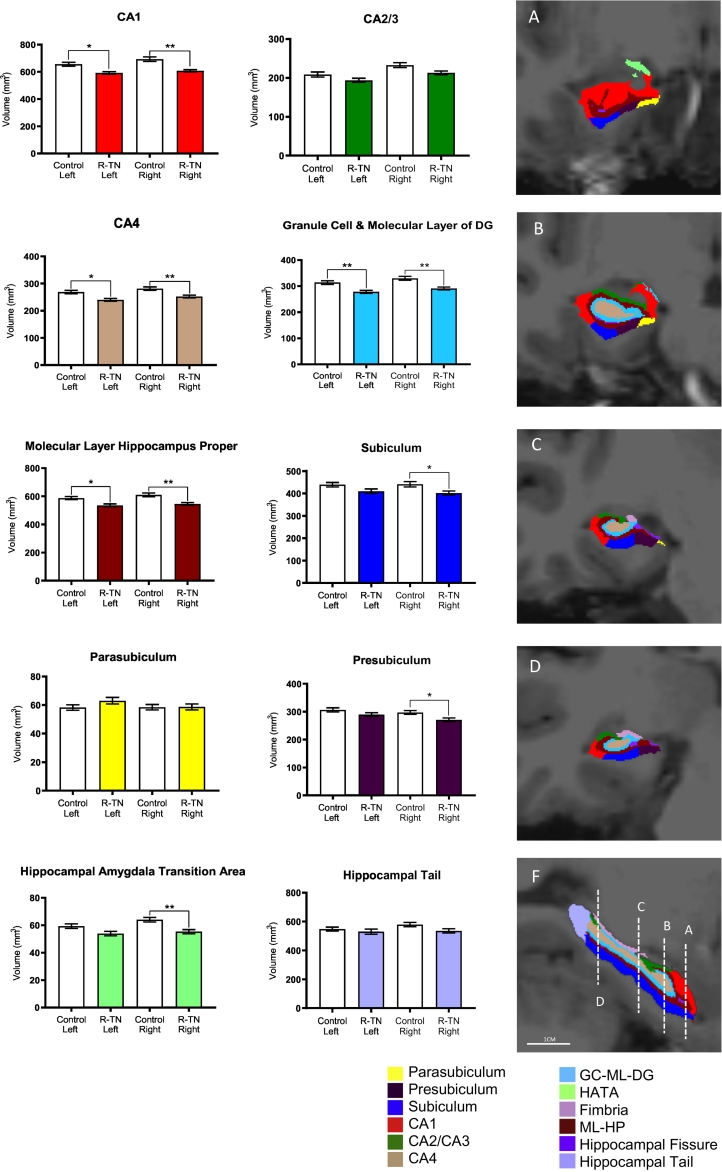
FreeSurfer 6.0 automated hippocampal segmentation protocol was successful in segmenting 12 regions of the left and right hippocampus in all R-TN subjects and matched controls, including the granule cell and molecular layer of the dentate gyrus (GC-ML-DG), CA1, CA2/CA3, CA4, subiculum, presubiculum, parasubiculum, molecular layer of hippocampus proper (ML-HP), fimbria, hippocampal tail, hippocampal fissure, and hippocampal-amygdala transition area (HATA) (Fig. 1). Segmentation results were visually inspected for errors by assessing alignment of subfield masks with processed T1 images, however, no manual edits were required. The right whole hippocampus was significantly smaller in R-TN subjects compared to control (p = .0024) (Fig. 2). The right whole hippocampus was significantly larger than the left whole hippocampus in the control but not the R-TN group (p = .0027) (Table 2, Fig. 2). We found specific subfield changes in the right, left and bilateral hippocampi, as follows. Right subfield volume reduction compared to controls was seen in the following: GC-ML-DG (p = .0012), CA4 (p = .0046), CA1 (p = .0014), subiculum (p = .0289), presubiculum (p = .0367), ML-HP (p = .0033), and the HATA (p = .0052) (Table 2, Fig. 3). Left subfields were significantly smaller in R-TN subjects compared to controls in GC-ML-DG (p = .0096), CA4 (p = .0204), CA1 (p = .0145), and ML-HP (p = .0196) (Fig. 3).
Fig. 2.
Automated hippocampal segmentation showing reduced ipsilateral whole hippocampal volume in R-TN patients compared to matched controls. * designates significant intergroup differences (R-TN vs. controls). θ designates significant intragroup differences. * p < .05, ** p < .01, with same value for θ.
Table 2.
Summary of automated hippocampal segmentation in R-TN and control subjects (* p < .05, ** p < .01).
| Subfield | Side | Mean volume R-TN (mm3) | Mean volume Control (mm3) | Difference (mm3) | Post-hoc P-value | Significance |
|---|---|---|---|---|---|---|
| Whole hippocampus | R | 3308.57 | 3676.00 | −367.42 | 0.0024 | ** |
| L | 3260.83 | 3529.70 | −268.86 | 0.0557 | ||
| GC-ML-DG | R | 291.06 | 329.98 | −38.92 | 0.0012 | ** |
| L | 277.90 | 313.62 | −35.72 | 0.0096 | ** | |
| CA4 | R | 252.04 | 281.29 | −29.25 | 0.0046 | ** |
| L | 240.07 | 268.42 | −28.35 | 0.0204 | * | |
| CA2/CA3 | R | 212.94 | 232.79 | −19.84 | 0.0767 | |
| L | 193.78 | 208.54 | −14.76 | 0.3607 | ||
| CA1 | R | 607.34 | 692.85 | −85.51 | 0.0014 | ** |
| L | 591.73 | 655.47 | −63.73 | 0.0145 | * | |
| Subiculum | R | 401.66 | 440.86 | −39.21 | 0.0289 | * |
| L | 409.81 | 439.00 | −29.19 | 0.1641 | ||
| Presubiculum | R | 270.94 | 297.07 | −26.13 | 0.0367 | * |
| L | 289.63 | 306.41 | −16.78 | 0.4390 | ||
| Parasubiculum | R | 58.66 | 58.49 | 0.17 | >0.9999 | |
| L | 63.02 | 58.28 | 4.74 | 0.7317 | ||
| ML-HP | R | 545.48 | 609.67 | −64.19 | 0.0033 | ** |
| L | 535.61 | 587.12 | −51.51 | 0.0196 | * | |
| Fimbria | R | 77.70 | 89.93 | −12.24 | 0.2470 | |
| L | 75.57 | 85.95 | −10.38 | 0.2734 | ||
| Hippocampal tail | R | 535.45 | 579.08 | −43.63 | 0.1963 | |
| L | 529.79 | 547.57 | 17.78 | >0.9999 | ||
| Hippocampal fissure | R | 142.60 | 144.24 | −1.64 | >0.9999 | |
| L | 139.73 | 134.68 | 5.05 | >0.9999 | ||
| HATA | R | 55.31 | 63.98 | −8.67 | 0.0052 | ** |
| L | 53.93 | 59.34 | −5.41 | 0.1459 |
Subfield volume difference calculated as: VOIadj of R-TN - ipsilateral VOIadj of matched control.
Abbreviations: GC-ML-DG: granule cell and molecular layer of the dentate gyrus; CA: cornu ammonis; ML-HP: molecular layer of hippocampus proper; HATA: hippocampal amygdala transition area.
Fig. 3.
Summary of automated hippocampal segmentation in 22 R-TN and matched control subjects. Right panels represent hippocampal segmentation. A-D are coronal views. F is a sagittal view. * p < .05, ** p < .01.
We also found specific asymmetries in the hippocampal subfields. Controls had a larger right subfield volume compared to the left side in the following 7 subfields: GC-ML-DG (p = .0053), CA4 (p = .0119), CA2/CA3 (p = .0007), CA1 (p = .0047), ML-HP (p = .0241), hippocampal tail (p = .0224), and HATA (p = .0045) (Table 3). In comparison, R-TN subjects had a larger right subfield volume compared to the left side in 3 of 12 subfields including the GC-ML-DG (p = .0153), CA4 (p = .0137), CA2/CA3 (p = .0034), and a larger left side presubiculum (p = .0017) (Table 3). Intergroup hippocampal volume abnormalities are reported in Fig. 3 and Table 2. Whole hippocampus findings are stated in Fig. 2.
Table 3.
Summary of automated hippocampal segmentation in left and right hemispheres in R-TN and control subjects (* p < .05, ** p < .01, *** p < .001).
| Subfield | Group | Mean volume right-side (mm3) | Mean volume left-side (mm3) | Difference (mm3) | Post-hoc P-value | Significance |
|---|---|---|---|---|---|---|
| Whole hippocampus | TN | 3308.57 | 3260.83 | 47.74 | 0.5120 | |
| CL | 3676.00 | 3529.70 | 146.30 | 0.0027 | ** | |
| GC-ML-DG | TN | 291.06 | 277.90 | 13.16 | 0.0153 | * |
| CL | 329.98 | 313.62 | 16.36 | 0.0053 | ** | |
| CA4 | TN | 252.04 | 240.07 | 11.97 | 0.0137 | * |
| CL | 281.29 | 268.42 | 12.87 | 0.0119 | * | |
| CA2/CA3 | TN | 212.94 | 193.78 | 19.16 | 0.0034 | ** |
| CL | 232.79 | 208.54 | 24.25 | 0.0007 | *** | |
| CA1 | TN | 607.34 | 591.73 | 15.61 | 0.6206 | |
| CL | 692.85 | 655.47 | 37.38 | 0.0047 | ** | |
| Subiculum | TN | 401.66 | 409.81 | −8.15 | 0.7095 | |
| CL | 440.86 | 439.00 | 1.86 | >0.9999 | ||
| Presubiculum | TN | 270.94 | 289.63 | −18.69 | 0.0017 | ** |
| CL | 297.07 | 306.41 | −9.34 | 0.4754 | ||
| Parasubiculum | TN | 58.66 | 63.02 | −4.36 | 0.0842 | |
| CL | 58.49 | 58.28 | 0.21 | >0.9999 | ||
| ML-HP | TN | 545.48 | 535.61 | 9.87 | 0.5087 | |
| CL | 609.67 | 587.12 | 22.55 | 0.0241 | * | |
| Fimbria | TN | 77.70 | 75.57 | 2.13 | >0.9999 | |
| CL | 89.93 | 85.95 | 3.98 | 0.9141 | ||
| Hippocampal tail | TN | 535.45 | 529.79 | 5.66 | >0.9999 | |
| CL | 579.08 | 547.57 | 31.51 | 0.0224 | * | |
| Hippocampal fissure | TN | 142.60 | 139.73 | 2.87 | >0.9999 | |
| CL | 144.24 | 134.68 | 9.56 | 0.1074 | ||
| HATA | TN | 55.31 | 53.93 | 1.38 | >0.9999 | |
| CL | 63.98 | 59.34 | 4.64 | 0.0045 | ** |
Subfield volume difference calculated as: VOIadj of ipsilateral (right) - VOIadj of contralateral (left).
Abbreviations: GC-ML-DG: granule cell and molecular layer of the dentate gyrus; CA: cornu ammonis; ML-HP: molecular layer of hippocampus proper; HATA: hippocampal amygdala transition area.
3.3. Sex dependent differences in hippocampal subfield volumes
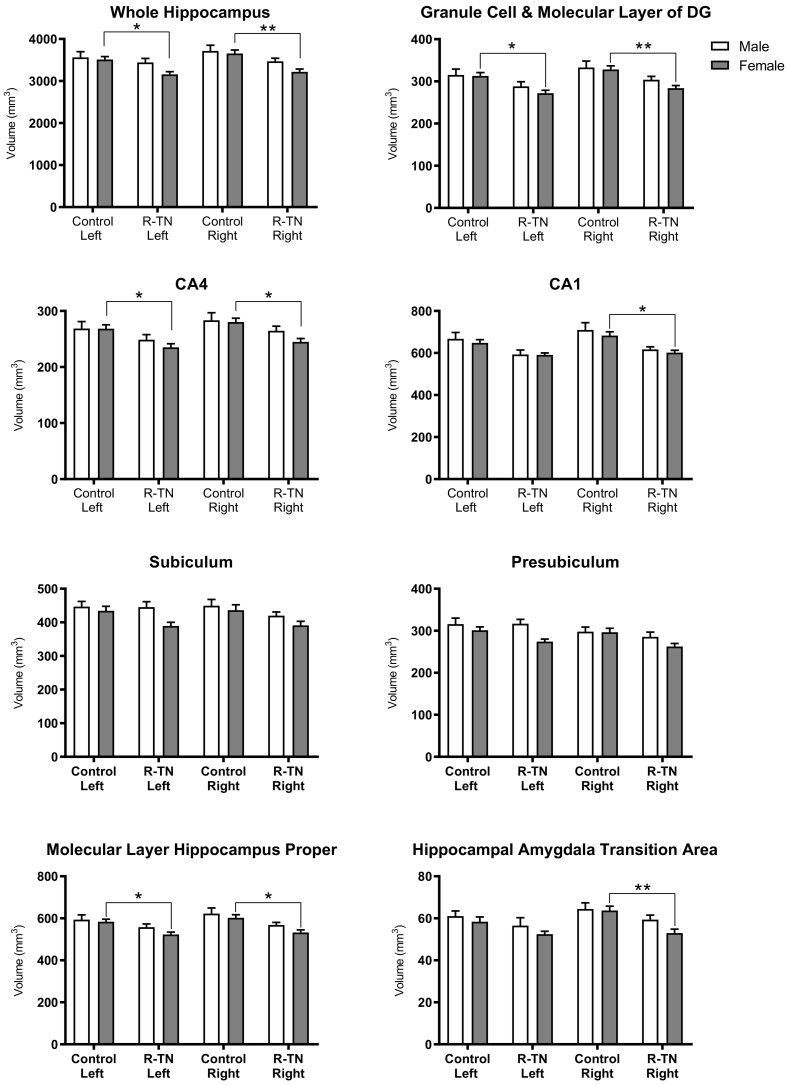
Female R-TN subjects had significantly smaller right and left whole hippocampal volume compared with female controls, however this change was not observed in males (Fig. 4). Female R-TN subjects showed reduced right subfield volumes compared to female controls in the GC-ML-DG (p = .0060), CA4 (p = .0209), CA1 (p = .0120), ML-HP (p = .0114), and the HATA (p = .0076). Additionally, female R-TN subjects showed smaller left subfields compared to controls in the GC-ML-DG (p = .0159), CA4 (p = .0380), and ML-HP (p = .0459). In comparison, male R-TN subjects did not show significant subfield volume changes compared to male controls. Subset analysis of hippocampal subfield volume by sex are shown in Fig. 4.
Fig. 4.
Hippocampal subfield volumetric changes in R-TN patients. Automated hippocampal segmentation stratified by sex shows hippocampal subfield reduction in female R-TNs compared to matched controls. * designates significant intergroup differences (R-TN vs. controls). * p < .05, ** p < .01.
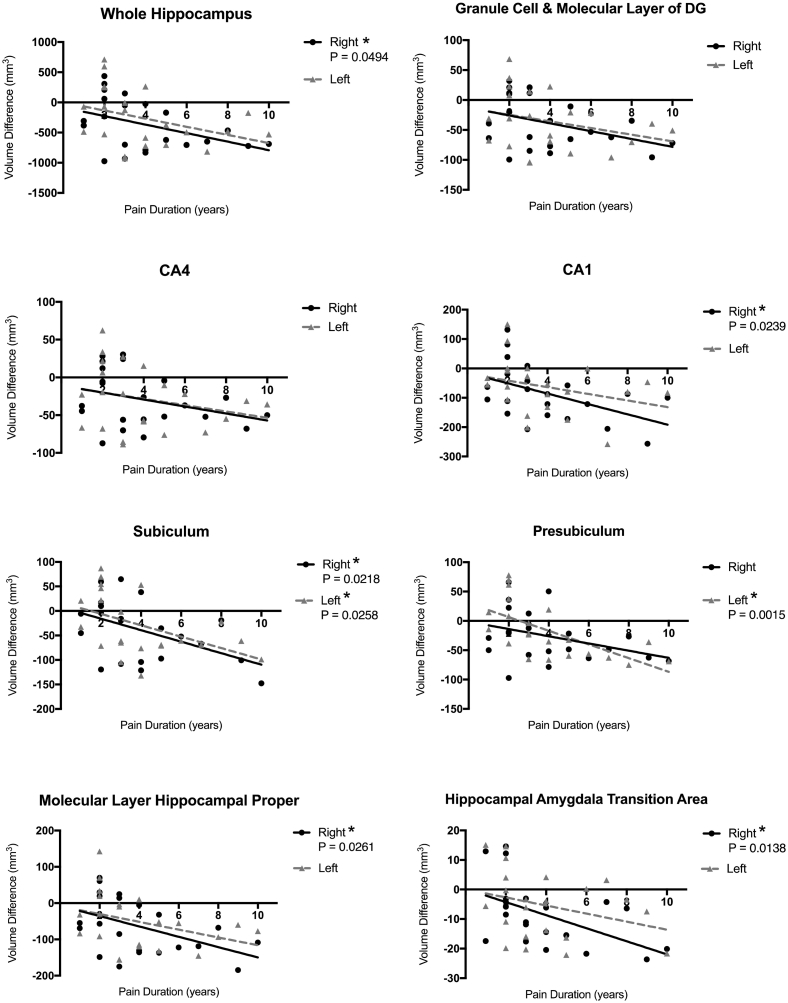
3.4. Correlation of hippocampal subfields changes and pain duration
Linear regression performed on the subfield volume differences over the reported pain duration showed significant correlation between pain duration and amount of hippocampal volume reduction in right hippocampus (p = .0494), but not the left (Fig. 5). The right CA1 (p = .0239), subiculum (p = .0218), ML-HP (p = .0261), and HATA (p = .0138), also demonstrated a significant correlation in volume reduction over time. Other subfields, including the GC-ML-DG and the CA4 showed a similar trend, but not statistically significant, towards reduction. Lastly, both the subiculum (p = .0258) and presubiculum (p = .0015) demonstrated correlation between left (contralateral) subfield reduction and pain duration.
Fig. 5.
Correlation between hippocampal subfield volume reduction and pain duration. Results of linear regression of pain duration and the volumetric differences between R-TN and matched controls. Subfield volume difference calculated as VOIadj in R-TN - ipsilateral VOIadj in controls. * p < .05.
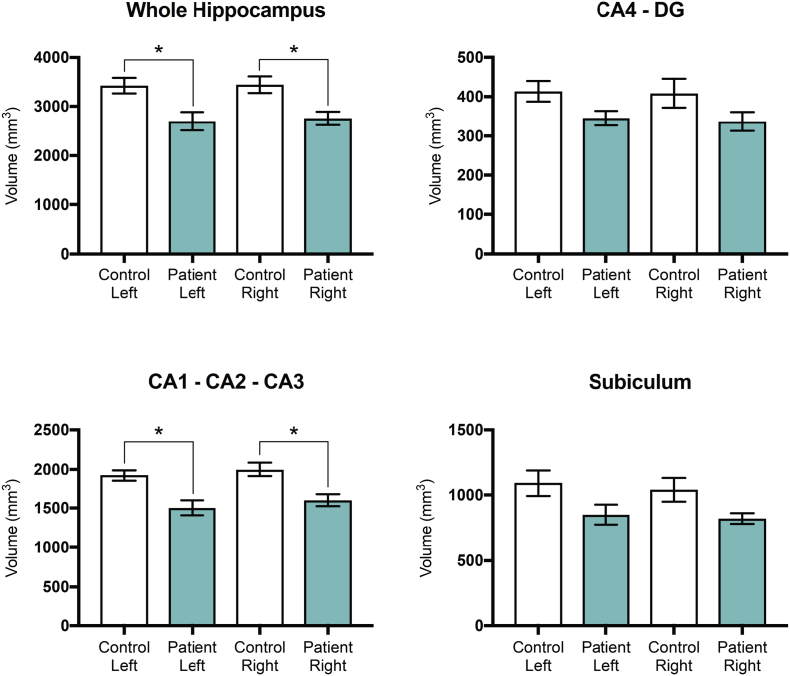
3.5. Manual hippocampal segmentation in R-TN subjects and controls
Manual hippocampal segmentation showed that the right whole hippocampus (p = .0337) and the right CA1-CA2-CA3 (p = .0166) subfield volume are significantly smaller in TN subjects compared to controls (Fig. 6). A similar difference was seen when comparing the left whole hippocampus (p = .0236) and left CA1-CA2-CA3 (p = .0199) subfield volume in TN subjects and controls. There was a trend towards bilateral volume reduction in the CA4-DG and subiculum segmentations of TN subjects compared to controls, however these were not significantly different. Manual segmentation findings are summarized in Table 4.
Fig. 6.
Manual hippocampal segmentation in 5 R-TNs and matched controls. Manual hippocampal segmentation shows volumetric reduction in the whole hippocampus as well as hippocampal subfields in R-TN patients compared to matched controls. * designates significant intergroup differences (R-TN vs. controls). * p < .05.
Table 4.
Summary of manual hippocampal segmentation in R-TN and their matched controls (* p < .05).
| Subfield | Side | Mean Volume R-TN (mm3) | Mean Volume Control (mm3) | Difference (mm3) | Post-hoc P-value | Significance |
|---|---|---|---|---|---|---|
| Whole Hippocampus | R | 2757.4 | 3445 | −687.6 | 0.0337 | * |
| L | 2699 | 3425.6 | −726.6 | 0.0236 | * | |
| CA4-DG | R | 336.8 | 408.4 | −71.6 | 0.3240 | |
| L | 345.4 | 413.8 | −68.4 | 0.3768 | ||
| CA1-CA2-CA3 | R | 1600 | 1997 | −397 | 0.0166 | * |
| L | 1503.8 | 1919.4 | −415.6 | 0.0119 | * | |
| Subiculum | R | 820.6 | 1039.6 | −219 | 0.2788 | |
| L | 849.8 | 1092.4 | −242.6 | 0.1875 |
Subfield volume difference calculated as: VOIadj of R-TN - ipsilateral VOIadj of matched control.
Abbreviations: DG: dentate gyrus; CA: cornu ammonis.
4. Discussion
There have been increasing reports examining the role of the CNS in contributing or maintaining TN pain (Desouza et al., 2013; Sarasa et al., 2018; Zhong et al., 2018). While these reports have primarily focused on white matter and neocortical structures, hippocampal abnormalities have not been linked to TN pain before. Yet TN is an excellent model for the study of the effect of neuropathic pain on the hippocampus, given its strictly unilateral representation of pain, as well as severity, leaving virtually no ambiguity as to the severity of the pain (Love and Coakham, 2001). Using TN as a model of neuropathic pain, our current study demonstrates significant changes in the hippocampus and its subregions. Specifically, we observe ipsilateral hippocampal whole and subregional volume reduction when compared with a cohort of healthy controls. The reduction in hippocampal subregions is further associated with duration of pain as well as sex, highlighting sex specific hippocampal plasticity in TN.
4.1. Hippocampal subfield volume reductions in R-TN subjects correlate with CNS circuits involved in pain processing
The hippocampus is composed of cytoarchitecturally and functionally distinct subfields with important roles such as the cognitive-affective processing of pain (Bushnell et al., 2013; Duvernoy et al., 2005; Simons et al., 2014). Reductions in hippocampal GM has previously been reported in other chronic pain diseases (Cauda et al., 2014), and recently in TN (Hayes et al., 2017). Considering the complex organization of the hippocampus and its varied functions, treating the entire structure of the hippocampus as a single volume likely ignores critical information now obtainable through MRI volumetric analysis techniques.
Previous studies have delineated the hippocampal afferent and efferent pathways involved in pain processing (for a detailed review please see Liu & Chen, 2009) (Liu and Chen, 2009). Afferents from the entorhinal cortex (EC) via the perforant path are composed of two distinct inputs. The first arises from layer II EC neurons that synapse on DG dendrites and input into the trisynaptic circuit (Dolorfo and Amaral, 1998). The second input, called the temporoammonic path, is comprised of layer II and III EC neurons that synapse directly on the distal dendrites of the CA1 and subiculum, and CA3, respectively (Empson and Heinemann, 1995; Kohler, 1985; Steward, 1976; Witter et al., 1988). We demonstrate that subfields directly innervated by the perforant path, including the DG, CA1, and subiculum, have reduced volume in TN. Interestingly, the CA2/CA3 volume is not reduced, which suggests the EC could relay pain stimuli preferentially through projections that input into the CA1, DG, and subiculum. These findings are in contrast with a study by Ezzati et al. 2014 (Ezzati et al., 2014), who report that older female subjects with chronic pain had smaller right CA2-CA3 and CA4-DG volumes. However, this study did not assess chronic pain from TN, and used an earlier version of FreeSurfer hippocampal segmentation with inferior accuracy (Iglesias et al., 2015; Schoene-Bake et al., 2014).
Additional circuits in pain processing may provide afferents to the EC or directly to the hippocampal formation (Liu and Chen, 2009). For instance, the Papez circuit, which contributes to emotional expression, connects the hypothalamus (mammillary bodies), anterior thalamic nuclei, cingulate cortex, and the hippocampus (Papez, 1995). Within this circuit, the anterior cingulate cortex (ACC) projects fibers directly to the subiculum and the EC (Henke, 1982). Our finding of reduced volume of the ipsilateral subiculum, presubiculum, and the subfields associated with perforant path inputs, align with this circuit. Additionally, this builds upon our previous findings of GM changes in affective circuits in TN subjects (DeSouza et al., 2014; Hayes et al., 2017).
Hippocampal outputs occur through two major pathways; the dorsal pathway involving the fimbria-fornix system, and the ventral pathway which connects the hippocampal formation and EC (Liu and Chen, 2009). Fibers of the fornix originate mainly from the CA1, whereas fibers of the fimbria originate in CA2, CA3, CA4 and the subicular complex (Meibach and Siegel, 1977; Swanson and Cowan, 1977). In TN subjects, the fimbria volume is unchanged, despite significant volume reduction of the CA4 and subiculum. This builds upon our prior work analyzing white matter changes with diffusion tensor imaging tractography in TN subjects where we found no changes associated with the structure of the fornix.
4.2. Bilateral hippocampal subfield reductions in R-TN female subjects
Female R-TN subjects have bilateral volume reduction in the GC-ML-DG, CA4, ML-HP, and whole hippocampus (Fig. 4). We have previously reported that bilateral hippocampal volume reduction occurs in patients with right-sided or left-sided TN, without stratifying by sex (Hayes et al., 2017). Currently, evidence conflicts as to whether chronic pain disorders produce bilateral (Cauda et al., 2014; Mutso et al., 2012), or unilateral (Ezzati et al., 2014) hippocampal volume reduction, with a similar discrepancy in animal studies (Belcheva et al., 2009; Mutso et al., 2012). Although TN produces ipsilateral pain symptoms, nociceptive inputs quickly cross the brainstem and present bilaterally (Dick and Rashiq, 2007). Our results show subfield volume reductions of greater significance ipsilaterally vs. contralaterally (Fig. 2), which coincides with the laterality of TN pain experience.
Chronic pain can lead to the development of a maladaptive stress and systemic hormonal changes via the hypothalamic–pituitary–adrenal (HPA) axis (McEwen and Kalia, 2010). The hippocampus regulates the HPA axis through feedback via mineralocorticoid and glucocorticoid receptors concentrated in the dentate gyrus (Galea et al., 2013). Recent evidence shows that elevated levels of cortisol through a maladaptive stress response in chronic pain is associated with smaller hippocampal volumes bilaterally (Vachon-Presseau et al., 2013). This may be another mechanism by which TN produces bilateral hippocampal subfield volume changes. Additionally, animal studies suggest that stress influences hippocampal neural plasticity differently in females compared to males (Galea et al., 2013), and sex hormones may explain differences in the hippocampal responses to aversive stimuli (Aloisi et al., 2000). Hormonal differences may contribute hippocampal subfield changes that are limited to female R-TN patients (Fig. 4). This finding supports a previous report demonstrating sex specific hippocampal volume reduction in chronic pain (Ezzati et al., 2014).
4.3. Aberrant neurogenesis as the substrate for volume loss in hippocampal subfields
GM volume reduction in the CNS as a result of chronic pain may manifest through changes in various substrates, including neuronal or glial turnover. A mechanism gaining increasing attention is the role of adult hippocampal neurogenesis (AHN). Disrupted AHN is associated with learning and memory deficits, and mood disorders. Depression and anxiety are highly comorbid with chronic pain (Gerrits et al., 2015), and patients often demonstrate learning and memory deficits (Dick and Rashiq, 2007). Apkarian et al. (Apkarian et al., 2016) demonstrated that AHN disruption in mice diminished persistent pain, while mice with AHN upregulation had prolonged persistent pain. Additionally, other studies reported decreased AHN in rodent models of chronic pain (Mutso et al., 2012). Thus, it has been proposed that downregulation of neurogenesis in chronic pain may serve to reduce pain-related memory formation as a self-protective mechanism (Eipe et al., 2016). Interestingly, we show that TN patients have bilateral volume reduction in the GC-ML-DG (Fig. 2), the primary location of AHN. It is possible that TN patients have aberrant AHN, which could contribute to volume reduction and maintenance of chronic pain. Vachon-Presseau et al. support this notion, suggesting that in patients with chronic back pain, elevated cortisol levels due to a maladaptive stress response may alter hippocampal circuitry via aberrant neurogenesis (Vachon-Presseau et al., 2013).
In agreement with others (Blankstein et al., 2010; Moayedi et al., 2011), we also demonstrate that GM volume reduction in R-TN subjects correlates with pain duration, specifically in the ipsilateral CA1, subiculum, ML-HP, HATA, and the whole hippocampus (Fig. 5). This supports the role of the CA1 and subiculum in pain processing (Liu and Chen, 2009), and the notion that chronic pain in the form of repetitive pain attacks can drive neuroplasticity (Teutsch et al., 2008). However, in the context of neurogenesis, we did not observe a similar correlation in the GC-ML-DG (although there was a trend of p = .0673). Although granule cells do not account for the entire DG structure, it is however plausible that a chronically reduced rate of neurogenesis could reach a steady state and stable DG volume over time. Future studies are required to delineate the role of AHN in chronic pain.
4.4. Study limitations
Our study utilizes FreeSurfer's hippocampal subfields segmentation protocol to investigate the effect of the neuropathic chronic pain in trigeminal neuralgia patients. Previous studies have reported hippocampal volumetric changes in chronic pain, in agreement with findings reported in this aspect of our current study (Apkarian et al., 2016; Mutso et al., 2012; Romero-grimaldi et al., 2015). Nonetheless, the interpretation of these findings, particularly the male alone cohort, may be limited by the sample size. Additionally, most TN patients were undergoing pharmacological therapy for TN, often with anticonvulsants such as carbamazepine (Table 1). A recent study suggests that chronic administration of gabapentin and carbamazepine may cause increase in neurodegenerative changes in the brain of animal models (Olaibi et al., 2014). Furthermore, long-term use of antiepileptics in patients with temporal lobe epilepsy results in reduced hippocampal betweenness centrality (a measure of connectivity) (Haneef et al., 2015). Although the aforementioned studies suggest that anticonvulsants may affect the hippocampal neuronal circuits, however, the direct effect of these medications on the hippocampus has not been previously studied and further investigation is required. Given the severity of TN pain, it is also not tenable to study a medication free patient cohort and the population investigated in our study therefore reflects the typical TN population.
5. Conclusions
Our results support our hypothesis of selective alterations to hippocampal GM in TN patients, with associated effects based on sex and pain duration. How these changes arise may be due to the distinct cytoarchitecture, and functions of hippocampal subfields. Pain duration can enhance stress and trigger alterations in affect-related circuitry, involving specific hippocampal subfields. This study contributes to the increasing body of evidence suggesting hippocampal involvement in pain modulation and future studies should be aimed at elucidating the potential dynamic nature of the structural changes in the hippocampus in chronic pain. Additionally, TN as a distinctive form of neuropathic facial pain with high pain severity should be considered as a unique model in investigating the role of the hippocampus in pain modulation.
Acknowledgments
Acknowledgements
The authors would like to thank Erika Wharton-Shukster and Jia-Yan Zhang for their assistance with chart reviews and data collection.
This study was supported by a Canadian Institutes of Health Research operating grant [grant number MOP130555], the Ontario Graduate Scholarship Program, and University of Toronto Centre for the Study of Pain.
Declaration of Competing Interests
The authors have no conflict of interest to declare.
References
- Acsády L., Káli S. Models, structure, function: the transformation of cortical signals in the dentate gyrus. In: Scharfman H.E., editor. Progress in Brain Research. vol. 163. Elsevier; 2007. pp. 577–599. [DOI] [PubMed] [Google Scholar]
- Aloisi A.M., Ceccarelli I., Herdegen T. Gonadectomy and persistent pain differently affect hippocampal c-Fos expression in male and female rats. Neurosci. Lett. 2000;281(1):29–32. doi: 10.1016/s0304-3940(00)00819-3. [DOI] [PubMed] [Google Scholar]
- Apkarian A.V., Mutso A.A., Centeno M.V., Kan L., Wu M., Levinstein M., Banisadr G., Gobeske K.T., Miller R.J., Radulovic J., Hen R., Kessler J.A. Role of adult hippocampal neurogenesis in persistent pain. Pain. 2016;157(2):418–428. doi: 10.1097/j.pain.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Fields H.L., Apkarian A.V. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Schnitzer T.J., Bauer W.R., Apkarian A.V. Brain morphological signatures for chronic pain. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva I., Ivanova M., Tashev R., Belcheva S. Differential involvement of hippocampal vasoactive intestinal peptide in nociception of rats with a model of depression. Peptides. 2009;30(8):1497–1501. doi: 10.1016/j.peptides.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Blankstein U., Chen J., Diamant N.E., Davis K.D. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138(5):1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Head D., Parker J., Fotenos A.F., Marcus D., Morris J.C., Snyder A.Z. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bushnell M.C., Ceko M., Low L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013;14(7):502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F., Palermo S., Costa T., Torta R., Duca S., Vercelli U., Geminiani G., Torta D.M. Gray matter alterations in chronic pain: a network-oriented meta-analytic approach. Neuroimage Clin. 2014;4:676–686. doi: 10.1016/j.nicl.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desouza D.D., Moayedi M., Chen D.Q., Davis K.D., Hodaie M. Sensorimotor and pain modulation brain abnormalities in trigeminal neuralgia: a paroxysmal, sensory-triggered neuropathic pain. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0066340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza D.D., Hodaie M., Davis K.D. Abnormal trigeminal nerve microstructure and brain white matter in idiopathic trigeminal neuralgia. Pain. 2014;155(1):37–44. doi: 10.1016/j.pain.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Dick B.D., Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesth. Analg. 2007;104(5):1223–1229. doi: 10.1213/01.ane.0000263280.49786.f5. (tables of contents) [DOI] [PubMed] [Google Scholar]
- Dolorfo C.L., Amaral D.G. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J. Comp. Neurol. 1998;398(1):25–48. [PubMed] [Google Scholar]
- Duvernoy H.M., Cattin E., Naidich T., Fatterpekar G.M., Raybaud C., Risold P.Y. Springer; Verlag, Berlin: 2005. The Human Hippocampus. [Google Scholar]
- Eipe N., Penning J., Yazdi F., Mallick R., Turner L., Ansari M.T. Hippocampal neurogenesis: does it relieve or worsen chronic pain? Pain. 2016;157(2):505–506. doi: 10.1097/j.pain.0000000000000418. [DOI] [PubMed] [Google Scholar]
- Empson R.M., Heinemann U. The perforant path projection to hippocampal area CA1 in the rat hippocampal-entorhinal cortex combined slice. J. Physiol. 1995;484(Pt 3):707–720. doi: 10.1113/jphysiol.1995.sp020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A., Zimmerman M.E., Katz M.J., Sundermann E.E., Smith J.L., Lipton M.L., Lipton R.B. Hippocampal subfields differentially correlate with chronic pain in older adults. Brain Res. 2014;1573:54–62. doi: 10.1016/j.brainres.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin N.J., Agster K.L., Eichenbaum H.B. Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 2002;5:458. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea L.A., Wainwright S.R., Roes M.M., Duarte-Guterman P., Chow C., Hamson D.K. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J. Neuroendocrinol. 2013;25(11):1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Gerrits M.M., van Marwijk H.W., van Oppen P., van der Horst H., Penninx B.W. Longitudinal association between pain, and depression and anxiety over four years. J. Psychosom. Res. 2015;78(1):64–70. doi: 10.1016/j.jpsychores.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Gwilym S.E., Filippini N., Douaud G., Carr A.J., Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62(10):2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- Haneef Z., Levin H.S., Chiang S. Brain graph topology changes associated with anti-epileptic drug use. Brain Connect. 2015;5(5):284–291. doi: 10.1089/brain.2014.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D.J., Chen D.Q., Zhong J., Lin A., Behan B., Walker M., Hodaie M. Affective circuitry alterations in patients with trigeminal neuralgia. Front. Neuroanat. 2017;11:73. doi: 10.3389/fnana.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache S The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Henke P.G. The telencephalic limbic system and experimental gastric pathology: a review. Neurosci. Biobehav. Rev. 1982;6(3):381–390. doi: 10.1016/0149-7634(82)90047-1. [DOI] [PubMed] [Google Scholar]
- Henry D.E., Chiodo A.E., Yang W. Central nervous system reorganization in a variety of chronic pain states: a review. PM R. 2011;3(12):1116–1125. doi: 10.1016/j.pmrj.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Hung P.S., Chen D.Q., Davis K.D., Zhong J., Hodaie M. Predicting pain relief: use of pre-surgical trigeminal nerve diffusion metrics in trigeminal neuralgia. Neuroimage Clin. 2017;15:710–718. doi: 10.1016/j.nicl.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker M.R., Kesner R.P. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18(9):955–964. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M., Roy N., Frosch M.P., McKee A.C., Wald L.L., Fischl B., Van Leemput K. Alzheimer's disease neuroimaging I. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner R.P., Hunsaker M.R. The temporal attributes of episodic memory. Behav. Brain Res. 2010;215(2):299–309. doi: 10.1016/j.bbr.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Kim S., Dede A.J.O., Hopkins R.O., Squire L.R. Memory, scene construction, and the human hippocampus. Proc. Natl. Acad. Sci. 2015;112(15):4767. doi: 10.1073/pnas.1503863112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C. A projection from the deep layers of the entorhinal area to the hippocampal formation in the rat brain. Neurosci. Lett. 1985;56(1):13–19. doi: 10.1016/0304-3940(85)90433-1. [DOI] [PubMed] [Google Scholar]
- Kulaga-Yoskovitz J., Bernhardt B.C., Hong S.J., Mansi T., Liang K.E., van der Kouwe A.J., Smallwood J., Bernasconi A., Bernasconi N. Multi-contrast submillimetric 3 Tesla hippocampal subfield segmentation protocol and dataset. Sci Data. 2015;2 doi: 10.1038/sdata.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.G., Chen J. Roles of the hippocampal formation in pain information processing. Neurosci. Bull. 2009;25(5):237–266. doi: 10.1007/s12264-009-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S., Coakham H.B. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(Pt 12):2347–2360. doi: 10.1093/brain/124.12.2347. [DOI] [PubMed] [Google Scholar]
- McCarberg B., Peppin J. 2019. Pain Pathways and Nervous System Plasticity: Learning and Memory in Pain. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. 2010;59(Suppl. 1):S9–15. doi: 10.1016/j.metabol.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Meibach R.C., Siegel A. Efferent connections of the hippocampal formation in the rat. Brain Res. 1977;124(2):197–224. doi: 10.1016/0006-8993(77)90880-0. [DOI] [PubMed] [Google Scholar]
- Moayedi M., Weissman-Fogel I., Crawley A.P., Goldberg M.B., Freeman B.V., Tenenbaum H.C., Davis K.D. Contribution of chronic pain and neuroticism to abnormal forebrain gray matter in patients with temporomandibular disorder. Neuroimage. 2011;55(1):277–286. doi: 10.1016/j.neuroimage.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Mutso A.A., Radzicki D., Baliki M.N., Huang L., Banisadr G., Centeno M.V., Radulovic J., Martina M., Miller R.J., Apkarian A.V. Abnormalities in hippocampal functioning with persistent pain. J. Neurosci. 2012;32(17):5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nurmikko T.J., Eldridge P.R. Trigeminal neuralgia—pathophysiology, diagnosis and current treatment. Br. J. Anaesth. 2001;87(1):117–132. doi: 10.1093/bja/87.1.117. [DOI] [PubMed] [Google Scholar]
- Olaibi O.K., Osuntokun O.S., Ijomone O.M. Effects of chronic administration of gabapentin and carbamazepine on the histomorphology of the hippocampus and striatum. Ann. Neurosci. 2014;21(2):57–61. doi: 10.5214/ans.0972.7531.210206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P.L., Zhong J.G., Shang H.F., Zhu Y.L., Xiao P.R., Dai Z.Y., Shi H.C. Quantitative meta-analysis of grey matter anomalies in neuropathic pain. Eur. J. Pain. 2015;19(9):1224–1231. doi: 10.1002/ejp.670. [DOI] [PubMed] [Google Scholar]
- Papez J.W. A proposed mechanism of emotion. 1937. J. Neuropsychiat. Clin. Neurosci. 1995;7(1):103–112. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Raecke R., Niemeier A., Ihle K., Ruether W., May A. Structural brain changes in chronic pain reflect probably neither damage nor atrophy. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0054475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T., Kesner R.P. A computational theory of hippocampal function, and empirical tests of the theory. Prog. Neurobiol. 2006;79(1):1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Romero-grimaldi C., Berrocoso E., Alba-Delgado C., Madrigal J., Perez-Nievas B., Carlos Leza J., Antonio Mico J. vol. 121. 2015. Stress Increases the Negative Effects of Chronic Pain on Hippocampal Neurogenesis. [DOI] [PubMed] [Google Scholar]
- Sanfilipo M.P., Benedict R.H., Zivadinov R., Bakshi R. Correction for intracranial volume in analysis of whole brain atrophy in multiple sclerosis: the proportion vs. residual method. Neuroimage. 2004;22(4):1732–1743. doi: 10.1016/j.neuroimage.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Sarasa T., Peter Shih-Ping H., Jidan Z., Mojgan H. Early postsurgical diffusivity metrics for prognostication of long-term pain relief after Gamma Knife radiosurgery for trigeminal neuralgia. J. Neurosurg. 2018:1–10. doi: 10.3171/2018.3.JNS172936. [DOI] [PubMed] [Google Scholar]
- Schoene-Bake J.C., Keller S.S., Niehusmann P., Volmering E., Elger C., Deppe M., Weber B. In vivo mapping of hippocampal subfields in mesial temporal lobe epilepsy: relation to histopathology. Hum. Brain Mapp. 2014;35(9):4718–4728. doi: 10.1002/hbm.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz D.A., Wideman T.H., Naso L., Hatami-Khoroushahi Z., Fallatah S., Ware M.A., Jarzem P., Bushnell M.C., Shir Y., Ouellet J.A., Stone L.S. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 2011;31(20):7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons L.E., Elman I., Borsook D. Psychological processing in chronic pain: a neural systems approach. Neurosci. Biobehav. Rev. 2014;39:61–78. doi: 10.1016/j.neubiorev.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood R.F., Laird A.R., Ramage A.E., Parkinson A.L., Lewis J., Clauw D.J., Williams D.A., Schmidt-Wilcke T., Farrell M.J., Eickhoff S.B., Robin D.A. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J. Pain. 2013;14(7):663–675. doi: 10.1016/j.jpain.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J. Comp. Neurol. 1976;167(3):285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Swanson L.W., Cowan W.M. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J. Comp. Neurol. 1977;172(1):49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Teutsch S., Herken W., Bingel U., Schoell E., May A. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage. 2008;42(2):845–849. doi: 10.1016/j.neuroimage.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E., Roy M., Martel M.O., Caron E., Marin M.F., Chen J., Albouy G., Plante I., Sullivan M.J., Lupien S.J., Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136(Pt 3):815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- Witter M.P., Griffioen A.W., Jorritsma-Byham B., Krijnen J.L. Entorhinal projections to the hippocampal CA1 region in the rat: an underestimated pathway. Neurosci. Lett. 1988;85(2):193–198. doi: 10.1016/0304-3940(88)90350-3. [DOI] [PubMed] [Google Scholar]
- Zhong J., Chen D.Q., Hung P.S.-P., Hayes D.J., Liang K.E., Davis K.D., Hodaie M. Multivariate pattern classification of brain white matter connectivity predicts classic trigeminal neuralgia. PAIN. 2018;159(10):2076–2087. doi: 10.1097/j.pain.0000000000001312. [DOI] [PubMed] [Google Scholar]