Abstract
The local metabolic hypothesis proposes that myocardial oxygen tension determines the degree of autoregulation by increasing the production of vasodilator metabolites as perfusion pressure is reduced. Thus, normal physiologic levels of coronary venous PO2, an index of myocardial oxygenation, are proposed to be required for effective autoregulation. The present study challenged this hypothesis through determination of coronary responses to changes in coronary perfusion pressure (CPP 140–40 mmHg) in open-chest swine in the absence (n = 7) and presence of euvolemic hemodilution (~ 50% reduction in hematocrit), with (n = 5) and without (n = 6) infusion of dobutamine to augment MVO2. Coronary venous PO2 decreased over similar ranges (~ 28–15 mmHg) as CPP was lowered from 140 to 40 mmHg in each of the groups. However, coronary venous PO2 was not associated with changes in coronary blood flow (r = − 0.11; P = 0.29) or autoregulatory gain (r = − 0.29; P = 0.12). Coronary zero-flow pressure (Pzf) was measured in 20 mmHg increments and determined to be directly related to vascular resistance (r = 0.71; P < 0.001). Further analysis demonstrated that changes in coronary blood flow remained minimal at Pzf > 20 mmHg, but progressively increased as Pzf decreased below this threshold value (r = 0.68; P < 0.001). Coronary Pzf was also positively correlated with autoregulatory gain (r = 0.43; P = 0.001). These findings support that coronary autoregulatory behavior is predominantly dependent on an adequate degree of underlying vasomotor tone, independent of normal myocardial oxygen tension.
Keywords: Coronary, Autoregulation, Zero-flow pressure, Swine
Introduction
The coronary circulation is actively regulated by a variety of mechanisms to maintain an adequate balance between myocardial oxygen delivery and metabolism. This point is demonstrated by the coronary response to a variety of physiologic perturbations, including alterations in perfusion pressure, cardiac workload, and tissue oxygenation [24]. Studies dating back to the 1950s definitively established that the coronary circulation has the innate ability to maintain relatively constant blood flow over a wide range of perfusion pressures [1] and that the overall level of this pressure–flow autoregulation adjusts to myocardial oxygen consumption (MVO2) [1, 22, 38]. The autoregulatory capacity of the coronary circulation is particularly important to compensate for intermediate degrees of stenosis (distal coronary pressures ≥ 60 mmHg) where, if absent, hypoperfusion can result in rapid reductions in cardiac function and/or myocardial injury [21, 24]. Despite the critical nature of coronary autoregulation, the mechanisms responsible for this physiologic phenomenon continue to be debated.
One of the most prominent theories to explain coronary pressure–flow autoregulation focuses on metabolic regulation of coronary microvascular resistance. The local metabolic hypothesis proposes that myocardial oxygen tension determines the degree of autoregulation by increasing the production of vasodilator metabolites as perfusion pressure is reduced [16, 22, 24]. This paradigm is supported by studies which have demonstrated that coronary venous PO2, a commonly used index of myocardial tissue PO2 [22], decreases with perfusion pressure [3, 8, 26, 42, 45] and that the overall autoregulatory capacity (i.e., gain) is directly dependent on normal physiologic levels of coronary venous PO2 [16]. Dole and Nuno documented that coronary autoregulation is only observed when coronary venous PO2 is below 25 mmHg and abolished when oxygen tension exceeds 32 mmHg. As such, local metabolic control is purported to be the dominant mechanism of coronary pressure–flow autoregulation [16]. However, efforts to elucidate specific metabolites or pathways that contribute to autoregulatory behavior have failed to show any role for putative dilators such as adenosine [17, 19, 25, 31], nitric oxide [40], and/or end-effector K+ channels [8, 19, 42].
Despite the attractiveness of the metabolic hypothesis of coronary autoregulation, interpretation of the inverse relationship between autoregulatory gain and coronary venous PO2 is confounded by effects of other vasoactive mechanisms, primarily the vascular smooth muscle response to alterations in intraluminal pressure [4]. Definitive evidence of the myogenic (Bayliss) response in the coronary circulation comes from studies which demonstrate pressure-dependent changes in the diameter of isolated pressurized coronary arterioles [33–35, 37]. Importantly, additional experiments also established that the overall degree of this intrinsic myogenic response decreases as underlying vasomotor tone is reduced [34, 37]. These findings have critical implications for prior in vivo observations in that increases in coronary venous PO2, typically induced by the administration of vasodilator agents (e.g., adenosine) and/or reductions in key determinants of MVO2 (e.g., heart rate) [3, 5, 8, 14, 16], would be predicted to diminish coronary autoregulatory capacity not via local metabolic pathways per se, but through the attenuation of a pressure-dependent myogenic mechanism. This contention is corroborated by earlier studies which documented that voltage-gated Ca2+ (CaV1.2) channels are critical for the coronary myogenic response [37] and that inhibition of CaV1.2 channels abolishes coronary autoregulation in vivo [8]. Accordingly, there is ample evidence to support the alternative hypothesis that autoregulation in the coronary circulation is predominantly myogenic in origin and thus more dependent on an adequate degree (threshold) of underlying coronary vasomotor tone rather than the prevailing level of myocardial oxygen tension.
The purpose of this investigation was to examine the metabolic hypothesis of coronary autoregulation through alterations in the overall vasomotor tone and/or MVO2, independent of the underlying differences in coronary venous PO2. Experiments were designed to determine the coronary responses to changes in perfusion pressure (140–40 mmHg) in the absence and presence of euvolemic hemodilution (~ 50% reduction in hematocrit), with and without infusion of dobutamine to augment MVO2. Hemodilution was utilized in these studies as reductions in hematocrit are well known to increase coronary blood flow and diminish vasodilator reserve with little/no change in coronary venous PO2 [11, 28, 36]. The effects of these conditions on coronary vasomotor tone were assessed through measurements of coronary pressure when coronary flow had ceased [i.e., zero-flow pressure (Pzf)] [5]; which has been shown to be predominantly determined by overall vascular smooth muscle tone [14, 15, 17, 30, 41, 47]. Data from these experiments offer novel insight into the fundamental question regarding the dominant mechanism(s) of coronary pressure–flow autoregulation.
Methods
This investigation was approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee and performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, Revised 2011). Adult ~ 50 kg male domestic swine were sedated with Telazol, xylazine, and ketamine (5.0, 2.5, and 2.5 mg/kg respectfully) prior to anesthesia with morphine (0.5 mg/kg) and intravenous α-chloralose (60 mg/kg).
Experimental preparation
Anesthetized swine were intubated and ventilated with O2-supplemented room air. Bilateral femoral cut downs were performed, and catheters placed in the femoral artery and vein. The right femoral artery catheter provided continuous measurement of systemic blood pressure and heart rate, while the venous catheter allowed for administration of anesthetic, dobutamine, and Hespan (6% hetastarch in 0.9% sodium chloride). The left femoral artery catheter supplied blood to an extracorporeal servo-controlled pump used to perfuse the left anterior descending (LAD) coronary artery at designated perfusion pressures, as previously described by our laboratory [8]. Arterial blood gases were analyzed periodically and adjustments to respiration were made to maintain parameters within physiological limits.
Succinylcholine (0.5 mg/kg) was administered prior to a thoracotomy in the left fifth intercostal space. Following isolation of the LAD and the administration of heparin (500 units/kg, iv), the LAD was cannulated with a steel tip cannula fed by the extracorporeal perfusion circuit. Coronary perfusion pressure (CPP) was regulated by a servo-controlled roller pump and coronary blood flow was continuously measured by an in-line Transonic Systems flow transducer (Ithaca, New York, USA). The anterior interventricular vein was cannulated to allow for sampling of venous blood from the LAD perfusion territory. Following a ~ 15 min stabilization period, data were continuously recorded on IOX data acquisition software (EMKA Technologies; Falls Church, VA).
Experimental protocol
Pigs were randomly assigned to one of the following groups: (1) control (n = 7); (2) hemodilution (n = 6); (3) hemodilution + dobutamine (n = 5). Hemodilution was performed by gradually replacing equal volumes of blood with the synthetic plasma expander, Hespan (6% hetastarch in 0.9% sodium chloride) at 37 °C until hematocrit was reduced ~ 50% from baseline [28]. Dobutamine was administered by an intravenous drip (250 mg/L in saline) that was titrated to increase heart rate ~ 75–100% above baseline levels.
Following a subsequent stabilization period of ~ 15 min, pressure–flow autoregulation was assessed by reducing coronary perfusion pressure (CPP) in increments of 10 mmHg from 140 to 40 mmHg. Arterial and coronary venous blood samples were simultaneously collected once hemodynamic parameters stabilized at each CPP. Coronary Pzf was assessed at 20 mmHg increments by clamping the perfusion circuit and allowing coronary blood flow to cease for ~ 4 s. Following completion of experimental protocols, hearts were fibrillated and excised as recommended by the American Veterinary Medical Association Guide on Euthanasia.
As previously reported by our laboratory and others [3, 8, 26], closed-loop autoregulatory gain (Gc) was calculated from the following formula:
where ∆F is the change in coronary blood flow, and F is the coronary flow measured at given perfusion pressure (P). Gc was determined in 20 mmHg increments over CPPs ranging from 120 to 60 mmHg. A Gc value of 1 reflects perfect autoregulation and values < 0 indicate no autoregulation. Coronary vascular resistance was calculated by dividing CPP by coronary blood flow.
Blood gas analyses
Arterial and coronary venous blood samples were collected, immediately sealed, and placed on ice. The samples were analyzed for pH, PCO2, PO2, glucose, lactate, and oxygen content with an Instrumentation Laboratories automatic blood gas analyzer (GEM Premier 3000) and CO-oximeter (682) system. LAD perfusion territory was estimated to be 30% of total heart weight, as previously described [23]. MVO2 was calculated by multiplying coronary blood flow by the coronary arterial–venous difference in oxygen content.
Statistical analysis
Data are presented as mean ± SE. Statistical comparisons for data presented in Table 1 were made by a two-way analysis of variance (ANOVA; factor A: CPP; factor B: treatment group). Differences were considered statistically significant when P < 0.05. If significance with ANOVA was detected, a Student–Newman–Keuls multiple comparison test was performed. Pearson correlation analysis was utilized to assess the relationship between coronary resistance, changes in coronary blood flow, and autoregulatory gain relative to coronary venous PO2 and Pzf. Lines of best fit are shown for significant associations with correlation coefficients (r) > 0.40. Statistical analyses were performed with Sigma Plot 11.0 software (Systat Software Inc., San Jose, CA, USA). Multiple linear regression analysis was performed with VassarStats (Arlington, New York, USA).
Table 1.
Hemodynamic and coronary responses to graded changes in perfusion pressure
| Coronary per-fusion pressure (mmHg) | 140 | 130 | 120 | 110 | 100 | 90 | 80 | 70 | 60 | 50 | 40 | CPP | Group | Interaction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean aortic pressure (mmHg) | ||||||||||||||
| Control | 87 ± 7 | 87 ± 6 | 88 ± 6 | 88 ± 6 | 88 ± 6 | 87 ± 6 | 88 ± 6 | 88 ± 6 | 87 ± 6 | 87 ± 6 | 85 ± 6 | P = 0.34 | P < 0.001 | P = 0.79 |
| Hemodilution | 86 ± 8 | 86 ± 8 | 81 ± 8 | 81 ± 7 | 80 ± 8 | 80 ± 8 | 80 ± 8 | 80 ± 8 | 84 ± 8 | 83 ± 8 | 82 ± 8 | |||
| Hemodilution + dobutamine | 91 ± 9 | 84 ± 8 | 74 ± 7 | 73 ± 6 | 66 ± 7† | 62 ± 6† | 60 ± 5† | 59 ± 4† | 59 ± 5† | 58 ± 5† | 58 ± 5† | |||
| Heart rate (beats/min) | ||||||||||||||
| Control | 74 ± 4 | 77 ± 3 | 75 ± 4 | 75 ± 4 | 77 ± 4 | 76 ± 4 | 77 ± 4 | 78 ± 4 | 78 ± 4 | 79 ± 4 | 80 ± 4 | P = 0.72 | P < 0.001 | P = 0.99 |
| Hemodilution | 75 ± 6 | 75 ± 6 | 72 ± 6 | 71 ± 5 | 72 ± 5 | 73 ± 5 | 73 ± 4 | 73 ± 4 | 77 ± 3 | 77 ± 3 | 78 ± 3 | |||
| Hemodilution + dobutamine | 152 ± 8† | 151 ± 8† | 134 ± 11† | 134 ± 9† | 142 ± 7† | 140 ± 6† | 139 ± 5† | 140 ± 5† | 142 ± 5† | 143 ± 5† | 140 ± 4† | |||
| Hematocrit (%) | ||||||||||||||
| Control | 32 ± 2 | 32 ± 2 | 32 ± 2 | 32 ± 2 | 33 ± 2 | 33 ± 2 | 33 ± 2 | 33 ± 2 | 33 ± 2 | 34 ± 2 | 33 ± 2 | P = 1.00 | P < 0.001 | P = 1.00 |
| Hemodilution | 17 ± 2† | 16 ± 2† | 16 ± 2† | 16 ± 2† | 16 ± 2† | 17 ± 2† | 17 ± 3† | 16 ± 2† | 16 ± 2† | 17 ± 2† | 16 ± 3† | |||
| Hemodilution + dobutamine | 18 ± 5† | 18 ± 6† | 21 ± 4† | 21 ± 4† | 21 ± 4† | 21 ± 4† | 20 ± 4† | 20 ± 4† | 19 ± 3† | 19 ± 4† | 19 ± 3† | |||
| Coronary blood flow (mL/min/g) | ||||||||||||||
| Control | 0.95 ± 0.09* | 0.83 ± 0.06* | 0.70 ± 0.05 | 0.67 ± 0.05 | 0.63 ± 0.04 | 0.58 ± 0.04 | 0.56 ± 0.04 | 0.50 ± 0.03 | 0.47 ± 0.03 | 0.38 ± 0.02* | 0.28 ± 0.03* | P < 0.001 | P < 0.001 | P < 0.001 |
| Hemodilution | 1.62 ± 0.15*† | 1.49 ± 0.12† | 1.43 ± 0.1† | 1.31 ± 0.07† | 1.25 ± 0.07† | 1.20 ± 0.06† | 1.08 ±s 0.08† | 1.02 ± 0.06† | 0.92 ± 0.05*† | 0.79 ± 0.04*† | 0.54 ± 0.05*† | |||
| Hemodilution + dobutamine | 3.44 ± 0.42† | 3.18 ± 0.44† | 3.01 ± 0.26† | 2.86 ± 0.26† | 2.63 ± 0.22† | 2.30 ± 0.13† | 1.99 ± 0.11*† | 1.76 ± 0.09*† | 1.51 ± 0.09*† | 1.23 ± 0.09*† | 0.87 ± 0.06*† | |||
| MVO2 (μL O2/min/g) | ||||||||||||||
| Control | 63 ± 2 | 61 ± 5 | 57 ± 6 | 62 ± 5 | 62 ± 6 | 62 ± 6 | 61 ± 5 | 58 ± 5 | 56 ± 4 | 49 ± 4 | 37 ± 5* | P < 0.001 | P < 0.001 | P = 0.16 |
| Hemodilution | 70 ± 12 | 69 ± 9 | 59 ± 8 | 58 ± 8 | 56 ± 4 | 53 ± 3 | 54 ± 3 | 51 ± 2 | 44 ± 4 | 40 ± 4 | 30 ± 2 | |||
| Hemodilution + dobutamine | 175 ± 28† | 159 ± 36† | 128 ± 29† | 130 ± 30† | 130 ± 24† | 104 ± 26† | 101 ± 24† | 98 ± 22† | 81 ± 14 | 73 ± 11 | 60 ± 9 | |||
| Coronary venous PO2 (mmHg) | ||||||||||||||
| Control | 28.7 ± 1.9 | 27.7 ± 3.0 | 25.0 ± 3.0 | 22.6 ± 2.5 | 20.1 ± 1.9 | 18.9 ± 2.4 | 17.6 ± 2.1 | 16.0 ± 2.0 | 15.4 ± 1.7 | 14.0 ± 1.9 | 13.4 ± 2.2 | P < 0.001 | P < 0.001 | P = 0.97 |
| Hemodilution | 24.8 ± 3.4 | 25.0 ± 3.3 | 28.7 ± 5.3 | 26.8 ± 4.5 | 21.2 ± 2.9 | 24.8 ± 4.5 | 19.8 ± 2.7 | 19.2 ± 2.7 | 17.2 ± 2.1 | 16.2 ± 2.5 | 15.6 ± 2.3 | |||
| Hemodilution + dobutamine | 26.0 ± 2.3 | 26.0 ± 1.7 | 29.0 ± 2.6 | 28.2 ± 2.4 | 26.4 ± 2.8 | 26.3 ± 2.0 | 24.7 ± 2.3 | 22.5 ± 2.2 | 20.3 ± 2.3 | 18.8 ± 2.2 | 16.8 ± 1.4 | |||
| Pzf (mmHg) | ||||||||||||||
| Control | 30.3 ± 1.6* | 27.0 ± 1.0 | 25.0 ± 0.7 | 22.9 ± 0.5 | 20.6 ± 0.4* | 18.4 ± 0.5* | P < 0.001 | P < 0.001 | P = 0.38s | |||||
| Hemodilution | 26.2 ± 1.9*† | 24.2 ± 1.8 | 21.2 ± 1.8† | 18.8 ± 0.7† | 16.2 ± 0.7*† | 15.2 ± 0.7*† | ||||||||
| Hemodilution + dobutamine | 16.0 ± 1.7† | 15.6 ± 1.5† | 14.8 ± 1.4† | 13.5 ± 1.1† | 11.7 ± 1.2† | 10.5 ± 1.2† | ||||||||
P < 0.05 vs CPP 100 mmHg within the group
P < 0.05 vs control at the same CPP
Results
Hemodynamic and coronary responses to alterations in perfusion pressure
Hemodynamic and coronary responses to graded reductions in CPP for each of the treatment groups are provided in Table 1. Blood gas values for control swine at CPP = 100 mmHg averaged: arterial pH (7.55 ± 0.02), PCO2 (39 ± 2 mmHg), and PO2 (177 ± 7 mmHg), and were not significantly altered by CPP, hemodilution, or the administration of dobutamine. Reducing hematocrit from ~ 33% (control) to ~ 17% (hemodilution) markedly increased coronary blood flow (P < 0.001), but did not significantly affect blood pressure (P = 0.07), heart rate (P = 0.21), or MVO2 (P = 0.44). Administration of dobutamine following hemodilution resulted in substantial increases in heart rate, MVO2, and coronary blood flow despite reductions in mean aortic pressure at CPP ≤ 100 mmHg (P < 0.001).
Effects of hemodilution ± dobutamine-induced increases in MVO2 on coronary blood flow and resistance as CPP was reduced from 140 to 40 mmHg are shown in Fig. 1. In untreated control swine, coronary resistance decreased linearly as CPP was reduced from 120 to 60 mmHg (Fig. 1b). Over this range of CPPs, the slope of the relationship between coronary blood flow and CPP equaled 0.0038 mL/min/g/mmHg (Fig. 1a) and autoregulatory gain averaged 0.36 ± 0.07. Significant reductions in coronary resistance (~ 50% relative to control) produced by hemodilution resulted in a modest increase in the slope of the coronary flow vs. CPP relationship (0.0085 mL/min/g/mmHg; P = 0.03), but did not significantly affect autoregulatory gain (0.33 ± 0.1; P = 0.78). Hemodilution + dobutamine caused further reductions in coronary resistance (> 75% relative to control), a marked increase in the slope of the relationship between coronary blood flow and CPP (0.025 mL/min/g/mmHg; P < 0.001), and a significant reduction in autoregulatory gain (− 0.01 ± 0.03; P < 0.01).
Fig. 1.
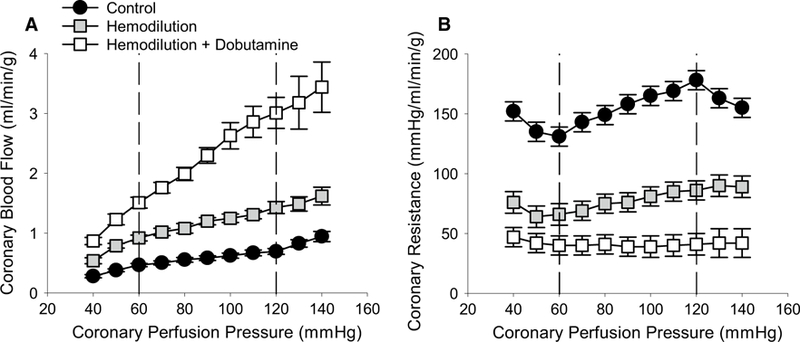
Effects of alterations in coronary tone and myocardial oxygen consumption on coronary pressure–flow autoregulation. a Coronary blood flow increased at a given CPP as vasomotor tone was decreased: control (n = 7) > hemodilution (n = 6) > hemodilution + dobutamine (n = 5). Relative to untreated control swine, the slope of flow–pressure relationship within the autoregulatory range (CPP 120–60 mmHg) was significantly increased by hemodilution (P = 0.03) and hemodilution + dobutamine (P < 0.001). b Average coronary vascular resistance was significantly reduced by hemodilution (P < 0.001) and hemodilution + dobutamine (P < 0.001)
Effects of coronary venous PO2 on coronary pressure–flow autoregulation
Coronary venous PO2 decreased as CPP was lowered from 140 to 40 mmHg in each of the treatment groups (Table 1). Although group differences in coronary venous PO2 were detected by ANOVA between hemodilution + dobutamine vs. control swine (P < 0.01), average values remained < 29.0 ± 2.6 mmHg in all groups and no differences were found by multiple comparison test at any given CPP. Regression analysis of all data revealed that differences in coronary resistance produced by hemodilution ± dobutamine were not predicted by underlying differences in myocardial oxygen tension, as coronary venous PO2 changed over very similar ranges in all groups (Fig. 2a). To assess the relationship between coronary venous PO2 and autoregulatory capacity, changes in coronary blood flow (20 mmHg increments from 140 to 40 mmHg) and autoregulatory gain (20 mmHg increments from CPP 120–60 mmHg) were plotted relative to their respective coronary venous PO2. Pearson correlation analyses determined that neither changes in coronary blood flow (Fig. 2b; P = 0.29) nor autoregulatory gain (Fig. 2c; P = 0.12) were significantly related to coronary venous PO2.
Fig. 2.
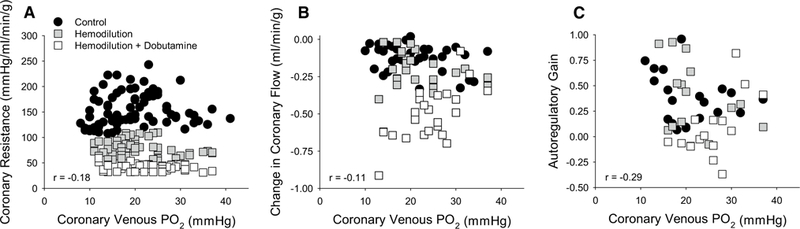
Relationship between coronary venous PO2 and coronary autoregulatory capacity. a Coronary venous PO2 decreased over similar ranges as CPP was lowered from 140 to 40 mmHg in all groups: control (n = 7); hemodilution (n = 6); hemodilution + dobutamine (n = 5). However, coronary venous PO2 was not predictive of coronary resistance. b Coronary venous PO2 was not associated with changes in coronary blood flow (20 mmHg increments) or c overall autoregulatory gain (CPP ranging from 120 to 60 mmHg) across treatment groups
Coronary Pzf, vascular smooth muscle tone, and autoregulatory capability
Representative tracings to demonstrate how coronary Pzf was determined at CPPs of 120 and 60 mmHg are provided in Fig. 3. Occlusion of the coronary perfusion circuit resulted in a rapid reduction in coronary blood flow and stabilization of coronary pressure at zero flow within ~ 3–4 s of the occlusion. Consistent with previous studies in the literature [14, 15, 17, 30, 41, 47], coronary Pzf was directly related with underlying coronary vascular tone as Pzf decreased from 25.0 ± 0.7 mmHg at CPP = 100 mmHg in control swine, to 21.2 ± 1.8 mmHg following hemodilution (P < 0.05), and to 14.8 ± 1.4 mmHg in the hemodilution + dobutamine group (P < 0.001) (Table 1). Coronary Pzf also decreased ~ 40% as CPP was lowered from 140 to 40 mmHg in each of the treatment groups (P < 0.001). Thus, Pzf was closely related to overall coronary vascular resistance (r = 0.71; P < 0.001) (Fig. 4). Examination of the relationship between coronary vascular tone (indexed by Pzf) and the change in coronary blood flow (20 mmHg increments from 140 to 40 mmHg) revealed relatively minimal changes in flow at Pzf > 20 mmHg and that the greatest changes in coronary flow occurred below this threshold value (r = 0.68; P < 0.001) (Fig. 4b). Coronary Pzf was also positively correlated with autoregulatory gain (r = 0.43; P = 0.001) (Fig. 4c).
Fig. 3.
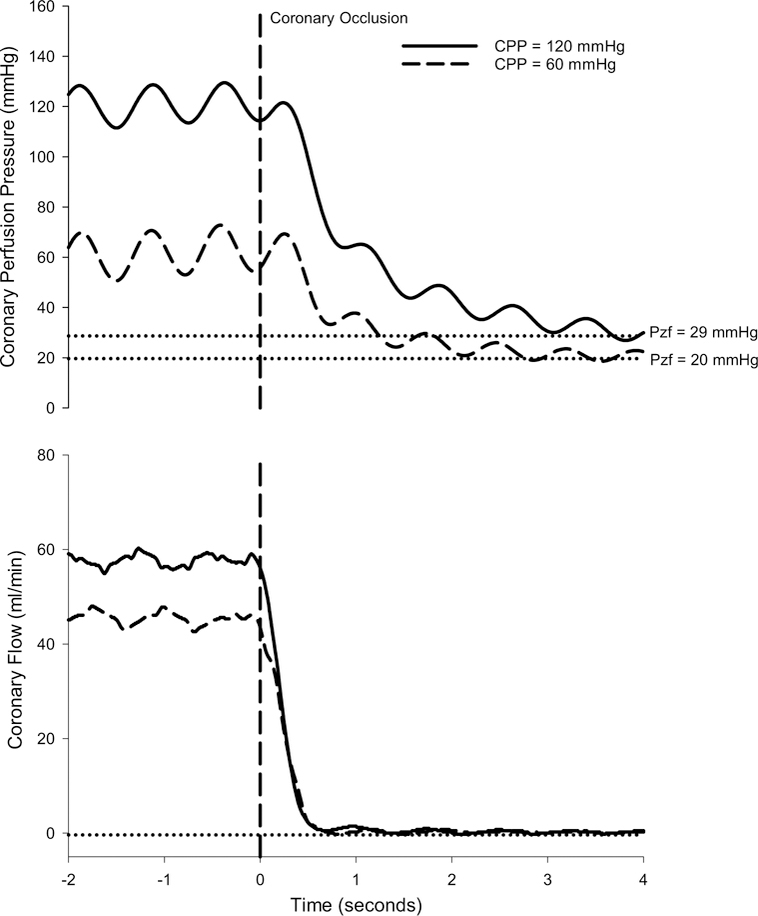
Representative tracings of coronary perfusion pressure and blood flow over time before and during a 4 s coronary artery occlusion to determine coronary zero-flow pressure (Pzf)
Fig. 4.
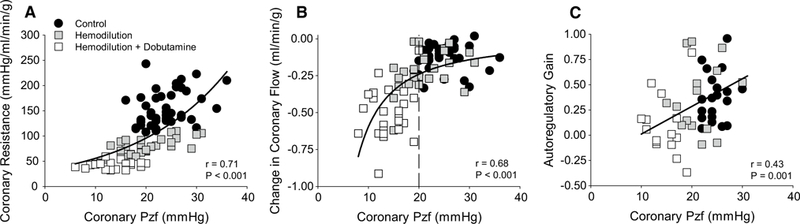
Relationship between coronary zero-flow pressure (Pzf) and coronary autoregulatory capacity. a Coronary Pzf was closely related to coronary vascular resistance as CPP was lowered from 140 to 40 mmHg in all groups: control (n = 7); hemodilution (n = 6); hemodilution + dobutamine (n = 5). b Changes in coronary blood flow (20 mmHg increments) remained modest at Pzf > 20 mmHg and significantly decreased below this threshold value. c Autoregulatory gain (CPP ranging from 120 to 60 mmHg) was positively correlated with coronary Pzf
Discussion
The question surrounding the mechanism(s) responsible for coronary pressure–flow autoregulation has been central to the field of coronary physiology since the 1950s [1, 7, 39]. The contention that myocardial oxygen tension determines the degree of autoregulatory behavior and thus that the primary mechanism of autoregulation is metabolic in nature has dominated since the seminal study of Dole and Nuno in 1986 [16]. However, we submit that there are reasons to challenge this hypothesis, in that coronary vasodilation not only augments coronary venous PO2, “uncoupling” the balance between flow and metabolism, but functionally antagonizes the intrinsic smooth muscle (myogenic) response to changes in pressure [34, 37]. We propose the necessary experiment to more directly examine this fundamental issue to establish conditions in which underlying coronary tone (resistance) is altered, without appreciable changes in coronary venous PO2. To achieve these states, we performed autoregulatory experiments (CPPs ranging from 140 to 40 mmHg) in the absence and presence of euvolemic hemodilution (~ 50% reduction in hematocrit), with and without the administration of dobutamine to augment MVO2. Findings from the present studies support that coronary autoregulatory behavior is predominantly dependent on an adequate degree of underlying vasomotor tone, independent of normal myocardial oxygen tension (coronary venous PO2).
Metabolic control and coronary autoregulatory capacity
The fundamental observation in support of the metabolic hypothesis of coronary autoregulation is the consistent result in this (Table 1) and prior studies [8, 26, 42, 45, 48] that coronary venous PO2 declines with reductions in CPP. This finding along with the inverse relationship between autoregulatory gain and coronary venous PO2 [3, 16] implicates the active production of dilator metabolites in proportion to pressure-dependent reductions in tissue oxygenation. However, an alternative interpretation of these findings is that the loss of autoregulation in vasodilated preparations is a consequence of the lack of an adequate vasomotor tone (or reserve) that results in “over-perfusion” and an increase in coronary venous PO2, as opposed to support of a causal role for metabolic control per se. Data from the present study offer a unique examination of these contrasting interpretations, as values of coronary venous PO2 remained quite similar between groups over the wide CPP range of 140–40 mmHg and stayed (on average) below the 32 mmHg autoregulatory threshold previously established by Dole and Nuno [16]. It is important to recognize that prior studies have consistently shown that the level of the steady state pressure–flow relationship directly adjusts to the level of MVO2; however, they have also definitively demonstrated that the strength of the autoregulatory response is independent of MVO2 [16, 18, 21, 38, 46]. As such, the complete loss of autoregulation in the hemodilution + dobutamine group (Fig. 1a) is neither predicted nor explained by the metabolic hypothesis. Further examination of the relationship between myocardial oxygen tension and the degree of autoregulation revealed that the level of coronary venous PO2 was not predictive of changes in coronary blood flow (Fig. 2b) or autoregulatory gain (Fig. 2c). Taken together, these findings directly refute that normal myocardial oxygen tension is requisite for coronary pressure–flow autoregulation. It should be recognized that this conclusion relies on the assumption that coronary venous PO2 provides a realistic estimate of myocardial tissue PO2 [22], which remains to be definitively established.
Coronary vasomotor tone, Pzf, and autoregulatory capability
To examine the effect of coronary vasomotor tone on autoregulatory capacity, we elected to measure coronary Pzf at 20 mmHg increments across all treatment groups. Our rationale was based on numerous earlier studies which established that Pzf was predominantly determined by underlying vascular smooth muscle tone [14, 15, 17, 30, 41, 47]. Pzf was determined in the present study by stopping/clamping the extracorporeal coronary perfusion circuit for ~ 4 s while the heart continued to beat (Fig. 3). As such, determination of Pzf in this manner relates to the decay of pressure as a function of resistance and capacitance of the system. It should be noted that prior measurements of coronary Pzf utilized a variety of different means, typically vagal stimulation (long diastole) but also by decreasing aortic or extracorporeal reservoir pressure, AV node ablation and pacing, and/or occlusion of perfusion circuit in both beating and non-beating hearts [2, 5, 14, 15, 20, 27, 29, 32, 43]. Although the values of coronary Pzf may differ between these conditions, comparison of Pzf within and between the current and previous studies confirms that Pzf varies linearly with CPP and coronary vascular tone (Table 1); i.e., Pzf was significantly reduced by hemodilution and hemodilution + dobutamine at a given CPP as well as diminished by reductions in CPP across all treatment groups. Therefore, while interpretation of coronary Pzf has been controversial [30, 41], there is strong evidence that measurements of Pzf serve as an objective and reliable index of underlying coronary vascular tone, as coronary Pzf was directly related to coronary vascular resistance across all treatment groups in this study (Fig. 4a). Use of an alternative estimate of coronary resistance (CPP minus Pzf divided by coronary blood flow) does not affect any of the relationships or conclusions of the study.
Analysis of individual data points from all treatment conditions demonstrates that coronary Pzf (vasomotor tone) is highly predictive of changes in coronary blood flow (Fig. 4b) and autoregulatory gain (Fig. 4c). More careful examination of these relationships reveals that the changes in blood flow were the greatest, and autoregulatory gain the lowest, when values of coronary Pzf fell below ~ 20 mmHg. These findings illustrate that coronary pressure–flow autoregulation is dependent on an adequate degree of underlying vasomotor tone and thus consistent with an intrinsic, pressure-dependent mechanism within the vasculature that is progressively impaired by reductions in overall resistance. Attenuation of the myogenic response with diminished coronary tone is evident in previous studies, which have compared changes in coronary diameter in isolated, pressurized arterioles with varying degrees of underlying vasomotor tone [34, 37]. To illustrate this point, we calculated a myogenic index (MI) on data from swine and human arterioles, in which the slope of the active pressure–diameter relationship at a given pressure was determined using the following equation:
where Di and Df are the initial and final diameters, while Pi and Pf are the initial and final intraluminal pressures. The more negative the myogenic index, the greater is the myogenic responsiveness [12]. Determination of the MI using data from Kuo et al. [34] and Miller et al. [37] demonstrates that vessels with less tone are less myogenically responsive (Fig. 5). The present findings are consistent with this paradigm, in that autoregulation is evident in the presence of hemodilution, i.e., when there was a sufficient level of tone (myogenic reserve), and absent following the administration of dobutamine, when there was an insufficient level of vasomotor reserve. It is important to recognize that values of coronary blood flow in the hemodilution + dobutamine group (highest average = 3.44 ± 0.42 mL/min/g) were far from established maximal levels of coronary flow which reach ~ 5.0 mL/min/g [44]. Thus, the loss of autoregulation in this group does not reflect the complete loss of tone or vasodilator reserve; i.e., a passive coronary vasculature.
Fig. 5.
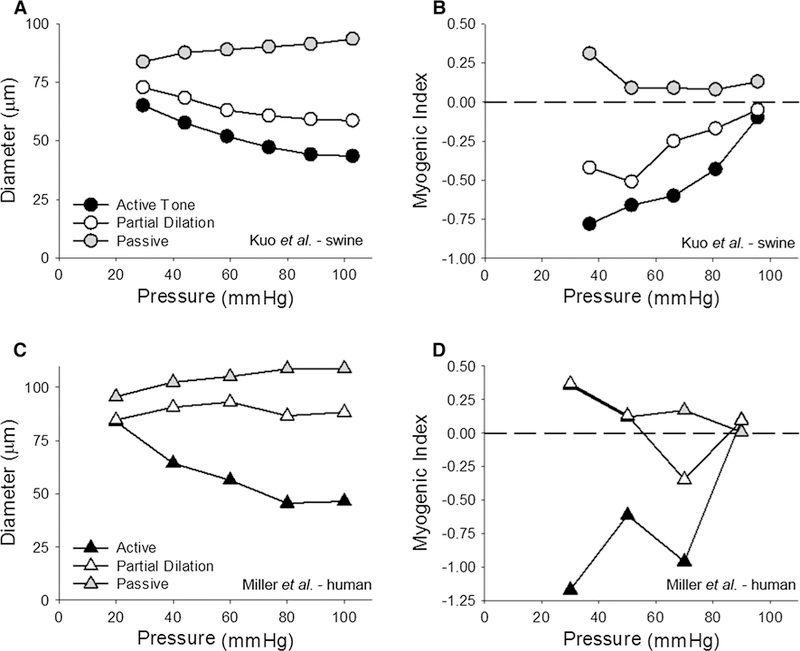
Myogenic reactivity is decreased by vasodilator influences. Data are from Kuo et al. [34] in a and b and from Miller et al. [37] in c and d. a, c The pressure–diameter relationship for coronary arterioles under three different conditions of tone: (a) in the active state (control); (b) when partially dilated; (c) passive (no tone). b, d Contain the respective analysis of myogenic index. The more negative the numbers, the more myogenically active an arteriole is. Positive values indicate pressure-induced dilation
Implications and conclusions
Findings from this investigation demonstrate that the local metabolic hypothesis as classically proposed is not sufficient to explain coronary autoregulatory behavior and suggest that the primary mechanism of coronary autoregulation is likely more myogenic in origin. This conclusion is consistent with prior studies which documented that CaV1.2 channels are critical for the coronary myogenic response [37] and that inhibition of these channels abolishes coronary autoregulation in vivo [8]. Furthermore, previous mathematical modeling studies also support that myogenic behavior is required for and independently necessary to explain pressure–flow autoregulation in the coronary circulation [9, 10, 13]. While our findings do not support myocardial tissue oxygen tension as an essential feedback signal for the autoregulatory response, they do not completely rule out a role for “metabolites” either. However, we submit that our understanding of metabolic control of coronary blood flow is sorely lacking and it is clear that prior studies which inhibited pathways implicated in maintaining myocardial oxygen supply/demand balance in response to exercise (H2O2/KV channels [8]; purine nucleotides/P2Y1 receptors [6]), anemia (KATP channels [19, 42]), or ischemia (adenosine [17, 19, 25, 31]; nitric oxide [40]) have all failed to show significant alterations in the coronary autoregulatory response. Nonetheless, the current results directly challenge that normal myocardial oxygen tension is required for coronary pressure–flow autoregulation and thus argue against a requisite role for local metabolic control of coronary resistance in response to changes in perfusion pressure.
Acknowledgements
The authors wish to thank Joshua Sturek for expert technical assistance. This study was supported by the National Institutes of Health U01HL118738.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Alella A, Williams FL, Bolene-Williams C, Katz LN (1955) Interrelation between cardiac oxygen consumption and coronary blood flow. Am J Physiol 183:570–582. 10.1152/ajplegacy.1955.183.3.570 [DOI] [PubMed] [Google Scholar]
- 2.Aversano T, Klocke FJ, Mates RE, Canty JM Jr (1984) Preload-induced alterations in capacitance-free diastolic pressure–flow relationship. Am J Physiol 246:H410–H417. 10.1152/ajpheart.1984.246.3.H410 [DOI] [PubMed] [Google Scholar]
- 3.Bai XJ, Iwamoto T, Williams AG Jr, Fan WL, Downey HF (1994) Coronary pressure–flow autoregulation protects myocardium from pressure-induced changes in oxygen consumption. Am J Physiol 266:H2359–H2368. 10.1152/ajpheart.1994.266.6.H2359 [DOI] [PubMed] [Google Scholar]
- 4.Bayliss WM (1902) On the local reactions of the arterial wall to changes of internal pressure. J Physiol 28:220–231. 10.1113/jphysiol.1902.sp000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy RF (1978) Diastolic coronary artery pressure–flow relations in the dog. Circ Res 43:92–101. 10.1161/01.RES.43.1.92 [DOI] [PubMed] [Google Scholar]
- 6.Bender SB, Berwick ZC, Laughlin MH, Tune JD (2011) Functional contribution of P2Y1 receptors to the control of coronary blood flow. J Appl Physiol 111:1744–1750. 10.1152/japplphysiol.00946.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berne RM (1959) Cardiodynamics and the coronary circulation in hypothermia. Ann N Y Acad Sci 80:365–383. 10.1111/j.1749-6632.1959.tb49217.x [DOI] [PubMed] [Google Scholar]
- 8.Berwick ZC, Moberly SP, Kohr MC, Morrical EB, Kurian MM, Dick GM, Tune JD (2012) Contribution of voltage-dependent K+ and Ca2+ channels to coronary pressure–flow autoregulation. Basic Res Cardiol 107:264 10.1007/s00395-012-0264-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelissen AJ, Dankelman J, VanBavel E, Spaan JA (2002) Balance between myogenic, flow-dependent, and metabolic flow control in coronary arterial tree: a model study. Am J Physiol Heart Circ Physiol 282:H2224–H2237. 10.1152/ajpheart.00491.2001 [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen AJ, Dankelman J, VanBavel E, Stassen HG, Spaan JA (2000) Myogenic reactivity and resistance distribution in the coronary arterial tree: a model study. Am J Physiol Heart Circ Physiol 278:H1490–H1499. 10.1152/ajpheart.2000.278.5.H1490 [DOI] [PubMed] [Google Scholar]
- 11.Crystal GJ, El-Orbany M, Zhou X, Salem MR, Kim SJ (2008) Hemodilution does not alter the coronary vasodilating effects of endogenous or exogenous nitric oxide. Can J Anaesth 55:507–514. 10.1007/BF03016670 [DOI] [PubMed] [Google Scholar]
- 12.Davis MJ (1993) Myogenic response gradient in an arteriolar network. Am J Physiol 264:H2168–H2179. 10.1152/ajpheart.1993.264.6.H2168 [DOI] [PubMed] [Google Scholar]
- 13.Dick GM, Namani R, Patel B, Kassab GS (2018) Role of coronary myogenic response in pressure–flow autoregulation in swine: a meta-analysis with coronary flow modeling. Front Physiol 10.3389/fphys.2018.00580 [DOI] [PMC free article] [PubMed]
- 14.Dole WP, Alexander GM, Campbell AB, Hixson EL, Bishop VS (1984) Interpretation and physiological significance of diastolic coronary artery pressure–flow relationships in the canine coronary bed. Circ Res 55:215–226. 10.1161/01.RES.55.2.215 [DOI] [PubMed] [Google Scholar]
- 15.Dole WP, Bishop VS (1982) Influence of autoregulation and capacitance on diastolic coronary artery pressure–flow relationships in the dog. Circ Res 51:261–270. 10.1161/01.RES.51.3.261 [DOI] [PubMed] [Google Scholar]
- 16.Dole WP, Nuno DW (1986) Myocardial oxygen tension determines the degree and pressure range of coronary autoregulation. Circ Res 59:202–215. 10.1161/01.RES.59.2.202 [DOI] [PubMed] [Google Scholar]
- 17.Dole WP, Yamada N, Bishop VS, Olsson RA (1985) Role of adenosine in coronary blood flow regulation after reductions in perfusion pressure. Circ Res 56:517–524 [DOI] [PubMed] [Google Scholar]
- 18.Drake-Holland AJ, Laird JD, Noble MI, Spaan JA, Vergroesen I (1984) Oxygen and coronary vascular resistance during autoregulation and metabolic vasodilation in the dog. J Physiol 348:285–299. 10.1113/jphysiol.1984.sp015110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncker DJ, van Zon NS, Ishibashi Y, Bache RJ (1996) Role of K+ ATP channels and adenosine in the regulation of coronary blood flow during exercise with normal and restricted coronary blood flow. J Clin Investig 97:996–1009. 10.1172/JCI118524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eng C, Jentzer JH, Kirk ES (1982) The effects of the coronary capacitance on the interpretation of diastolic pressure–flow relationships. Circ Res 50:334–341. 10.1161/01.RES.50.3.334 [DOI] [PubMed] [Google Scholar]
- 21.Feigl EO (1989) Coronary autoregulation. J Hypertens Suppl 7:S55–S58 (discussion S59) [PubMed] [Google Scholar]
- 22.Feigl EO (1983) Coronary physiology. Physiol Rev 63:1–205. 10.1152/physrev.1983.63.1.1 [DOI] [PubMed] [Google Scholar]
- 23.Feigl EO, Neat GW, Huang AH (1990) Interrelations between coronary artery pressure, myocardial metabolism and coronary blood flow. J Mol Cell Cardiol 22:375–390. 10.1016/0022-2828(90)91474-L [DOI] [PubMed] [Google Scholar]
- 24.Goodwill AG, Dick GM, Kiel AM, Tune JD (2017) Regulation of coronary blood flow. Compr Physiol 7:321–382. 10.1002/cphy.c160016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanley FL, Grattan MT, Stevens MB, Hoffman JI (1986) Role of adenosine in coronary autoregulation. Am J Physiol 250:H558–H566. 10.1152/ajpheart.1986.250.4.H558 [DOI] [PubMed] [Google Scholar]
- 26.Hoffman JI, Spaan JA (1990) Pressure–flow relations in coronary circulation. Physiol Rev 70:331–390. 10.1152/physrev.1990.70.2.331 [DOI] [PubMed] [Google Scholar]
- 27.Kajiya F, Tsujioka K, Ogasawara Y, Wada Y, Hiramatsu O, Goto M, Nakai M, Tadaoka S, Matsuoka S, Sha Y (1988) Effect of packed cell volume on diastolic coronary artery pressure–flow relations in the dog. Cardiovasc Res 22:545–554. 10.1093/cvr/22.8.545 [DOI] [PubMed] [Google Scholar]
- 28.Kiel AM, Goodwill AG, Noblet JN, Barnard AL, Sassoon DJ, Tune JD (2017) Regulation of myocardial oxygen delivery in response to graded reductions in hematocrit: role of K(+) channels. Basic Res Cardiol 112:65 10.1007/s00395-017-0654-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkeeide R, Puschmann S, Schaper W (1981) Diastolic coronary pressure–flow relationships investigated by induced long-wave pressure oscillations. Basic Res Cardiol 76:564–569. 10.1007/BF01908362 [DOI] [PubMed] [Google Scholar]
- 30.Klocke FJ, Mates RE, Canty JM Jr, Ellis AK (1985) Coronary pressure–flow relationships. Controversial issues and probable implications. Circ Res 56:310–323. 10.1161/01.RES.56.3.310 [DOI] [PubMed] [Google Scholar]
- 31.Komaru T, Lamping KG, Dellsperger KC (1994) Role of adenosine in vasodilation of epimyocardial coronary microvessels during reduction in perfusion pressure. J Cardiovasc Pharmacol 24:434–442 [DOI] [PubMed] [Google Scholar]
- 32.Kroll K, Hendriks FF, Schipperheyn JJ (1979) Extracorporeal circulation system for coronary artery perfusion in the closed-chest dog. Am J Physiol 236:H652–H656. 10.1152/ajpheart.1979.236.4.H652 [DOI] [PubMed] [Google Scholar]
- 33.Kuo L, Chilian WM, Davis MJ (1990) Coronary arteriolar myogenic response is independent of endothelium. Circ Res 66:860–866. 10.1161/01.RES.66.3.860 [DOI] [PubMed] [Google Scholar]
- 34.Kuo L, Chilian WM, Davis MJ (1991) Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol 261:H1706–H1715. 10.1152/ajpheart.1991.261.6.H1706 [DOI] [PubMed] [Google Scholar]
- 35.Kuo L, Davis MJ, Chilian WM (1990) Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol 259:H1063–H1070. 10.1152/ajpheart.1990.259.4.H1063 [DOI] [PubMed] [Google Scholar]
- 36.Levy PS, Kim SJ, Eckel PK, Chavez R, Ismail EF, Gould SA, Ramez Salem M, Crystal GJ (1993) Limit to cardiac compensation during acute isovolemic hemodilution: influence of coronary stenosis. Am J Physiol 265:H340–H349. 10.1152/ajpheart.1993.265.1.H340 [DOI] [PubMed] [Google Scholar]
- 37.Miller FJ Jr, Dellsperger KC, Gutterman DD (1997) Myogenic constriction of human coronary arterioles. Am J Physiol 273:H257–H264. 10.1152/ajpheart.1997.273.1.H257 [DOI] [PubMed] [Google Scholar]
- 38.Mosher P, Ross J Jr, McFate PA, Shaw RF (1964) Control of coronary blood flow by an autoregulatory mechanism. Circ Res 14:250–259. 10.1161/01.RES.14.3.250 [DOI] [PubMed] [Google Scholar]
- 39.Osher WJ (1953) Pressure–flow relationship of the coronary system. Am J Physiol 172:403–416. 10.1152/ajplegacy.1953.172.2.403 [DOI] [PubMed] [Google Scholar]
- 40.Smith TP Jr, Canty JM Jr (1993) Modulation of coronary autoregulatory responses by nitric oxide. Evidence for flow-dependent resistance adjustments in conscious dogs. Circ Res 73:232–240. 10.1161/01.RES.73.2.232 [DOI] [PubMed] [Google Scholar]
- 41.Spaan JA (1985) Coronary diastolic pressure–flow relation and zero flow pressure explained on the basis of intramyocardial compliance. Circ Res 56:293–309. 10.1161/01.RES.56.3.293 [DOI] [PubMed] [Google Scholar]
- 42.Stepp DW, Kroll K, Feigl EO (1997) K + ATP channels and adenosine are not necessary for coronary autoregulation. Am J Physiol 273:H1299–H1308. 10.1152/ajpheart.1997.273.3.H1299 [DOI] [PubMed] [Google Scholar]
- 43.Traverse JH, Chen Y, Crampton M, Voss S, Bache RJ (2001) Increased extravascular forces limit endothelium-dependent and -independent coronary vasodilation in congestive heart failure. Cardiovasc Res 52:454–461. 10.1016/S0008-6363(01)00392-3 [DOI] [PubMed] [Google Scholar]
- 44.Tune JD (2014) Coronary circulation Morgan & Claypool Life Sciences, Williston [Google Scholar]
- 45.van de Hoef TP, Nolte F, Rolandi MC, Piek JJ, van den Wijngaard JP, Spaan JA, Siebes M (2012) Coronary pressure–flow relations as basis for the understanding of coronary physiology. J Mol Cell Cardiol 52:786–793. 10.1016/j.yjmcc.2011.07.025 [DOI] [PubMed] [Google Scholar]
- 46.Vergroesen I, Noble MI, Wieringa PA, Spaan JA (1987) Quantification of O2 consumption and arterial pressure as independent determinants of coronary flow. Am J Physiol 252:H545–H553. 10.1152/ajpheart.1987.252.3.H545 [DOI] [PubMed] [Google Scholar]
- 47.Westerhof N, Boer C, Lamberts RR, Sipkema P (2006) Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev 86:1263–1308. 10.1152/physrev.00029.2005 [DOI] [PubMed] [Google Scholar]
- 48.Yonekura S, Watanabe N, Caffrey JL, Gaugl JF, Downey HF (1987) Mechanism of attenuated pressure–flow autoregulation in right coronary circulation of dogs. Circ Res 60:133–141. 10.1161/01.RES.60.1.133 [DOI] [PubMed] [Google Scholar]


