Abstract
The goal of the present study was to evaluate the effects of SGLT2i on cardiac contractile function, substrate utilization, and efficiency before and during regional myocardial ischemia/reperfusion injury in normal, metabolically healthy swine. Lean swine received placebo or canagliflozin (300 mg PO) 24 h prior to and the morning of an invasive physiologic study protocol. Hemodynamic and cardiac function measurements were obtained at baseline, during a 30-min complete occlusion of the circumflex coronary artery, and during a 2-h reperfusion period. Blood pressure, heart rate, coronary flow, and myocardial oxygen consumption were unaffected by canagliflozin treatment. Ventricular volumes remained unchanged in controls throughout the protocol. At the onset of ischemia, canagliflozin produced acute large increases in left ventricular end-diastolic and systolic volumes which returned to baseline with reperfusion. Canagliflozin-mediated increases in end-diastolic volume were directly associated with increases in stroke volume and stroke work relative to controls during ischemia. Canagliflozin also increased cardiac work efficiency during ischemia relative to control swine. No differences in myocardial uptake of glucose, lactate, free fatty acids or ketones, were noted between treatment groups at any time. In separate experiments using a longer 60 min coronary occlusion followed by 2 h of reperfusion, canagliflozin increased end-diastolic volume and stroke volume and significantly diminished myocardial infarct size relative to control swine. These data demonstrate that SGLT2i with canagliflozin preserves cardiac contractile function and efficiency during regional myocardial ischemia and provides ischemia protection independent of alterations in myocardial substrate utilization.
Keywords: Pig, SGLT2 inhibition, Myocardial ischemia, Cardiac function, Fuel selection, Infarct
Introduction
The type 2 sodium–glucose cotransporter (SGLT2) is located in the S1 and S2 segments of the renal proximal tubules and is responsible for ~ 90% of glucose reabsorption in the kidney. In chronic hyperglycemia, SGLT2 activity is increased as a result of upregulation, exacerbating increases in plasma glucose concentration [31]. Inhibitors of this cotransporter (SGLT2i) reduce glucose reabsorption in the kidneys, resulting in increased urinary glucose excretion and a reduction in plasma glucose levels [22]. Cardiovascular outcome studies have demonstrated that SGLT2i also significantly reduces major adverse cardiovascular events in subjects with type 2 diabetes mellitus (T2DM) [3, 35, 46]. These cardiovascular benefits do not appear to be mediated by direct effects of these agents on the SGLT2 transporter in cardiovascular tissues, as SGLT2 protein expression is not detected in the heart or vasculature [7, 20, 21]. These benefits are also not explained by improved glycemic control, as antidiabetes agents in other classes have provided similar improvements in glucose without parallel beneficial cardiac effects [30, 35, 46]. Furthermore, SGLT2i have been shown to improve cardiac function and mitigate myocardial infarct size in metabolically normal animal models [28, 39]. Improved arterial stiffness [8] and reduced blood pressure [9] have been reported with chronic SGLT2i treatment; however, it is unclear whether these effects are sufficient to explain the observed cardiovascular benefits.
In this context, the ‘thrifty fuel’ hypothesis has been proposed to explain the cardiovascular benefits of SLGT2i [33]. In particular, inhibition of SGLT2 has been demonstrated to augment circulating ketone bodies via alterations in hepatic fuel metabolism [15, 34]. Since ketone transport via the monocarboxyl transporter depends simply on the transmembrane gradient, increased ketone availability could serve to shift myocardial fuel utilization away from free fatty acids toward ketones, which require less oxygen per mole of ATP produced [5]. Some support for the plausibility of this ketone hypothesis in improving cardiac function and efficiency in response to pathologic stimuli such as ischemia has been presented [14]. However, studies to directly assess the effects of SGLT2i on myocardial substrate utilization during ischemia are presently lacking.
The goal of the present study was to evaluate the effects of SGLT2i on cardiac contractile function, substrate utilization, and efficiency before and during regional myocardial ischemia/reperfusion injury in normal, metabolically healthy swine. We tested the hypothesis that SGLT2i improves cardiac function and efficiency during ischemia/reperfusion injury via shifts in myocardial substrate selection toward ketone utilization. To exclude contributions of effects of chronic exposure, we elected to perform studies following very short-term (24 h) exposure to SGLT2i. Invasive methodologies were used to obtain high-quality and precise in vivo measures of hemodynamics and cardiac function, including gold standard measurements of contractility. Findings from this study provide novel insight in to the acute, cardioprotective effects of SGLT2i during ischemic injury.
Methods
Animal model and surgical preparation
All experiments involving animals were approved by an Institutional Animal Care and Use Committee and performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication. No. 85–23, Revised 2011). Male domestic swine (~ 50 kg) were placed on a standard chow (5L80, Purina Test Diet, Richmond, IN, USA) and randomly assigned into Control or Treatment groups. Treatment animals were given canagliflozin (300 mg, oral) 24 h prior to and approximately 2 h before the acute/terminal procedure. Control animals received no treatment.
Left circumflex ischemia protocol
Following an overnight fast (~ 16 h), control (n = 7), and canagliflozin-treated (n = 8) swine were anesthetized with telazol (5 mg/kg), ketamine (3 mg/kg), and xylazine (2.2 mg/kg) cocktail (i.m.), and then, anesthesia was maintained with morphine (3 mg/kg) and α-chloralose (100 mg/kg, i.v). Depth of anesthesia was monitored by observing continuous measurements of arterial blood pressure and heart rate as well as regular (15-min intervals) reflex tests (corneal, jaw, and limb withdrawal), beginning after induction of anesthesia and continuing throughout the experimental protocol. Periodic supplementation with α-chloralose was performed every 1.5 h to maintain a level, stage 3 plane of anesthesia. The right femoral artery and vein were isolated and catheterized to allow measurement of systemic arterial pressure and maintain venous access, respectively. The heart was exposed by a left lateral thoracotomy. The proximal region of both the left anterior descending artery (LAD) and left circumflex artery (LCX) was isolated for placement of perivascular flow probes (Transonic Systems Inc., Ithaca, NY, USA) and a snare occluder was placed around the left circumflex artery. A catheter was then placed in the intraventricular vein to allow sampling of coronary venous blood from the area supplied by the LAD. A SciSense pressure/volume admittance catheter (Transonic Scisense, London Ontario, Canada) was passed through a hemostatic control valve placed directly into the left ventricle via a transmural stab near the base of the left ventricle and secured with a purse string suture. Measurements were obtained at baseline, during a 30 min complete occlusion of the LCX, and during a 2-h reperfusion. At the beginning of the study, a total of 20 animals were enrolled (10 per group; randomized). Three swine from the control group and two swine from the canagliflozin group fibrillated during the coronary occlusion portion of the protocol and were thus not included in the study.
Metabolic analysis
Arterial (5 mL) and coronary venous (5 mL) blood samples were collected simultaneously into untreated syringes, immediately sealed, and placed on ice. These samples were collected every 15 min during baseline and the coronary occlusion, and every 30 min during reperfusion; total blood samples across the 2–3 h experimental protocol summed to < 100 mL from an estimated ~ 4 to 5 L total circulating blood volume. The samples were analyzed immediately upon collection for pH, PCO2, PO2, O2 content, glucose, lactate, and hematocrit with an Instrumentation Laboratories automatic blood gas analyzer (GEM Premier 4000). Free fatty acids (NEFA—Non-Esterified Fatty Acids) were measured colorimetrically (WAKO Life Sciences, City State) and ketones were analyzed with a chemistry analyzer (Roche Hitachi Modular P, Indianapolis, IN, USA), reporting concentrations of β-hydroxybutyrate and acetone as total ketone measure. Myocardial oxygen consumption (MvO2;μL O2/min/g) was calculated using the Fick principle [coronary blood flow × (arterial O2 content−coronary venous O2 content)]. For these calculations, LAD perfusion territory was estimated to be 30% of total heart weight, as previously described [13]. The Fick principle was also used to calculate substrate uptake (glucose, lactate, free fatty acids, and ketone), [coronary blood flow × (arterial substrate content−coronary venous substrate content)]. Accordingly, the term “uptake” refers to the result of the Fick calculation and it should be recognized that this variable does not account for the degree to which substrate production by the tissue (e.g., lactate) contributes to the reported value. Cardiac efficiency was calculated as cardiac work (product of cardiac output and mean arterial pressure) divided by MvO2.
Infarct protocol
Additional studies to assess the effects of SGLT2i on myocardial infarct size were also performed in control (n = 8) and canagliflozin-treated (n = 8) swine. Owing to the fact that historic attempts at proximal occlusions of the LCX have resulted in high mortality rates with prolonged ischemic duration as well as variable access to distal LCX territories, these studies were performed in the distal LAD region, as previously described [17, 18]. In these experiments, the distal LAD was subjected to a 60 min occlusion followed by 2 h of reperfusion. Durations of ischemia and reperfusion were selected based on literature support indicating that the stimulus would be adequate and appropriate for induction of regional myocardial infarction [17, 18]. Four swine fibrillated during the LAD coronary occlusion portion of this protocol (n = 2 in each group) and thus were not included in infarct analyses. Therefore, for infarct analyses, the final n = 6 for each group.
Hemodynamic data were collected in these animals as well. However, due to a failure of the pressure volume catheter, we were unable to reliably obtain pressure volume loops for two animals in the infarct study (n = 1 per group). As a result, the n for metrics associated with the pressure–volume catheter (left ventricular pressures, volumes, and derived measures) is 5 per group.
Infarct quantification
At the completion of each experiment, hearts were fibrillated by direct application of a 9 V battery and excised. Hearts were then sliced into 1-cm-thick sections and incubated in 1% 2,3,5-triphenyltetrazolium chloride (TTC) solution for 20 min at 37°C. Following incubation with TTC solution, a digital image of each heart slice was captured by a Canon Eos-Rebel sl1 with an associated 18–55 mm lens, capturing 18.0 MP images in RAW format. No measurable infarct was detected in animals subjected to LCX occlusion regardless of treatment (control n = 3; SGLT2i n = 4) and those animals are, accordingly, not included in infarct analyses. Measurable infarct was present and consistent in animals who received the longer (60 min) occlusion of the distal LAD. In those animals, quantification of infarct area (unstained) vs. viable myocardium (stained) was performed using the ImageJ software (National Institutes of Health). Each heart slice was evaluated by three separate investigators who were blinded to experimental treatment group. Data are presented as averaged area for left ventricle (stained) and infarct area (unstained); the coefficient of variation between the three investigators was 7% for the total left ventricle area and 9% for the infarcted area.
RNA isolation and cDNA synthesis
Tissue mRNA was prepared using a miRNeasy Mini Kit (Qiagen Cat#1038703). Flash frozen (500 mg) pig heart (n = 5) and kidney (n = 5) samples from domestic swine were homogenized in 2 mL Qiazol with a Polytron homogenizer (Kinematica Model PT3100) at 1000 rpm until clear homogenate was observed (1–2 min in cold room on ice bath). The homogenate was vigorously shaken for 15 s with 380 μL chloroform. Total RNA was isolated from the upper aqueous phase according to the kit protocol using on-column DNA digestion. cDNA was synthesized from 500 ng total RNA in a 20 μL reaction using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems Cat#4368814).
RT-PCR
The pig SGLT1 RT-PCR assay was obtained from ThermoFisher (Applied Bioscience catalog # Ss03374377_ m1). The SGLT2 assay was designed on the basis of whole genome shotgun sequence published in NCBI (Reference Sequence: NC_010445.4) and made by Integrated DNA Technologies, Inc. (Skokie, Illinois) as a custom assay. Primer/Probe sequences were: Pig SGLT2 Assay 1, reverse primer GAA TCC AGC TGA GCC ACT C, forward primer GGG AAG CGA TAC TGT CAG ATC, probe TGG AAA AGG ACC CCT CTG GAT TTG G.
The pig SGLT2 Assay 2, reverse primer GGA CAG GTA GAG GCG AAT G, forward primer CTC TTC GTG CCA GTG TAC C, probe CGC CGG TGTCAT TAC CAT GCC. cDNA was diluted 1:5 and RT-PCR was accomplished with Taqman 2X Universal PCR Master Mix (Applied Biosytems, catalog #4304437) in ABI Prism 7900HT Sequence Detection System (Applied Biosystems). Cycles for SGLT1 and 2 were normalized to housekeeping gene, peptidylpropyl isomerase A.
Statistical analyses
Data were analyzed using the SigmaPlot statistical package (version 11 Systat Software Inc, San Jose, CA, USA) and SPSS (version 22 IBM, Chicago, IL, USA). Data are presented as mean ± standard error. Comparisons were assessed by t test or two-way ANOVA; [factor A = treatment (time control/SGLT2i); factor B = condition (baseline/occlusion/reperfusion)] as appropriate. When significance was established with ANOVA, Student–Newman–Keuls post-hoc testing was performed to identify pairwise differences between treatment groups and conditions. Statistical significance was declared when P < 0.05.
Results
mRNA expression of SGLT1 vs. SGLT2 in swine heart and kidney
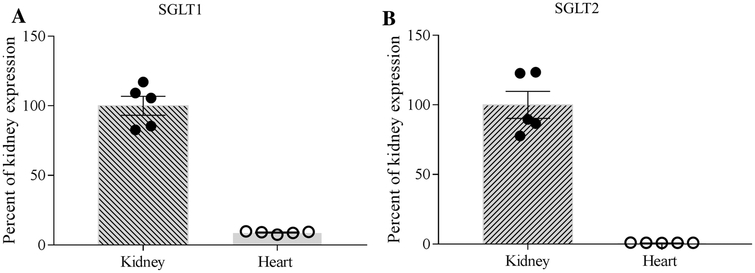
Determination of the relative abundance of SLC5A1 transcript (the gene-encoding SGLT1 protein) and SLC5A2 transcript (the gene encoding SGLT2 protein) was assessed by qPCR in kidney and left ventricular biopsies from domestic swine (n = 5). These analyses detected the presence of SGLT1 (Fig.1a) and SGLT2 (Fig.1b) mRNA in both heart and kidney. However, the abundance of both these transcripts was approximately 100-fold higher in kidney tissue than that of heart tissue.
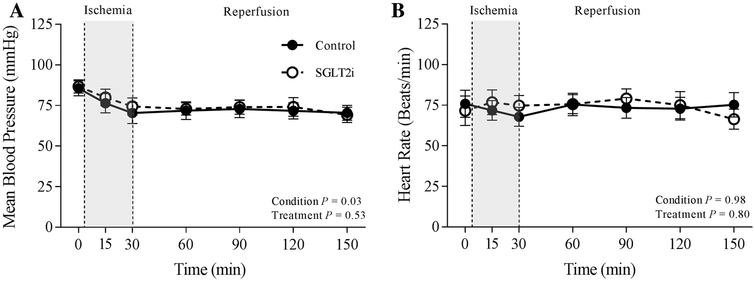
Fig. 2.
Effects of ischemia/reperfusion injury on mean blood pressure (a) and heart rate (b) in control (n = 7) and SGLT2i (canagliflozin)-treated (n = 8) swine
Systemic effects of SGLT2i during ischemia/reperfusion injury of the LCX
Hemodynamic and blood gas data for control (n = 7) and canagliflozin (n = 8)-treated swine at rest and during LCX ischemia/reperfusion injury are provided in Table 1. The 24 h exposure to canagliflozin did not significantly affect baseline resting systolic arterial pressure (P = 0.16), diastolic arterial pressure (P = 0.93), or coronary blood flow (P = 0.56) (Table 1). Although canagliflozin-related decreases in arterial pH (P < 0.001) and hematocrit (P < 0.001) and increases in arterial PO2 (P < 0.001) were detected by ANOVA, values of these variables remained within physiologic limits throughout the experimental protocol (Table 1).
Table 1.
Effects of canagliflozin on systemic hemodynamics, cardiac contractile function, and blood gas parameters
| Parameter | Treatment | Condition | Condition P value | Treatment P value | Interaction P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Ischemia | Reperfusion | |||||||||
| 0 min | 15 min | 30 min | 60 min | 90 min | 120 min | 150 min | |||||
| Arterial pH | Control | 7.57 ± 0.01 | 7.56 ± 0.01 | 7.55 ± 0.01 | 7.54 ± 0.01 | 7.53 ± 0.01 | 7.55 ± 0.01 | 7.55 ± 0.10 | 0.753 | <0.001 | 0.895 |
| SGLT2i | 7.52 ± 0.01 | 7.52 ± 0.01 | 7.52 ± 0.01 | 7.49 ± 0.02 | 7.52 ± 0.02 | 7.52 ± 0.01 | 7.50 ± 0.03† | ||||
| Arterial PCO2 (mmHg) | Control | 38 ± 2 | 38 ± 2 | 39 ± 2 | 40 ± 2 | 41 ± 2 | 39 ± 2 | 39 ± 2 | 0.993 | 0.953 | 0.907 |
| SGLT2i | 40 ± 1 | 39 ± 1 | 39 ± 1 | 39 ± 1 | 38 ± 1 | 38 ± 1 | 41 ± 1 | ||||
| Arterial PO2 (mmHg) | Control | 143 ± 12 | 143 ± 11 | 143 ± 11 | 136 ± 9 | 138 ± 10 | 139 ± 10 | 134 ± 9 | 0.978 | <0.001 | 0.873 |
| SGLT2i | 159 ± 11 | 164 ± 9 | 167 ± 10 | 163 ± 7 | 168 ± 7 | 155 ± 13 | 179 ± 15† | ||||
| Coronary venous PO2 (mmHg) | Control | 16 ± 1 | 14 ± 2 | 15 ± 1 | 15 ± 2 | 15 ± 2 | 15 ± 2 | 15 ± 1 | 0.982 | 0.132 | 1.000 |
| SGLT2i | 16 ± 1 | 15 ± 1 | 16 ± 1 | 17 ± 1 | 17 ± 1 | 16 ± 1 | 17 ± 2 | ||||
| HCT (%) | Control | 34 ± 1 | 35 ± 1 | 35 ± 1 | 35 ± 1 | 35 ± 1 | 34 ± 1 | 34 ± 1 | 0.978 | <0.001 | 0.988 |
| SGLT2i | 32 ± 1 | 32 ± 1 | 32 ± 1 | 33 ± 1 | 32 ± 1 | 33 ± 1 | 33 ± 1 | ||||
| Arterial O2 content (mL/dL) | Control | 15 ± 1 | 15 ± 1 | 15 ± 1 | 16 ± 1 | 15 ± 1 | 15 ± 1 | 15 ± 1 | 0.637 | 0.774 | 0.710 |
| SGLT2i | 15 ± 1 | 15 ± 1 | 15 ± 1 | 16 ± 1 | 15 ± 1 | 15 ± 1 | 16 ± 1 | ||||
| Coronary venous O2 content (mL/dL) | Control | 3.4 ± 0.4 | 2.9 ± 0.4 | 3. 0 ± 0.4 | 3.1 ± 0.5 | 3.1 ± 0.5 | 3.2 ± 0.5 | 3.2 ± 0.5 | 0.907 | 0.292 | 0.975 |
| SGLT2i | 3.3 ± 0.3 | 2.9 ± 0.3 | 3.0 ± 0.2 | 3.8 ± 0.9 | 3.9 ± 0.9 | 3.6 ± 0.7 | 3.6 ± 0.8 | ||||
| Myocardial oxygen consumption (μLO2/min/g) | Control | 47 ± 5 | 49 ± 5 | 43 ± 5 | 43 ± 6 | 44 ± 6 | 41 ± 5 | 39 ± 5 | 0.673 | 0.214 | 1.000 |
| SGLT2i | 50 ± 7 | 53 ± 6 | 48 ± 6 | 47 ± 6 | 45 ± 6 | 45 ± 6 | 43 ± 4 | ||||
| Systolic pressure (mmHg) | Control | 105 ± 7 | 94 ± 8 | 87 ± 8 | 88 ± 7 | 91 ± 8 | 90 ± 7 | 89 ± 7 | 0.362 | 0.02 | 0.989 |
| SGLT2i | 127 ± 17 | 111 ± 14 | 106 ± 14 | 103 ± 14 | 101 ± 11 | 99 ± 11 | 95 ± 10 | ||||
| Diastolic pressure (mmHg) | Control | 71 ± 4 | 64 ± 5 | 58 ± 5 | 60 ± 4 | 61 ± 4 | 60 ± 4 | 59 ± 4 | 0.095 | 0.468 | 0.995 |
| SGLT2i | 71 ± 3 | 67 ± 5 | 63 ± 5 | 62 ± 3 | 63 ± 5 | 63 ± 5 | 57 ± 4 | ||||
| Systemic vascular resistance (mmHg × min/mL) | Control | 0.02 ± 0.00 | 0.03 ± 0.00 | i 0.04 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.832 | 0.03 | 0.557 |
| SGLT2i | 0.03 ± 0.00 | 0.02 ± 0.00 | i 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | ||||
| Coronary blood flow (mL/min/g) | Control | 0.40 ± 0.05 | 0.38 ± 0.05 | 0.34 ± 0.05 | 0.35 ± 0.05 | 0.35 ± 0.05 | 0.34 ± 0.05 | 0.33 ± 0.05 | 0.673 | 0.063 | 1.000 |
| SGLT2i | 0.44 ± 0.06 | 0.44 ± 0.05 | 0.40 ± 0.05 | 0.40 ± 0.04 | 0.39 ± 0.04 | 0.39 ± 0.03 | 0.36 ± 0.03 | ||||
| Ejection fraction (%) | Control | 59 ± 3 | 45 ± 5 | 47 ± 7 | 49 ± 7 | 48 ± 5 | 52 ± 4 | 52 ± 5 | 0.069 | 0.146 | 0.984 |
| SGLT2i | 64 ± 4 | 50 ± 6 | 48 ± 4 | 50 ± 5 | 56 ± 3 | 57 ± 4 | 55 ± 4 | ||||
| Left ventricular end-diastolic pressure (mmHg) | Control | 14 ± 2 | 16 ± 2 | 16 ± 2 | 15 ± 2 | 15 ± 2 | 14 ± 2 | 13 ± 2 | 0.886 | 0.624 | 0.999 |
| SGLT2i | 13 ± 2 | 16 ± 2 | 15 ± 2 | 14 ± 2 | 13 ± 2 | 14 ± 2 | 14 ± 3 | ||||
| dP/dtmax (mmHg/s) | Control | 1748 ± 138 | 1358 ± 191 | 2296 ± 895 | 1368 ± 158 | 1330 ± 143 | 1405 ± 131 | 1322 ± 112 | 0.120 | 0.066 | 0.487 |
| SGLT2i | 1522 ± 191 | 1307 ± 140 | i 1235 ± 125 | 1212 ± 134 | 1264 ± 125 | 1262 ± 131 | 1336 ± 99 | ||||
| dP/dtmin (mmHg/s) | Control | − 1613 ± 168 | − 1251 ± 168 | − 1162 ± 131 | − 1246 ± 229 | − 1164 ± 171 | − 1161 ± 192 | − 1101 ± 163 | 0.031 | 0.641 | 0.446 |
| SGLT2i | − 1708 ± 183 | − 1471 ± 109 | − 1199 ± 109 | − 1127 ± 102 | − 1111 ± 109 | − 1155 ± 133 | − 999 ± 102 | ||||
| τ1/2 (ms) | Control | 30 ± 3 | 38 ± 5 | 41 ± 5 | 37 ± 5 | 40 ± 8 | 43 ± 9 | 45 ± 10 | 0.234 | 0.414 | 0.857 |
| SGLT2i | 31 ± 3 | 30 ± 1 | 34 ± 3 | 39 ± 4 | 39 ± 5 | 35 ± 5 | 37 ± 3 | ||||
| τ1/e (ms) | Control | 21 ± 2 | 25 ± 3 | 28 ± 4 | 25 ± 3 | 28 ± 6 | 30 ± 7 | 32 ± 8 | 0.214 | 0.382 | 0.870 |
| SGLT2i | 21 ± 2 | 20 ± 1 | 23 ± 2 | 27 ± 3 | 27 ± 3 | 24 ± 3 | 26 ± 2 | ||||
Values are mean ± SE for Control (n = 7) and SGLT2i (canagliflozin) (n = 8)
P < 0.05 vs. baseline value (same treatment)
Mean arterial pressure decreased from ~ 85 ± 5 mmHg at baseline to ~ 70 ± 5 mmHg following 30 min ischemia/reperfusion injury in control-and canagliflozin-treated swine (Fig.2a; Condition: P = 0.03; treatment: P = 0.53). Heart rate averaged ~ 75 beats/min throughout the entire protocol in both untreated and treated swine (Fig.2b; Condition: P = 0.98; treatment group: P = 0.80). None of the hemodynamic or blood gas parameters were significantly affected by 30 min ischemia/reperfusion (Table 1).
Effects of SGLT2i on cardiac contractile function during ischemia/reperfusion injury of the LCX
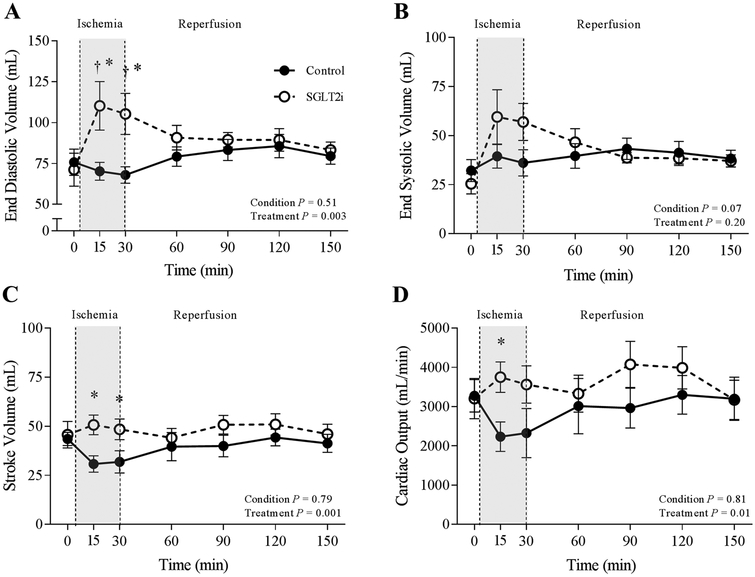
Under baseline conditions, canagliflozin had no effect on left ventricular end-diastolic volume (P = 0.69), end-systolic volume (P = 0.47), stroke volume (P = 0.76), or cardiac output (P = 0.92) (Fig.3). However, following the onset of regional myocardial ischemia (LCX occlusion), canagliflozin treatment was associated with a significant and acute increase in left ventricular end-diastolic volume (Fig.3a; P = 0.002) and left ventricular end-systolic volume (Fig.3b; P = 0.03). These changes in cardiac volumes corresponded with a maintenance of stroke volume (Fig.3c; P = 0.03) and cardiac output (Fig.3d; P = 0.05) throughout the ischemic time period and were not observed in control animals. Canagliflozin did not affect load-dependent measures of cardiac function, including ejection fraction (P = 0.14), dP/dtmax (P = 0.06), dP/dtmin (P = 0.30), or τ1/2 (P = 0.31) (Table 1). MvO2 of the non-occluded anterior coronary vascular bed was unaffected by canagliflozin or by 30 min ischemia/reperfusion in either group (Table 1).
Fig. 3.
Effect of ischemia/reperfusion injury on end-diastolic pressure (a), end-systolic volume (b), stroke volume(c) and cardiac output (d) in control (n = 7) and SGLT2i (canagliflozin)-treated (n = 8) swine. *P <0.05 vs. control (same timepoint), †P <0.05 vs. baseline (same treatment)
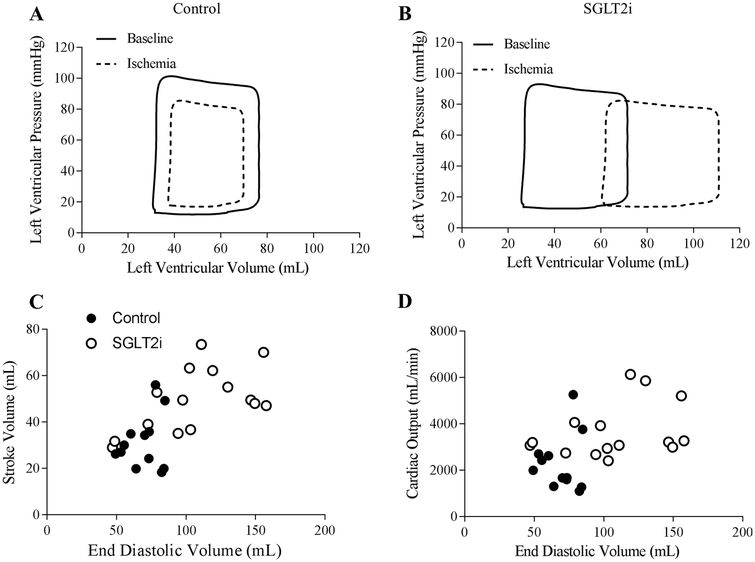
Schematic representations of average steady-state left ventricular pressure–volume loops illustrating the effect of regional myocardial ischemia in control vs. canagliflozin-treated swine are provided in Fig.4. In control swine, these pressure–volume relationships demonstrate an expected modest reduction of left ventricular end-diastolic volume and systolic pressure generation during regional ischemia (Fig. 4a). This was notably different in the canagliflozin-treated animals, which showed a substantial right shift of the pressure–volume relationship (Fig. 4b). Comparisons of the relationship between stroke volume vs. end-diastolic volume and cardiac output vs. end-diastolic volume (Frank–Starling relationship) during ischemia in control and canagliflozin-treated swine are provided in Fig.4. These plots reveal that canagliflozin-mediated increases in stroke volume (Fig. 4c) and cardiac output (Fig. 4d) were directly related to increases in end-diastolic volume (preload). Left ventricular end-diastolic pressure was unaffected by 30 min ischemia/reperfusion or SGLT2i with canagliflozin (Table 1).
Fig. 4.
Representative pressure–volume loops of average steady-state conditions at baseline and during regional myocardial ischemia in control (n = 7) (a) and SGLT2i (canagliflozin) (n = 8)-treated (b) swine. Relationship between stroke volume (c) and cardiac output (d) and end-diastolic volume during ischemia in control (n = 8) and SGLT2i (canagliflozin) (n = 7)-treated swine
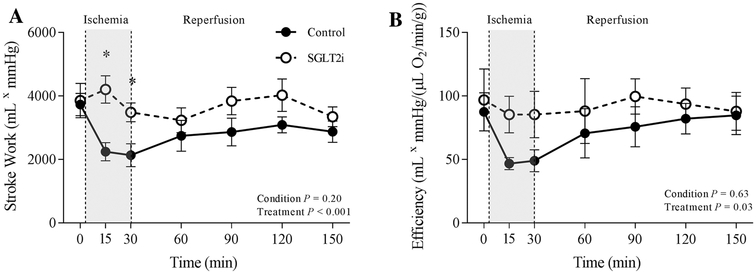
Stroke work [stroke volume (mL) × mean arterial pressure (mmHg)] and efficiency [cardiac output (mL/min) × mean arterial pressure (mmHg)]/MvO2 (μL O2/min/g) were unaffected by canagliflozin treatment under baseline conditions (Fig. 5). However, stroke work (Fig.5a; P < 0.001) and efficiency (Fig. 5b; P = 0.03) were significantly increased by canagliflozin administration during regional myocardial ischemia compared to control swine.
Fig. 5.
Effect of ischemia/reperfusion injury on cardiac stroke work (a), efficiency (b) in control (n = 7), and SGLT2i (canagliflozin)-treated (n = 8) swine. *P <0.05 vs. control (same timepoint)
Effects of SGLT2i on myocardial substrate selection during ischemia/reperfusion injury of the LCX
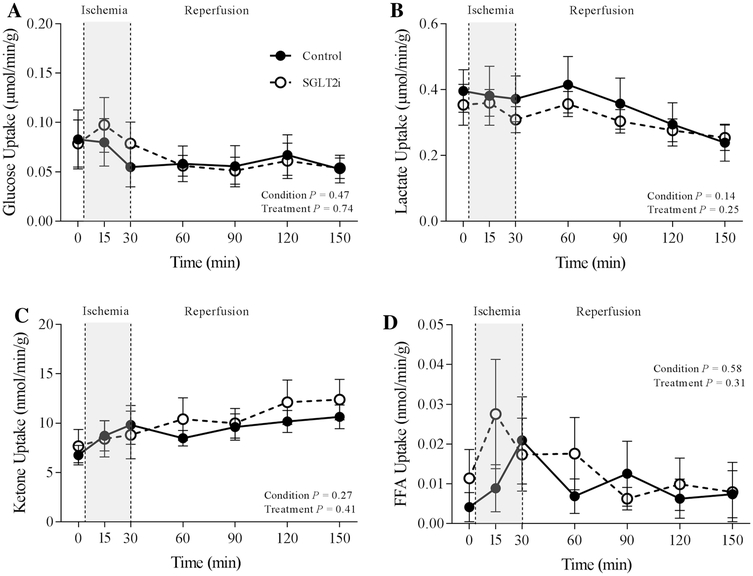
Administration of canagliflozin augmented arterial free fatty acid concentration (Table 2; P = 0.017). However, circulating concentrations of other cardiac substrates including glucose (P = 0.087), lactate (P = 0.336) and total ketone bodies (acetoacetone and 3-hydroxybutyrate) (P = 0.74) were unaffected by SGLT2i (Table 2). Canagliflozin did not significantly influence uptake of glucose (P = 0.75), lactate (P = 0.26), ketones (P = 0.41), or free fatty acids (P = 0.31) (Fig. 6), under resting conditions, during the 30 min ischemia, or during reperfusion.
Table 2.
Effects of canagliflozin on circulating substrate concentrations
| Parameter | Treatment | Condition | Condition P value | Treatment P value | Interaction P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Ischemia | Reperfusion | |||||||||
| 0 min | 15 min | 30 min | 60 min | 90 min | 120 min | 150 min | |||||
| Arterial glucose (mg/dL) | Control | 104 ± 14 | 110 ± 21 | 105 ± 19 | 120 ± 24 | 119 ± 26 | 112 ± 21 | 109 ± 20 | 1.000 | 0.087 | 0.990 |
| SGLT2i | 102 ± 25 | 96 ± 20 | 93 ± 20 | 91 ± 20 | 84 ± 18 | 89 ± 20 | 89 ± 19 | ||||
| Arterial lactate (mmol/L) | Control | 2.0 ± 0.4 | 2.5 ± 0.7 | 2.8 ± 0.9 | 3.1 ± 0.9 | 3.0 ± 0.8 | 2.7 ± 0.7 | 2.4 ± 0.6 | 0.754 | 0.336 | 1.000 |
| SGLT2i | 1.8 ± 0.4 | 2.1 ± 0.5 | 2.2 ± 0.5 | 2.8 ± 0.8 | 2.5 ± 0.7 | 2.5 ± 0.6 | 2.2 ± 0.5 | ||||
| Arterial ketone (|amol/L) | Control | 31 ± 5 | 41 ± 8 | 48 ± 10 | 47 ± 8 | 50 ± 9 | 53 ± 8 | 59 ± 10 | 0.002 | 0.74 | 0.953 |
| SGLT2i | 31 ± 4 | 34 ± 5 | 31 ± 6 | 46 ± 6 | 49 ± 6 | 59 ± 7 | 63 ± 8* | ||||
| Arterial FFA (mmol/L) | Control | 0.32 ± 0.06 | 0.32 ± 0.05 | 0.31 ± 0.05 | 0.34 ± 0.06 | 0.34 ± 0.05 | 0.35 ± 0.06 | 0.36 ± 0.05 | 0.995 | 0.017 | 0.999 |
| SGLT2i | 0.43 ± 0.05 | 0.41 ± 0.05 | 0.44 ± 0.05 | 0.44 ± 0.06 | 0.47 ± 0.06 | 0.49 ± 0.07 | 0.50 ± 0.08 | ||||
Values are mean ± SE for control (n = 7) and SGLT2i (canagliflozin) (n = 8)
P < 0.05 vs. control (same timepoint)
Fig. 6.
Effect of ischemia/reperfusion injury on myocardial uptake of glucose (a), lactate (b), ketones (c), and free fatty acids (FFA) d in control (n = 7) and SGLT2i (canagliflozin)-treated (n = 8) swine
Effect of SGLT2i on myocardial infarct size and cardiac function in response to 60 min LAD occlusion
No evidence of myocardial infarction was detected in either control (n = 3) or SGLT2i (n = 4)-treated hearts following the 30 min LCX occlusion protocol. Therefore, to better assess potential effects on magnitude of myocardial infarction, a 60 min occlusion with 2 h reperfusion was performed in a separate cohort of animals in the distal LAD region. The rationale for a change in perfusion territory was based on historic attempts at longer term proximal occlusions of the LCX which resulted in marked increases in mortality [17, 18]. Therefore, our additional cohort of animals was modeled after established protocols demonstrating reliable induction of myocardial infarction with higher survival rates following 60 min occlusion of the distal LAD in swine [17, 18].
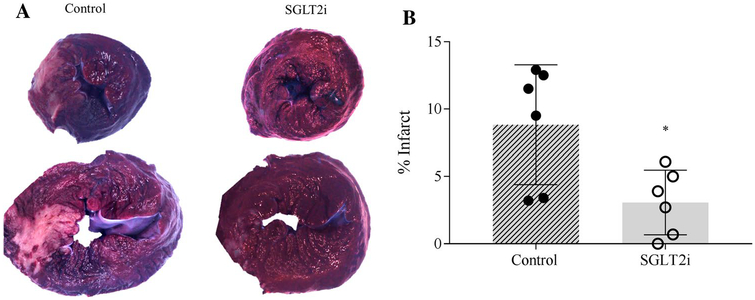
In contrast to data from the 30 min LCX occlusion, TTC staining of hearts from the 60 min LAD occlusion protocol revealed that treatment with canagliflozin (n = 6) significantly reduced infarct size by ~ 60% compared to control (n = 6) swine (Fig. 7; P = 0.03). In addition, canagliflozin treatment produced similar effects following the onset (15 and 30 min) of the distal LAD occlusion, including a significant increase in left ventricular end-diastolic volume and stroke volume (Supplemental Figure; P = 0.01) compared to control swine. Heart rate was significantly lower in the SGLT2i group at the later ischemic timepoints (≥ 30 min occlusion) (Supplemental Table). However, this reduction was not associated with alterations in end-diastolic volume or stroke volume at either 45 or 60 min occlusion.
Fig. 7.
Images in a show representative transmural sections of left ventricular slices from control (n = 6) and canagliflozin (n = 6)-treated swine. Quantification of total infarct area relative to total left ventricular area is presented in b. *P <0.05 vs. control
Discussion
There is a growing body of evidence supporting beneficial cardiovascular effects of SGLT2i in the setting of obesity/type 2 diabetes [3, 35, 46] and in normal, metabolically healthy animal models [28, 39]. However, there was no a priori reason to expect such beneficial effects of these glucosuric agents, and the mechanisms underpinning these unexpected effects are unknown. As such, whether effects of SGLT2i are the result of direct action on cardiomyocytes or other cardiac cells, or are secondary to extra-cardiac effects of SGLT2i remains undetermined. A proposed mechanism to explain SGLT2i-associated improvements in major adverse cardiovascular events as well as reductions in myocardial infarct size [3, [28] centers around the “thrifty fuel hypothesis” [5, 16, 34, 42]. This hypothesis is based on a recognized effect of SGLT2i to induce ketone production, which by providing ketones to the heart may improve cardiac efficiency by shifting myocardial substrate utilization away from free fatty acids [5, 14]. Accordingly, we set out to evaluate the effects of the SGLT2i canagliflozin on cardiac contractile function, substrate utilization, and efficiency before and during regional myocardial ischemia/reperfusion injury in normal, metabolically healthy swine. We observed effects of canagliflozin to augment cardiac function during regional myocardial ischemia and reduce myocardial infarct size, but these effects were independent of alterations myocardial substrate utilization. These observations argue against contributions of ketone-induced shifts in fuel selection to the cardioprotective effects of SGLT2i.
Cardiac effects of SGLT2i
Here, we show for the first time that SGLT2i produces a prominent and sustained increase in left ventricular volumes during regional myocardial ischemia in otherwise normal, metabolically healthy swine. There was no evidence of these effects under baseline conditions, and they abated during reperfusion (Fig. 4). Furthermore, these effects were not associated with any changes in arterial blood pressure (afterload) or heart rate (ventricular filling time) (Fig. 2). These data are consistent with recent rodent investigations which demonstrate that SGLT2i was able to preserve cardiac function in pressure-overload-induced heart failure mice independent of changes in afterload, as cardioprotection was evident both in vivo and in subsequent isolated heart studies [6].
Significant increases in end-diastolic volume could be interpreted as potentially deleterious, as volume overload is commonly associated with dilated cardiomyopathy and/or exacerbation of myocardial infarction. In this study, however, changes in ventricular volume handling were seen only during ischemia and reversed with relief of ischemia, at least under these acute exposure conditions. The timeline of the ischemia in this study (minutes) would not allow for the necessary remodeling associated with a dilated cardiomyopathy phenotype. Furthermore, interpretation of this study is simplified, as effects were evident in the absence of measurable myocardial infarction, as measured by TTC staining in the 30 min LCX occlusion study. The lack of myocardial infarction following a 30 min coronary occlusion is consistent with previously published studies in swine [17, 23, 24, 40]. Therefore, changes in ventricular volume handling must occur independent of ventricular remodeling and/or infarction. Our observations of unchanged end-diastolic pressure suggest that SGLT2i enhanced ventricular compliance; however, no significant differences in global indices of diastolic function (dP/dtmin, τ) were noted.
Although we did not observe any evidence of myocardial infarction following the 30 min LCX occlusion protocol, insights into potential infarct mitigating behavior of SGLT2i are fundamentally valuable. Therefore, we performed additional studies in a separate cohort of animals, wherein we subjected conditionally identical swine to a 60 min coronary artery occlusion. The LAD was occluded during these experiments, as longer duration LCX occlusion is associated with decreased survivability [17]. In these animals, we did observe significant SGLT2i-associated reduction in infarct size (Fig. 7). This finding is consistent with prior studies in rodent models which documented reductions in myocardial infarct size in response to ischemia/reperfusion injury [3, 28].
We were able to amplify small amounts of SGLT1 and SGLT2 mRNA from swine myocardial samples (Fig. 1), consistent with observations in human samples [7, 20, 21]. However, it is unclear whether the SGLT2i-mediated effects in this study are the result of direct and/or indirect actions on the heart. As initial dosing was 24 h prior to the acute study, the potential for paracrine/humoral mediation exists. The 300 mg oral dosing regimen utilized in this study was selected to directly parallel the “high-dose” utilized clinically. Prior studies indicate that a single oral dose of canagliflozin (300 mg) achieves a plasma Cmax of 3480 ng/mL (7.67 μM) in humans with tmax of 1.5 h and a half-life of 14.9 h [11, 12, 41]. We estimate (using allometric scaling) that the maximal plasma concentration of canagliflozin in ~ 50 kg swine such as those used in the present study to be ~ 3757 ng/mL (assuming no plasma protein binding), which is comparable to exposure in humans [11, 12, 41]. Given our present observations of clear effects of canagliflozin on myocardial function, it seems plausible that a myocardial receptor system might exist that is responsive to this agent but not from the SGLT2 protein family; e.g., sodium hydrogen exchanger-1 (NHE-1) discussed below. Therefore, further studies to determine dose dependence and direct vs. indirect actions of SGLT2i are needed.
Fig. 1.
qPCR for SGLT1 (a) and SGLT2 (b) in kidney (n = 5) vs. heart (n = 5) biopsies from domestic swine
Potential mechanisms underlying cardiac effects of SGLT2i
SGLT2i therapies have been demonstrated to augment circulating ketone bodies in type 2 diabetes, thereby suggesting the “thrifty fuel hypothesis” of cardioprotection. We found that canagliflozin increased free fatty acid concentrations modestly, but did not affect arterial levels of lactate or ketone bodies (Table 2). Arterial glucose levels tended to be lower in the treatment group (P = 0.08); however, ischemia/reperfusion status had no effect on glucose levels on either group (P = 1.0). These data are consistent with prior studies of SGLT2i treatment in metabolically healthy humans who also exhibited no change in fasting plasma glucose or ketone concentrations [1]. Despite the modest changes in circulating substrate concentration that we observed, no changes in myocardial uptake of glucose, lactate, ketones, or free fatty acid were seen (Fig. 6). Thus, while interventions such as insulin and malonyl CoA inhibition have been shown to improve contractile function during ischemia via promotion of the utilization of thirty fuels [16, 27, 43], this does not appear to be the explanation for the currently observed effects of SGLT2i. A caveat of the present study is that our findings were made in a metabolically normal animal model, under conditions of acute and controlled ischemia. Therefore, whether our findings extend to the setting of chronic treatment in patients with type 2 diabetes with or without acute ischemia merits future study.
It is well recognized that SGLT2i influence circulating blood volume, promoting osmotic diuresis, and natriuresis and reductions in plasma volume [8, 25]. Although we did not directly measure plasma volume, there was a modest, but significant decrease in hematocrit in SGLT2i treated swine which runs counter to anticipated effects of diuresis. Furthermore, the current observations are not consistent with beneficial effects of diuresis, as any reduction in plasma volume would be expected to reduce overall ventricular filling volumes, in contrast to our observed ~ 50% increase in end-diastolic volume during ischemia. In addition, no differences in hemodynamics or cardiac volumes were noted at baseline or during the reperfusion period (Fig. 3). Cardiovascular benefits associated with diuretic-induced reductions in plasma volume are understood to result from preload lowering and associated decreased wall tension within the ventricle and direct reductions in Frank–Starling-mediated cardiac force generation [10], again in contrast to what we observed. Together, these data argue against a role of diuresis in the observed effects of canagliflozin following short-term (single-day) therapy and acute ischemic insult.
An alternate hypothesis to potentially explain SGLT2i mediated cardioprotection is the “sodium hypothesis”. Recent evidence suggests that the SGLT2i-mediated effects could work via direct inhibition of NHE-1, as has been demonstrated in isolated cardiomyocytes from rats and rabbits [4, 44]. In conditions leading to cellular acidosis such as myocardial ischemia, NHE-1 has been shown to be activated resulting in a reversal of the Na+/Ca2+ exchanger and increase intracellular calcium [36, 37]. By extension, inhibition of NHE-1 would act to lessen Ca2+ overload in cardiomyocytes during myocardial ischemia. Improvement in cytosolic Ca2+ handling also produces myriad effects on regulatory and contractile proteins within the cardiomyocyte [2, 19, 29, 38, 45]. As the interplay between developed tension and resting cardiomyocyte length can determine Starling effects [32], a mechanism that directly alters intracellular Ca2+ handling could potentially contribute to the effects seen in this investigation. Previous studies have demonstrated a reduction in infarct size in a swine model of ischemia/reperfusion with NHE-1 inhibitors [26]. These data are directionally consistent with our present observations, and suggest a direction for further systematic in vivo and in vitro studies to explore the potential mechanisms of SGLT2i-mediated cardioprotection.
Conclusion
Data from these studies demonstrate that canagliflozin preserves cardiac contractile function and efficiency during acute regional myocardial ischemia through acute effects on cardiac volume regulation that cannot be explained by myocardial fuel switching. Furthermore, SGLT2i reduces infarct size following 60 min ischemia. Findings from this study highlight the therapeutic potential for SGLT2i in metabolically normal subjects [39] and demonstrate the need for further investigations to both elucidate the mechanisms responsible for improvements in cardiac function during ischemia and to extend these studies into metabolically deranged cohorts as well as cohorts with normal glucose metabolism but abnormal cardiac function.
Supplementary Material
Acknowledgements
The authors wish to thank Joshua Sturek for his assistance in performing the described studies.
Funding This work was funded by Eli Lilly and Company. Eli Lilly and Company had no role in study design, data collection, data analysis, data interpretation, or writing of the report. BS and SL were supported by T35HL110854. AK was supported by T32DK101001.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00395-019-0733-2) contains supplementary material, which is available to authorized users.
Conflict of interest No conflicts of interest to disclose for AK, KM, JD, SL, BS, CE, and AG. HB is a full-time graduate student at Indiana University and an employee of Eli Lilly and Co., but received no compensation from Eli Lilly for the work performed in this study. AR and WR are both employees of Eli Lilly and Co.
References
- 1.Al Jobori H, Daniele G, Adams J, Cersosimo E, Triplitt C, DeFronzo RA, Abdul-Ghani M (2017) Determinants of the increase in ketone concentration during SGLT2 inhibition in NGT, IFG and T2DM patients. Diabetes Obes Metab 19:809–813. 10.1111/dom.12881 [DOI] [PubMed] [Google Scholar]
- 2.Amende I, Bentivegna L, Morgan JP (1992) Ventricular function and calcium handling during ischemia. J Cardiovasc Pharmacol 20(Suppl 5):S42 10.1097/00005344-199206205-00007 [DOI] [PubMed] [Google Scholar]
- 3.Andreadou I, Efentakis P, Balafas E, Togliatto G, Davos CH, Varela A, Dimitriou CA, Nikolaou PE, Maratou E, Lambadiari V, Ikonomidis I, Kostomitsopoulos N, Brizzi MF, Dimitriadis G, Iliodromitis EK (2017) Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects. Front Physiol 8:1077 10.3389/fphys.2017.01077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baartscheer A, Schumacher CA, Wust RC, Fiolet JW, Stienen GJ, Coronel R, Zuurbier CJ (2017) Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia 60:568–573. 10.1007/s00125-016-4134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayeva M, Gheorghiade M, Ardehali H (2013) Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol 61:599–610. 10.1016/j.jacc.2012.08.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne NJ, Parajuli N, Levasseur JL, Boisvenue J, Beker DL, Masson G, Fedak PWM, Verma S, Dyck JRB (2017) Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. JACC Basic Transl Sci 2:347–354. 10.1016/j.jacbts.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Williams S, Ho S, Loraine H, Hagan D, Whaley JM, Feder JN (2010) Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther 1:57–92. 10.1007/s13300-010-0006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, Woerle HJ, von Eynatten M, Broedl UC (2014) The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 13:28 10.1186/1475-2840-13-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE (2015) Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 17:1180–1193. 10.1111/dom.12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow MS (1993) Assessing the treatment of congestive heart failure: diuretics, vasodilators, and angiotensin-converting enzyme inhibitors. Pharmacotherapy 13:82S–87S [PubMed] [Google Scholar]
- 11.Devineni D, Curtin CR, Polidori D, Gutierrez MJ, Murphy J, Rusch S, Rothenberg PL (2013) Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol 53:601–610. 10.1002/jcph.88 [DOI] [PubMed] [Google Scholar]
- 12.Devineni D, Polidori D (2015) Clinical pharmacokinetic, pharmacodynamic, and drug-drug interaction profile of canagliflozin, a sodium–glucose co-transporter 2 inhibitor. Clin Pharmacokinet 54:1027–1041. 10.1007/s40262-015-0285-z [DOI] [PubMed] [Google Scholar]
- 13.Feigl EO, Neat GW, Huang AH (1990) Interrelations between coronary artery pressure, myocardial metabolism and coronary blood flow. J Mol Cell Cardiol 22:375–390 [DOI] [PubMed] [Google Scholar]
- 14.Ferrannini E, Mark M, Mayoux E (2016) CV protection in the EMPA-REG OUTCOME trial: a “Thrifty Substrate” hypothesis. Diabetes Care 39:1108–1114. 10.2337/dc16-0330 [DOI] [PubMed] [Google Scholar]
- 15.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ (2014) Metabolic response to sodium–glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Investig 124:499–508. 10.1172/JCI72227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fillmore N, Lopaschuk GD (2014) Malonyl CoA: a promising target for the treatment of cardiac disease. IUBMB Life 66:139–146. 10.1002/iub.1253 [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Dorado D, Theroux P, Elizaga J, Galinanes M, Solares J, Riesgo M, Gomez MJ, Garcia-Dorado A, Fernandez Aviles F (1987) Myocardial reperfusion in the pig heart model: infarct size and duration of coronary occlusion. Cardiovasc Res 21:537–544 [DOI] [PubMed] [Google Scholar]
- 18.Gent S, Skyschally A, Kleinbongard P, Heusch G (2017) lschemic preconditioning in pigs: a causal role for signal transducer and activator of transcription 3. Am J Physiol Heart Circ Physiol 312:H478–H484. 10.1152/ajpheart.00749.2016 [DOI] [PubMed] [Google Scholar]
- 19.Gorski PA, Ceholski DK, Hajjar RJ (2015) Altered myocardial calcium cycling and energetics in heart failure—a rational approach for disease treatment. Cell Metab 21:183–194. 10.1016/j.cmet.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Y, Cho YE, Ayon R, Guo R, Youssef KD, Pan M, Dai A, Yuan JX, Makino A (2015) SGLT inhibitors attenuate NO-dependent vascular relaxation in the pulmonary artery but not in the coronary artery. Am J Physiol Lung Cell Mol Physiol 309:L1027–1036. 10.1152/ajplung.00167.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hediger MA, Kanai Y, You G, Nussberger S (1995) Mammalian ion-coupled solute transporters. J Physiol 482:7S–17S. 10.1113/jphysiol.1995.sp020559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ (2016) Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134:752–772. 10.1161/CIRCULATIONAHA.116.021887 [DOI] [PubMed] [Google Scholar]
- 23.Heusch G, Skyschally A, Schulz R (2011) The in-situ pig heart with regional ischemia/reperfusion - ready for translation. J Mol Cell Cardiol 50:951–963. 10.1016/j.yjmcc.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 24.Heyndrickx GR, Amano J, Patrick TA, Manders WT, Rogers GG, Rosendorff C, Vatner SF (1985) Effects of coronary artery reperfusion on regional myocardial blood flow and function in conscious baboons. Circulation 71:1029–1037 [DOI] [PubMed] [Google Scholar]
- 25.Kawasoe S, Maruguchi Y, Kajiya S, Uenomachi H, Miyata M, Kawasoe M, Kubozono T, Ohishi M (2017) Mechanism of the blood pressure-lowering effect of sodium–glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol Toxicol 18:23 10.1186/s40360-017-0125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein HH, Pich S, Bohle RM, Lindert-Heimberg S, Nebendahl K (2000) Na(+)/H(+) exchange inhibitor cariporide attenuates cell injury predominantly during ischemia and not at onset of reperfusion in porcine hearts with low residual blood flow. Circulation 102:1977–1982 [DOI] [PubMed] [Google Scholar]
- 27.Klein LJ, Visser FC (2010) The effect of insulin on the heart: Part 2: effects on function during and post myocardial ischaemia. Neth Heart J 18:255–259. 10.1007/bf03091772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim VG, Bell RM, Arjun S, Kolatsi-Joannou M, Long DA, Yellon DM (2019) SGLT2 inhibitor, canagliflozin, attenuates myocar-dial infarction in the diabetic and nondiabetic heart. JACC Basic Transl Sci. 10.1016/j.jacbts.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lou Q, Janardhan A, Efimov IR (2012) Remodeling of calcium handling in human heart failure. Adv Exp Med Biol 740:1145–1174. 10.1007/978-94-007-2888-2_52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, Desai M, Matthews DR, Group CPC (2018) Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 137:323–334. 10.1161/CIRCULATIONAHA.117.032038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martens P, Mathieu C, Verbrugge FH (2017) Promise of SGLT2 inhibitors in heart failure: diabetes and beyond. Curr Treat Options Cardiovasc Med 19:23 10.1007/s11936-017-0522-x [DOI] [PubMed] [Google Scholar]
- 32.Moss RL, Fitzsimons DP (2002) Frank–Starling relationship: long on importance, short on mechanism. Circ Res 90:11–13. 10.1161/res.90.1.11 [DOI] [PubMed] [Google Scholar]
- 33.Mudaliar S, Alloju S, Henry RR (2016) Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME Study? A unifying hypothesis. Diabetes Care 39:1115–1122. 10.2337/dc16-0542 [DOI] [PubMed] [Google Scholar]
- 34.Mudaliar S, Polidori D, Zambrowicz B, Henry RR (2015) Sodium–glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care 38:2344–2353. 10.2337/dc15-0642 [DOI] [PubMed] [Google Scholar]
- 35.Neal B, Perkovic V, Matthews DR (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:2099 10.1056/NEJMc1712572 [DOI] [PubMed] [Google Scholar]
- 36.Odunewu-Aderibigbe A, Fliegel L (2014) The Na(+)/H(+) exchanger and pH regulation in the heart. IUBMB Life 66:679–685. 10.1002/iub.1323 [DOI] [PubMed] [Google Scholar]
- 37.Perez NG, de Hurtado MC, Cingolani HE (2001) Reverse mode of the Na+–Ca2+ exchange after myocardial stretch: underlying mechanism of the slow force response. Circ Res 88:376–382. 10.1161/01.res.88.4.376 [DOI] [PubMed] [Google Scholar]
- 38.Saini HK, Dhalla NS (2005) Defective calcium handling in cardiomyocytes isolated from hearts subjected to ischemia-reperfusion. Am J Physiol Heart Circ Physiol 288:H2260–2270. 10.1152/ajpheart.01153.2004 [DOI] [PubMed] [Google Scholar]
- 39.Santos-Gallego CG, Garcia-Ropero A, Mancini D, Pinney SP, Contreras JP, Fergus I, Abascal V, Moreno P, Atallah-Lajam F, Tamler R, Lala A, Sanz J, Fuster V, Badimon JJ (2019) Rationale and design of the EMPA-TROPISM Trial (ATRU-4): are the “Cardiac Benefits” of empagliflozin independent of its hypoglycemic activity? Cardiovasc Drugs Ther. 10.1007/s10557-018-06850-0 [DOI] [PubMed] [Google Scholar]
- 40.Schaper W, Gorge G, Winkler B, Schaper J (1988) The collateral circulation of the heart. Prog Cardiovasc Dis 31:57–77 [DOI] [PubMed] [Google Scholar]
- 41.Scheen AJ (2014) Evaluating SGLT2 inhibitors for type 2 diabetes: pharmacokinetic and toxicological considerations. Expert Opin Drug Metab Toxicol 10:647–663. 10.1517/17425255.2014.873788 [DOI] [PubMed] [Google Scholar]
- 42.Staels B (2017) Cardiovascular protection by sodium glucose cotransporter 2 inhibitors: potential mechanisms. Am J Med 130:S30–S39. 10.1016/j.amjmed.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 43.Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85:1093–1129. 10.1152/physrev.00006.2004 [DOI] [PubMed] [Google Scholar]
- 44.Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, Zuurbier CJ (2018) Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia 61:722–726. 10.1007/s00125-017-4509-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wehrens XH, Lehnart SE, Reiken SR, Marks AR (2004) Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res 94:e61–70. 10.1161/01.RES.0000125626.33738.E2 [DOI] [PubMed] [Google Scholar]
- 46.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.