Abstract
Background
In September 2018 the FDA provided a draft guidance on master protocols reflecting an increased interest in these designs by industry. Master protocols refer to a single overarching protocol developed to evaluate multiple hypotheses and may be further categorized as basket, umbrella, and platform trials. However, inconsistencies in reporting persist in the literature. We conducted a systematic review to describe master protocol reporting with the goal of facilitating the further development and spread of these innovative trial designs.
Methods
We searched MEDLINE, EMBASE, and CENTRAL from inception to April 25, 2019 for English articles on master protocols. This was supplemented by hand searches of trial registries and of the bibliographies of published reviews. We used the FDA's definitions of master protocols as references and compared them to self-reported master protocols.
Results
We identified 278 master protocol publications, consisting of 228 protocols and 50 reviews. Sixty-six records provided unique definitions of master protocol types. We observed considerable heterogeneity in definitions of master protocols, and over half (54%) used oncology-specific language. The majority of self-classified master protocols (57%) were consistent with the FDA's definitions of master protocols.
Conclusion
The terms ‘master protocol’, ‘basket trial’, ‘umbrella trial’, and ‘platform trial’ are inconsistently described. Careful treatment of these terms and adherence to the definitions set forth by the FDA will facilitate better understanding of these trial designs and allow them to be used broadly and to their full potential in clinical research. We encourage trial methodologists to use these trial designations when applicable.
Keywords: Master protocols, Basket trials, Umbrella trials, Platform trials, Nomenclature
1. Introduction
Over the last decade, the concept of master protocols has developed complementary to the precision oncology movement, an emerging field of research designed to target cancers with treatments according to genetic mutations (i.e. targeted therapies) [1]. Master protocols generally refer to a single overarching design divided into sub-studies to evaluate multiple hypotheses, with the goal of improving overall efficiency in clinical trial research [[1], [2], [3], [4]]. Master protocols emphasize the use of a common infrastructure and standardized procedures in order to promote uniformity and are often categorized as basket trials, umbrella trials and platform trials [[1], [2], [3], [4]]. However, researchers have highlighted a lack of standardization and inconsistency within master protocol nomenclature in the published literature [4,5].
The United States (US) Food and Drug Administration (FDA) is one of the leading agencies encouraging and supporting a wider dissemination of master protocols, as demonstrated by their recently released (September 2018) draft guidance on master protocols [2] and a 2017 editorial published by the Directors of Drug Evaluation and Research (CDER) at the FDA in the New England Journal of Medicine [1]. The definitions presented in these papers reflect those that will likely be used by the American research industry in the coming years (Table 1). These definitions are not restricted to a certain field (e.g. oncology), making them widely accessible to a great variety of researchers.
Table 1.
FDA definitions and key attributes of master protocols.
| Trial Type | Definitions | Key attributes |
|---|---|---|
| Master protocol | “…[A] protocol designed with multiple substudies, which may have different objectives and [involve] coordinated efforts to evaluate one or more investigational drugs in one or more disease subtypes within the overall trial structure.” |
|
| Basket trial | “A master protocol designed to test a single investigational drug or drug combination in different populations defined by disease stage, histology, number of prior therapies, genetic or other biomarkers, or demographic characteristics.” |
|
| Umbrella trial | “A master protocol designed to evaluate multiple investigational drugs administered as single drugs or as drug combinations in a single disease population.” |
|
| Platform trial | “[A trial designed to] study multiple targeted therapies in the context of a single disease in a perpetual manner, with therapies allowed to enter or leave the platform on the basis of a decision algorithm”. |
|
As the adoption of these innovative trial methods continues to spread, ensuring the standardization of master protocol nomenclature will be critically important in order to facilitate communication, regulatory procedures, and patient safety. Efforts towards standardized reporting, such as the CONSORT statement, have historically led to endorsement by journals and improved publication quality [6]. Several reviews of master protocols have already been published [1,3,4,7,8] but, to our knowledge, none have systematically evaluated the reporting of master protocol definitions and the compliance of existing master protocols to existing definitions. We conducted a comprehensive systematic literature review on both individual studies and published reviews to assess the extent to which definitions of master protocols and reporting of these trial types have differed. The purpose of our review is to encourage standardization in the theoretical foundation of master protocols and to facilitate further development and spread of these innovative trial designs.
2. Methods
2.1. Selection strategy and criteria
Any English-language guidance documents from regulatory agencies, review articles on non-original work (e.g. editorials, descriptive or systematic reviews, commentary, and etc.), and original research articles (e.g. registered or published master protocols outlining methods and/or results) pertaining to master protocols were eligible for inclusion (see Supplementary Table 5). We included original research articles that self-classified as a master protocol or that were classified by another source (e.g. a review or methods paper). This study was conducted in accordance with PRISMA reporting guidelines for systematic literature reviews [9]. Draft guidance published by the FDA [2] was reviewed, alongside recent review and guidance publications in this field [[1], [2], [3], [4],10,11], to inform the eligibility criteria and to develop robust search strategies (Supplementary Tables 1–4). We searched MEDLINE, EMBASE and CENTRAL for master protocol publications published from database inception to April 25, 2019. We supplemented this with hand searches of trial registries (ClinicalTrials.gov and ISRCTN registry) to increase the sensitivity of the literature search. Finally, the reference lists of publications identified through either database searches or trial registries were reviewed.
2.2. Study selection and data extraction
Three independent reviewers (EGS, JJHPand MJZ) reviewed all abstracts and proceedings identified in the literature searches. The full-text publications of potentially relevant abstracts were retrieved and assessed for eligibility by EGS and JJHP independently and in duplicate. Two independent reviewers screened the bibliographies of the published reviews of master protocols (EGS and JJHP) and trial registries (JJHP and LD). Discrepancies in study selection were resolved by discussion or by a third investigator (KT or EJM), when necessary. Data extraction was completed by two independent reviewers (EGS and JJHP) into a standardized, piloted form. Cross-checking for consistency was conducted by other independent reviewers (MJZ and LD). Definitions of master protocols and their sub-types were extracted. In addition, we extracted whether and how each trial self-classified as a master protocol. A “self-classifying trial” was considered to be any trial calling itself a master protocol, basket trial, umbrella trial or platform trial in its protocol. We also classified all trials according to the FDA master protocol definitions.
2.3. Data synthesis
We used descriptive statistics to summarize literature identified through this review. We focused on key themes of master protocols as summarized in prior reviews, such as the types of adaptive trials designs, number of biomarkers studied, number of diseases studied, and number of interventions studied for each master protocol type [[1], [2], [3], [4], [5],11]. We used the FDA definitions as our reference definitions as they represent the definitions that will be used by industry and may therefore already be considered ‘standardized’ [1,2]. The Woodcock et al. paper also represents the most highly cited recent review of master protocols. We considered protocols to be consistent with the FDA definitions only if they hit all ‘key attributes’ of the FDA definitions as presented in Table 1.
3. Results
3.1. Selection of articles
Our search of the literature returned 5346 abstracts, with an additional 150 records identified through trial registries and hand searches. Of 696 abstracts selected for full text review, 278 publications satisfied all eligibility criteria (Supplementary Fig. 1). There were 50 review papers and 228 original research articles pertaining to 99 trials (Supplementary Tables 6 and 7). Eighty-nine of these trials self-classified as master protocols (Supplementary Table 13), while the remaining trials were identified as such by other sources (e.g. reviews or methods papers).
3.2. Definitions of master protocols
Reported definitions of master protocol and their sub-types are presented in Supplementary Tables 8–11. Of the 278 records included in this review, 68 records included at least one unique definition of master protocols or its sub-types, resulting in 106 total definitions. Publications that did not report a unique definition typically cited the other review papers included in this study. The most commonly defined trial design were basket trials (n = 35), while definitions of master protocols were reported the least often (n = 20). Reported individual definitions of master protocols (Supplementary Table 8), basket trials (Supplementary Table 9), umbrella trials (Supplementary Table 10), and platform trials (Supplementary Table 11) are provided in the Appendix.
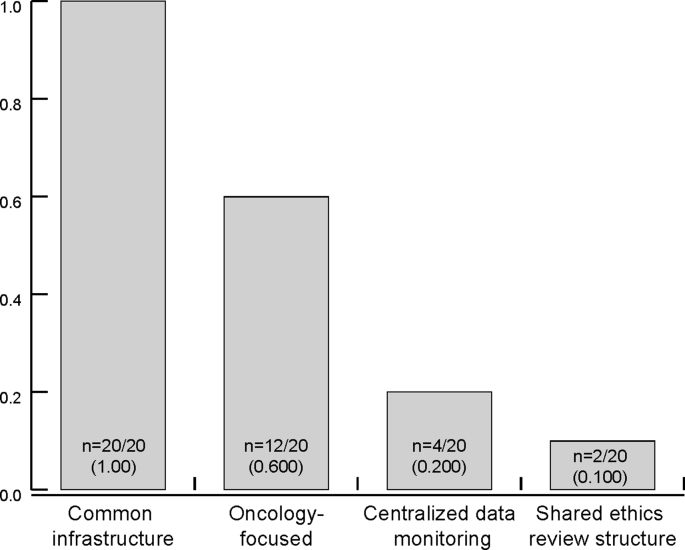
There were four key characteristics found among the 20 unique definitions of master protocols (Fig. 1) [[12], [13], [14], [15], [16]] [[1], [2], [3], [4], [5],8,[17], [18], [19], [20], [21], [22]]. While all sources consistently highlighted that common infrastructure is essential for master protocols, there were some variation with respect to scope, shared ethics review, and centralized data monitoring. Twelve definitions included oncology-specific terms in their definitions (i.e. the reported definitions pertained to oncology or a specific area in oncology only), limiting master protocols to the field of oncology. Only a few definitions specified shared ethics review structures (n = 2) [13,22] and centralized data monitoring (n = 4) [1,2,16,20] as integral to master protocols.
Fig. 1.
Master protocol definition characteristics. A summary of key characteristics of the definitions of ‘master protocol’. In this figure, the 20 definitions of master protocols identified were compared based four key attributes of common infrastructure, scope (i.e. oncology focused), centralized data monitoring, and shared ethics review structure.
3.3. Definitions of basket trials, umbrella trials, and platform trials
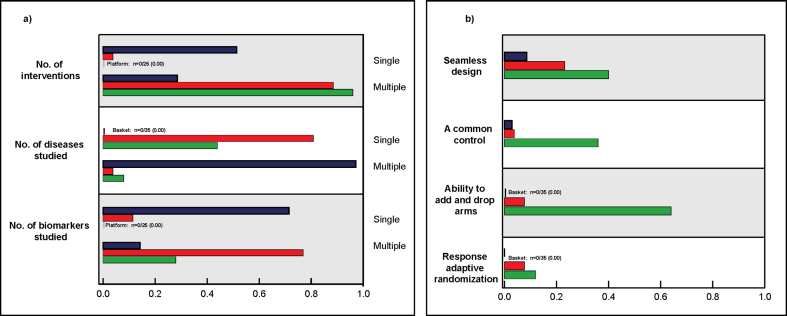
The characteristics of basket, umbrella, and platform trial definitions are illustrated in Fig. 2. While basket trial definitions (n = 35) [[1], [2], [3], [4], [5],11,13,15,18,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]] were consistent on some aspects, such as that basket trials study multiple disease types (n = 34), other features such as the number of interventions to be studied were inconsistent. Approximately half of the reviewed publications (n = 18) [1,2,4,5,11,13,15,25,30,31,33,35,38,39,41,42,45,49] specified that basket trials are designed to evaluate a single intervention, while around a third (n = 10) [3,18,23,[26], [27], [28], [29],36,40,43] stated that they can evaluate multiple interventions. The remaining definitions (n = 7) specified both [37,44,47] or neither [32,34,46,48]. The number of biomarkers or subgroups studied in a basket trial also varied, with the majority of definitions describing these studies as including only a single biomarker (n = 25) [1,[3], [4], [5],11,13,15,23,[26], [27], [28], [29],[31], [32], [33], [34], [35],[37], [38], [39], [40], [41], [42], [43],45] and considerably fewer including multiple (n = 5) [2,18,25,30,49]. Three basket trials specified the need for seamless design [3,4,39] and one for common control [41].
Fig. 2.
Key characteristics of basket trial, umbrella trial, and platform trial definitions. Theree were 35 definitions of basket trials identified, 26 umbrella trial definitions and 25 for platform trial definitions. These different definitions are summarized based on the number of interventions, diseases, and biomarkers involved are illustrated in the left panel (2a), and features of trial designs included in these definitions are mentioned in the right panel (2b). Basket trials are illustrated in color blue, color red for umbrella trials, and color green for platform trials. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Umbrella trials (n = 26) were more consistent than basket trials with regards to content (Fig. 2) [[1], [2], [3], [4], [5],11,13,15,18,19,27,28,[31], [32], [33],35,36,38,[45], [46], [47],[50], [51], [52], [53], [54]]. For example, the majority described umbrella trials as limited to a single disease (n = 21) [[1], [2], [3],11,13,15,18,27,[31], [32], [33],35,36,38,[45], [46], [47],[50], [51], [52], [53]], while a single publication proposed the opposite [19]. The remaining four trials specified both [4] or neither [5,28,54]. Most definitions(n = 20) [1,[3], [4], [5],11,15,18,28,[31], [32], [33],35,38,[45], [46], [47],[50], [51], [52],54] described umbrella trials as considering multiple biomarkers with only three [13,27,53] asserting that umbrella trials are restricted to a single biomarker. Moreover, in terms of adaptive trial design, one publication specified the need for seamless design and the ability to add and drop arms [51], and another specified the need for adaptive randomization [55].
Definitions of platform trials (n = 25) consistently referenced the investigation of multiple interventions [[1], [2], [3], [4], [5],7,19,22,30,34,[56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69]]. References to the number of diseases or biomarkers studied were not included in the definitions as often as for basket or umbrella trials. Moreover, compared to basket and umbrella trials definitions, the definitions of platform trials were much more likely to reference design features, with nine requiring a common control [3,5,19,58,60,63,64,66] and ten mentioning plans for subsequent stages [30,57,58,[61], [62], [63],[65], [66], [67],70]. The ability to add and drop arms was generally consistent across definitions (n = 16/25) [1,3,4,7,19,22,30,56,[58], [59], [60], [61], [62],64,68,70], though two publications specified the ability to drop but not add [65,66] and three the ability to add but not drop [63,67,69]. In addition, although the majority (n = 14/26) [1,4,5,7,30,[56], [57], [58], [59],[62], [63], [64],66,69] described platform trials as perpetual, a minority (n = 3/26) [57,65,70] asserted that platform trials are of finite duration.
3.4. Oncology-specific definitions
Basket trial definitions and umbrella trial definitions were equally likely to be oncology-specific, with about three quarters of both basket trial definitions (n = 27/35) and umbrella trial definitions (n = 20/26) using oncology-specific language and thus restricting the scope of their definitions. No platform trial definitions were oncology-specific.
3.5. Concurrence of self-classifications with FDA definitions
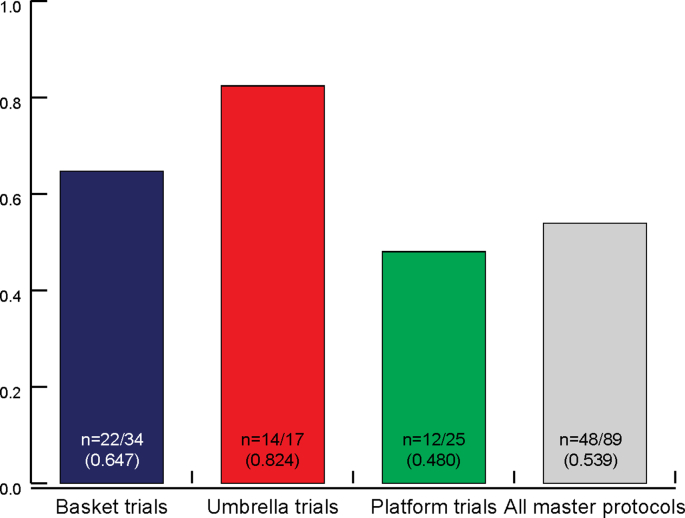
Overall, only slightly over half of trials self-classifying as a ‘master protocol’, ‘basket trial’, ‘umbrella trial’ or platform trial’ (n = 48/89) were consistent with the FDA definitions (Table 1). Several inconsistencies between the definitions identified in the literature and with the FDA definitions were observed (Fig. 3).
Fig. 3.
Proportion of trials classified consistently with FDA definitions. The proportion of master protocols consistent with the FDA definitions are shown in this figure. We compared all self-classified master protocols with the FDA definitions and their attributes (outlined in Table 1). In total, this review identified 89 self-classified master protocols (34 basket trials, 17 umbrella trials, 25 platform trials). 13 protocols self-identified as master protocols and therefore were included under ‘all master protocols’ only.
Of the 41 ‘inconsistent’ trials, four were ‘unclear’; that is, they did not provide enough information for us to designate them as master protocols [[71], [72], [73], [74]]. The majority of these were records from registries that provided insufficient detail with respect to trial design. Four other protocols contained the words ‘master protocol’ in their titles but were in no way master protocols [[75], [76], [77], [78]]. Ten protocols were diagnostic rather than treatment-based [14,16,[79], [80], [81], [82], [83], [84], [85]].
Three trials were misclassified based on FDA definitions; one was listed as a platform trial instead of as a master protocol [86] and one was listed as a platform trial but had no ability to add or drop arms [87]. Others had adaptive designs such as MAMS design (n = 6/32) [[88], [89], [90], [91], [92], [93]] or seamless design (n = 1/32) [94] but were not master protocols. Four protocols enrolled only one arm (n = 4/32) [[95], [96], [97], [98]], while two self-classified as basket trials but contained only one disease population [99,100]. Finally, five trials were classified as basket trials but contained multiple biomarker or intervention-defined cohorts [25,29,[101], [102], [103]]. We have marked these as ‘multi-basket’ trials in Supplementary Table 13.
4. Discussion
In this study, we used a systematic approach to describe the definitions of master protocols and their sub-types basket trials, umbrella trials, and platform trials that have been reported in the literature, and also to assess the consistency of how different individual studies have self-classified themselves as master protocols. There are considerable variations in the definitions proposed for master protocols and their sub-types, and also considerable inconsistencies in how different trials self-classified themselves as being a master protocol.
Among the reported definitions of master protocols, there were considerable variabilities in terms of the trial designs between the definitions of basket, umbrella, and platform trials. For instance, the definitions of basket and umbrella trials tended to be more biomarker-focused and exploratory than the definitions of platform trials. Only a few basket and umbrella trials definitions specified the need for a common control and seamless design, where the trial could transition seamlessly from an early exploratory or feasibility phase into later pivotal phase (e.g. confirmatory phase) [104,105]. A larger proportion of platform trial definitions, on the other hand, included the use of a common control and pre-specified abilities to add and drop arms during the trial. There were a small number of platform trial definitions that specifically mentioned the use of responsive adaptive randomization [7] [4,61,66], where allocation ratio can be adjusted to favor the arms over the course of the trial based on interim data [104,105]; however, it should be noted the responsive adaptive randomization is a type of adaptive trial designs that can also be applied to non-master protocols.
Our review also indicates that 52% (n = 48/93) of definitions were specific to oncology. Expanding the definitions of master protocols to be non-disease specific will be important to improve adoption of master protocols to the disease areas outside of oncology. Use of non-oncology-specific definitions, such as those presented by the FDA [2] and Butler et al. [64], allow for broader use of these terms, facilitating their spread to other fields in need of efficient trial design such as infectious diseases [16,59,64] Alzheimer's [106] and autoimmune diseases [107,108].
When we compared the consistency of master protocol classifications, only 56% (n = 41/73) of self-classified master protocols were fully consistent with the master protocol definitions proposed by the FDA. We decided to use the FDA definitions as a common reference since the 2017 NEJM editorial by Woodcock and LaVange was the most commonly cited paper. However, it may be important to broaden and expand upon these definitions, as broadening the flexibility of these definitions may contribute to better adherence. For instance, the FDA defined basket trials as having a single intervention, while Table 2 shows that many other basket trial definitions allow for multiple interventions. This issue might be solved by either permitting basket trials to have multiple interventions or by specifying an additional trial type such as a ‘multi-basket’. We therefore propose the cautious adoption of the FDA definitions as standardized definitions, with an understanding that these definitions should continue to be refined. It may be also worth considering whether platform trials need to be perpetual, or whether it may also be possible to have a finite platform trial with the capacity to add and drop arms up to a certain limit.
There are strengths and limitations to our study. The strength of our review lies in the comprehensiveness of our search, which included a systematic query of medical literature databases and clinical trial registries as well as a hand search of relevant reviews and methods papers. However, it is possible that our review may have been limited by variability of indexing and lack of MeSH terms that currently exist for master protocols. The creation of MeSH terms based on standardized definitions for ‘master protocol’, ‘basket trial’, ‘umbrella trial’ and ‘platform trial’ will be an important step to ensure that researchers can efficiently access and build on existing master protocol research. It is in the best of interests of all of those impacted by clinical trials to contribute to the development and maintenance of consistent and inclusive definitions.
Our review confirms that there is considerable work to be done towards the standardization of these terms, confirming the conclusions of previous reviews [4,5]. The standardization of master protocol nomenclature will become increasingly important as countries such as Canada and the US look towards drug approval using these trial designs [2,109,110]. A common understanding of these terms will be necessary to ensure that regulatory boards and potential trial participants know which questions to ask of trial administrators pertaining to patient safety and trial power. Standardizing master protocol definitions may also strengthen collaboration and facilitate further research attributed to more uniform data collection and trial documentation.
The framework of master protocols promotes clinical research that is highly efficient, making the best use of trial infrastructures, resources, and time. However, the terms, ‘master protocol’, basket trial’, ‘umbrella trial’ and platform’ have been inconsistently used and defined in the literature to date. Adoption of and careful adherence to the FDA definitions and will facilitate better understanding of these important new trial design frameworks and encourage them to be used broadly and to their full potentials. We encourage trial design methodologists to use these designations whenever applicable.
Funding
JP was financially supported by the Mitacs Accelerate Fellowship PhD grant. The other authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of interest
The authors have no conflicts of interest to report.
Acknowledgements
JP thanks the Mitacs Program for the Mitacs Accelerate Fellowship PhD grant(IT10703).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100406.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Woodcock J., LaVange L.M. Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med. 2017;377(1):62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 2.U.S Department of health and human services food and Drug administration, master protocols: efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance for industry (Draft Guidance) 2018. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM621817.pdf
- 3.Renfro L.A., Sargent D.J. Statistical controversies in clinical research: basket trials, umbrella trials, and other master protocols: a review and examples. Ann. Oncol. 2017;28(1):34–43. doi: 10.1093/annonc/mdw413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirakawa A., Asano J., Sato H., Teramukai S. Master protocol trials in oncology: review and new trial designs. Contemp Clin Trials Commun. 2018;12:1–8. doi: 10.1016/j.conctc.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry D.A. The Brave New World of clinical cancer research: adaptive biomarker-driven trials integrating clinical practice with clinical research. Mol Oncol. 2015;9(5):951–959. doi: 10.1016/j.molonc.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner L., Shamseer L., Altman D.G., Schulz K.F., Moher D. Does use of the CONSORT Statement impact the completeness of reporting of randomised controlled trials published in medical journals? A Cochrane reviewa. Syst. Rev. 2012;1(1):60. doi: 10.1186/2046-4053-1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry S.M., Connor J.T., Lewis R.J. The platform trial: an efficient strategy for evaluating multiple treatments. J. Am. Med. Assoc. 2015;313(16):1619–1620. doi: 10.1001/jama.2015.2316. [DOI] [PubMed] [Google Scholar]
- 8.Redman M.W., Allegra C.J. The master protocol concept. Semin. Oncol. 2015;42(5):724–730. doi: 10.1053/j.seminoncol.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 10.Berry S.M., Connor J.T., Lewis R.J. The platform trial: an efficient strategy for evaluating multiple TreatmentsPlatform trials to evaluate multiple TreatmentsPlatform trials to evaluate multiple treatments. J. Am. Med. Assoc. 2015;313(16):1619–1620. doi: 10.1001/jama.2015.2316. [DOI] [PubMed] [Google Scholar]
- 11.Parmar M.K., Sydes M.R., Cafferty F.H., Choodari-Oskooei B., Langley R.E., Brown L., Phillips P.P., Spears M.R., Rowley S., Kaplan R. Testing many treatments within a single protocol over 10 years at MRC Clinical Trials Unit at UCL: multi-arm, multi-stage platform, umbrella and basket protocols. Clin. Trials. 2017;14(5):451–461. doi: 10.1177/1740774517725697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bando H., Takebe N. Perspectives on research activity in the USA on cancer precision medicine. Jpn. J. Clin. Oncol. 2016;46(2):106–110. doi: 10.1093/jjco/hyv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst R.S., Gandara D.R., Hirsch F.R., Redman M.W., LeBlanc M., Mack P.C., Schwartz L.H., Vokes E., Ramalingam S.S., Bradley J.D., Sparks D., Zhou Y., Miwa C., Miller V.A., Yelensky R., Li Y., Allen J.D., Sigal E.V., Wholley D., Sigman C.C., Blumenthal G.M., Malik S., Kelloff G.J., Abrams J.S., Blanke C.D., Papadimitrakopoulou V.A. Lung master protocol (Lung-MAP)-A biomarker-driven protocol for accelerating development of therapies for squamous cell lung cancer: SWOG S1400, clinical cancer research. Off. J. Am.Assoc.Cancer.Res. 2015;21(7):1514–1524. doi: 10.1158/1078-0432.CCR-13-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid H.-P., Morant R., Bernhard J., Maibach R. Prostate specific antigen doubling time as auxiliary end point in hormone refractory prostatic carcinoma. Eur. Urol. 2003;43(1):28–30. doi: 10.1016/s0302-2838(02)00539-0. [DOI] [PubMed] [Google Scholar]
- 15.Lam V.K., Papadimitrakopoulou V. Master protocols in lung cancer: experience from Lung Master Protocol. Curr. Opin. Oncol. 2018;30(2):92–97. doi: 10.1097/CCO.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 16.Manca C., Hill C., Hujer A.M., Patel R., Evans S.R., Bonomo R.A., Kreiswirth B.N., Laboratory G. Center of the antibacterial resistance leadership, leading antibacterial laboratory research by integrating conventional and innovative approaches: the laboratory center of the antibacterial resistance leadership group. Clin. Infect. Dis. 2017;64(suppl_1):S13–S17. doi: 10.1093/cid/ciw826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadimitrakopoulou V., Redman M., Gandara D.R., Hirsch F.R., Mack P., Borghaei H., Langer C., Wade J., Edelman M., Albain K., Lara P., Aggarwal C., Socinski M., Gettinger S., Bazhenova L., Malik S., Miller V., McDonough S., Sigal E.V., Kelly K., Herbst R., Lung-MAP S1400) lung master protocol: accrual and genomic screening updates. J. Thorac. Oncol. 2017;12(1 Supplement 1):S439–S440. [Google Scholar]
- 18.Ventz S., Barry W.T., Parmigiani G., Trippa L. Bayesian response-adaptive designs for basket trials. Biometrics. 2017;73(3):905–915. doi: 10.1111/biom.12668. [DOI] [PubMed] [Google Scholar]
- 19.Saville B.R., Berry S.M. Efficiencies of platform clinical trials: a vision of the future. Clin. Trials. 2016;13(3):358–366. doi: 10.1177/1740774515626362. [DOI] [PubMed] [Google Scholar]
- 20.Patel R., Tsalik E.L., Petzold E., Fowler V.G., Jr., Klausner J.D., Evans G S. Antibacterial resistance leadership, MASTERMIND: bringing microbial diagnostics to the clinic. Clin. Infect. Dis. : an official publication of the Infectious Diseases Society of America. 2017;64(3):355–360. doi: 10.1093/cid/ciw788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledford H. Master protocol' aims to revamp cancer trials: pilot project will bring drug companies together to test targeted lung-cancer therapies. Nature. 2013;498(7453):146–148. doi: 10.1038/498146a. [DOI] [PubMed] [Google Scholar]
- 22.Khan T., Stewart M., Blackman S., Rousseau R., Donoghue M., Cohen K., Seibel N., Fleury M., Benettaib B., Malik R., Vassal G., Reaman G. Accelerating pediatric cancer drug development: challenges and opportunities for pediatric master protocols. Ther Innov Regul Sci. 2019 Mar;53(2):270–278. doi: 10.1177/2168479018774533. Epub 2018 May. [DOI] [PubMed] [Google Scholar]
- 23.Barroilhet L., Matulonis U. The NCI-MATCH trial and precision medicine in gynecologic cancers. Gynecol. Oncol. 2018;148(3):585–590. doi: 10.1016/j.ygyno.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 24.A Phase II Study of Avelumab Monotherapy in PD-L1 Positive or EBV Positive or MSI-H or POLE/POLD1 Mutated Advanced Solid Tumor (Part of K-BASKET Trial; Korea-Biomarker-Driven Multi-Arm Drug-Screening, Knowledge and Evidence-Generating Targeted Trial) 2018. [Google Scholar]
- 25.Slosberg E.D., Kang B.P., Peguero J., Taylor M., Bauer T.M., Berry D.A., Braiteh F., Spira A., Meric-Bernstam F., Stein S., Piha-Paul S.A., Salvado A. Signature program: a platform of basket trials. Oncotarget. 2018;9(30):21383–21395. doi: 10.18632/oncotarget.25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ornes S. Core Concept: basket trial approach capitalizes on the molecular mechanisms of tumors. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113(26):7007–7008. doi: 10.1073/pnas.1608277113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore K.N., Mannel R.S. Is the NCI MATCH trial a match for gynecologic oncology? Gynecol. Oncol. 2016;140(1):161–166. doi: 10.1016/j.ygyno.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Mandrekar S.J., Dahlberg S.E., Simon R. Am Soc Clin Oncol Educ Book; 2015. Improving clinical trial efficiency: thinking outside the box; pp. e141–e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Chavez A., Thomas A., Rajan A., Raffeld M., Morrow B., Kelly R., Carter C.A., Guha U., Killian K., Lau C.C., Abdullaev Z., Xi L., Pack S., Meltzer P.S., Corless C.L., Sandler A., Beadling C., Warrick A., Liewehr D.J., Steinberg S.M., Berman A., Doyle A., Szabo E., Wang Y., Giaccone G. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J. Clin. Oncol. : Off.J. Am. Soc. Clin. Oncol. 2015;33(9):1000–1007. doi: 10.1200/JCO.2014.58.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J., Bunn V. Comparison of multi-arm multi-stage design and adaptive randomization in platform clinical trials. Contemp. Clin. Trials. 2017;54:48–59. doi: 10.1016/j.cct.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Lih C.J., Takebe N. Considerations of developing an NGS assay for clinical applications in precision oncology: the NCI-MATCH NGS assay experience. Curr. Probl. Cancer. 2017;41(3):201–211. doi: 10.1016/j.currproblcancer.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Simon R. Genomic alteration-driven clinical trial designs in oncology. Ann. Intern. Med. 2016;165(4):270–278. doi: 10.7326/M15-2413. [DOI] [PubMed] [Google Scholar]
- 33.Kourie H.R., Aoun F. Why balls are not put inside the basket? A reflection on testicular cancer clinical trial design. Investig. New Drugs. 2016;34(4):513–514. doi: 10.1007/s10637-016-0338-7. [DOI] [PubMed] [Google Scholar]
- 34.Hobbs B.P., Landin R. Bayesian basket trial design with exchangeability monitoring. Stat. Med. 2018;37(25):3557–3572. doi: 10.1002/sim.7893. [DOI] [PubMed] [Google Scholar]
- 35.Beckman R.A., Antonijevic Z., Kalamegham R., Chen C. Adaptive design for a confirmatory basket trial in multiple tumor types based on a putative predictive biomarker. Clin. Pharm. Ther. 2016;100(6):617–625. doi: 10.1002/cpt.446. [DOI] [PubMed] [Google Scholar]
- 36.Trusheim M.R., Shrier A.A., Antonijevic Z., Beckman R.A., Campbell R.K., Chen C., Flaherty K.T., Loewy J., Lacombe D., Madhavan S., Selker H.P., Esserman L.J. PIPELINEs: creating comparable clinical knowledge efficiently by linking trial platforms. Clin. Pharm. Ther. 2016;100(6):713–729. doi: 10.1002/cpt.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao J.J., Schram A.M., Hyman D.M. Basket studies: redefining clinical trials in the era of genome-driven oncology. Annu. Rev. Med. 2018;69:319–331. doi: 10.1146/annurev-med-062016-050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steuer C.E., Papadimitrakopoulou V., Herbst R.S., Redman M.W., Hirsch F.R., Mack P.C., Ramalingam S.S., Gandara D.R. Innovative clinical trials: the LUNG-MAP study. Clin. Pharm. Ther. 2015;97(5):488–491. doi: 10.1002/cpt.88. [DOI] [PubMed] [Google Scholar]
- 39.Derhaschnig U., Gilbert J., Jager U., Bohmig G., Stingl G., Jilma B. Combined integrated protocol/basket trial design for a first-in-human trial. Orphanet J. Rare Dis. 2016;11(1):134. doi: 10.1186/s13023-016-0494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hainsworth J.D., Meric-Bernstam F., Swanton C., Hurwitz H., Spigel D.R., Sweeney C., Burris H., Bose R., Yoo B., Stein A., Beattie M., Kurzrock R. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J. Clin. Oncol. 2018;36(6):536–542. doi: 10.1200/JCO.2017.75.3780. [DOI] [PubMed] [Google Scholar]
- 41.Diamond E.L., Subbiah V., Craig Lockhart A., Blay J.Y., Puzanov I., Chau I., Raje N.S., Wolf J., Erinjeri J.P., Torrisi J., Lacouture M., Elez E., Martinez-Valle F., Durham B., Arcila M.E., Ulaner G., Abdel-Wahab O., Pitcher B., Makrutzki M., Riehl T., Baselga J., Hyman D.M. Vemurafenib for BRAF V600-mutant erdheim-chester disease and langerhans cell histiocytosis analysis of data from the histology-independent, phase 2, open-label VE-BASKET study. J. Am. Med. Assoc.Oncol. 2018;4(3):384–388. doi: 10.1001/jamaoncol.2017.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunanan K.M., Iasonos A., Shen R., Begg C.B., Gonen M. An efficient basket trial design. Stat. Med. 2017;36(10):1568–1579. doi: 10.1002/sim.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conley B.A., Doroshow J.H. Molecular analysis for therapy choice: NCI MATCH. Semin. Oncol. 2014;41(3):297–299. doi: 10.1053/j.seminoncol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y., Chi P. Basket trial of TRK inhibitors demonstrates efficacy in TRK fusion-positive cancers. J. Hematol. Oncol. 2018;11(1):78. doi: 10.1186/s13045-018-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunanan K.M.I., Shen A., Gonen M R. Variance prior specification for a basket trial design using Bayesian hierarchical modeling. Clin. Trials. 2019;16(2):142–153. doi: 10.1177/1740774518812779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galot R., Machiels J.P., Kong A., Govaerts A.S., Caballero C., Fortpied C., Staelens D., Bogaerts J., Rodegher L., Raveloarivahy T., Laes J.F., Le Tourneau C., Saada-Bouzid E., Guigay J., Licitra L., Tinhofer I. Personalized biomarker-based treatment strategy for patients with squamous cell carcinoma of the head and neck: EORTC position and approach. Ann. Oncol. 2018;29(12):2313–2327. doi: 10.1093/annonc/mdy452. [DOI] [PubMed] [Google Scholar]
- 47.Janiaud P.S., Ioannidis Stylianos, John P.A. New clinical trial designs in the era of precision medicine: an overview of definitions, strengths, weaknesses, and current use in oncology. Cancer Treat Rev. 2019;73:20–30. doi: 10.1016/j.ctrv.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Peron J.M., Staelens S., Raveloarivahy D., Nzokirantevye T., Flament A., Steuve J., Lia J., Collette M., Schoffski L., P. A multinational, multi-tumour basket study in very rare cancer types: the European Organization for Research and Treatment of Cancer phase II 90101 'CREATE' trial. Eur. J. Cancer. 2019;109:192–195. doi: 10.1016/j.ejca.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 49.FOCUS4-N Protocol: A randomised controlled comparison of capecitabine against active monitoring after first-line treatment for patients with colorectal cancer, 2018. http://www.focus4trial.org/media/1470/focus4-n-protocol-v40-_05apr2018_final-clean.pdf
- 50.Lee J. Biomarker-based Bayesian adaptive designs for targeted agent development. J. Thorac. Oncol. 2011:S91–S92. [Google Scholar]
- 51.Kaplan R. The FOCUS4 design for biomarker stratified trials. Chin. Clin. Oncol. 2015;4(3):35. doi: 10.3978/j.issn.2304-3865.2015.02.03. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan R., Maughan T., Crook A., Fisher D., Wilson R., Brown L., Parmar M. Evaluating many treatments and biomarkers in oncology: a new design. J. Clin. Oncol. 2013;31(36):4562–4568. doi: 10.1200/JCO.2013.50.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrarotto R., Redman M.W., Gandara D.R., Herbst R.S., Papadimitrakopoulou V.A. Lung-MAP--framework, overview, and design principles. Chin. Clin. Oncol. 2015;4(3):36. doi: 10.3978/j.issn.2304-3865.2015.09.02. [DOI] [PubMed] [Google Scholar]
- 54.Papadimitrakopoulou V.R., Gandara M.W., Hirsch D.R., Mack F.R., Langer P.C., Edelman C.J., Aggarwal M.J., Socinski C., Gettinger M.A., Waqar S.N., Griffin S.N., Leighl K., Owonikoko N.B., Bradley T.K., Ramalingam J.D., Stinchcombe S.S., Blanke T., Kelly C.D., Herbst K., R.S. First comprehensive report of impact of genomic alterations, chemotherapy, targeted therapy and immunotherapy on outcomes in the genomics driven squamous master protocol LungMAP. J. Clin. Oncol. 2018;36(15 Supplement 1) [Google Scholar]
- 55.Lee J.J., Xuemin G., Suyu L. Bayesian adaptive randomization designs for targeted agent development. Clin. Trials. 2010;7(5):584–596. doi: 10.1177/1740774510373120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butler C.C., Connor J.T., Lewis R.J., Broglio K., Saville B.R., Cook J., van der Velden A., Verheij T. Answering patient-centred questions efficiently: response-adaptive platform trials in primary care. Br. J. Gen. Pract. 2018;68(671):294–295. doi: 10.3399/bjgp18X696569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walter R.B., Michaelis L.C., Othus M., Uy G.L., Radich J.P., Little R.F., Hita S., Saini L., Foran J.M., Gerds A.T., Klepin H.D., Hay A.E., Assouline S., Lancet J.E., Couban S., Litzow M.R., Stone R.M., Erba H.P. Intergroup LEAP trial (S1612): a randomized phase 2/3 platform trial to test novel therapeutics in medically less fit older adults with acute myeloid leukemia. Am. J. Hematol. 2018;93(2):E49–E52. doi: 10.1002/ajh.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilson C., Chowdhury S., Parmar M.K.B., Sydes M.R., Investigators S. Incorporating biomarker stratification into STAMPEDE: an adaptive multi-arm, multi-stage trial platform. Clinical oncology (Royal College of Radiologists (Great Britain)) 2017;29(12):778–786. doi: 10.1016/j.clon.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berry S.M., Petzold E.A., Dull P., Thielman N.M., Cunningham C.K., Corey G.R., McClain M.T., Hoover D.L., Russell J., Griffiss J.M., Woods C.W. A response adaptive randomization platform trial for efficient evaluation of Ebola virus treatments: a model for pandemic response. Clin. Trials. 2016;13(1):22–30. doi: 10.1177/1740774515621721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rugo H.S., Olopade O.I., DeMichele A., Yau C., van 't Veer L.J., Buxton M.B., Hogarth M., Hylton N.M., Paoloni M., Perlmutter J., Symmans W.F., Yee D., Chien A.J., Wallace A.M., Kaplan H.G., Boughey J.C., Haddad T.C., Albain K.S., Liu M.C., Isaacs C., Khan Q.J., Lang J.E., Viscusi R.K., Pusztai L., Moulder S.L., Chui S.Y., Kemmer K.A., Elias A.D., Edmiston K.K., Euhus D.M., Haley B.B., Nanda R., Northfelt D.W., Tripathy D., Wood W.C., Ewing C., Schwab R., Lyandres J., Davis S.E., Hirst G.L., Sanil A., Berry D.A., Esserman L.J., Investigators I.S. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N. Engl. J. Med. 2016;375(1):23–34. doi: 10.1056/NEJMoa1513749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexander B.M., Ba S., Berger M.S., Berry D.A., Cavenee W.K., Chang S.M., Cloughesy T.F., Jiang T., Khasraw M., Li W., Mittman R., Poste G.H., Wen P.Y., Yung W.K.A., Barker A.D., Network G.A. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin. Cancer Res. 2018;24(4):737–743. doi: 10.1158/1078-0432.CCR-17-0764. [DOI] [PubMed] [Google Scholar]
- 62.Richman S.D., Adams R., Brown E., Brown L., Butler R., Falk S., Fisher D., Kaplan R., Middleton G., Quirke P., Samuel L., Seligmann J., Seymour M.T., Shiu K.K., Wasan H., Wilson R., Maughan T. FOCUS4: MAMS trial design in action. Early closure of FOCUS4-D (Pan-HER 1, 2 and 3 inhibitor versus placebo) in advanced colorectal cancer (acrc) patients, with tumours wildtype (WT) for KRAS, NRAS, BRAF and PIK3CA. J. Pathol. 2017;243(Supplement 1):S13. [Google Scholar]
- 63.Sydes M.R., Parmar M.K.B., James N.D., Clarke N.W., Dearnaley D.P., Mason M.D., Morgan R.C., Sanders K., Royston P. Issues in applying multi-arm multi-stage methodology to a clinical trial in prostate cancer: the MRC STAMPEDE trial. Trials. 2009;10:39. doi: 10.1186/1745-6215-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Butler C.C., Coenen S., Saville B.R., Cook J., van der Velden A., Homes J., de Jong M., Little P., Goossens H., Beutels P., Ieven M., Francis N., Moons P., Bongard E., Verheij T. A trial like ALIC4E: why design a platform, response-adaptive, open, randomised controlled trial of antivirals for influenza-like illness? ERJ Open Research. 2018;4(2):00046–02018. doi: 10.1183/23120541.00046-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bratton D.J., Phillips P.P., Parmar M.K. A multi-arm multi-stage clinical trial design for binary outcomes with application to tuberculosis. BMC Med. Res. Methodol. 2013;13:139. doi: 10.1186/1471-2288-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phillips P.P., Gillespie S.H., Boeree M., Heinrich N., Aarnoutse R., McHugh T., Pletschette M., Lienhardt C., Hafner R., Mgone C., Zumla A., Nunn A.J., Hoelscher M. Innovative trial designs are practical solutions for improving the treatment of tuberculosis. J. Infect. Dis. 2012;205(Suppl 2):S250–S257. doi: 10.1093/infdis/jis041. [DOI] [PubMed] [Google Scholar]
- 67.PRISM . 2018. A platform protocol for the treatment of relapsed/refractory aggressive non-hodgkin's lymphoma. [Google Scholar]
- 68.Kaizer A.M.H., Koopmeiners Brian P., Joseph S. A multi-source adaptive platform design for testing sequential combinatorial therapeutic strategies. Biometrics. 2018;74(3):1082–1094. doi: 10.1111/biom.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tolles J.L., Roger J. Adaptive and platform trials in remote damage control resuscitation. J. Trauma Acute.Care.Surg. 2018;84(6S Suppl 1):S28–S34. doi: 10.1097/TA.0000000000001904. [DOI] [PubMed] [Google Scholar]
- 70.Hobbs B.P., Chen N., Lee J.J. Controlled multi-arm platform design using predictive probability. Stat. Methods Med. Res. 2018;27(1):65–78. doi: 10.1177/0962280215620696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.An Open-Label Multicenter. Participants with Advanced And/or Metastatic Solid Tumors. ClinicalTrials.com; 2017. Phase II study to evaluate the therapeutic activity of RO6874281, an immunocytokine, consisting of interleukin-2 variant (IL-2v) targeting fibroblast activation protein-Α (FAP), in combination with atezolizumab (Anti-PD-L1), administered intravenously. [Google Scholar]
- 72.Forster M.D., Moreno V., Boni V., Guerra E., Poveda A., Kristeleit R., Kahatt C.M., Lardelli P., Lopez Vilarino J.A., Cuevas N.M., Soto-Matos A., Calvo E. Activity of lurbinectedin (PM01183) as single agent and in combination in patients with endometrial cancer. J. Clin. Oncol. 2017;35(15_suppl) 5586-5586. [Google Scholar]
- 73.The P10s Basket Trial of A Carbohydrate Mimotope-Based Vaccine with MONTANIDE™ ISA 51 VG Combined with Standard Treatment for Metastatic Cancer. ClinicalTrials; 2016. [Google Scholar]
- 74.Metronomic Oral Vinorelbine Plus Anti-PD-L1/Anti-CTLA4 ImmunothErapy in Patients with Advanced Solid Tumours (MOVIE) ClinicalTrials.com; 2018. [Google Scholar]
- 75.Safety and Effectiveness of Four Anti-HIV Drug Combinations in HIV-Infected Children and Teens. ClinicalTrials.com; 2000. [Google Scholar]
- 76.ClinicalTrials.com; 2019. Platform trial evaluating safety and efficacy of BI 754091 anti- PD-1 based combination therapies in PD-(L)1 naïve and PD- (L)1 pretreated patient populations with advanced/metastatic solid tumours. [Google Scholar]
- 77.Comparison of New Anti-HIV Drug Combinations in HIV-Infected Children Who Have Taken Anti-HIV Drugs, 2001.
- 78.Renal Adjuvant MultiPle Arm Randomised Trial (RAMPART), ClinicalTrials.com, 2018.
- 79.Panc Precision. ISRCTN Registry; 2018. Advancing Personalised Medicine Treatment Strategies for Pancreatic Cancer. [Google Scholar]
- 80.Jao J.Y., Patel W., Miller K., Karalius T.L., Geffner B., DiMeglio M.E., Mirza L.A., Chen A., Silio J.S., McFarland M., Van Dyke E.J., Jacobson R.B., Pediatric D. H.I.V.Aids Cohort Study Adolescent Master Protocol study, Improvement in lipids after switch to boosted atazanavir or darunavir in children/adolescents with perinatally acquired HIV on older protease inhibitors: results from the Pediatric HIV/AIDS Cohort Study. HIV Med. 2018;19(3):175–183. doi: 10.1111/hiv.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.al R.e. Optimization of a digital medicine system in psychiatry. J. Clin. Psychiatry. 2016;77(9) doi: 10.4088/JCP.16m10693. [DOI] [PubMed] [Google Scholar]
- 82.Evans S.R., Hujer A.M., Jiang H., Hill C.B., Hujer K.M., Mediavilla J.R., Manca C., Tran T.T.T., Domitrovic T.N., Higgins P.G., Seifert H., Kreiswirth B.N., Patel R., Jacobs M.R., Chen L., Sampath R., Hall T., Marzan C., Fowler V.G., Jr., Chambers H.F., Bonomo R.A. Informing antibiotic treatment decisions: evaluating rapid molecular diagnostics to identify susceptibility and resistance to carbapenems against acinetobacter spp. in PRIMERS III. J. Clin. Microbiol. 2016;55(1):134–144. doi: 10.1128/JCM.01524-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee J., Kim S.T., Mortimer P.G., Hollingsworth S.J., Harrington E.A., Shepherd C., Kilgour E., Park S.H., Lee H., Oh S.Y., Kang J.H., Park J.O., Park Y.S., Lim H.Y., Kim K.-M., Kang W.K. VIKTORY trial: report on AZD1775/paclitaxel in TP53 mutation (+) GC, selumetinib/paclitaxel in ras aberrant GC, AZD5363/paclitaxel in PIK3CA mt and biomarker negative, savolitinib/docetaxel in met (+), and vistusertib/paclitaxel in RICTOR(+) GC. J. Clin. Oncol. 2017;35(15_suppl) 4024-4024. [Google Scholar]
- 84.The Antibacterial Resistance Leadership G., Evans S., Jacobs M.R., Perez F., Sampath R., Marzan C., Hall T., Fowler V., Chambers H., Tran T.T.T., Hujer A., Hill C., Domitrovic T.N., Hujer K.M., Bonomo R.A., Mediavilla J.R., Chen L., Manca C., Kreiswirth B.N., Patel R. vol. 3. Open Forum Infectious Diseases; 2016. (Choosing Ceftazidime/Avibactam and Ceftolozane/Tazobactam as Empiric Therapies against Pseudomonas aeruginosa (Pa) Using Rapid Molecular Diagnostics (RMDs): PRIMERS IV). suppl_1. [Google Scholar]
- 85.Evans S.R., Hujer A.M., Jiang H., Hujer K.M., Hall T., Marzan C., Jacobs M.R., Sampath R., Ecker D.J., Manca C., Chavda K., Zhang P., Fernandez H., Chen L., Mediavilla J.R., Hill C.B., Perez F., Caliendo A.M., Fowler V.G., Jr., Chambers H.F., Kreiswirth B.N., Bonomo G R.A. Antibacterial resistance leadership, rapid molecular diagnostics, antibiotic treatment decisions, and developing approaches to inform empiric therapy: PRIMERS I and II. Clin. Infect. Dis. : an official publication of the Infectious Diseases Society of America. 2016;62(2):181–189. doi: 10.1093/cid/civ837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sebag-Montefiore D., Adams R., Bell S., Berkman L., Gilbert D., Glynne-Joones R., Goh V., Gregory W., Harrison M., Kachnic L., Lee M., McParland L., Muirhead R., O'Neil B., Hutchins G., Rao S., Renehan A., Smith A., Velikova G., Hawkins M. Optimising RT dose for anal cancer-the development of three clinical trials in one platform. Radiother. Oncol. 2017;123(Supplement 1):S685–S686. [Google Scholar]
- 87.Hitchcock C.G., O'Leary Siobhan, Rodrigues Cliodhna, Wright Evangeline, Griffiths Isobel, Gillard Kirsty, Watson Julia, Hammond Peter, Werner-Seidler Emily, Tim Aliza Dalgleish. Study protocol for a randomised, controlled platform trial estimating the effect of autobiographical Memory Flexibility training (MemFlex) on relapse of recurrent major depressive disorder. BMJ open. 2018;8(1):e018194. doi: 10.1136/bmjopen-2017-018194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Two-month Regimens Using Novel Combinations to Augment Treatment Effectiveness for Drug-Sensitive Tuberculosis (TRUNCATE-TB) ClinicalTrials.com; 2018. [Google Scholar]
- 89.Preventing Recurrence of Endometriosis by Means of Long Acting Protestogen Therapy. ISRCTN Registry; 2014. [Google Scholar]
- 90.A Trial Investigating Multiple Potential Interventions for Increasing Uptake of HIV Testing and Linkage into Care for Male Partners of Pregnant Women Attending Antenatal Clinic through HIV Self-Testing. ISRCTN Registry; 2016. [Google Scholar]
- 91.Evaluation of SQ109 . ClinicalTrials.com; 2013. High-dose Rifampicin, and Moxifloxacin in Adults with Smear-Positive Pulmonary TB in a MAMS Design. [Google Scholar]
- 92.ADAPT-BLADDER . ClinicalTrials.com; 2017. Modern Immunotherpy in BCG-Relapsing Urothelial Carcinoma of the Bladder. [Google Scholar]
- 93.Comparing Alternative Regimens for Escalating Treatment of Intermediate and High-Risk Oropharyngeal Cancer. ISRCTN Registry; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prevention of Delirium in Intensive Care by Melatonin (DEMEL) ClinicalTrials.com; 2019. [Google Scholar]
- 95.A Study of GSK2256098 and Trametinib in Advanced Pancreatic Cancer. ClinicalTrials.com; 2016. [Google Scholar]
- 96.Capmatinib in Patients with Non-small Cell Lung Cancer Harboring cMET Exon14 Skipping Mutation. ClinicalTrials.com; 2018. [Google Scholar]
- 97.Banerjee S.N., Lewsley L.-A., Clamp A.R., Gabra H., Herbertson R., Green C., Orbegoso C., Wilson C., Banerji U., Hanif A., McNeish I.A., Paul J. OCTOPUS: a randomised, multi-centre phase II umbrella trial of weekly paclitaxel+/- novel agents in platinum-resistant ovarian cancer—vistusertib (AZD2014) J. Clin. Oncol. 2017;35(15_suppl) TPS5609-TPS5609. [Google Scholar]
- 98.Dodd L.E., Proschan M.A., Neuhaus J., Koopmeiners J.S., Neaton J., Beigel J.D., Barrett K., Lane H.C., Davey R.T., Jr. Design of a randomized controlled trial for ebola virus disease medical countermeasures: PREVAIL II, the ebola MCM study. J. Infect. Dis. 2016;213(12):1906–1913. doi: 10.1093/infdis/jiw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kok M., Voorwerk L., Horlings H., Sikorska K., van der Vijver K., Slagter M., Warren S., Ong S., Wiersma T., Russell N., Lalezari F., de Maaker M., Kemper I., Mandjes I.A., Chalabi M., Sonke G.S., Salgado R., Linn S.C., Schumacher T., Blank C.U. Adaptive phase II randomized trial of nivolumab after induction treatment in triple negative breast cancer (TONIC trial): final response data stage I and first translational data. J. Clin. Oncol. 2018;36(15_suppl) 1012-1012. [Google Scholar]
- 100.Systemic Therapy with or without Local Consolidative Therapy in Treating Participants with Oligometastatic Solid Tumor. ClinicalTrials.com; 2018. [Google Scholar]
- 101.Skamene T.S., Renouf L.L., Laskin D.J., Bedard J.J., Jones P.L., Ferrario S.J.M., Whitlock C., Petrie J., Sullivan J., Malone P., Nomikos E.R., Chen D., Dancey B.E. J., Canadian profiling and targeted agent utilization trial (CAPTUR/PM.1): a phase II basket precision medicine trial. J. Clin. Oncol. 2018;36(15 Supplement 1) [Google Scholar]
- 102.European Proof-Of-Concept Therapeutic Stratification Trial of Molecular Anomalies in Relapsed or Refractory Tumors (ESMART) ClinicalTrials.gov; 2016. [Google Scholar]
- 103.Domchek S.M.P.-V., Im S., Hee Park S.A., Delord Y., Italiano J.P., Alexandre A., You J., Bastian B., Krebs S., Waqar M.G., Lanasa S., Angell M., Tang H.K., Gresty M., Opincar C., Herbolsheimer L., Kaufman P., B. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): updated results in patients with germline BRCA-mutated (gBRCAm) metastatic breast cancer (MBC) Cancer Res. 2019;79(4 Supplement 1) [Google Scholar]
- 104.Park J.J., Thorlund K., Mills E.J. Critical concepts in adaptive clinical trials. Clin. Epidemiol. 2018;10:343–351. doi: 10.2147/CLEP.S156708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thorlund K., Haggstrom J., Park J.J., Mills E.J. Key design considerations for adaptive clinical trials: a primer for clinicians. BMJ. 2018;360:k698. doi: 10.1136/bmj.k698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bateman R.J., Benzinger T.L., Berry S., Clifford D.B., Duggan C., Fagan A.M., Fanning K., Farlow M.R., Hassenstab J., McDade E.M., Mills S., Paumier K., Quintana M., Salloway S.P., Santacruz A., Schneider L.S., Wang G., Xiong C. D.-T.P.C.f.t.D.I.A. Network, The DIAN-TU Next Generation Alzheimer's prevention trial: adaptive design and disease progression model, Alzheimer's & dementia. J.Alzheimer's.Assoc. 2017;13(1):8–19. doi: 10.1016/j.jalz.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Benedetti F., Anton J., Gattorno M., Lachmann H., Kone-Paut I., Ozen S., Frenkel J., Simon A., Zeft A., Ben-Chetrit E., Hoffman H.M., Joubert Y., Lheritier K., Speziale A., Guido J. Efficacy and safety of Canakinumab in patients with periodic fever syndromes (colchicine-resistant fmf, hids/mkd and traps): results from a phase 3, pivotal, umbrella trial. Pediatr. Rheumatol. 2017;15(Supplement 1) [Google Scholar]
- 108.De Benedetti F., Anton J., Gattorno M., Lachmann H., Kone-Paut I., Ozen S., Frenkel J., Simon A., Zeft A., Ben-Chetrit E., Hoffman H.M., Joubert Y., Lheritier K., Speziale A., Guido J., Xu X. Pharmacokinetics and pharmacodynamics of canakinumab in patients with periodic fever syndromes (colchicine-resistant FMF, HIDS/MKD and TRAPS): results from a phase III pivotal umbrella trial. Pediatr. Rheumatol. 2017;15(Supplement 1) [Google Scholar]
- 109.CADTH . 2018. Adaptive and Novel Trial Designs: an Overview of Key Methodologies and Issues in Critical Appraisa. [Google Scholar]
- 110.Zwierzyna M., Davies M., Hingorani A.D., Hunter J. Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005. BMJ. 2018;361:k2130. doi: 10.1136/bmj.k2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.